Review Article - Imaging in Medicine (2012) Volume 4, Issue 1
Preoperative functional MRI of motor and sensory cortices: how imaging can save vital functions
Christina Stathopoulos, Nicole Brennan, Kyung K Peck & Andrei I Holodny*Department of Radiology, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA
- Corresponding Author:
- Andrei I Holodny
Department of Radiology
Memorial Sloan-Kettering Cancer Center
1275 York Avenue, New York, NY 10065, USA
Tel: +1 212 639 3182
Fax: +1 917 432 2345
E-mail: holodnya@mskcc.org
Abstract
Blood oxygen level-dependent functional MRI (fMRI) has been used to enhance the understanding of neuroanatomy and functions of the brain and is becoming an accepted brain-mapping tool for clinicians, researchers and basic scientists alike. fMRI has an ever-growing list of clinical applications, including presurgical mapping of motor, language and memory functions, and has no known risks as a noninvasive procedure. fMRI data gives the neurosurgeon the opportunity to make more informed decisions regarding the approach to the tumor and the necessity for invasive mapping procedures. This article reviews the applicability of fMRI to clinical neurosurgical practice, describes the optimization of paradigm design and delivery for motor and sensory mapping and illustrates artifacts and other clinically relevant pitfalls of fMRI.
Keywords
brain tumors ▪ functional MRI ▪ intraoperative mapping ▪ motor and sensory ▪ paradigm
Clinical importance
The ability to visualize brain activity and to determine which parts of the brain are responsible for which cognitive processes has revolutionized the neurosciences [1–3]. The use of blood oxygen leveldependent (BOLD), functional MRI (fMRI) in the clinical setting benefits patients insomuch as it allows neurosurgeons to be aware of and to navigate the precise location of patient-specific eloquent cortices and any structural anomalies that may have developed from a tumor [4–6]. This anatomic/functional preview facilitates the creation of more effective patient-specific treatment plans [7]. Before the use of fMRI, the preoperative location of eloquent cortices and their relationship to the lesion was determined based on the historical localization of function and performing intraoperative mapping using direct cortical stimulation, which unfortunately has several drawbacks. First, this procedure requires a craniotomy. Should intraoperative mapping reveal a relationship of the eloquent cortex to the lesion than is different from what was assumed using historical data, an adjustment of the operative plan and possible expected complications may ensue. Furthermore, patients sometimes have difficulty cooperating with task performance during cortical stimulation, especially when attempting to map higher functions, such as language. As a result, operations can be terminated early because of inadequate intraoperative mapping results that might have been foreseen and avoided with a reliable preoperative mapping technique such as fMRI. A lack of preoperative functional information may also cause a lesion to be unnecessarily deemed inoperable [8]. In addition, mapping is restricted to the exposed surface of the brain and as a result the cortex in the deep sulci cannot be mapped. Lastly, invasive mapping procedures, such as cortical stimulation, do not aid the neurosurgeon in presurgical risk assessment and patient counseling [7].
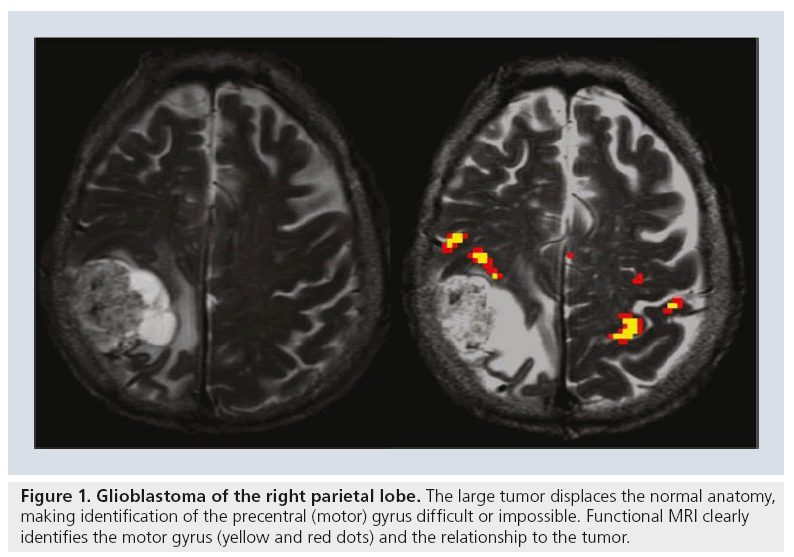
Preoperative fMRI, however, by defining eloquent motor, language and/or memory areas, allows a surgeon to optimize perioperative planning to maximize tumor resection [4–6] while minimizing damage to surrounding areas. Planning a surgical resection relies heavily on the anatomic location of a tumor relative to the eloquent cortices. However, the locations of the functional cortices vary with individuals and may be affected by the lesion itself and/or associated cortical reorganization and thus may not match the predicted anatomic locations [8,9]. fMRI allows for the clarification of anatomy in specific patients and detection of any irregularities. These details may become even more important when the eloquent cortices are infiltrated by a tumor (Figure 1) [8,9].
Figure 1: Glioblastoma of the right parietal lobe. The large tumor displaces the normal anatomy, making identification of the precentral (motor) gyrus difficult or impossible. Functional MRI clearly identifies the motor gyrus (yellow and red dots) and the relationship to the tumor.
In addition, fMRI data can help a surgeon decide either for or against resection by providing information about tumors in high-risk locations (e.g., involving Broca’s area, Wernicke’s area) without invasive mapping, sparing the patient awake anesthesia and associated potential complications. If a surgeon decides to perform surgery, fMRI may help the surgeon to choose the best trajectory for resection, such as taking a more posterior approach to bypass an important functional area. Other benefits of using fMRI before surgery include gathering data to guide intraoperative direct cortical stimulation or other mapping procedures, and decreasing the operation time. fMRI can also aid the neurosurgeon in deciding whether or not to perform ‘awake mapping’ in patients due to undergo tumor resection. Awake mapping is used to identify essential areas of higher cortical function (e.g., language function) adjacent to a tumor to be resected by the use of direct cortical stimulation. This procedure is complicated and time consuming and involves bringing the patient out of deep anesthesia following craniotomy and exposure of the surface of the brain. To identify language function, in this setting, the brain is stimulated while the patient is undergoing language testing and the areas of speech arrest or other language dysfunction are deemed essential language areas. If fMRI can unequivocally identify that the patient’s dominant language areas are contralateral to the lesion to be resected, the neurosurgeon may decide to forego awake mapping and direct cortical stimulation [7].
Theoretic basis
fMRI uses deoxyhemoglobin as an endogenous contrast agent to create functional maps derived from changes in cerebral blood flow, cerebral blood volume and cerebral metabolic rate of oxygen [1,2,10,11]. This occurs secondary to an increase in neuronal activity and the changes in these vascular parameters together yield the BOLD effect. The BOLD effect is a phenomenon resulting from an overshoot in the amount of diamagnetic oxyhemoglobin (from the increase in cerebral blood flow in response to task-related demand) relative to the paramagnetic deoxyhemoglobin (from the increase in oxygen consumption). As the deoxyhemoglobin is diluted by the overshoot in diamagnetic oxyhemoglobin, the fMRI signal actually increases in the active area. Therefore, fMRI signal is derived from the relative differences in the magnetic susceptibility of these two states of hemoglobin [1,2,10,11].
Relevant anatomy
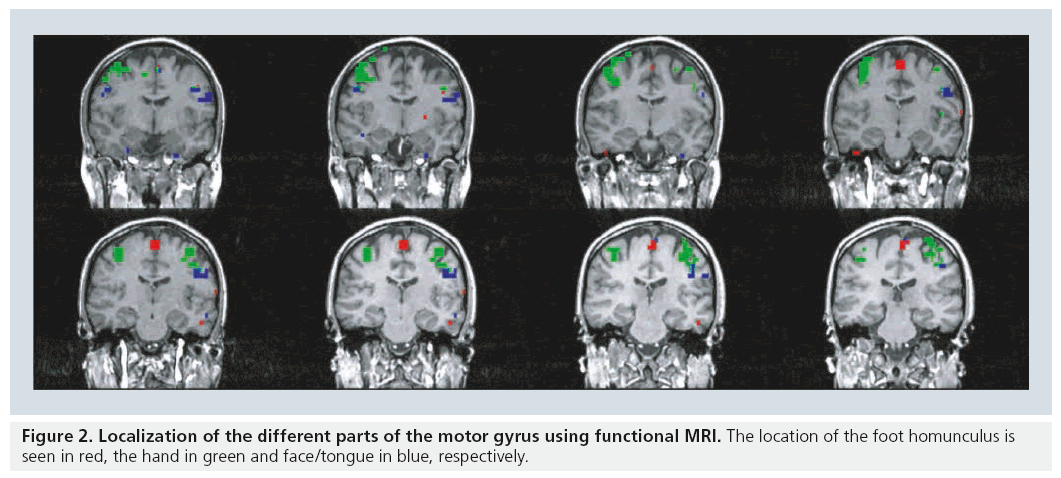
Motor function is organized topographically within the precentral gyrus in a pattern referred to as the homunculus while sensory function is topographically organized in a similar manner in the sensory gyrus. A brief review of basic anatomy of the motor and sensory system facilitates the discussion of paradigm selection. The motor and sensory systems both have topographic organizations that are mapped to specific locations of the cortex [12–14]. The foot and leg are represented along the interhemispheric fissure, the hand is lateral to that of the foot and leg, and the tongue and face are lateral to that of the hand (Figure 2).
Voluntary movement is performed through a network of different motor areas: the primary motor cortex (M1), the supplementary motor area (SMA), the lateral premotor cortex and the superior parietal lobules. The M1 area is involved in actually performing movement. The SMA is involved in motor planning and organization [12– 18]. The SMA is located in the superior frontal gyrus and consists of two parts: the rostral pre- SMA and the caudal SMA proper. The pre-SMA functions in cognitive tasks and language. The SMA proper – anatomically closer to primary motor and sensory areas – is involved in sensory, motor planning and word articulation [12–18].
Paradigm design
Paradigms, task designs that elicit neuronal activity, are used to produce fMRI maps of cerebral function. Paradigms are either event related or block design. Event-related paradigms better mirror the hemodynamic response but their limitations include long acquisition times, low statistical power and complicated statistical signal analyses. Block-design paradigms are able to detect subtle BOLD signal changes across time and are able to reduce artifacts from physiological variations in signal [15,19]. Block-design paradigms are more commonly used in the clinical setting.
In the preoperative setting, the most commonly requested fMRI examinations are language and motor/sensory related. Paradigms often help neurosurgeons and neurologists offer the best care to patients. They frequently provide physicians with critical information, which can facilitate the evalutation risk/benefit ahead of neurosurgery and other treatments. Appropriate paradigm selection has two requirements: clinical evaluation of the patient’s deficit(s) and the inspection of the anatomic images to determine the proximity of the lesion to the eloquent cortex [15]. Several factors must be considered, including a patient’s handedness and clinical ability. Most motor tasks suitable for fMRI are limited to a few areas of the body [15].
Common paradigm designs include blockdesign and event-related paradigms. To acquire meaningful results a patient typically performs the fMRI task (the ‘ON‘ state) three- to ten-times, with breaks (‘OFF‘ states) between each ON state [5,15]. When performing a block paradigm patients alternate between ON and OFF periods of equal or unequal duration (nonperiodic task delivery). For example, to identify the hand motor area, a block paradigm might consist of alternating finger tapping and resting periods, each lasting 20 s [5,20]. Nonperiodic task delivery often proves effective in decreasing noise from the scanner, heartbeat and breathing [5,21]. In event-related paradigms a patient performs a single event, such as swallowing or clenching a fist, with an ON period that has a shorter duration than that of a block design and an OFF period that is the same duration. This type of paradigm is used to investigate neuronal or hemodynamic response to a specific single event [15,20]. In event-related paradigms, statistical power for the events being measured is compromised because these designs typically require more images to regain the same accuracy obtained with a block paradigm [20,22]. The advantage is that the hemodynamic parameters, such as the time to peak, full of width half maximum and return to baseline, can be estimated in a way that is not possible with block designs [22]. Although of theoretical importance, the possible advantages of the temporal profile of the hemodynamic response obtained through eventrelated designs is usually not clinically applicable and therefore of limited clinical importance. In our experience, block design is also easier for patients who may have impaired function or cognitive capacity to comply with and perform an event-related design.
Baseline tasks for the rest period should be chosen carefully because the goal is to maximize the contrast of neural response between rest and activity periods. For example, a baseline task in which a patient passively views nonsense objects has been shown to improve the detection of signal in the Broca’s area when performed alongside sentence generation tasks [23].
There is currently no standardization of paradigms to be used for all clinical scenarios. A helpful list of suggested paradigms with recommendations and comments that are continuously updated is available on the webpage of the American Society of Functional Neuroradiology [101].
Patient preparation & monitoring
Task familiarity before imaging is important to ensure accurate fMRI responses. Accuracy is improved through the use of detailed instructions and reassurance during task performance. Patients should arrive early to receive an explanation of the fMRI procedure and the timing of the paradigms. It is essential to practice the paradigm with the patient prior to placing the patient in the scanner. This is vital especially in patients with neurological deficits, such as weakness, deafness or cognitive disorders, which often compromise the optimal performance of the necessary paradigm while in the scanner.
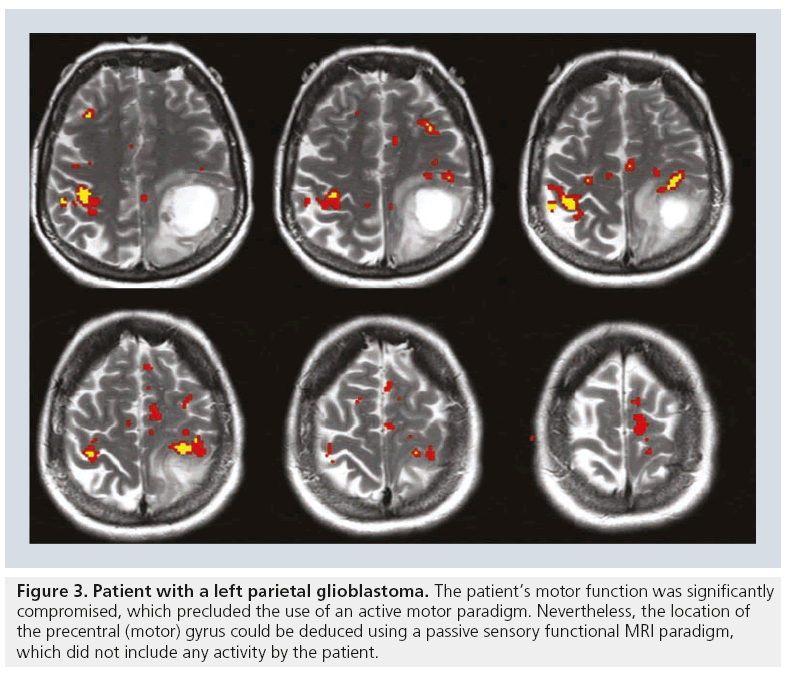
Paradigms may also need to be modified to better suit the patient’s neurologic limitations [5,6,15]. For example, if the neurosurgeon is interested in the location of the hand motor homunculus but the patient is paralyzed, asking the patient to tap his/her fingers will not yield a useful fMRI result. In such a scenario a sensory paradigm may be substituted, which will entail sensory stimulation of the patient’s hand thereby circumventing the patient’s motor deficit. Sensory paradigms can identify the sensory cortex or can be used to determine the location of the motor cortex in paretic patients. A sensory paradigm may include brushing, squeezing or touching the patient’s foot or hand [6,15]. In terms of analyzing the data, one is almost always able to infer the location of the motor homunculus from the sensory fMRI results. Even without motor homunculus fMRI activation during a sensory paradigm, the location of the motor gyrus can often be deduced from the sensory information (Figure 3).
Figure 3: Patient with a left parietal glioblastoma. The patient’s motor function was significantly compromised, which precluded the use of an active motor paradigm. Nevertheless, the location of the precentral (motor) gyrus could be deduced using a passive sensory functional MRI paradigm, which did not include any activity by the patient.
It is important to properly position the patient in the scanner and to properly select the scanning plane. Regardless of the paradigm used, it is important that it is designed to minimize any type of unwanted head or body motion to achieve ideal results. For example, a toe paradigm should be performed with no ankle motion, and a tongue paradigm should be performed with a closed mouth [9,15,24]. Monitoring motor tasks is easy although methods such as video monitoring or using squeeze balls to monitor motor response are occasionally used [15].
Sensory & motor mapping
Functional mapping of the sensory and motor systems is more reliable than language mapping. The somatosensory cortex is located in the postcentral gyrus just posterior to the central sulcus, while the motor cortex resides in the precentral gyrus just anterior to the central sulcus. Direct electrocortical stimulation can be used to identify the central sulcus during surgery. fMRI provides useful information before surgery that can be used to modify surgical planning, guide electrocortical stimulation mapping and predict the risk of motor and/or sensory functional loss. fMRI mapping of the central sulcus has proved resistant to potentially limiting artifacts from head motion, patient anxiety and abnormal vasculature. fMRI mapping also aids in identifying the foot and leg motor cortex, which can be difficult to identify by electrocortical stimulation mapping [7]. Sensory and motor mapping often entails simple paradigms that use the previously described ON/OFF method [5,6].
Mapping of the motor cortex involves finger, toe and/or tongue movement paradigms help identify the relative locations of the lesion and regions of the motor homunculus [15]. Paradigms chosen should determine the position of the lesion with regard to the different areas of the motor strip. For lesions close to the midline, leg or foot paradigms are used [5,15] because the foot and leg motor areas are represented along the interhemispheric fissure.
Data analysis, correction & interpretation
fMRI data analysis seeks to determine active areas. This is done through identification of voxels in the brain with statistically significant changes in the MRI signal from baseline to activation [6]. The goal is to pinpoint voxels that show changes in signal related to the paradigms performed (the timing of the ON/OFF periods) [6,15]. However, when interpreting fMRI data or planning an operation based on such data, it is essential to understand that artifacts do exist and can occasionally lead to spurious results [25].
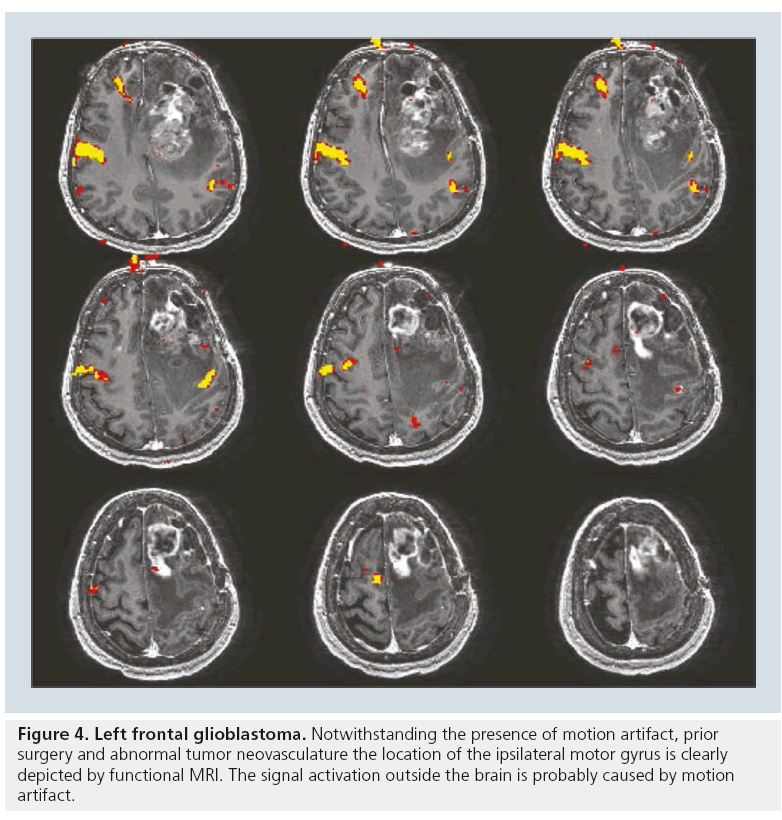
The accuracy of fMRI results has been validated by a variety of techniques. When compared with results of the sodium amobarbital procedure (also known as the Wada test), fMRI for language lateralization has shown excellent concordance with determination of hemispheric dominance [26]; however, fMRI for memory function is currently limited owing to prominent susceptibility artifacts from the bone–air interface in the temporal bones, which often overwhelms the BOLD fMRI signal in the temporal lobes and hippocampi. Studies have also shown that data collected by both intraoperative electrocorticography and direct cortical stimulation are comparable to those collected by fMRI motor mapping [27–29]. Dropout artifact may truncate the magnitude of the response; however, what little signal remains is almost always correct in its localization of the motor gyrus (Figure 4).
Coregistration of neuronavigational systems
After analyzing the fMRI data and determining active voxels in a functional MR image, these low-resolution data (typically 64 × 64 matrix) are coregistered onto high-resolution anatomical images (typically 256 × 256 matrix) to reveal the precise location in the brain in which these signal changes occurred. The coregistered images are downloaded to the neurosurgical navigational system in the operating room and these images allow the surgeon to view the relationship of the lesion to the adjacent eloquent cortex in real time intraoperatively. Using the coregistered data, the neurosurgeon can also visualize the 3D relationship between the lesion and the adjacent eloquent cortices during the resection itself, allowing for more precise patient-specific functional localization [7,23,27–30].
One of the problems with coregistration of fMRI data to neurosurgical navigational systems is that of brain shift. However, the majority of the brain mapping done by direct cortical simulation to corroborate the BOLD fMRI data is done directly after the craniotomy and prior to commencement of the tumor resection. Hence, the brain shift encountered following resection of a large volume of tumor is avoided. In this scenario, brain shift is limited to the usually mild protrusion of the swollen brain through the craniotomy.
Artifacts & other pitfalls in clinical fMRI
fMRI has limitations that at times can make it difficult to acquire consistent, accurate and meaningful data. The BOLD signal comprises three components: true neuronal activity, susceptibility artifacts that can create false-positive or false-negative signals, and neurovascular uncoupling effects that can create false-negative signals on the fMRI map.
Holodny and colleagues first described neurovascular uncoupling in a patient with glioblastoma involving the left precentral gyrus, with muted fMRI activation of the left motor cortex [31]. The BOLD fMRI effect is based on an intact vasculature, which is capable of the normal neurovascular response. In malignant gliomas, the tumor neovasculature is abnormal and lacks the normal neurovascular response to various stimuli. Hence, this abnormal tumor neovasculature does not respond in the normal manner to an increase in neuronal activity (e.g., due to finger tapping), which leads to a muted BOLD fMRI response [30]. In addition, in a malignant tumor, which exhibits hypoxia, the baseline blood flow may already be maximal that will preclude and further increase in blood flow (the basis of the BOLD fMRI response) owing to an increase in neuronal activity.
Artifacts from various sources can affect the results of fMRI data. Head motion can inadvertently move voxels of a high signal intensity to locations of low signal intensity [32]. This often leads to false-negative/false-positive results. Whereas types of motion can be compensated for by software, the stimulus-correlated motion (e.g., when the patient bobs his/her head in time with a finger-tapping paradigm) often cannot be compensated for because the artifactual signal looks deceptively similar to the real fMRI signal.
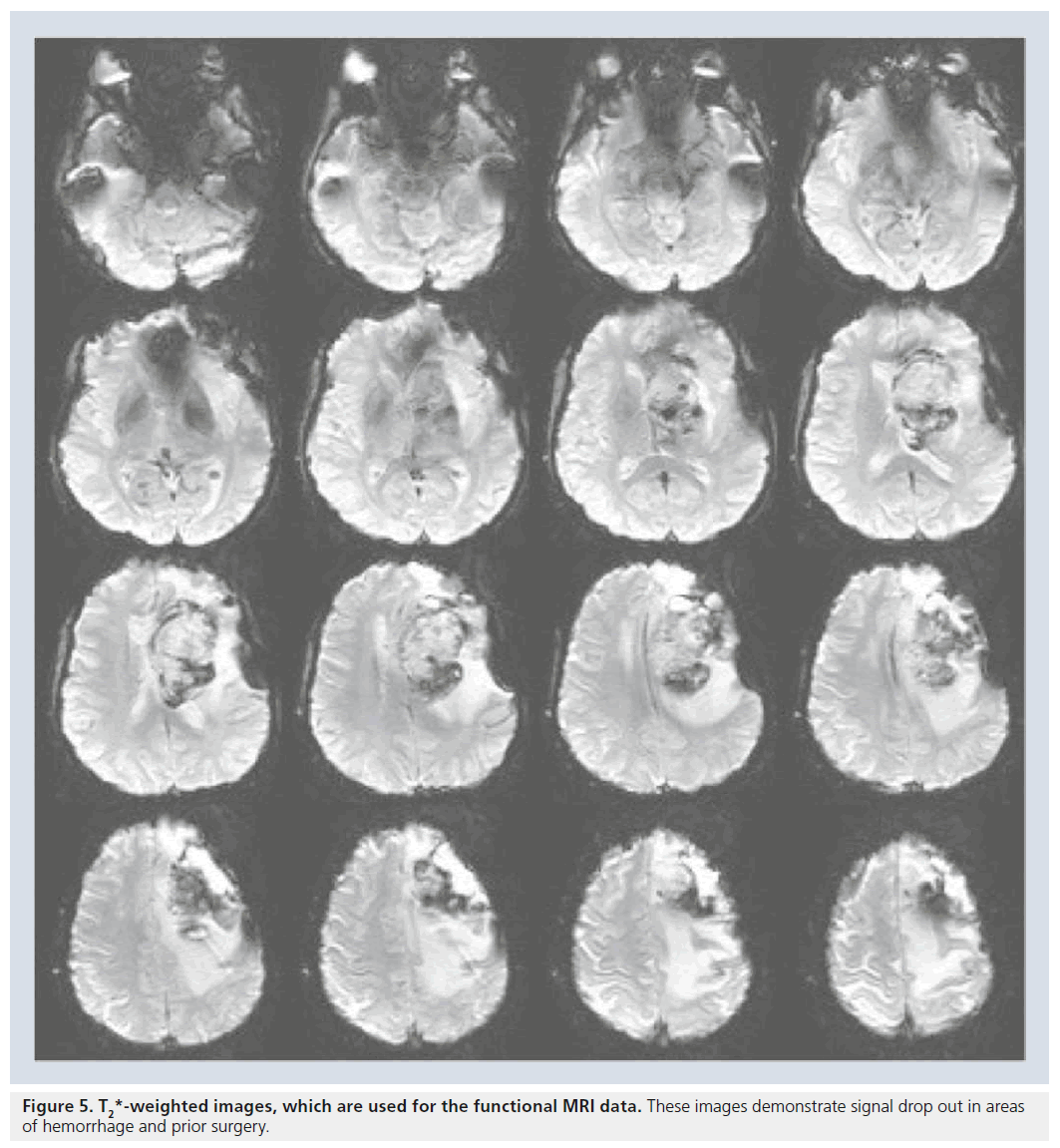
The MR sequence used to acquire fMRI data (usually the echoplanar imaging sequences) is used to maximize the subtle difference in susceptibility between oxyhemoglobin and deoxyhemoglobin. An unfortunate consequence is that susceptibility artifacts from both endogenous and exogenous sources are also magnified, which leads to a compromise of the BOLD fMRI signal. These false-negative results can lead to inaccuracies and need to be acknowledged for the purpose of neurosurgical planning. In patients without prior surgery, susceptibility artifacts are usually located at air–tissue interfaces, near cavities, or near moving tissues (Figure 5) [23]. Susceptibility artifacts pose an even larger issue for patients who have previously had neurosurgery. Staples, titanium plates and residue from skull drills increase the risk of susceptibility artifacts and may affect the accuracy of the fMRI data [25]. Hence, it is important to interpret fMRI signals in the context of these susceptibility artifacts, understanding that the lack of signal or maps in this region is uninterpretable as there are no useful signals in these regions. Another issue encountered in preoperative mapping is the problem of ‘vascular point spread’. It is essential to remember that BOLD fMRI identifies changes in the oxyhemoglobin/ deoxyhemoglobin ratios in the blood vessels, rather then neuronal activity. Therefore, BOLD fMRI depicts signals from the microvasculature near the active area as well as signal from venules and large draining veins [5,15,25]. The BOLD activation of the microvasculature accurately depicts the site of neuronal firing; however, BOLD activation also identifies draining veins at some, rather small, distance from the area of actual neuronal firing. In interpreting BOLD fMRI studies for neurosurgical planning one tends to be more inclusive in identifying possible eloquent areas adjacent to tumors, tending to err on the side of false-positive rather than false-negative results. This approach highlights possible eloquent areas adjacent to the area of resection and maximizes the attention of the operating neurosurgeon to these areas. In our experience, this conservative approach (in general as well as in the questions of the contribution of draining veins to the BOLD fMRI signal) has served us well in guiding neurosurgery and limiting damage to eloquent cortices adjacent to brain tumors.
Similarly, there is no consensus currently on the optimal threshold to use to interpret the preoperative BOLD fMRI data, as the extent of eloquent cortices as well the laterality indices can change with different statistical thresholds. As in the above paragraph, we tend to be more inclusive when interpreting BOLD fMRI data for the presurgical patient. We tend to lower the statistical threshold to include more areas of possible activation on our BOLD fMRI maps, thinking that false-positive errors are preferable to false-negative errors in preoperative mapping. Needless to say, our colleague neurosurgeons are clearly aware of and appreciate our strategy in erring on the side of maximizing patient safety.
Cortical plasticity/reorganization
Brain plasticity or cortical reorganization refers to the transfer of function to a different area of the brain, often the opposite hemisphere, in the setting of an insult to the brain, for example, the growth of a brain tumor. Cortical reorganization is thought to occur when an area of the brain is no longer able to complete its function therefore causing another area of the brain to attempt to compensate in an effort to maintain function [8,32]. It is likely that the details of the injury, the type of injury (e.g., tumor vs stroke), the extent of brain damage and the function affected greatly determine the resultant pattern of interhemispheric or intrahemispheric compensation [5,8]. For example, when a primary motor area is damaged, other areas (such as the SMA) may start to play a larger role in normal motor function and movement execution [16]. Studies have shown that in normal humans the SMA does in fact activate temporally before M1 [33,34]. The SMA was shown to assume a larger role in motor planning and movement execution, classically thought of as an M1 function, in patients with tumors for whom high-grade tumors were affecting normal M1 function. The mechanism by which such reorganization occurs in the motor strip is being investigated but may involve a decrease in the ipsilateral functional activity and increased activity in the contralateral hemisphere [5,32].
Applications in treatment & therapy
It has been suggested that neuroimaging could be used to develop new methods of therapy for movement disorders and pain management and to better understand existing methods [35,36,102]. This is currently being explored in deep brain stimulation, where electrodes are implanted deep within the brain in an attempt to relieve the patient’s neurological problem, such as Parkinson’s disease, chronic pain, tremor or dystonia [37]. Although each patient must be considered individually before deep brain stimulation therapy, overall stimulation of the tissue that is dorsal, lateral and posterior to the centroids of the subthalamic nucleus is thought to have the best therapeutic results [38]. Advanced neuroimaging techniques, including fMRI [39] and diffusion tractography [40], have been proposed to improve the localization of these small structures, the location of which varies in different individuals. fMRI may ultimately facilitate the prediction of both positive and negative effects of treatment of these neurologic diseases [6,41]. The ability of fMRI to provide individualized care for patients is an exciting prospect [42].
Future perspective
In the near future, essentially all brain tumor neurosurgery will be guided by imaging. This will include fMRI and diffusion tractography to help identify areas to be avoided by the neurosurgeon as well as molecular markers (e.g., new PET tracers) that will identify those areas of the tumor that are necessary to resect. Current neurosurgical navigational systems allow all of this multimodal information to be loaded onto a neurosurgical navigational computer and visualized during brain tumor surgery. With increased use and understanding of the technique, fMRI-guided presurgical planning concerning atypical anatomy (e.g., disruptions caused by tumors and tumor neovasculature) will improve and become more reliable [102]. In addition, fMRI is also proving to be an important tool in studies of cortical plasticity, as well as in the clinical setting, providing neurosurgeons with valuable information that may influence their decision on whether or not to perform surgery [5,6]. Another area of active fMRI research relevant to neurosurgical planning is the body specificity of fMRI data and how an individual’s movement habits, experiences and medical history affect the results of motor imagery fMRI [42]. This 2009 study concluded that because the imagination of movement is most likely to be envisioned based on the way than an individual would normally perform a motion, motor imagery, fMRI results reflects one’s lifestyle, including handedness, movement, habits and patterns [43].
Conclusion
fMRI is important to the understanding of brain mechanisms, function and structure as well as to the localization of critical motor, speech and cognitive function. It has many advantages when compared with other methods of neuroimaging and functional mapping: it is noninvasive, it does not entail the use of radioactive isotopes, it is easily repeatable and it has no known risks [2]. The use of fMRI use in presurgical mapping tasks, such as locating motor, sensory, language and/or memory functional areas in relation to a lesion, is becoming increasingly widespread and accepted [4]. Current research is investigating other exciting uses of the technique, such as more patient-specific functional mapping.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.

References
Papers of special note have been highlighted as:
• of interest
• • of considerable interest
- Ogawa S, Lee TM. Magnetic resonance imaging of blood vessels at high fields: in vivo and in vitro measurements and image simulation. Magn. Reson. Med. 16(1), 9–18 (1990).
- Ogawa S, Lee TM, Nayak AS et al. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn. Reson. Med. 14(1), 68–78 (1990).
- Ogawa S, Lee TM, Kay AR et al. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl Acad. Sci. USA 87(24), 9868–9887 (1990).
- Holodny A, Hou B. Physical principles of BOLD fMRI – what is important for the clinician. In: Functional Neuroimaging: A Clinical Approach. Holodny AI (Ed.). Informa Healthcare Inc., NY, USA, 1–12 (2008).
- Bogomolny DL, Petrovich NM, Hou BL et al. Functional MRI in the brain tumor patient. Top. Magn. Reson. Imaging 15(5), 325–335 (2004).
- Peck KK, Holodny AI. fMRI clinical applications. In: Magnetic Resonance Tomography. Reiser MF, Semmler W, Hricak H (Eds). Springer Verlag, Berlin, Germany, 1308–1331 (2007).
- Brennan CW, Petrovich Brennan NM. Functional image-guided neurosurgery. In: Functional Neuroimaging: A Clinical Approach. Holodny AI (Ed.). Informa Healthcare Inc., NY, USA, 91–106 (2008).
- Holodny AI. Cortical plasticity. In: Functional Neuroimaging: A Clinical Approach. Holodny AI (Ed.). Informa Healthcare Inc., NY, USA (2008).
- Krings T, Reinges MH, Erberich S et al. Functional MRI for presurgical planning: problems, artifacts, and solution strategies. J. Neurol. Neurosurg. Psychiat. 70(6), 749–760 (2001).
- Buxton RB, Uludag K, Dubowitz DJ et al. Modeling the hemodynamic response to brain activation. Neuroimage 23(1), 220–233 (2004).
- Logothetis NK, Wandell BA. Interpreting the BOLD signal. Ann. Rev. Physiol. 66, 735–769 (2004).
- Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb. Cortex 6(3), 342–353 (1996).
- Roland PE, Zilles K. Functions and structures of the motor cortices in humans. Curr. Opin. Neurobiol. 6(6), 773–781 (1996).
- Vorobiev V, Govoni P, Rizzolatti G et al. Parcellation of human mesial area 6: cytoarchitectonic evidence for three separate areas. Eur. J. Neurosci. 10(6), 2199–2203 (1998).
- Brennan NP. Preparing the patient for the fMRI study and optimization of paradigm selection and delivery. In: Functional Neuroimaging: A Clinical Approach. Holodny AI (Ed.). Informa Healthcare Inc., NY, USA, 13–21 (2008).
- Peck KK, Bradbury MS, Hou BL et al. The role of the supplementary motor area (SMA) in the execution of primary motor activities in brain tumor patients: functional MRI detection of time-resolved differences in the hemodynamic response. Med. Sci. Monit. 15(4), 55–62 (2009).
- Peck KK, Bradbury M, Psaty EL et al. Joint activation of the supplementary motor area and presupplementary motor area during simultaneous motor and language functional MRI. Neuroreport 20(5), 487–491 (2009).
- Alario FX, Chainay H, Lehericy S et al. The role of the supplementary motor area (SMA) in word production. Brain Res. 1076(1), 129–143 (2006).
- Kennan RP, Zhong J, Gore JC. Intravascular susceptibility contrast mechanisms in tissues. Magn. Reson. Med. 31(1), 9–21 (1994).
- Kesavadas C, Thomas B. Clinical applications of functional MRI in epilepsy. Indian J. Radiol. Imaging 18(3), 210–217 (2008).
- Veltman DJ, Mechelli A, Friston KJ et al. The importance of distributed sampling in blocked functional magnetic resonance imaging designs. Neuroimage 17(3), 1203–1206 (2002).
- Birn RM, Cox RW, Bandettini PA. Detection versus estimation in event-related fMRI: choosing the optimal stimulus timing. Neuroimage 15(1), 252–264 (2002).
- Peck KK, Wierenga CE, Moore AB et al. Comparison of baseline conditions to investigate syntactic production using functional magnetic resonance imaging. Neuroimage 23(1), 104–110 (2004).
- Hoeller M, Krings T, Reinges MH et al. Movement artifacts and MR BOLD signal increase during different paradigms for mapping the sensorimotor cortex. Acta Neurochir. (Wien) 144(3), 279–284 (2002).
- Kaufman JF, Maldjian JA. Fact or artifact? In: Functional Neuroimaging: A Clinical Approach. Holodny AI (Ed.). Informa Healthcare Inc., NY, USA, 37–49 (2008).
- Desmond JE, Sum JM, Wagner AD et al. Functional MRI measurement of language lateralization in Wada-tested patients. Brain 118(6), 1411–1419 (1995).
- Jack CR Jr, Thompson RM, Butts RK et al. Sensory motor cortex: correlation of presurgical mapping with functional MR imaging and invasive cortical mapping. Radiology 190(1), 85–92 (1994).
- Lehericy S, Duffau H, Cornu P et al. Correspondence between functional magnetic resonance imaging somatotopy and individual brain anatomy of the central region: comparison with intraoperative stimulation in patients with brain tumors. J. Neurosurg. 92(4), 589–598 (2000).
- Puce A, Constable RT, Luby ML et al. Functional magnetic resonance imaging of sensory and motor cortex: comparison with electrophysiological localization. J. Neurosurg. 83(2), 262–270 (1995).
- Ulmer JL, Hacein-Bey L, Mathews VP et al. Lesion-induced pseudo-dominance at functional magnetic resonance imaging: implications for preoperative assessments. Neurosurgery 55(3), 569–579 (2004).
- Holodny AI, Schulder M, Liu WC et al. Decreased BOLD functional MR activation of the motor and sensory cortices adjacent to a glioblastoma multiforme: implications for image-guided neurosurgery. Am. J. Neuroradiol. 20(4), 609–612 (1999).
- Matthews PM, Honey GD, Bullmore ET. Applications of fMRI in translational medicine and clinical practice. Nat. Rev. Neurosci. 7(9), 732–744 (2006).
- Peck KK, Sunderland A, Peters AM et al. Cerebral activation during a simple force production task: changes in the time course of the haemodynamic response. Neuroreport 12(13), 2813–2816 (2001).
- Richter W, Andersen PM, Georgopoulos AP et al. Sequential activity in human motor areas during a delayed cued finger movement task studied by time-resolved fMRI. Neuroreport 8(5), 1257–1261 (1997).
- Jech R. Functional imaging of deep brain stimulation: fMRI, SPECT, and PET. In: Deep Brain Stimulation in Neurological and Psychiatric Disorders. Tarsy D, Vitek JL, Starr PA (Eds). Humana Press, NJ, USA, 179–201 (2008).
- Brunenberg EJ, Platel B, Hofman PA, Ter Haar Romeny BM, Visser-Vandewalle V. Magnetic resonance imaging techniques for visualization of the subthalamic nucleus. J. Neurosurg. 115(5), 971–984 (2011).
- Kringelbach ML, Jenkinson N, Owen SLF, Aziz TZ. Translational principles of deep brain stimulation. Nat. Rev. Neuro. 8, 623–635 (2007).
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch. Gen. Psychiat. 62(7), 761–768 (2005).
- Cheng K. Recent progress in high-resolution functional MRI. Curr. Opin. Neurol. 24(4), 401–408 (2011).
- Coenan VA, Aller N, Mädler B. A role of diffusion tensor imaging fiber tracking in deep brain stimulation surgery: DBS of the dentate-rubro-thalamic tract (drt) for the treatment of therapy-refractory tremor. Acta Neurochir. (Wien) 153(8), 1579–1585 (2011).
- Young RJ, Knopp EA. Brain MRI: tumor evaluation. J. Magn. Reson. Imaging 24(4), 709–724 (2006).
- Bernad DM, Doyon J. The role of noninvasive techniques in stroke therapy. Int. J. Biomed. Imaging 2008, 672582 (2008).
- Willems RM, Toni I, Hagoort P et al. Body-specific motor imagery of hand actions: neural evidence from right- and left-handers. Front. Hum. Neurosci. 3, 39 (2009).
- American Society of Functional Neuroradiology. www.asfnr.org
- Columbia University Program for Imaging and Cognitive Sciences. About functional MRI. www.fmri.org/fmri.htm
• Studies what causes the blood oxygen level-dependent effect and how the effect provides maps of blood oxygenation in the brain.
• Discusses in detail the current and potential future clinical applications of functional MRI (fMRI).
• • Summarizes the current understanding of cortical plasticity in response to brain tumor growth.
• Suggests that in patients with high-grade brain tumors invading the primary motor cortex, the supplementary motor area may assume a greater role in the execution of primary motor activities.
• Discusses the influence of fMRI results on seizure teams’ decisions regarding diagnosis and therapy and the issues involved in fMRI of epilepsy patients.
• Discusses the emergence of real-time high-resolution fMRI as an indispensable tool for surgical planning, diagnosis of neurological disease and targeting of deep brain stimulation.
• Discusses how motor imagery is influenced by the way people habitually perform motor actions with their particular bodies.
■ Websites