Research Article - International Journal of Clinical Rheumatology (2022) Volume 17, Issue 3
Prevalence and predictors of gout in lagos: a community based study
Ohagwu Kenneth Arinze1*, Kenneth Arinze Ohagwu1, Olufemi Oladipo Adelowo2,Innocent Ijezie Chukwuonye1
1Division of Rheumatology, Department of Internal Medicine, Federal Medical Centre Umuahia, Abia state, Nigeria
2Division of Rheumatology, Department of Internal Medicine, Lagos State University Teaching Hospital, Lagos State, Nigeria
- *Corresponding Author:
- Ohagwu Kenneth Arinze
Division of Rheumatology, Department of Internal Medicine, Federal Medical Centre Umuahia, Abia state, Nigeria
E-mail: arysdon@yahoo.com
Received: 28-Jul-2019, Manuscript No. IJCR-19-693; Editor assigned: 31-Jul-2019, PreQC No. IJCR-19-693(PQ); Reviewed: 26-Aug-2019, QC No. IJCR-19-693; Revised: 11-Mar-2022, Manuscript No. IJCR-19-693(R); Published: 16-Mar-2022, DOI: 10.37532/1758-4272.2022.17(3).068-077
Abstract
Background: Gout is an inflammatory disease caused by reversible deposition of monosodium urate crystals in joints and other tissues. There is dearth of data regarding the prevalence of gout in Nigeria, necessitating this study.
Methods: The study was a descriptive cross – sectional study aimed at determining the prevalence and predictors of gout at Agbowa, a semi – urban community in Lagos state. The community was randomly selected. Four hundred adults aged 18year and above, were involved in the study.Questionnaires were used to obtain relevant information from the subjects. Anthropometric measurements were taken and body mass index calculated. Blood samples were collected for relevant investigations.
Results: One hundred and seventy nine (179) males and 221 females participated in the study giving male: female ratio of 1:1.2. Mean age of the participants was 46.5±16.0 years. One hundred and eleven subjects (27.8%) had associated joint pain, mostly involving the lower back and the knees. The prevalence of gout and hyperuricemia was 2.25% and 21.5% respectively. Male gender, increasing age, diuretic use, alcohol consumption, low dose aspirin, hypertension, serum uric acid, high serum triglycerides and decreased glomerular filtration rate were factors associated with gout on bivariate logistic regression.
Conclusion: Gout prevalence is low in the community, however the prevalence hyperuricemia is high, and about one person in every five persons in the community has hyperuriceamia.
Keywords:
gout • hyperuricemia • prevalence
Introduction
Gout is an inflammatory disease that is caused by the deposition of monosodium urate (MSU) crystals in joints and other tissues [1]. It is associated with impaired quality of life. Gout is associated with elevated serum uric acid and accompanying precipitation of uric acid crystals in the synovial joints [2]. Hyperuricemia results from either overproduction of uric acid, or under excretion of uric acid or a combination of both conditions [3]. When the serum level exceeds a given threshold, 6.8mg/dl, super saturation of body fluids with urate occur resulting in precipitation of urate crystals [4,5]. The deposition of MSU crystals is reversible. Not all subjects with hyperuricemia will develop gout. The probability of developing gout is directly proportional to the duration and the degree of hyperuricemia [6,7]. Serum uric acid level in normal persons varies widely across the globe and varies between the two sexes.
Gouty arthritis is inflammatory in nature and is associated with renal and cardiovascular disease [8]. In recent years, evidence has shown the association of hyperuricemia and the metabolic syndrome thus emphasizing the importance of asymptomatic hyperuricemia [8,9].The deposition of these crystals in the synovium causes tissue and joint damage with chronic pain. Overall, gout increases morbidity and mortality, from cardiovascular events and premature atherosclerosis, cerebrovascular events and renal failure.
In Nigeria, there are few data on the community prevalence of hyperuricemia. Available data regarding the prevalence of gout in Nigeria are hospital-based studies that do not represent the prevalence of gout in the general population. Therefore, a community based prevalence study of gout, its risk factors and associated co-morbidities was undertaken.
Methodology
Study design and area
The methodology used in this study has been described in an earlier publication.
The study was a community based descriptive study carried out in Agbowa community in Ikosin Ejirin local council development area in Lagos state, Nigeria. The community is a semi – urban community.
Study population
The study population was made up of adults aged 18 years and above living in the study area during the study period. A previous study on the prevalence of gout by Zhu et al in which a prevalence rate of 3.9% [10] reported was used. Using the modified Fishers formula [11] and 95% confidence interval, a minimum sample size of 57 subjects was arrived at. This was increased to 400 subjects. Subjects less than 18 years of age, those not resident in the community and those that failed to give informed consent were excluded from the study.
Sampling technique
Systematic sampling technique was used. One local council development area (LCDA) out of 37 in the state was selected randomly by balloting. This was Ikosi-Ejirin Local Council Development area, Lagos. A semi – urban community in the LCDA was randomly selected by balloting. This was Agbowa community. A central location in the community was used as study centre. This was Agbowa town hall. Every fourth subject that met the inclusion criteria was recruited for the study. This was arrived at by dividing the target population by the sample size.
Study protocol
The study was carried out using the standardized World Health Organization/International League against Rheumatism’s Community Oriented Programme on the Control of Rheumatic Diseases (COPCORD) standardized protocol. Ten research assistants were trained to assist in the study. These assistants were paramedics and nurses working in the community. They had good command of both English as well as Yoruba languages. The training included basic methods of taking anthropometric measurements, blood pressure and completion of questionnaires. The researchers made sure that all assistants adhered to protocol.
A researcher – administered questionnaire was completed per subject.
Blood pressure was taken using the left arm after about 5 minutes rest.
Every subject had weight and height measured and body mass index (BMI) calculated. Musculoskeletal examination was done for each respondent by the researchers.
Blood samples were taken for the following tests: Serum uric acid estimation, creatinine, triglycerides, total cholesterol and blood glucose.
Estimated GFR was calculated from serum creatinine using the CKD-EPI formula [12]. Hyperuriceamia was defined as serum uric acid >420 μmol/L (7 mg/dL) in men and >357 μmol/L (5.8mg/dL) in women [10]. Hypercholesterolemia was defined as serum cholesterol greater than 200mg/dl [13]. Serum Triglyceride values greater than 150mg/dl was taken for hypertriglyceridemia [13].
Basis for diagnosis of gout
The diagnosis of gout was based the standard COPCORD protocol using the 1977 ACR classification criteria for gout.
Data analysis
Data was analyzed using Statistical Package for Social Sciences (SPSS) version 21.0 IBM SPSS® 2012 for windows by IBM USA, Amonk NY 10504. Demographic and clinical data of respondents were summarized using frequencies, percentages and proportions. Mean and standard deviation were calculated for continuous variables. Student’s T test was used to compare continuous variables while Chi square test was used to compare categorical variables. Bivariate logistic regression was used to determine factors associated with hyperuricemia and gout. P value less than 0.05 was regarded as being statistically significant.
Ethical consideration
The participants did not incur any harm for participating in the study. The only form of pain was during venipuncture for blood sample collection. There was no financial cost to the participants. Confidentiality was maintained.
Ethical approval was obtained from the ethical and research committee of Lagos State University Teaching Hospital, Lagos.
Results
There were 221 females and 179 males. The male: female ratio of the subjects was 1:1.2. The age range of the subjects was 18-88 years. Mean age was 46.5±16.0 years. Subjects aged between 36 - 45 years constituted majority of the study population (N=121, 30.2%) while those aged more than 75 years constituted the minority (N=23, 5.8%). There was no statistically significant difference between the mean age of male and female subjects (t=1.822, p=0.069). Majority of the participants were traders, artisans and farmers while 60(15%) were unemployed. Most of the subjects had received a form of formal education. Other socio-demographic parameters are as in Table 1.
| Characteristics | Number (N) | Percentage (%) | |
|---|---|---|---|
| Sex | Male | 179 | 44.7 |
| Female | 221 | 55.3 | |
| Age range (years) |
≤25 | 39 | 9.7 |
| 26 – 35 | 50 | 12.5 | |
| 36 – 45 | 121 | 30.2 | |
| 46 – 55 | 81 | 20.3 | |
| 56 – 65 | 48 | 12.0 | |
| 66 – 75 | 38 | 9.5 | |
| >75 | 23 | 5.8 | |
| Marital status | Single | 75 | 18.8 |
| Married | 297 | 74.2 | |
| Divorced/Separated | 6 | 1.5 | |
| Widow/widower | 22 | 5.5 | |
| Religion | Islam | 164 | 41.0 |
| Christianity | 228 | 57.0 | |
| Others | 8 | 2.0 | |
| Postmenopausal (n=221) | Yes | 73 | 33.0 |
| No | 148 | 67.0 | |
| Education | No formal education | 65 | 16.3 |
| Primary level | 121 | 30.2 | |
| Secondary level | 177 | 44.3 | |
| Tertiary level | 37 | 9.2 | |
| Occupation | Trader | 126 | 31.5 |
| Artisan | 102 | 25.5 | |
| Clerical officers | 6 | 1.5 | |
| Professionals | 22 | 5.5 | |
| Clergy | 8 | 2.0 | |
| Farming | 65 | 16.2 | |
| Unemployed | 60 | 15.0 | |
| Retired | 9 | 2.3 | |
| Student | 2 | 0.5 | |
Table 1. Socio-demographic characteristics of the subjects.
Thirty-six (9%) of the subjects were known hypertensive patients while three (0.3%) were known diabetes mellitus patients. Thirty one (7.8%) subjects were on various diuretics of which hydrochlorothiazide/amiloride combination was the commonest (62.5%) (Table 2). Thirty four (8.5%) of the subjects were on low dose aspirin. Other drugs that the subjects were using mostly included amlodipine (N=16;4%), various non - steroidal anti-inflammatory drugs (N=24;6%) and paracetamol (N=13;3.3%) (Table 2). There was significant difference between the number of men that take alcohol and the number of women that take alcohol (41 males versus 13 females [X2=24.669, p=0.001]) (Table 3). Two hundred and seventy seven (69.3%) of the subjects gave history of soft drink consumption while 14(3.5%) gave history of shellfish consumption (Table 2).
| Item | Number | Percentage | |
|---|---|---|---|
| History of hypertension | Yes | 36 | 9.0 |
| No | 364 | 91.0 | |
| History of diabetes mellitus | Yes | 3 | 0.8 |
| No | 397 | 99.2 | |
| Diuretic use(n=31) | Frusemide | 5 | 15.6 |
| Hydrochlorothiazide(HCT) | 5 | 15.6 | |
| Frusemide+HCT | 2 | 6.3 | |
| Amiloride+HCT | 20 | 62.5 | |
| Other drugs | Herbal | 4 | 1.0 |
| Amlodipine | 16 | 4.0 | |
| NSAID | 24 | 6.0 | |
| Lisinopril | 2 | 0.5 | |
| Aspirin | 34 | 8.5 | |
| Tramadol | 3 | 0.8 | |
| Paracetamol | 13 | 3.3 | |
| Alcohol use | Do not use | 341 | 85.2 |
| Uses alcohol | 59 | 14.8 | |
| Alcohol type(n=59) | Beer | 35 | 59.3 |
| Wine | 4 | 6.8 | |
| Local brew | 7 | 11.9 | |
| Whisky | 5 | 8.5 | |
| Gin | 8 | 13.5 | |
| Soft drink intake | Yes | 277 | 69.3 |
| No | 123 | 30.7 | |
| Shellfish consumption | Yes | 14 | 3.5 |
| No | 386 | 96.5 | |
Table 2. Medical history.
| Item | Number | X2 | P value | |
|---|---|---|---|---|
| Alcohol intake | Men | 41 | 24.669 | 0.001 |
| Women | 13 | |||
| Obesity | Men | 16 | 19.435 | <0.001 |
| Women | 33 | |||
Table 3. Gender differences in alcohol intake, obesity and hyperuricemia.
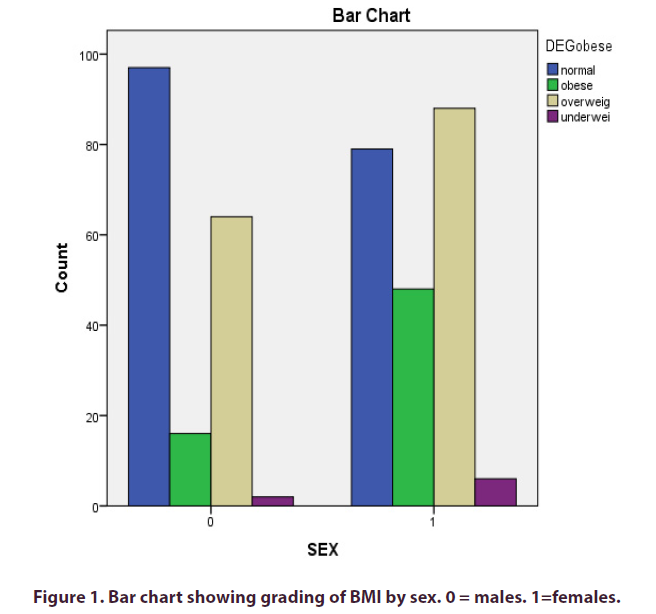
One hundred and fifty two {152(38%)} had elevated blood pressure and were classified as having hypertension. Nineteen subjects (4.7%) had blood glucose level higher than normal and were classified as having diabetes mellitus. Sixty four subjects (16%) had body mass index (BMI) ≥ 30kg/M2. Females were found to be significantly more obese than males (48 versus 16, X2 = 19.435, p<0.001). More females were also overweight (88 versus 64). This is shown in Figure 1.
Laboratory Results
Serum cholesterol was done on 370 sera. Of these, 80(21.6%) had hypercholesterolaemia. The difference in serum cholesterol between the two sexes was not statistically significant. Serum triglyceride was done on 382 sera. Sixty-eight (17.8%) subjects had hypertriglyceridemia. The difference in serum triglyceride between the two sexes was not statistically significant. Serum creatinine was elevated in 14(3.6%) subjects and 30 (7.5%) subjects had estimated Glomerular filtration rate (eGFR) below 60ml/min using the chronic kidney disease epidemiology formula. Using the respective sex – specific cut – off values for serum uric acid, 53(13.3%) males and 33(8.2%) females had hyperuricemia, giving a prevalence of 21.5% (Table 4). Males had higher serum uric acid levels than females and this difference was found to be statistically significant (p <0.001) (Table 4). A total of nine subjects were diagnosed with gout giving a prevalence rate of 2.25%. These comprised eight males (88.9%) and one female (11.1%). All the subjects had in the past experienced an intense joint pain that got to maximum intensity within 24 hours. In eight subjects, joint pain started at night, the affected joints were swollen, warm and affected their ability to walk. Three subjects could not bear to touch the joints. Five subjects had experienced podagra in the past. Gouty arthritis was monoarticular in one (11.1%) subject involving the elbow, oligoarticular in 7 (77.8%) subjects and polyarticular in one (11.1%) subject. The affected joints were ankles (n=7), knees (n=5), elbows (n=6), shoulder (n=1) and toe (n=5). Five (55.6%) of the subjects with gouty arthritis had experienced acute intermittent gout in the past but were in the intercritical period during the study period. Four (44.4%) subjects had chronic tophaceous gout with tophi involving the olecranon and the malleoli.Six (66.7%) out of the 9 subjects with gout had associated hyperuricemia, with all the subjects having serum uric acid level ≥5.8mg/dl. Other characteristics of the gouty arthritis subjects are as presented in Table 5.
| n(%) | X2 | p-value | ||
|---|---|---|---|---|
| Hypercholesterolemia | Male | 30(8.1) | 2.703 | 0.259 |
| Female | 50(13.5) | |||
| Hypertriglyceridemia | Male | 27(7.1) | 1.758 | 0.454 |
| Female | 41(10.7) | |||
| High serum creatinine | Male | 7(1.8) | 0.162 | 0.688 |
| Female | 7(1.8) | |||
| Hyperuricemia | Male | 53(13.3) | 21.635 | <0.001 |
| Female | 33(8.2) | |||
Table 4. Laboratory parameters.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Sex | Male | Male | Female | Male | Male | Male | Male | Male | Male |
| Age(years) | 80 | 54 | 63 | 71 | 68 | 60 | 49 | 58 | 56 |
| HTN | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes |
| DM | No | No | No | No | No | No | No | No | No |
| BMI(kg/m2) | 30.1 | 19.1 | 23.3 | 31 | 25.7 | 27.3 | 20.2 | 27.7 | 26.4 |
| Diuretic | HCT | HCT+AMI | Nil | Nil | Nil | HCT+AMI | HCT | HCT | HCT+AMI |
| Aspirin | Yes | No | No | No | No | Yes | Yes | Yes | Yes |
| Alcohol | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Soft drink | Yes | Yes | Yes | Yes | No | Yes | No | No | No |
| Joints affected | Knee, Ankles, toe | Shoulder, knee, ankle, toe | Knee,elbow | Elbow, ankle, Toe | Knee, ankle | Knee, ankles, elbows, toe | Ankle | Ankle, Elbow | Elbow,toe |
| Podagra | Yes | Yes | No | Yes | No | Yes | No | No | Yes |
| Episodes | 4 | 5 | 2 | 3 | 5 | 4 | 2 | 3 | 2 |
| Duration of each(days) | 20 | 14 | 14 | 28 | 14 | 28 | 21 | 14 | 10 |
| Intercritical period(days) | 60 | 140 | 180 | 90 | 60 | 120 | 360 | 90 | 180 |
| EGFR(ml/min/1.73m2) | 44 | 79 | 49 | 65 | 101 | 110 | 98 | 85 | 82 |
| SUA(mg/dl) | 8.5 | 7.3 | 7.5 | 7.2 | 12.8 | 6.5 | 5.8 | 6 | 7.7 |
| TG(mg/dl) | 92 | 160 | 152 | 167 | 160 | - | 131 | 129 | 120 |
| CHOL(mg/dl) | 121 | 123 | 139 | 265 | 144 | 178 | 112 | 232 | 200 |
| HTN = Hypertension. HCT = Hydrochlorothiazide. CHOL = serum cholesterol DM = Diabetes Mellitus. TG = serum triglycerides. EGFR = estimated Glomerular filtration rate. SUA = Serum uric acid. AMI = Amiloride. BMI = Body Mass Index (Kg/M2) |
|||||||||
Table 5. Characteristics of gouty arthritis subjects.
Factors associated with gout and hyperuricemia
The following variables were subjected to bivariate logistic regression to determine factors associated with gout and hyperuricemia: age, sex, menopause, diuretic use, alcohol intake, use of aspirin, intake of soft drinks, consumption of shellfish, overweight and obesity, hypertension, diabetes mellitus, hyperuricemia, estimated Glomerular filtration rate, hypercholesterolemia and hypertriglyceridemia. Increasing age, diuretic use, alcohol intake, aspirin use, hypertension, hyperuricemia, hypertriglyceridemia, eGFR <60ml/min and male sex were associated with gout (Table 6).
| Variable | Odds ratio | 95% Confidence interval | P value |
|---|---|---|---|
| Age >45 years | 6.01 | 1.82 – 19.82 | 0.003* |
| Sex(male gender) | 10.29 | 1.27 – 83.80 | 0.029* |
| Diuretic use | 17.45 | 4.41 – 68.93 | 0.001* |
| Aspirin use | 5.81 | 1.38 – 24.35 | 0.016* |
| Alcohol consumption | 8.62 | 2.24 – 33.21 | 0.002* |
| Soft drink intake | 1.55 | 0.32 – 7.57 | 0.588 |
| Shellfish consumption | 0.01 | 0.00 – 0.10 | 0.999 |
| Hypertension | 14.52 | 3.71 – 56.97 | 0.001* |
| Hyperuricemia | 32.10 | 3.96 – 260.48 | 0.001* |
| Overweight and obesity | 1.11 | 0.29 – 4.19 | 0.879 |
| Reduced eGFR | 11.23 | 2.84 – 44.35 | 0.001* |
| Hypertriglyceridemia | 15.09 | 2.98 – 76.54 | 0.001* |
| Hypercholesterolemia | 1.01 | 0.375 – 2.56 | 0.761 |
Table 6. Bivariate analysis of factors associated with gout.
Increasing age, menopause, diuretic use, aspirin use, consumption of alcohol, overweight and obesity, reduced estimated glomerular filtration rate <60ml/min, consumption of soft drinks, male gender and serum triglycerides were associated with hyperuricemia (Table 7).
| Variable | Odds Ratio | 95% confidence interval | P value |
|---|---|---|---|
| Reduced eGFR | 8.27 | 3.01 – 22.76 | 0.001* |
| High Triglycerides | 5.28 | 3.01 – 9.27 | 0.001* |
| High Total cholesterol | 1.65 | 0.93 – 2.94 | 0.084 |
| Hypertension | 1.38 | 0.85 – 2.25 | 0.183 |
| Overweight and obesity | 2.15 | 1.30 – 3.56 | 0.003* |
| Sex (male gender) | 2.6 | 3.32 – 12.8 | 0.001* |
| Age >45 years | 2.45 | 1.50 – 4.01 | 0.001* |
| Hyperglycemia | 1.88 | 0.68 – 5.18 | 0.218 |
| Shellfish consumption | 0.59 | 0.13 – 2.71 | 0.503 |
| Soft drink intake | 6.36 | 4.05 – 18.64 | 0.002* |
| Alcohol intake | 4.63 | 2.86 – 5.10 | 0.032* |
| Aspirin intake | 2.85 | 1.37 – 5.93 | 0.004* |
| Diuretic use | 3.37 | 1.58 – 7.16 | 0.002* |
| Menopause | 5.98 | 2.24 – 6.31 | 0.005* |
Table 7. Bivariate analysis of factors associated with hyperuricemia.
Discussion
The prevalence of gout in this study was 2.25%. This is significantly higher than the finding by in Jos, Nigeria by Courage et al [14] who carried out a study on the prevalence of musculoskeletal in a rural community using the Community – Oriented Program for the Control of Rheumatic Diseases (COPCORD) protocol. The prevalence of gout in the community was found to be 0.1%. In the study, subjects who gave history of possible rheumatological disease were later seen in a specialist rheumatology clinic where further investigations were done to adequately classify the diagnoses. This might explain the lower prevalence of gout in the community. Also, the prevalence of gout in this study is higher than findings in Shantou in China (1.08%) [15], Tehran in Iran (0.13%) [16] and Vietnam (0.14%) [17]. These studies mostly used the 1977 ACR criteria for the diagnosis of gout. In the study in Tehran the diagnosis of gout was based on clinical judgment only. This may partly explain the lower prevalence in the study.
The 2.25% prevalence of gout in this study is lower than the finding in the Nutrition and Health Survey in Taiwan from 1993 – 1996 and 2005 – 2008 where the prevalence of gout in 1993 – 1996 and 2003 – 2005 were 4.74% and 8.21% among men and 2.19% and 2.33% among women [18]. The reason for this may be because the diagnosis of gout in the survey was by report by the participants, not by the researchers. The prevalence of gout in this study is similar to that in the NHANES III study (between 1988 – 1994) in USA (2.7%) [19] and also the NHANES 2007 – 2008 study (3.9%) [10]. The pooled prevalence of gout in China as reported by Rui et al [20] was 1.1% which is lower than the finding in this index study. The finding is also higher than that reported in United Kingdom and Germany (1.4%) [21].
The prevalence of hyperuricemia in this study is similar to the finding by Alikor et al [22] in a community in the Niger Delta region of Nigeria but lower than the finding by Ewenighi et al in Abakaliki [23]. The reason may be because the study in Abakaliki was carried out only among men [23]. The prevalence of hyperuricemia in this study is also lower than that reported by Kamdem et al [24] in Cameroun who reported a prevalence of 31.8% among newly diagnosed, treatment naïve subjects. The higher prevalence in that study may be explained by the fact that all the subjects were hypertensive and hypertension is known to be a modifiable risk factor for hyperuricemia and gout. It is also higher than the finding in Taiwan by Shao-Yuan et al [18] who found prevalence of hyperuricemia among both sexes to be 15.6% and also higher than the pooled prevalence of hyperuricemia in China (13.3%) [20]. However, Shao-Yuan et al used a higher cut – off values to define hyperuricemia (>7.7mg/dl in men and >6.6mg/dl in women) and this might have underestimated the prevalence of hyperuricemia. The pooled prevalence of hyperuricemia in China by Rui et al was a systemic review and the prevalence ranged from 5.5 – 23.6%. In the NHANES 2007 – 2008 survey in USA [10], the prevalence of hyperuricemia was 21.4% which is similar to the finding in this study. The cut – off values for serum uric acid is similar in both studies (7.0mg/dl for men and 5.8mg/dl for women).
Gout mostly affected more than one joint in this study as only one subject had monoarticular manifestation. This is similar to the finding by Mody and Naidoo in 1984 in South African blacks [25]. This finding is at variance from the finding by Adelowo et al [26] where more than 50% of the patients had monoarticular presentation. The reason may be because of the relatively small number of gout subjects in this study.
Gout is known to be associated with comorbidities especially renal impairment and cardiovascular risk factors [27]. Seven of the nine subjects (77.8%) that had gout also had hypertension. This is higher than the finding by Esmaeilzadeh et al in Iran who found that among gout patients attending a hospital in Imam, 34% were hypertensive [28]. It is also higher than 58% reported by Emilio Gonzalez in 2012 [29]. The reason for this higher prevalence of hypertension in this index study may be because of the difference in study population as idiopathic hypertension has a higher prevalence among blacks. Adelowo et al reported the frequency of hypertension among gouty arthritis patients that attended a private rheumatology clinic in Lagos to be 72/146(49.3%) [26]. This is lower than the finding in this index study. The reason for this difference is not clear but may not be unrelated to the relatively few gout cases in this study.
Six out of the nine subjects (66.9%) had evidence of hyperlipidemia. This is similar to the report by Adelowo et al where 53.3% of gout patients had hypercholesterolemia and 11.4% had hypertriglyceridemia [26]. It is also similar to the finding in Iran where 64% of gout patients were found to have associated hyperlipidemia [28]. It is lower than the report by Emilio where he reported that 45% of gout patients had dyslipidemia and 33% had a combination of hypertension and dyslipidemia [29].
Diabetes mellitus is an important co – morbid disease in gout. Surprisingly in this study, none of the nine patients with gout had associated diabetes mellitus. This is contrary to the study by Adelowo et al where 4.1% of gout patients had diabetes mellitus [26]. It is also at variance with the report from Kenya by Oyoo in 2004 where 7.7% of gouty arthritis patients attending a rheumatology clinic in Nairobi had diabetes mellitus [30]. Mody and Naidoo also reported that 10.5% of South African blacks with gout had associated diabetes mellitus [25]. Also, a study from Iran showed that 9% of gout patients had diabetes [28]. The reason for this difference may be because of the relatively few gout cases identified in this study.
Obesity is a non-communicable disease that has been associated with increased morbidity and mortality, from increased cardiovascular events and stroke. Various studies have shown increased frequency (or prevalence) of overweight and obesity among gout patients. This study revealed that 22.2% of the gout subjects had overt obesity while 44.4% were overweight. This supports the earlier report be Adelowo et al where 12.7% of gout patients were obese [26]. The study on gout patients in Iran showed that 50% of the patients were overweight while 9% were obese [28]. Obesity was more common among gout patients in Nairobi as Oyoo reported that 90.5% of gout patients were obese [30]. Report from Togo by Mijinyawa is also similar to the finding in this index study where 38% of gouty arthritis patients were obese [31].
Alcohol consumption is known to increase serum uric acid, trigger acute attacks of gout and also precipitate recurrent attacks. In this study, significant number (n=7) of the subjects with gout consumes alcohol. This is higher than the finding by Adelowo et al where only 17.8% of gout patients took alcohol [26]. The reason for this disparity is not clear. However, studies from Togo and Nairobi reported similar high alcohol consumption rate among gout patients. Mijinyawa reported alcohol consumption rate of 74% among gout patients in Lome, Togo [31] while Oyoo reported even a higher percentage, 100% in Nairobi [30]. Esmaeilzadeh reported alcohol consumption among gout patients in Imam, Iran to be only 35.7% [28]. This difference may be because the study population in Imam, Iran was made up of predominantly Muslims who abhor alcohol consumption.
Lucia et al reported that alcohol consumption, hypertriglyceridemia, obesity, psoriasis, chronic kidney disease and diuretic use are all associated with gout [32]. Mahmoud et al in Sudan reported that among 101 patients with gout that were seen in clinic in Khartoum state, 81.2% were males [33]. Also, in a cohort of 72 Italian patients with gout that were studied by Zampogna et al, 87.5% were males [34]. These two studies support the finding in this index study that being male is associated with increased probability of developing gout. Numerous studies have shown that males are more likely to have higher uric acid levels than females [18,20,22] This is similar to the finding in this index study. Alikor et al reported an associated between serum uric acid and male gender [22]. Ewenighi et al reported an association between serum uric acid, body mass index and age among hyperuricemic male subjects in Abakaliki [23]. Kamdem et al reported an association between age greater than 55 years, obesity, high low density lipoprotein, high serum triglycerides and hyperuricemia [24]. Consumption of soft drink was found to be negatively associated with hyperuricemia. This is different from earlier studies by Choi et al [35,36] and the reason for this difference is not known.
The subjects that were diagnosed with gout in this study were all older than 45 years of age and as much as 55.6% were aged ≥60 years. This shows that the probability of developing gout in this setting increases with age. This finding is similar to findings in USA [10], Taiwan [18], China [37] and the United Kingdom [38] where population – based studies showed that the prevalence of gout increased with advancing age.
Conclusion
This study has revealed that gout is a significant ailment in Agbowa community of Lagos state Nigeria with a prevalence of 2.25%. Hyperuricemia was also prevalent in the community. Most of the gouty arthritis subjects had associated hyperuricemia with a prevalence of 66.7%. Hypertension, dyslipidemia, overweight and obesity were the co-morbid conditions identified among gout subjects. The factors found to be associated with gout were older age, male gender, use of diuretics, aspirin and alcohol, hypertriglyceridemia, hypertension, hyperuricemia and reduced eGFR. These factors, in addition to overweight and obesity, menopause, intake of soft drinks were associated with hyperuricemia. Gouty arthritis was mostly oligoarticular and associated co – morbidities identified were hypertension, overweight and obesity and hyperlipidemia.
Limitations
- Inability to do joint aspiration, synovial biopsy or tophus examination for identification of MSU crystals made definitive diagnosis of gout difficult. The ACR criteria cannot replace polarized microscopy or DECT for MSU identification. The use of ACR criteria for the diagnosis of gout in this study might have overestimated the prevalence of gout.
- Recall bias on the part of the respondents.
- Small number of gout subjects identified might have affected the overall pattern of presentation of gout in this study.
References
- Hyon K Choi. Epidemiology of gout. In: Marc C Hochberg, Alan J Silman, Josef S Smolen et al. Rheumatology (6th edn). Philadelphia. Elsevier. (2015).
- Singh JA. When gout goes to the heart:does gout equal a cardiovascular disease risk factor?Ann Rheum Dis. 74,631-4 (2015).
- Choi HK, Mount DB RA. Pathogenesis of gout. Ann intern Med. 143,499-516 (2005).
- Wells AF, Macdonald PA, Chefo S et al. African American patients with gout:efficacy and safety of febuxostat vs allopurinol. BMC Musculoskelet Disord. 13,15 (2012).
- Lachy McLean, Nicola Dalbeth. Etiology and pathogenesis of gout. In: Marc C Hochberg, Alan J Silman, Josef S Smolen et al. Rheumatology (6th edn). Philadelphia, Elsevier. (2015).
- Annemans L, Spaepen E, Gaskin M et al. Gout in the UK and Germany: prevalence,comorbidities and management in general practice 2000-2005. Ann Rheum Dis. 67,960-966 (2008).
- Hall AP, Barry PE, Dawber TR et al. Epidemiology of gout and hyperuricemia, a long term population study. Am J Med. 42,27-37 (1967).
- Kuo CF, Grainge MJ, Mallen C et al. Comorbidities in patients with gout prior to and following diagnosis:case-control study. Ann rheum dis. 10,1-8 (2014).
- Luk AJ, Simkin PA. Epidemiology of Hyperuricemia and Gout. Am J Manag Care. 11,435-442 (2005).
- Zhu Y, Pandya BJ, Choi HK. Prevalence of Gout and Hyperuricemia in the US General Population The National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 63,3136-3141 (2011).
- Charan J, Biswas T. How to Calculate Sample Size for Different Study Designs in Medical Research? Indian J Psychol Med. 35(2),121-126 (2013).
- Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 150,604-612 (2009).
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 15,539-553 (1998).
- Courage UU, Stephen DP, Lucius IC et al. Prevalence of musculoskeletal diseases in a semi-urban Nigerian community: results of a cross-sectional survey using COPCORD methodology. Clin Rheumatol. 36(11),2509-2516 (2017).
- Zeng S, Gong Y, Zhang Y et al. Changes in the Prevalence of Rheumatic Diseases in Shantou , China , in the Past Three Decades:A COPCORD Study. PLoS One. 10(9),1-15 (2015).
- Davatchi F, Jamshidi A, Banihashemi AT et al. WHO-ILAR COPCORD Study (Stage 1 , Urban Study) in Iran. J Rheumatol. 35(7),1384-1390 (2008).
- Tran Thi Minh Hoa, John Darmawan, Shun Le Che et al. Prevalence of the rheumatic diseases in urban Vietnam: a WHO-ILAR COPCORD study. J Rheumatol. 30,2252-2256 (2003).
- Chuang SY, Lee S, Hsieh YT et al. Trends in hyperuricemia and gout prevalence:Nutrition and Health Survey in Taiwan from 1993-1996 to 2005- 2008. Asia Pac J Clin Nutr. 20(2),301-308 (2011).
- Lawrence RC, Helmick CG, Arnett FC et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 41,778-799 (1998).
- Liu R, Han C, Wu D et al. Prevalence of Hyperuricemia and Gout in Mainland China from 2000 to 2014:A Systematic Review and Meta-Analysis. Biomed Res Int. 2015,1-12 (2015).
- Ciancio G, Bortoluzzi A, Govoni M. Epidemiology of gout and chondrocalcinosis. Reumatismo. 63(4),207-220 (2011).
- Alikor CA, Emem-Chioma PC, Odia OJ. Prevalence of hyperuricaemia in a rural population of Nigerian Niger Delta region. Niger J Med. 22(3),187-192 (2013).
- O EC, Uchechukwu D, Uchechukwu E et al. Prevalence of hyperuricemia and its risk factors in healthy male adults from Abakaliki metropolis, Nigeria. J mol pathophysiol. 4(3),94-98 (2015).
- Kamdem F, Doualla MS, Kemta Lekpa F et al. Prevalence and factors associated with hyperuricaemia in newly diagnosed and untreated hypertensives in a sub-Saharan African setting. Arch Cardiovasc Dis. 109,527-532 (2016).
- Mody G M NP. Gout in South African blacks. Ann Rheum Dis. 6,394-397 (1984).
- Adelowo OO, Umar A OS. Gouty Arthritis in Nigerians: clinical and laboratory correlates. Afr J Rheumatol. 2,23-28 (2014).
- Richette P, Clerson P, Périssin L et al. Revisiting comorbidities in gout: a cluster analysis. Ann Rheum Dis. 74,142-147 (2013).
- Esmaeilzadeh M, Sorkhani AB, Rad MT et al. Epidemiology and Clinical Manifestation of GOUT in Imam Khomeini Hospital in Tehran, Iran. Iran Red Crescent Med J. 11,259-264 (2009).
- Gonzalez EB. An update on the pathology and clinical management of gouty arthritis. Clin Rheumatol. 31,13-21 (2012).
- Oyoo GO. Gout in patients attending a Rheumatology clinic in Nairobi, Kenya. Health Line. 8,67-70 (2004).
- Mijinyawa M. Gout in patients attending the rheumatology unit of Lome hospital. Br J Rheum. 34,843-846 (1995).
- Cea Soriano L, Rothenbacher D, Choi HK et al. Contemporary epidemiology of gout in the UK general population. Arthritis Res Ther. 13,R39 (2011).
- Mudawi MME, Mukhtar FSM. Assessment of awareness about management and drugs used for treatment of gout in Khartoum State, Sudan. World J Pharm Sci. 2,443-448 (2014).
- Zampogna G, Andracco R, Parodi M et al. Clinical features of gout in a cohort of Italian patients. Reumatismo. 61(1),41-47 (2009).
- Choi JWJ, Ford ES, Gao X et al. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 59,109-116 (2008).
- Choi HK, Atkinson K, Karlson EW et al. Alcohol intake and risk of incident gout in men: a prospective study. Lancet (London, England). 363,1277-1281 (2004).
- Yu JW,Yang TG,Diao WX et al. Epidemiological study on hyperuricemia and gout in Foshan areas,Guangdong province. Chinese J Epidemiol. 31,860-862 (2010).
- Mikuls TR, Farrar JT, Bilker WB et al. Gout epidemiology: results from the UK General Practice Research Database, 1990-1999. Ann rheum dis. 64,267-272 (2005).
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref