Review Article - Interventional Cardiology (2021) Volume 13, Issue 3
Prenatal detected Isolated Congenital Coronary Artery Fistula (ICCAF) characteristics and impact on the fetal hemodynamic situation: Systematic literature review
- Corresponding Author:
- Ulrich Gembruch
Department of Obstetrics and Prenatal Medicine,
University Hospital Bonn,
Venusberg-Campus 1, 53127 Bonn,
Germany,
E-mail: ulrich.gembruch@ukbonn.de
Received date: April 12, 2021; Accepted date: April 26, 2021; Published date: May 03, 2021
Abstract
Background: Detection of an Isolated Congenital Coronary Artery Fistula (ICCAF) is rarely seen prenatally. We aimed to describe prenatal characteristic and try identify possible prognostic parameters that could guide prenatal assessment and improve counseling.
Methods and Findings: A systematic literature review was performed. PubMed database was reviewed for publications using the terms “isolated (congenital) coronary artery fistula, coronary-cameral fistula”. Papers with full description of the parental fistula characteristics were included for evaluation. 22 publications fulfilled search criteria describing a total of 25 pregnancies. Mean gestational age at diagnosis was 25.4 weeks of gestation (range: 19 and 38). ICCAF predominantly drained into the right side of the heart (81.5%) and in most cases into the right atrium in 52.4% (11/21). Prenatal sonographic findings included: Enlargement of the originated coronary artery in all cases (100%), enlargement of the draining structure in 8 (32%), cardiomegaly in 5 (20%), turbulent jet in 6 (24%), to-and-fro flow in the ascending aorta in 6 (24%) and a hydrops fetalis in 2 (8%), of the reviewed subjects. Prenatal findings of high volume shunting through the fistula, hydrops fetalis or aneurysm were associated with a more serious negative impact on fetal outcome.
Conclusion: Prenatal diagnosis of an ICCAF requires an examination of the affected fetal hemodynamic situation by the fistula. Significant derangements in cardiac function, may have relevant postpartale consequences. Evaluation of the coronary artery circulation and possible prognostic parameters as the presence of a cardiomegaly, isolated cardiac chamber enlargement, to-and-fro flow in the aortic arch, dilatation of one of the great vessels, the size, tortuosity, length of the fistula and hydrops fetalis should be examined and targeted management be performed.
Keywords
Congenital heart diseases • Coronary artery • Fetal heart • Prenatal detection • Prenatal heart failure • Prenatal high cardiac output
Introduction
Isolated Congenital Coronary Artery Fistula (ICCAF) is rare in prenatal diagnosis [1]. With an incidence of 1: 50.000 live births, it describes one of the most common congenital anomalies of the coronary arterial circulation that counts for 0.2% to 0.4% of all congenital heart diseases [2].
ICCAF is defined as an abnormal communication between the coronary artery, bypassing the myocardial capillary bed and terminating either to one of the four cardiac chambers or any of the great vessels [3]. Depending on the structures connected by the fistula, a hemodynamic overload may occur already during fetal period [1,4].
Affected fetuses might be at risk to develop congestive heart failure or myocardial ischemia that that may deteriorate already prenatally or shortly after birth and require an immediate intervention. Prenatal detection of an ICCAF may therefore have a potential impact on outcome, counseling and perinatal management, especially as further serious complications like prenatally, cardiac arrhythmia or hydrops fetalis and postnatal complications such as, thromboembolic events, aneurysm, pulmonary hypertension or further cardiac arrhythmias may occur [5].
Increasing sophistication of ultrasound technology allows prenatal detection of such malformations [6,7]. Targeted management may be realized, as knowledge of the malformation and its intrauterine course enables categorization of follow-up, intrauterine counselling and planning multidisciplinary therapy by pediatric cardiologists and obstetricians.
However, ultrasound examination of the coronary arteries is not standard clinical practice and prenatal diagnosis of ICCAF in an otherwise structurally unremarkable heart uncommon [8]. Diagnostic hints that could indicate a detailed prenatal examination of the coronary arteries and evaluate possible prognostic parameters for a more accurate prenatal counseling are still missing.
For this, we performed a systematic literature review and compared previous published data. We aimed to summarize prenatal characteristics and fetal hemodynamic implications in fetuses with prenatally detected ICCAF. We also try to evaluate possible prognostic parameters in order to guide a structural prenatal examination.
Literature Review
Methods
A systematic review of the literature reported between 1996 and 2021 was performed. PubMed database was reviewed for publications relevant to the following keywords: isolated (congenital) coronary artery fistula, coronary-cameral fistula, fetal, prenatal or prenatally detected. References list of selected publications were examined to identify further studies that might be suitable for inclusion. Cases that had been reported previously, were excluded, as well as cases with abnormal karyotype combined with multiple (>2) malformations (n=2) or cases showing the presence of concurrent major congenital cardiac malformation (n=15). Reports were included if diagnosis of ICCAF was made prenatally and a full and detailed description of the prenatal and postnatal course was available.
Prenatal characteristics and fetal hemodynamic implications in fetuses with prenatally diagnosed ICCAF were evaluated by background, prenatal and postnatal data. The following items were considered for assessment: Maternal age, referral reason, gestational age at diagnosis, fistula characteristics (main echocardiographic features, origin, drainage, fistula size and Doppler velocity), additional findings, gestational age at birth, mode of delivery, survival at birth, intervention age, type and clinical outcome.
Statistical analysis was performed using SPSS (v23.0, IBM, Armonk, NY, US). Outcomes were quantified as means with Standard Deviation (SD) for continuous variables, median with range, and percentages for categorical variables. Pearson correlation coefficient was calculated for the relationship between sonographic findings and time of intervention and prenatal outcome. A p value below 0.05 was considered significant.
Results
Literature review revealed a total of 39 hits. Only 22 publication were eligible for data analysis, including 21 case reports and one case series. Selected studies described a total of 25 pregnancies. Mean maternal age was 30.3 years (range: 23 and 43). Mean gestational age at diagnosis was 25.4 weeks of gestation (range: 19 and 38). Reasons for referral were suspected nonspecific Congenital Heart Disease (CHD) in 80.0% (16/20), including atrial septal defects (ASD) (n=1), or unclear tricuspid regurgitation (n=1). In 10.0% (2/20) a Hypoplastic Left Heart syndrome (HLH) was presumed followed by an anomalous pulmonary venous connection, intracardial suspected tumor and a family history of CHD each in one case.
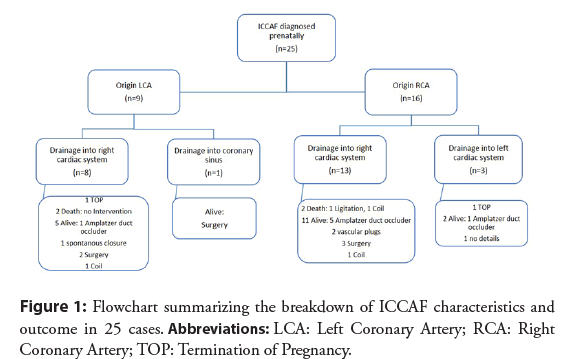
Included cases showed only unilateral fistulas. Distribution of the fistula formation and postnatal outcome and treatment are demonstrated in Figure 1. Published cases predominantly drained into the right side of the heart (81.5%): Into the right atrium in 52.4% (11/21) and the right ventricle in 47.6% (10/21). In only 3 cases fistulae terminated into the left heart side (in all cases into the left ventricle).
Different prenatal echocardiographic findings are summarized in Table 1. In all cases, at least two sonographic abnormalities were present. Both fetuses with prenatally diagnosed hydrops fetalis, and the two with prenatally diagnosed ventricular aneurysm died in the postnatal course. No correlation was observed between the presence of cardiomegaly or a to-and-fro flow in the ascending aorta and time of intervention or prenatal outcome.
| Prenatal echocardiographic findings | References | n=25 (%) |
|---|---|---|
| Enlargement of the originated coronary artery | All cases | 25 (100) |
| Enlargement of the draining structure | 27-34 | 8 (32.0) |
| Cardiomegaly | 5,7,16,27,29 | 5 (20) |
| Hydrops fetalis | 31,33 | 2 (8) |
| Aneurysm | 26,34 | 2 (8) |
| Turbulent jet in the fistula or into the cardiac chamber/great arteries | 7,8,17,26,30,34 | 6 (24.0) |
| to-and-fro flow in the ascending aorta | 16,18,20,27,30,32 | 6 (24.0) |
Table 1: Prenatal echocardiographic findings with corresponding references.
Obstetric data and postnatal echocardiographic findings are outlined in Table 2.
| Parameter | Value |
|---|---|
| GA at delivery (weeks) | 37.9 (32-40) |
| Mode of delivery | |
| Vaginal (%) | 9 (47.4) |
| Caesarian delivery (%) | 10 (52.6) |
| Postnatal anomalous findings | |
| ASD (%) | 3 (14.2) |
| VSD (%) | 3 (14.2) |
| PDA (%) | 6 (28.6) |
| CoA (%) | 3 (14.2) |
| Pulmonary hypertension (%) | 3 (14.2) |
| Cardiac arrhythmia (%) | 3 (14.2) |
Abbreviations: GA: Gestational Age; ASD: Atrial Septal Defect; VSD: Ventricular Septal Defect; PDA: Patent Ductus Arteriosus; CoA: Coarcation of the Aorta.
Table 2: Obstetric data and postnatal echocardiographic findings.
Presence of an ICCAF can cause significant derangements in cardiac function and coronary blood flow already during fetal period. Prenatal detection should be mandatory as it might change management and outcome in affected fetuses (Table 3).
| Author/year/ Reference | Origin of ICCAF (diameter, mm) | Diameter of fistula (mm) | Drainage | Blood flow velocity in the fistula (m/s) | Cardiomegaly (CTR) | To-and-fro Flow in the aortic arch | CHF | Hydrops |
|---|---|---|---|---|---|---|---|---|
| Zeng (2016) [8] | LCA (2.2) | - | RA | 1.08 | +(0.4) | - | - | |
| RCA (4.0) | - | LV | 1.36 | - | - | - | ||
| Sharland (1996) [31] | RCA | 3 | RV | 1.8 | + | + | + | + |
| Sharland (2016) [20] | RCA | 1.5 | RA | 0.48 | + | |||
| RCA | 2 | RA | 0.6 | + | ||||
| RCA | 1.8 | LV | 0.45 | |||||
| LCA | 1 | RA | 0.5 | |||||
| RCA | 0.8 | RA | 0.5 | + | ||||
| Cotton (2000) [21] | LCA | RV | - | - | - | |||
| Mielke (2002) [5] | LCX (2) | RA | 2.3 | -(0.55) | - | - | ||
| Khan (2003) [29] | RCA | 4 | RA | +(0.65) | + | - | ||
| Hung (2006) [35] | RCA | 3.8 | RA | + | + | - | ||
| Karagoz (2008) [36] | RCA | 6 | RV | + | + | - | ||
| Hayashi (2012) [16] | RCA (5) | 4 | RV | 3.1 | +(0.4) | + | - | - |
| Tekesin (2018) [7] | LCA (2.1) | 1.4 | RA | 3.1 | +(0.52) | - | - | |
| Chae (2018) [32] | LCA | CS | - | + | - | - | ||
| Oztunce (2013) [33] | LCA | RV | 1.1 | + | + | + | ||
| Zhao (2019) [22] | RCA (2.6) | 3 | LV | 2.1 | -(0.29) | - | - | |
| Wacker-G.(2018) [26] | LCA (2.9) | 2.9 | RV | + | + | |||
| Daniel (2010) [28] | RCA | 6 | RV | + | + | - | ||
| Walter (2021) [27] | RCA (10.4) | 9 | RA | 3.7 | +(0.69) | + | + | - |
| Zhao (2012) [30] | RCA (9) | 5 | RV | 3.6 | ||||
| Li (2020) [17] | RCA (2.3) | 1.6 | RV | |||||
| Cui (2020) [34] | RCA (4) | RA | ||||||
| Matsumoto (2012) [19] | LCA (8) | 12 | RV |
Abbreviations: CS: Coronary Sinus; LCA: Left Coronary Artery; RCA: Right Coronary Artery; CTR: Cardio-Thoracic-Ratio; CHF: Congestive Heart Failure.
Table 3: Review of Literature of prenatal findings of fetuses affected by an ICCAF.
Etiology: Focusing on an isolated congenital Coronary Artery Fistula (CAF), presumed etiology involves the persistence of sinusoidal connections between the lumens of the primitive tubular heart that supply myocardial blood flow in the early embryologic period [6,9]. Defining a CAF to be isolated implies the fistula to be the only cardiac malformation or associated with abnormalities like Patent Ductus Arteriosus (PDA) or Atrial Septal Defect (ASD) [3].
Otherwise, CAF’s may be complicated by major congenital cardiac malformations, as seems in outflow tract obstructions, such as pulmonary valve atresia with intact ventricular septum or Hypoplastic Left Heart (HLH) [10-12]. Cardiac malformation appears to be responsible for the main hemodynamic impediment and the fistula results consequently. These ventriculo-coronary communications may provide a conduit to release intraventricular pressures and therefore differ embryological from ICCAF’s. Hence, ventriculo-coronary communications should be distinguished from an ICCAF [6].
Anatomy: Postnatal in most cases CAF involves a single coronary artery and less often multiple branches. As indicated by postnatal studies, fistula originates from the RCA in 56%, from the LCA in 36% and from both CA’s in only 5% [1,13]. Right side of the heart is the predominant drainage side (92%) into the right ventricle in 39%-41%, into the right atrium in 26%-33%, followed by pulmonary artery in 17%, coronary sinus in 7%, left atrium in 5%, left ventricle in 3% and superior vena cava in 1% 1,14. Our literature search retrieved prenatal detected ICCAF opening into the coronary sinus in only 3.8% and no opening into the LA or to the pulmonary artery. Possible reasons for disparities may be the difficulty in detection of an ICCAF when terminating to the LA, due to simultaneous confluence of the pulmonary veins and the possible small shunting volume of the fistula when terminating to the pulmonary arteries, as vascular resistance is prenatally high. Additional ICCAF may be mistaken for typical differential diagnosis or poor adjustment or the coronary arteries as evaluation of coronary arteries requires examiner expertise and knowledge on optimal diagnostic modalities.
Diagnostic modalities: Examination of coronary arteries can be primary achieved by color flow imaging and pulsed waved Doppler, although it remains difficult due to the small vessel size and movement of the fetal heart [6]. Mainstem right and left coronary arteries are ideally examined in a long-axis view of the left ventricular outflow tract and ascending aorta or a modified short-axis view of the aortic root [6,14,15]. Typically, the affected coronary artery presents dilated or tortuous, which might be the first prenatal diagnostic sign of an ICCAF, according to the current review. Color Doppler should be used to verify the presence of high velocity and turbulent flow 6. In addition it facilitate monitoring of a possible progress [16]. Pulsed waved Doppler can assist to characterize blood flow throughout the cardiac cycle and determine the draining structure. For Example, continuous flow is seen when an ICCAF drains to the right atrium and bidirectional flow when it terminates into the right ventricle [15]. Modern ultrasound technologies as 4D color Doppler ultrasound High- Definition flow (HD-flow) or Spatiotemporal Image Correlation (STIC) may further improve the visualization, as reported by Li et al. [17].
Prenatal sonographic findings: Fetal echocardiography typically reveals normal four chamber view and myocardial motion. Visualization of the coronary artery characteristically shows dilatation of the originating, possible tortuous CA as well as a dilatation of the draining structure [18,19]. Further clinical presentation may include a turbulent jet lasting the whole cardiac cycle, to-and-fro flow of the aortic arch and congestive heart failure leading to possible a hydrops fetalis [20].
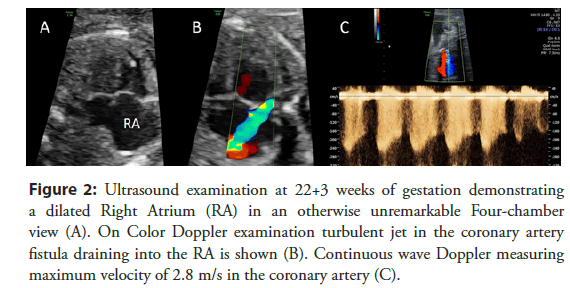
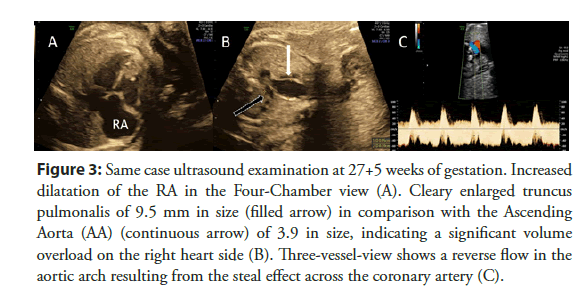
Therefore, the presence of a coronary fistula should be considered, especially in case of an unclear isolated dilatation of one of the four cardiac chambers, an unclear, turbulent flow or jet by Color Doppler examination or an unexplained cardiomegaly (Figures 2 and 3).
Figure 2: Ultrasound examination at 22+3 weeks of gestation demonstrating a dilated Right Atrium (RA) in an otherwise unremarkable Four-chamber view (A). On Color Doppler examination turbulent jet in the coronary artery fistula draining into the RA is shown (B). Continuous wave Doppler measuring maximum velocity of 2.8 m/s in the coronary artery (C).
Figure 3: Same case ultrasound examination at 27+5 weeks of gestation. Increased dilatation of the RA in the Four-Chamber view (A). Cleary enlarged truncus pulmonalis of 9.5 mm in size (filled arrow) in comparison with the Ascending Aorta (AA) (continuous arrow) of 3.9 in size, indicating a significant volume overload on the right heart side (B). Three-vessel-view shows a reverse flow in the aortic arch resulting from the steal effect across the coronary artery (C).
Differential diagnosis: Nevertheless, an ICCAF might be mistaken for Ventricular Septal Defects (VSD), as dilated coronary sinus, or as valve regurgitation [21,22]. In such cases using color and pulse Doppler can help in the more detailed assessment. In case of suspected VSD, abnormal communication between the both ventricles could be identified [23]. In case of dilated coronary sinus low-velocity venous blood flow would suggest where ICCAF typically represents high-velocity flow [5,24]. Referring to this review, severe Tricuspid Regurgitation (TR) was the most commonly suspected differential diagnosis (XX%). Only precise consideration of the cardiac cycle allowed to confirm diagnosis, as TR is seen in systole whereas ICCAF occurs during diastole [25].
Hemodynamic implications and prognostic parameters: The physiological derangement depends primarily on the resistance of the fistula connection and the location of the fistula termination [18,26]. As resistance is determined by its size, tortuosity and length, evaluation of these parameters may be possibly used as prenatal prognostic parameters, by correlation to the shunted volume [20]. Sharland et al. was able to indicate, that fetuses with short distance between the main coronary artery and the terminating structure appear to develop congestive heart failure earlier after birth, in some cases even antenatally, compared to those with long distance and a more tortuous course of the fistula [20].
Further, assessment of the terminating structure is crucial, as drainage into a low-pressure system can result in a large left-to-right shunt, which is particularly seen in artery-atrial communications [8,27]. These fetuses are especially at risk of heart failure progressing soon after birth, as peripheral vascular resistance, consequently cardiac workload and left-to-right shunting through the fistula augments. Further the distribution of the coronary arterial blood flow is changed, as the fistula competes with the normal coronary circulation. Coronary artery blood flow preferentially enters the fistula, since normal coronary artery circulation has a greater resistance to flow [6]. Leading to this “coronary steal” phenomenon, fetuses are at risk of myocardial ischemia, as postnatal coronary perfusion competes with system perfusion, especially if a PDA is found [27]. Intervention might than be required already during neonatal period although in most cases later intervention is sufficient [8]. Referring to all published cases, consequently an intervention was required shortly after birth, if duct occlusion has failed [27,28].
Further, the presence of a to-and-fro flow of the aortic arch may also results from the steal effect across the coronary fistula. This sonographic finding should similarly be associated with a huge blood volume flow in the fistula, leading to the risk of high cardiac output and to a possible postnatal risk of developing a Coarcation of the Aorta (CoA) due to a prenatally reduced flow through the aortic arch, as seen in previous published cases [20,27,29].
In summarize, communication of the fistula to the left heart of side, can cause an effective left-to-left shunt volume, overloading the left heart only, whereas by connection to the right side, the left-to-right shunt increases primary flow to the pulmonary circulation and ultimately to the left heart. As specific result of these fistulas with an increases flow to the pulmonary circulation, fetuses might be of risk to develop pulmonary hypertension in the postnatal period [16,30-36]. Nevertheless as in both cases fistulas consequently lead to a volume overload, heart failure with consequent hydrops fetalis may occur.
Discussion and Conclusion
Prenatal detection of an ICCAF should be attempted as it might change management and outcome in affected patients. Although there is still a lack of systematic evidence based medical basis for a structural prenatal examination and prognosis evaluation, we were able to summarize the needed diagnostic modalities and further mentioned possible prognostic parameters. In a structural prenatal examination determining connected structures of the fistula should be of major importance as they may lead to fetal short and long-term health defects. Possible prognostic parameters as the presence of a cardiomegaly, cardiac chamber enlargement, and a to-and-fro flow in the aortic arch, the size, tortuosity and length of the fistula should be examined. Needed follow-up examination might therefore not be overlooked and delivery optimal timed. Although later interventional treatment of the fistula is sufficient, as most cases ICCAF’s typically represents asymptomatic, we think delivery should be performed in a perinatal center with a pediatric cardiology unit. If hydrops fetalis or a centralization of fetal blood flow and signs of a beginning heart failure caused by ICCAF is prenatally observed, delivery, even preterm should be inducted. Further intervention may be required shortly after birth, if duct occlusion has failed.
Conflict of Interest
There are no conflicts of interest to be declared.
Funding
None
Disclosure
None
References
- Schumacher G, Roithmaier A, Lorenz HP, et al. Congenital coronary artery fistula in infancy and childhood: diagnostic and therapeutic aspects. Thorac Cardiovasc Surg. 45(6): 287-294 (1997).
- Kardos A, Babai L, Rudas L, et al. Epidemiology of congenital coronary artery anomalies: A coronary arteriography study on a central European population. Cathet Cardiovasc Diagn. 42(3): 270-275 (1997).
- Fernandes ED, Kadivar H, Hallman GL, et al. Congenital malformations of the coronary arteries: The Texas Heart Institute experience. Ann Thorac Surg. 54(4): 732-740 (1992).
- Cetiner N, Altunyuva Usta S, Akalın F. Coronary arteriovenous fistula causing hydrops fetalis. Case Rep Obstet Gynecol. 2014: 487281 (2014).
- Mielke G, Sieverding L, Borth-Bruns T, et al. Prenatal diagnosis and perinatal management of left coronary artery to right atrium fistula. Ultrasound Obstet Gynecol. 19(6): 612-615 (2002).
- Baschat AA, Gembruch U. Evaluation of the fetal coronary circulation. Ultrasound Obstet Gynecol. 20(4): 405-412 (2002).
- Tekesin I, Uhlemann F. Prenatal diagnosis of coronary artery fistula using 2D and 3D/4D ultrasound. Ultrasound Obstet Gynecol. 51(2): 274-275 (2018).
- Zeng S, Zhou Q, Tian L, et al. Isolated coronary artery fistula in fetal heart: Case reports and literature review. Fetal Pediatr Pathol. 35(5): 348-352 (2016).
- Dimitrakakis G, Von Oppell U, Luckraz H, et al. Surgical repair of triple coronary-pulmonary artery fistulae with associated atrial septal defect and aortic valve regurgitation. Interact Cardiovasc Thorac Surg. 7(5): 933-934 (2008).
- Axt-Fliedner R, Tenzer A, Kawecki A, et al. Prenatal assessment of ventriculocoronary connections and ventricular endocardial fibroelastosis in hypoplastic left heart. Ultraschall Med. 35(4):357-363 (2014).
- Chaoui R, Tennstedt C, Göldner B, et al. Prenatal diagnosis of ventriculo-coronary communications in a second-trimester fetus using transvaginal and transabdominal color Doppler sonography. Ultrasound Obstet Gynecol. 9(3): 194-197 (1997).
- Baschat AA, Love JC, Stewart PA, et al. Prenatal diagnosis of ventriculocoronary fistula. Ultrasound Obstet Gynecol. 18(1): 39-43 (2001).
- Lowe JE, Sabiston DC. Surgical correction of congenital malformations of the coronary circulation. South Med J. 75(12): 1508-1516 (1982).
- Sunder KR, Balakrishnan KG, Tharakan JA, et al. Coronary artery fistula in children and adults: A review of 25 cases with long-term observations. Int J Cardiol. 58(1): 47-53 (1997).
- Gembruch U, Baschat AA. Demonstration of fetal coronary blood flow by color-coded and pulsed wave Doppler sonography: A possible indicator of severe compromise and impending demise in intrauterine growth retardation. Ultrasound Obstet Gynecol. 7(1): 10-16 (1996).
- Hayashi G, Inamura N, Kayatani F, et al. Prenatal diagnosis of a left coronary artery to left atrial fistula. Prenat Diagn. 32(2): 194-196 (2012).
- Li T-G, Ma B, Nie F, et al. An unusual case of prenatal diagnosis of right coronary artery to right ventricle fistula with HD-flow render mode and spatiotemporal image correlation (STIC). Echocardiography. 37(7): 1105-1108 (2020).
- Nagiub M, Mahadin D, Gowda S, et al. Prenatal diagnosis of coronary artery fistula: A case report and review of literature. AJP Rep. 4(2): e83-86 (2014).
- Matsumoto Y, Hoashi T, Kagisaki K, et al. Successful surgical treatment of a gigantic congenital coronary artery fistula immediately after birth. Interact Cardiovasc Thorac Surg. 15(3): 520-522 (2012).
- Sharland GK, Konta L, Qureshi SA. Prenatal diagnosis of isolated coronary artery fistulas: Progression and outcome in five cases. Cardiol Young. 26(5): 915-920 (2016).
- Cotton JL. Diagnosis of a left coronary artery to right ventricular fistula with progression to spontaneous closure. J Am Soc Echocardiogr. 13(3): 225-228 (2000).
- Zhao L, Wang Y, Wang M, et al. Prenatal diagnosis of fetal isolated right coronary artery to left ventricle fistula. Echocardiography. 36(5): 1009-1013 (2019).
- Axt-Fliedner R, Schwarze A, Smrcek J, et al. Isolated ventricular septal defects detected by color Doppler imaging: Evolution during fetal and first year of postnatal life. Ultrasound Obstet Gynecol. 27(3): 266-273 (2006).
- Karl K, Kainer F, Knabl J, et al. Prenatal diagnosis of total anomalous pulmonary venous connection into the coronary sinus. Ultrasound Obstet Gynecol. 38(6):729-731 (2011).
- Gembruch U, Smrcek JM. The prevalence and clinical significance of tricuspid valve regurgitation in normally grown fetuses and those with intrauterine growth retardation. Ultrasound Obstet Gynecol. 9(6): 374-382 (1997).
- Wacker-Gussmann A, Esser T, Lobmaier SM, et al. Prenatally diagnosed isolated coronary arterial fistula leading to severe complications at birth. Case Rep Cardiol. 2018: 2509502 (2018).
- Walter A, Calite E, Engels AC, et al. Prenatal detection of a giant isolated coronary fistula-Impact on the fetal haemodynamic situation. Clinical Case Reports. 9(3): 15 (2021).
- Daniel M, Mavroudis C, Preminger T, et al. Prenatal diagnosis and neonatal surgical management of a giant proximal right coronary artery to right ventricular fistula. World J Pediatr Congenit Heart Surg. 1(2): 243-248 (2010).
- Khan MD, Qureshi SA, Rosenthal E, et al. Neonatal transcatheter occlusion of a large coronary artery fistula with Amplatzer duct occluder. Catheter Cardiovasc Interv. 60(2): 282-286 (2003).
- Zhao X, Yang Y, Li R. A large hemodynamically significant right coronary artery fistula to right ventricle: Prenatal detection and progression. Echocardiography. 29(7): E173-175 (2012).
- Sharland GK, Tynan M, Qureshi SA. Prenatal detection and progression of right coronary artery to right ventricle fistula. Heart. 76(1): 79-81 (1996).
- Chae U, Lee M-Y, Kim H, et al. Prenatal diagnosis of isolated coronary arteriovenous fistula. Obstet Gynecol Sci. 61(1): 161-164 (2018).
- Oztunc F, Gokalp S, Yuksel MA, et al. Prenatal diagnosis of left coronary artery to right ventricle fistula. J Clin Ultrasound. 43(2): 129-131 (2015).
- Cui C, Liang W, Fan T, et al. Prenatal diagnosis of a right coronary artery to right atrial fistula with a giant coronary artery aneurysm: A case report. J Clin Ultrasound. 48(8): 489-492 (2020).
- Hung J-H, Lu J-H, Hung J, et al. Prenatal diagnosis of a right coronary-cameral fistula. J Ultrasound Med. 25(8): 1075-1078 (2006).
- Karagöz T, Ozkutlu S, Celiker A. Percutaneous closure of a prenatally diagnosed large coronary artery fistula with an Amplatzer vascular plug immediately after delivery. Acta Cardiol. 63(3): 405-408 (2008).