Case Report - Clinical Investigation (2018) Volume 8, Issue 1
Preferences and ease of use of preservative-free IOP-lowering eye drop containers: A comparison of two multi-dose bottles
- *Corresponding Author:
- Kai Kaarniranta
Department of Ophthalmology
University of Eastern Finland and Kuopio University Hospital, Kuopio, Finland
E-mail: kai.kaarniranta@kuh.fi
Submitted Date: 20 December 2017; Accepted Date: 03 January 2018; Published Date: 08 January 2018
Abstract
Key points
When prescribing intraocular pressure (IOP)-lowering medication for glaucoma, physicians’ considerations of a patient’s age, preferences and prior experience using different types of eye drop containers could potentially improve adherence, prevent glaucomatous progression and ultimately improve outcomes.
Keywords
Novelia® preservative-free multi-dose bottle; 3K®-System pump; usability; characteristics, convenience of use, patient preferences and adherence
Introduction
Glaucoma is the leading cause of permanent blindness worldwide [1]. Although disease progression can be delayed with intraocular pressure (IOP)-lowering agents [2], patient adherence still remains a barrier to effective treatment [3-5]. Patient adherence is multifactorial, with tolerability and convenience factors both playing an important role [2,6]. To address the tolerability issues associated with the use of preservatives, preservative-free (PF) IOP agents have been developed; however, this approach raises some safety concerns, as nonpreserved eye drops in multi-use bottles could potentially increase the risk of contamination and infection [7]. Of note, the risk may be higher in elderly patients owing to poor sight and limited dexterity. Understanding optimal methods of administering IOP-lowering drops is challenging, as there are multiple factors that can impact upon the patient’s ability to successfully use a drug delivery device.
To assess this issue, we conducted an open-label, single-centre study, in which we compared the usability characteristics, convenience of use and user preferences of two different preservative-free multi-dose (PFMD) eye drop containers: the Novelia® bottle (Nemera) and the 3K®-System pump (Ursatec), both containing dorzolamide (20 mg/ml) and timolol (5 mg/ml) eye drops solution (Figure 1). The study population consisted of patients with glaucoma or ocular hypertension. The Novelia® bottle features a multi-dose closing tip system that removes the need for preservatives and prevents bacterial contamination over the duration of treatment [8]. Specifically, the sterile solution flows through the one-way valve preventing any contamination ingress, while the PureFlow® technology allows air, but not bacteria, to enter back into the bottle. In addition, the Novelia® bottle has a blue tip that can be used as a focus point, allowing for improved precision when targeting the eye [9]. The 3K®-System pump is a ‘non-airless’ dosing pump that can be combined with glass and plastic containers to dispense PF formulations. The completely preassembled pump is snapped on the primary container after the aseptic filling process and fits hermetically with the container [10].
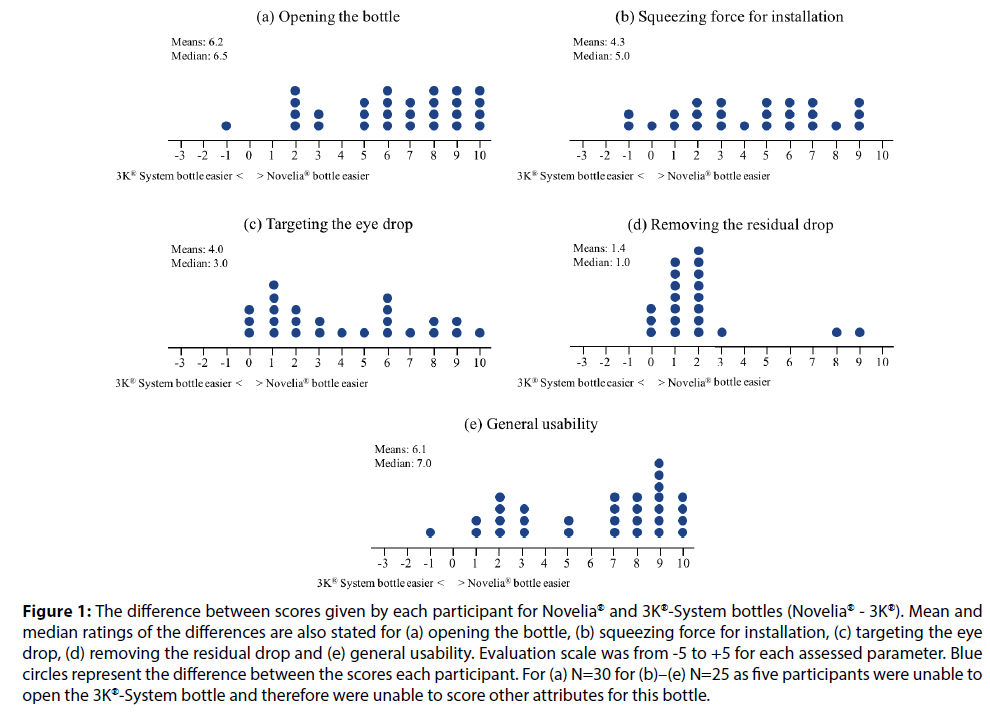
Figure 1:The difference between scores given by each participant for Novelia® and 3K®-System bottles (Novelia® - 3K®). Mean and median ratings of the differences are also stated for (a) opening the bottle, (b) squeezing force for installation, (c) targeting the eye drop, (d) removing the residual drop and (e) general usability. Evaluation scale was from -5 to +5 for each assessed parameter. Blue circles represent the difference between the scores each participant. For (a) N=30 for (b)–(e) N=25 as five participants were unable to open the 3K®-System bottle and therefore were unable to score other attributes for this bottle.
Methods
The study was conducted at the Department of Ophthalmology, Kuopio University Hospital, Finland in accordance with the good clinical practices and ethical principles of the Declaration of Helsinki. Eligible participants were 50 years of age or older with glaucoma or ocular hypertension and with a minimum experience of 6 months in the use of eye drops (unassisted). Users with active inflammation or infection in the eye(s) or eyelid(s) were excluded from this study. All participants tested both bottles. Each eligible participant was given a unique number, assigned chronologically from S01 to S30. A Latin square then defined the order of testing: participants with uneven numbers started with the Novelia® PFMD bottle and participants with even numbers started with the 3K®- System PFMD pump bottle.
The users were provided with safety glasses to prevent eye drops and bottle tip reaching their eyes. Prior to initiating administration, participants were asked to familiarise themselves with the dropping instructions of both container types and after opening each bottle discarded a few drops. Participants then instilled the eye drop(s) onto the protective glasses above each eye in turn at their convenience, and removed the residual drop from the tip of the bottle after the instillations. Usability of each eye drop container was evaluated using an 11-point grading system from -5 to +5, ranging from ‘extremely difficult’ to ‘extremely easy’. The following parameters were evaluated: opening of the container, squeezing force needed for drop administration, targeting the eye, drop control, removal of the residual drop and general usability of the container. The number of drops instilled was evaluated using a 5-point grading system from 0 (zero drops) to 4 (a spurt). In addition, the users were also asked about their preferences between the two eye drop containers. The results were analysed using standard statistical tests for paired data: paired t-tests, Wilcoxon signed-rank tests and sign tests for the primary analyses, and Wilcoxon rank sum tests for the subgroup analyses.
Results
A total of 30 participants were included in the study. The mean age of the participants was 71 years (range 52– 83 years), with two-thirds (19/30, 63%) being 70 years of age or older. The majority of the participants were female (23/30, 77%) and had prior experience of using both an eye drop bottle and pipette (16/30, 53%). Nine participants (9/30, 30%) had prior experience of using an eye drop bottle only and five participants (5/30, 17%) had prior experience of using unit-dose pipettes only.
The Novelia® bottle outperformed the 3K®-System pump in that it was significantly easier to open, squeeze, target, remove the residual drop and general usability (P<0.001 for all comparisons) (Figures 1a–1d). Five participants were not able to open the 3K®-System bottle and rated the 3K®-System as being extremely difficult to open (-5.0). Therefore, these participants could not instil the eye drops and evaluate the remaining usability characteristics. Additionally, the Novelia® bottle had superior eye drop control compared with the 3K®-System (P<0.001) (Table 1).
| Bottle | Novelia® bottle (N=30) | 3K®-Pump system (N=25*) | ||||||
|---|---|---|---|---|---|---|---|---|
| Eye | Left eye | Right eye | Left eye | Right eye | ||||
| Drops | N | % | N | % | N | % | N | % |
| 0 | 0 | 0.0% | 0 | 0.0% | 2 | 8.0% | 3 | 12.0% |
| 1 | 30† | 100.0% | 30† | 100.0% | 19† | 76.0% | 20† | 80.0% |
| 2 | 0 | 0.0% | 0 | 0.0% | 3 | 12.0% | 1 | 4.0% |
| ≥3 | 0 | 0.0% | 0 | 0.0% | 1 | 4.0% | 1 | 4.0% |
| A spurt | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
*Five participants were unable to open the bottle.
†Frequencies representing median scores.
Table 1: Number of eye drops instilled on the protective glasses
All the participants (30/30) in the study managed to instil one drop onto the protective glasses over both eyes using the Novelia® PFMD bottle, compared with only 72% of participants (18/25) using the 3K®-System PFMD pump (P=0.016). Of the remaining seven participants using the 3K®-System PFMD pump, 12% (3/25) instilled one drop above either eye or 16% (4/25) above neither.
Overall, the Novelia® PFMD bottle was rated as the eye drop container of choice by 96.7% of participants (29/30), compared with only one participant in favour of the 3K®-System PFMD pump (Figure 1e).
Discussion
Both the Novelia® bottle and the 3K®-System pump are PFMD eye drop containers; however, differences in the design of these eyedroppers may account for differences in the convenience of use and user preferences reported in this study. The Novelia® bottle has a blue tip that patients can use as a focus point, thus allowing for a better precision when targeting the eye. This feature may explain why 88% of participants in our study reported that targeting the eye drop was easier using the Novelia® PFMD bottle, with 12% of participants giving an equal rating to both eye drop containers and none giving the 3K®-System pump a better rating.
In our study, 88% of participants reported that it was easier to squeeze the Novelia® bottle compared with 3K®-System pump, one participant gave an equal rating to both eye drop containers and a further two participants gave the 3K®-System pump a better rating. A recent survey investigating patient preferences regarding ease of use of glaucoma bottles revealed that the most frequent patient suggestions related to the usability characteristics of the eye drop bottles, particularly improving ease and reliability of squeezing and dispensing a single drop [8]. Moore et al. investigated the force requirements to dispense a single drop from 21 commonly prescribed brand and generic glaucoma medications and correlated these findings with pinch strength in 53 consecutive patients with glaucoma. A statistically significant variability in the force required to squeeze a drop from common glaucoma medications was found, while the enrolled patients reported that the force requirements of several bottle designs were particularly challenging, thus urging the need for standardisation of topical glaucoma drug delivery and design [2]. Drew and Wolffsohn determined the force needed to extract a drop from 13 multi-dose or uni-dose latanoprost eye droppers and how this could be related to the comfortable and maximum pressure the participants could exert. One of the main findings was that the force required to expel a drop differed significantly between dropper designs ranging from 6.4 Newtons to 23.4 Newtons. In addition, the force needed to exert successive drops and storing droppers in the fridge further increased the force required. The authors concluded that prostaglandin monotherapy eye droppers for glaucoma treatment vary in the force required to extract a drop, which may affect treatment compliance and efficacy [11]. Taking patients’ age, preference and prior experience into consideration when prescribing IOP-lowering medication can minimise the occurrence of use errors and inaccurate dosing that can potentially compromise optimal treatment outcomes [12]. In our study, over 10% of participants using the 3K®-System pump were unsuccessful in instilling eye drops onto protective glasses above either eye. Had these patients been in a real-world setting, this barrier of use would have precluded any subsequent therapeutic benefit. This is of particular importance in the elderly patient population, where difficulties in properly instilling the eye drops-often a result of unsteady hands and inability to grip and squeeze the eye drop container-can negatively impact on adherence. It is important to note that even experienced patients with glaucoma often instil more than one drop. Hennessy and colleagues evaluated the ability of visually disabled experienced patients with glaucoma to successfully administer a single drop onto their eye. One of the main findings was that only 71% of patients were able to get any number of drops onto the ocular surface, even with multiple attempts. In addition, only 52% of patients were successful at instilling a single drop onto the ocular surface. Interestingly, this number further dropped to 39% when the definition of ‘success’ was modified to patients instilling just one drop onto the eye without touching the ocular surface [13].
A study limitation is the differing experience of study participants in using the containers prior to study entry, with just over one-half of patients having prior experience with using both types of containers. Our study was not designed to compare effectiveness of the Novelia® bottle and the 3K®-System pump; all the participants were wearing safety glasses to prevent eye drops and bottle tips reaching patients’ eyes and therefore, real-life usage is not reflected. Although this study showed the superiority of Novelia® compared with the 3K®-System PFMD container in the opening and squeezing of the bottle, targeting the eye, removal of the residual drop and general usability, care must be taken when translating the subjective assessment of these parameters to the overall adherence and treatment effectiveness.
Conclusion
This study showed that the Novelia® eye drop bottle had superior eye drop control compared with the 3K®-System pump. Novelia® out-rated the 3K®- System in being significantly easier to open, squeeze, target, usability and removal of residual drops from the tip. Overall, the Novelia® bottle was rated as the eye drop container of choice by 29 participants out of the 30, with only one participant favouring the 3K®-System pump. Given the ageing glaucoma population, the need for care and effective IOPlowering eye drop delivery devices continues to be of importance. Physicians should consider patients’ age, preference, prior experience and comorbidities when prescribing IOP-lowering medication to increase usability. This could potentially increase patient adherence, prevent disease progression and ultimately improve outcomes.
Acknowledgements
The study was funded by Santen Oy. Editorial support was provided by Virgo Health and funded by Santen. The authors would like to thank Dr Jouni Vuorinen (senior statistician, 4Pharma Ltd) for being part of the research team, and research nurse Helvi Käsnänen for assistance during this study.
Conflict of Interest
Kai Kaarniranta received honoraria from Santen Oy for lecturing fees. Auli Ropo is an employee of Santen Oy.
Contributions to the Manuscript
Hillevi Aalto and Auli Ropo designed and supervised this study, reviewed and edited the draft and approved the final version of this manuscript. Kai Kaarniranta conducted the study at his centre in Finland, provided feedback at all stages of development and approved the final version of this manuscript.
References
- EGS. Terminology and Guidelines for Glaucoma, 4th edition, 2014.
- Moore DB, Hammer JD, Akhtari R, Beck J, Sanders S, Kryscio RJ. Squeeze Me if You Can: Variability in Force Requirements to Extract a Drop from Common Glaucoma Bottles. J Glaucoma 25: 780–784 (2016).
- Hommer A. Managing Primary Open-angle Glaucoma – Ocular Tolerability, Compliance, Persistence and Patient Outcomes. Eur Ophthal Rev 3: 19–22 (2009).
- Robin A, Grove DS. Compliance and Adherence in Glaucoma Management. Indian J Ophthalmol 59: 93–96 (2011).
- Skalicky SE, Goldberg I. Adherence and Persistence: The Challenges for Glaucoma Medical Therapy. Asia-Pac J Ophthalmol 2: 356–361 (2013).
- Morse AR. Improving Medication Adherence to Reduce Vision Loss in Patients with Glaucoma: Low Hanging Fruit? Ophthalmology 122:1280–1282 (2015).
- Bagnis A, Papadia M, Scotto R, Traverso CE. Antiglaucoma Drugs: The Role of Preservative-free Formulations. Saudi J Ophthalmol 25: 389–394 (2011).
- Nemera Novelia® flyer, 2015. Available from: http://www.nemera.net/wp-content/uploads/2015/06/Flyer-NOVELIA_April20151.pdf [Accessed December 2017].
- URSATEC 3K®-System Product Profile. Available from: http://www.wirth.se/products/Produktprofil%203K-System.pdf [Accessed December 2017].
- Marando CM, Seibold LK, SooHoo JR, Pantcheva MB, Ramulu PY, Kahook MY. The Utility of Cap Color and Bottle Characteristics for Topical Glaucoma Therapy. Ophthalmology 122: 2577–2578 (2015).
- Drew T, Wolffsohn JS. Usability of Prostaglandin Monotherapy Eye Droppers. Br J Ophthalmol 99: 1251–1254 (2015).
- Med Device Online. Keeping the Patient at the Centre of Drug Delivery Devices [online] January 2016.
- Hennessy AL, Katz J, Covert D, Protzko C, Robin AL. Videotaped Evaluation of Eye Drop Instillation in Glaucoma Patients with Visual Impairment or Moderate to Severe Visual Field Loss. Ophthalmology 117: 2345–2352 (2010).