Perspective - Interventional Cardiology (2009) Volume 1, Issue 2
Prevention and treatment of complications during carotid artery stenting
- Corresponding Author:
- Francesco Bedogni
Department of Interventional Cardiology & Cardiovascular Interventional Radiology
Istituto Clinico S. Ambrogio, Gruppo S. Donato, Milan, Italy
Tel: +39 02 3312 7714
Fax: +39 02 3312 7038
E-mail: francesco.bedogni@ grupposandonato.it
Abstract
Keywords
carotid, complications, prevention, stent, treatment
Since its introduction in the early 1980s, carotid artery stenting (CAS) acquired progressive diffusion worldwide. Technical advances allowed the percutaneous treatment to be a valid alternative to the traditional surgical treatment, carotid endarterectomy (CEA), which had already proved to be effective in preventing major complications with respect to medical treatment in both symptomatic and asymptomatic patients [1,2]. The advantages of endovascular treatment over CEA are reduced invasiveness, local anesthesia, shorter hospitalization and therefore better tolerability, preserved cerebral perfusion during the procedure in the majority of cases and the possibility of a combined treatment of common and internal carotid artery lesions. However, despite these advantages, the aim of CAS is to reach the same level of safety as CEA stated by the guidelines (major complications rate <6 and <3% in symptomatic and asymptomatic patients, respectively) [3]. In particular, the risk of any type of prophylactic intervention should not exceed the inherent risk of the natural history, therefore safety must be the absolute end point during carotid stenting.
Available data from randomized, controlled trials, as well as from study registries in both high- and low-risk populations, are controversial [4–12]. Indeed, early published positive results [4–9] have been questioned by more recent data [10–13] in which a high rate of complications has been reported. Of note, is that among possible drawbacks of all the available evidences are the extremely diverse operators’ experience, and the availability in most of the studies of only one specific device. Following these negative results, the foreseen growth of CAS over CEA did not take place. The key for success of CAS is the reduction of both incidence and consequences of procedural as well as periprocedural complications by means of adequate prevention and treatment, respectively.
The aim of this article is to summarize the most common complications, and provide a perspective on their prevention and treatment using different strategies and devices.
General considerations
In order to keep the rate of complications as low as possible, the first crucial step in performing a CAS procedure is to recognize possible factors associated with an increased risk of complications. A number of publications over recent years have tried to assess which are the main predictors of the overall risk of the procedure. Different publications highlighted different features; however, it is pivotal to consider that several of the known risk factors are usually combined in the same patient, particularly in the elderly. Main predictors can be summarized as clinical [14–18], anatomical [19–22] and operator strategy and experience (Box 1) [16–18].
Box 1: Predictors of complications during coronary artery stenting.
A cautious approach, especially at an early stage of the learning curve of the first operator, and a tailored approach are mandatory to reduce the incidence of complications. It is difficult to suggest a threshold number of cases beyond which it is reasonable to define an ‘expert operator’; moreover, there is no training requirement/ level at which patient safety is guaranteed. The Society for Vascular Surgery is signatory to a joint society document that specifies 30 carotid angiograms and 25 carotid stents as the recommended prerequisite for credentialing in CAS [21]. This cut-off is obviously arbitrary.
While complications might occur, a prompt and adequate diagnosis along with the right therapeutic choice are extremely important to reduce the damage.
The systematic overview of complications (i.e., their presentations, signs, symptoms and solutions) we are presenting might be helpful for a wide readership aiming at improving prevention and management.
Different complications, grouped according to different stages of the procedure, are listed in Box 2.
Access-site complications
Femoral arterial access is commonly used for CAS. As in any other percutaneous procedure, it has its own set of complications. Pre-existing peripheral vascular disease may predispose to complications such as hematoma, pseudoanuerism, arteriovenous fistulae, thrombosis, iatrogenic dissection and retroperitoneal hematoma. Such complications range from local, asymptomatic to life-threatening conditions.
Bleeding complications are largely more common, considering the mandatory use of dual antiplatelet therapy in every CAS procedure and the intraoperative administration of weight‑adjusted unfractioned heparin.
▪ Local hematoma
Growing hematomas are the most common complication. They require a treatment (transfusion or surgical approach) in less than 0.5% of the cases [22].
▪ Pseudoaneurism & arteriovenous fistulae
Because of the concomitant administration of antiplatelet drugs, these complications often require a percutaneous or surgical treatment [23]. The majority of small, asymptomatic psuedoaneurisms can be successfully managed conservatively with a compressive dressing of the groin [24]. Larger pseudoaneurisms have to be treated. Options are ultrasound-guided compression [25] or ultrasound-guided thrombin injection [26]. In a few cases (4.7%), surgical repair is necessary [23].
▪ Retroperitoneal hematoma
Retroperitoneal hematoma is a very rare but life-threatening complication, with an incidence of 0.15%. Hemodynamic instability up to a hemorrhagic shock are the typical signs. CT scan is mandatory if retroperitoneal bleeding is suspected.
Identified risk factors for access-site complications are: hypertension, high body mass index, improper site arterial puncture, inadequate compression, excessive anticoagulation and use of a large diameter sheath [27,28].
Prevention of these complications consists of correct puncture of the common femoral artery, use of a 6 F sheath, its early removal and appropriate use of closure devices. When a femoral approach is difficult, radial or brachial approach can be considered.
Catheterization of the common carotid artery
Catheter navigation in the aortic arch as well as the selective engagement of the common carotid artery (CCA) are crucial points. Carotid access is not protected. Atheromatous aortic arch and CCA disease, angulated CCA take-off and tortuosity are responsible for most procedural failures. Furthermore, approximately 40% of the major complications during CAS procedures occur during the selective catheterization phase [29].
Moreover, type 2 and 3 aortic arches are the main predictors of failure and complications [19]. In particular, the rate of failure and neurological complications is fourfold higher in patients with abnormal arch anatomy compared with those with normal anatomy [30]. Thus, it is mandatory to comprehensively assess the anatomical features of the aortic arch before starting the procedure.
A CT scan or an aortic arch angiography are necessary to recognize access hurdles and plan the best and least aggressive approach. For example, in some situations such as the ‘bovine arch’, radial or brachial approaches are the preferable options. Prolonged and aggressive manipulation should be avoided considering that a failure is better than a complication and CEA remains an option.
There are basically two different approaches: the first requires a deep intubation of the CCA with a long sheath or guiding catheter landing close to the lesion; the second consists of the placement of the guiding catheter at the ostium of the CCA far from the lesion (‘cardiologists technique’). The latter has to be chosen in the presence of a diffuse CCA disease, or when the external carotid artery is occluded, thus preventing the placement of a stiff wire. In all the other cases the former is preferable to achieve a better support and minimize the size of the sheath.
Availability of different tip angles and shapes of the guiding catheters allows correct engagement regardless of the specific anatomy of the patient. Gentle manipulation aiming at keeping coaxiality is mandatory in order to avoid common complications such as embolism and dissection.
▪ Embolism
Cerebral embolization during the catheterization phase may be due to air injection, thrombus from the aortic arch or the catheter, or atherosclerotic debris. The latter condition is the most dangerous as there is no treatment and it may lead to a large area of cerebral infarct. Prevention of any kind of embolization consists of upstream administration of dual antiplatelet treatment, intraoperative administration of anticoagulant, continuous pressure monitoring at the tip of the catheter, use of a soft-tip sheath or use of a catheter with telescopic technique. Treatment of the embolism from the aortic arch is complicated and has a poor chance of success. First of all, it is mandatory to get a prompt diagnosis, then in the case of air injection is important to maintain an adequate blood pressure to protect the cerebral perfusion, and ischemia should disappear quickly. If neurological impairment persists, hyperbaric oxygen therapy may be considered [31]. Differential diagnosis with thrombus and atherosclerotic debris is difficult. If the embolus is recognized as coming from a thrombus, possible treatment options include dilation, aspiration and thrombus retrieval with specific devices, or alternatively, waiting and monitoring. As ongoing dual antiplatelet therapy and unfractioned heparin administered intraoperatively are the current standard practice, thrombolysis appears obsolete or at best feasible in a very small percentage of cases. In the presence of an embolic complication, an intracranial angiography should be performed to assess the extension of the involved area.
▪ Dissection
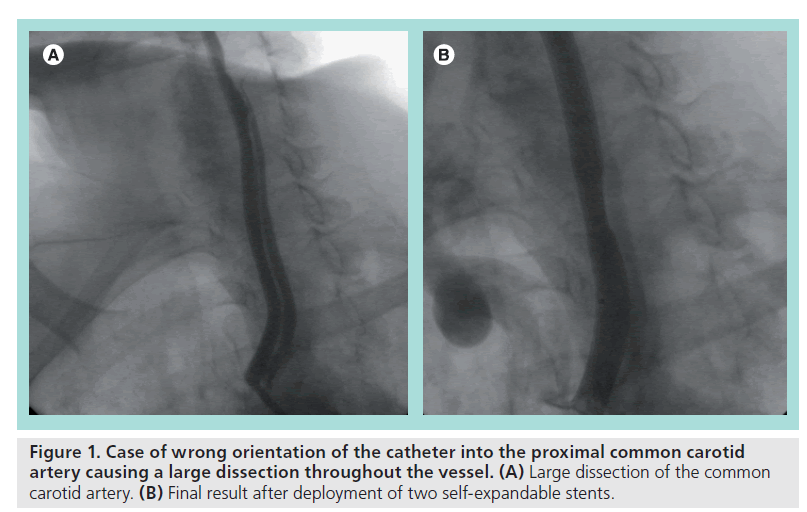
Common carotid artery dissection is a less frequent complication during the catheterization phase compared with embolization. It is basically due to an aggressive manipulation of the guiding catheter against the arterial wall and/or without coaxiality. Dissection may impair blood flow, determine a mechanical occlusion and predispose to thrombosis. Significant dissections always require prompt treatment by means of stent implantation (Figure 1 shows an improper approach to the aortic arch causing a large CCA dissection treated with the implantation of two self-expandable stents).
Treatment of the lesion
Once the hurdles of the access site and engagement of the CCA are resolved, it is necessary to face all the issues related to the treatment of the internal carotid artery (ICA) lesion. Complications may occur during every step of the treatment: lesion crossing, predilation, stent deployment, postdilation and embolic protection device (EPD) retrieval.
Plaque characteristics and proximal and distal vessel tortuosity are the main features to bear in mind when planning the stenting strategy.
Consistent reports demonstrate that use of EPDs is associated with a lower rate of complications compared with an unprotected procedure [32]. However, EPD-related complications have been reported with a not negligible incidence of 1.1% of the cases [33]. Recognized complications include: spasm, dissection, embolization, thrombosis, plaque prolapse and stent underexpansion (Box 2).
▪ Spasm
Occurrence of ICA spasm is the most frequent but least severe complication. It usually occurs after EPD placement, in particular when the device is oversized and placed in a tortuous segment [34]. It can also occur at the distal edge of the stent. It may impair the blood flow up to an overt occlusion. Therefore, it is crucial to recognize the spasm among other possible causes of occlusion, which are occlusion of the filter by clot, thrombosis or dissection.
A clear knowledge of the particular ICA anatomy and tortuosity before the EPD placement is absolutely pivotal.
After diagnosis, treatment is not mandatory if well tolerated. Nitrates can be helpful, but the blood flow usually significantly improves only after the retrieval of the EPD.
▪ Dissection
Dissection of ICA is a rare complication. It may occur when using stiff wires or filters in a sharply angulated vessel, and with overinflated distal balloon occlusion systems. Treatment of this complication is the deployment of a stent in order to seal the intimal flap and improve the flow. There are essentially two options: self-expandable or coronary balloon-expandable stents. The choice has to be made according to the length of the dissection, the size and the shape of the vessel, the trackability of the stent, tortuousity and its ease of deployment.
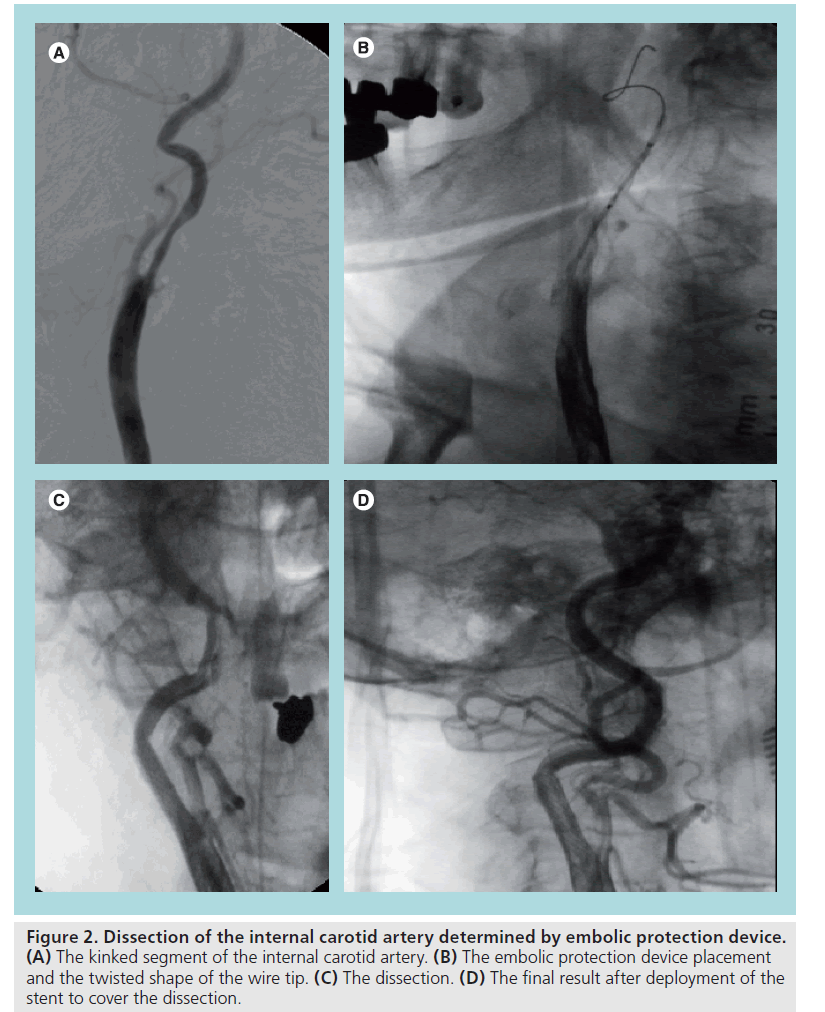
A specific classification of different types of dissection is not available; however, we can use the classification made for the coronary dissection (NHLBI). All of the flow limiting and/or significant dissections must be treated. It is questionable whether a small intimal tear has to be covered (Figure 2 shows a dramatic case of ICA dissection due to the placement of an EPD in a kinked vessel causing a severe cerebral ischemia). The ‘golden rule’ is to avoid the use of stiff filters in the presence of severe tortuousity and prefer proximal occlusive protection devices (MOMA™, GORE™ or distal balloon occlusive devices such as Twin One Theron™). Use of IIb/IIIa inhibitors or anticoagulants can be considered despite the lack of supporting data.
Figure 2: Dissection of the internal carotid artery determined by embolic protection device. (A) The kinked segment of the internal carotid artery. (B) The embolic protection device placement and the twisted shape of the wire tip. (C) The dissection. (D) The final result after deployment of the stent to cover the dissection.
▪ Embolization
This is the most frequent complication during CAS procedures despite the frequent use of EPDs at present. Transcranial Doppler studies have demonstrated that the phenomenon of ‘microembolic shower’ can occur during every phase of the procedure [35,36]. Some degree of embolization can be well tolerated when a preserved cerebral reserve is present. Prior stroke, microangiopathy, insufficient collateral circulation or dementia due to reduced cerebrovascular reserve are predictors of a higher risk of experiencing neurological deficits after CAS. A plaque without risk of embolization does not exist.
Predisposing clinical factors have been recognized: symptoms within 6 months prior, older age (>80 years) and impaired cerebral reserve. Noninvasive as well as invasive imaging tools such as echocolor Doppler and angiography may show anatomical predisposing factors. It has been demonstrated that echolucent plaques of the ICA carry a higher risk of embolization [37–39], as do highly calcified plaques [16]. There are some angiographic criteria that are independent predictors of an increased risk of embolization: more than 15-mm long lesions (large plaque burden) and the involvement of the ostium of the ICA. It is still debated if lesions with the ‘string sign’ might be associated with a higher risk. Of note is that it is mandatory to keep the stability of the filter in order to avoid intimal damage and increase the amount of particles released by even very slight movement.
When clinical and/or anatomical predictors of a higher risk of embolization are present, the choice of the correct device is even more crucial, despite Iyer et al. suggesting that the type of EPD appears to be unrelated to the clinical outcome [40]. Among the drawbacks of every distal filter, the possibility of embolization during the crossing of the lesion and also the potentially incomplete capture of atheroslcerotic debris must be taken into account. Therefore, we believe that when a high-risk lesion is present, the use of a proximal occlusion device creating a reverse flow is safest (MOMA, GORE and PARODI™).
Besides the choice of EPD, the choice of the right stent is also pivotal. Bosier et al. demonstrated that in symptomatic patients, the free cell area is directly correlated with the risk of neurological events. Thus, it is reasonable to advocate the use of a closed cell stent in the presence of a high‑risk plaque.
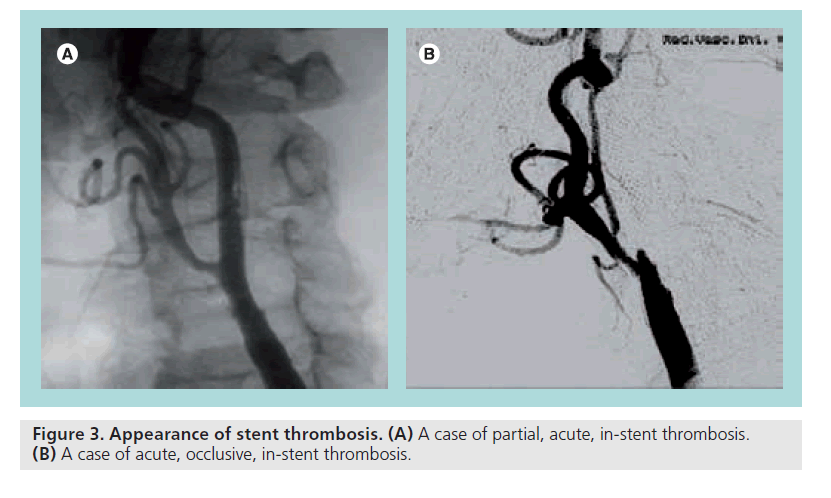
▪ Thrombosis
Thrombosis is a rare but significant complication, which can partially occupy the lumen or be occlusive (Figure 3). Predisposing factors can be prolonged slow flow caused by spasm, dissection, apposition of large debris in the filter, inadequate antiplatelet/anticoagulant therapy and pre-existing hypercoagulable status that must be known before starting the procedure. Treatment must be rapid and effective to reduce the ischemic damage. Pharmacological approaches include thrombolysis or IIb/IIIa inhibitors. Mechanical approaches include urgent surgical removal, thrombus aspiration, thrombus retrieving catheter, stent-in-stent and reverse flow. The case depicted in Figure 3A, a thrombus apposition within the stent struts has been treated with thrombo aspiration, IIb/IIIa inhibitors and ‘closed cell’ stent deployment with a good angiographic result but major neurological consequences.
In Figure 3B, a case of occlusive thrombosis is shown. It has been quickly and successfully treated surgically.
▪ Plaque prolapse
This is probably very frequent but largely underdiagnosed. Soft, large plaques are probably prone to this complication. It is likely that plaque prolapse is responsible for the high rate of neurological complications observed after the procedure, during the hospital stay. Even high resolution angiography is an inadequate tool to diagnose plaque prolapse. There are often images of doubtful interpretation.
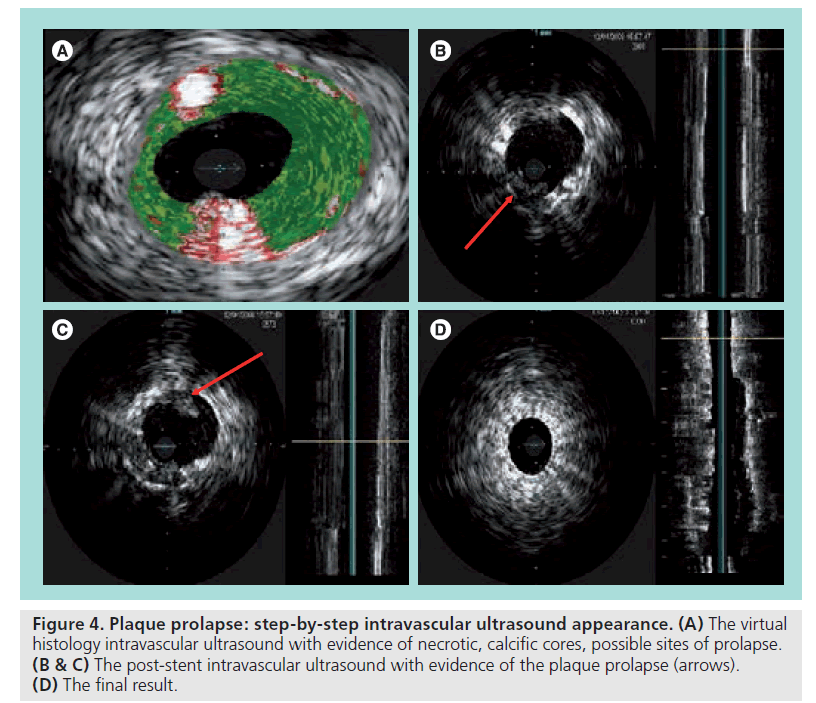
In our experience, intravascular ultrasound and virtual histology (IVUS, VH‑IVUS) have demonstrated good reliability in the qualitative and quantitative assessment of this phenomenon; moreover, the use of an open cell stent in soft lesions with large plaque burdens and necrotic cores at the VH-IVUS may predispose to a plaque prolapse, in particular if large diameter balloons for postdilation are used. Thus, in the presence of predicting factors, the use of a closed cell stent is advisable. This point is still debated; however, emerging evidence and cases as shown in Figure 4 seem to support this hypothesis [41,42].
Figure 4: Plaque prolapse: step-by-step intravascular ultrasound appearance. (A) The virtual histology intravascular ultrasound with evidence of necrotic, calcific cores, possible sites of prolapse. (B & C) The post-stent intravascular ultrasound with evidence of the plaque prolapse (arrows). (D) The final result.
No data are available suggesting whether this condition should always be treated; however, considering the lack of any type of classification of the severity of plaque prolapse, we believe that in the presence of evident prolapsing material at IVUS it is advisable to cover the area with another closed cell stent with an EPD in place – it is a simple and effective procedure. We usually check the final result with a further IVUS.
▪ Stent underexpansion
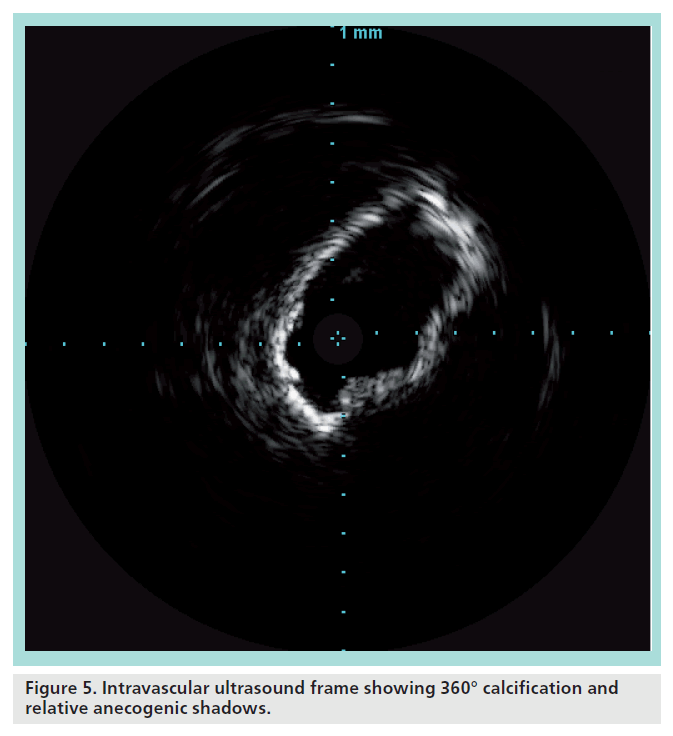
The impossibility of obtaining an adequate stent expansion (i.e., a sufficient minimum lumen diameter, affecting the long-term result) is frequent when treating highly calcified lesions. Accurate imaging before the intervention should identify those situations, even if the resolution and quality of the ecocolor Doppler image is greatly affected by anecogenic shadows caused by the calcium itself. In our experience, preprocedural IVUS has proven to be helpful in clearly visualizing the longitudinal and circumferential extent of the calcification.
When the calcification involves most of the circumference of the vessel (Figure 5), predilation with a cutting balloon is mandatory. In these cases, a stent with high radial force (i.e., nitinol closed cell) should be preferred. In these cases, postdilation should be carried out with a slightly undersized, noncompliant balloon. A residual stenosis of 30% is largely acceptable.
▪ Filter retrieval failure
Failure of filter retrieval is frequent, but rarely impossible to overcome percutaneously. Vessel tortuosity and underexpansion of the proximal edge of the stent are the main causes of a difficult filter retrieval. Abrupt manipulations can result in the entrapment of the filter within the stent struts and sometimes in the detachment of the filter element. Careful manipulation is crucial.
In failed filter retrieval, it may be helpful to move the patient’s head, have the patient swallow or chose a higher torquability retrieval sheath. In the unlucky case of filter fracture, we recommend ‘jailing’ the filter against the arterial wall with an additional stent.
▪ Dislodgement of material during filter retrieval
This complication is very rare. It usually occurs when a large amount of debris is entrapped in the filter and can cause distal embolization, thus appearing as an impaired blood flow. Soft, large plaques are probably prone to this complication and the use of large balloons may be a predisposing factor.
Future perspective
The reduction of complications is the main issue with CAS. A complication is often the result of multiple elements, ranging from inadequate patient monitoring and radiological imaging equipment, to improper indications or insufficient experience of the operator. A correct strategy planned before the procedure is crucial, especially in high-risk situations.
The next step to improve the currently good result of CAS is to facilitate the ability to select the right tools from the wide range of available technologies: the ‘tailored approach’. IVUS is an extremely useful procedural tool in several conditions and may help reduce the rate of complications by guiding the decision-making process. Moreover, an honest and lucid analysis of complications should always serve to help us avoid the same mistakes in the future by learning from them.
Executive summary
▪ The reduction of complications is the main issue with carotid artery stenting.
▪ As the risk of any type of prophylactic intervention should not exceed the inherent risk of the natural history, safety must be the absolute end point during carotid stenting.
▪ The first crucial step in performing a carotid artery stenting procedure is to recognize possible factors associated with an increased risk of complications, which can be summarized as: clinical, anatomical and operator’s strategy and experience.
▪ A proper learning curve is mandatory, and different threshold number of cases have been suggested; however, there is no training requirement/level at which patient safety is guaranteed.
▪ A correctly planned strategy along with a thorough knowledge of all the available tools are key for the necessary ‘tailored approach’.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪of interest
▪▪ of considerable interest
- North American Symptomatic Carotid Endarterectomy Trial Collaborators: Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N. Engl. J. Med. 325, 445–453 (1991).
- Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA 273, 1421–1428 (1995).
- Goldstein LB, Adams R, Alberts MJ et al.; American Heart Association/American Stroke Association Stroke Council; Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; Quality of Care and Outcomes Research Interdisciplinary Working Group; American Academy of Neurology: Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline. Stroke 37, 1583–1633 (2006).
- Wholey MH, Wholey M, Mathias K et al.: Global experience in cervical carotid artery stent placement. Catheter Cardiovasc. Interv. 50(2), 160–167 (2000).
- Yadav JS, Wholey MH, Kuntz RE et al.; Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators: Protected carotid-artery stenting versus endarterectomy in high-risk patients. N. Engl. J. Med. 351(15), 1493–1501 (2004).
- Roubin GS, New G, Iyer SS et al.: Immediate and late clinical outcomes of carotid artery stenting in patients with symptomatic and asymptomatic carotid artery stenosis: a 5‑year prospective analysis. Circulation 103(4), 532–537 (2001).
- CaRESS Steering Committee: Carotid Revascularization Using Endarterectomy or Stenting Systems (CaRESS) Phase I clinical trial: 1‑year results. J. Vasc. Surg. 42(2), 213–219 (2005).
- CAVATAS Investigators: Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet 357, 1729–1737 (2001).
- Gray WA, Yadav JS, Verta P et al.; CAPTURE Trial Collaborators: The CAPTURE registry: predictors of outcomes in carotid artery stenting with embolic protection for high surgical risk patients in the early post-approval setting. Catheter Cardiovasc. Interv. 70, 1025–1033 (2007).
- Hobson RW 2nd, Howard VJ, Roubin GS et al.; CREST Investigators: Carotid artery stenting is associated with increased complications in octogenarians: 30‑day stroke and death rates in the CREST lead-in phase. J. Vasc. Surg. 40, 1106–1111 (2004).
- Mas JL, Chatellier G, Beyssen B et al.; EVA-3S Investigators: Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N. Engl. J. Med. 355(16), 1660–1671 (2006).
- Ringleb PA, Allenberg J, Brückmann H et al.; SPACE Collaborative Group: 30 day results from the SPACE trial of stentprotected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet 368, 1239–1247 (2006).
- Coward LJ, Featherstone RL, Brown MM: Safety and efficacy of endovascular treatment of carotid artery stenosis compared with carotid endarterectomy: a Cochrane systematic review of the randomized evidence. Stroke 36(4), 905–911 (2005).
- Hofmann R, Niessner A, Kypta A et al.: Risk score for peri-interventional complications of carotid artery stenting. Stroke 37, 2557–2561 (2006).
- Chiam PT, Roubin GS, Panagopoulos G et al.: One-year clinical outcomes, midterm survival, and predictors of mortality after carotid stenting in elderly patients. Circulation 119(17), 2343–2348 (2009).
- Chiam PT, Roubin GS, Iyer SS et al.: Carotid artery stenting in elderly patients: importance of case selection. Catheter Cardiovasc. Interv. 72(3), 318–324 (2008).
- Roubin GS, Iyer S, Halkin A, Vitek J, Brennan C: Realizing the potential of carotid artery stenting: proposed paradigms for patient selection and procedural technique. Circulation 113(16), 2021–2030 (2006).
- Sayeed S, Stanziale SF, Wholey MH, Makaroun MS: Angiographic lesion characteristics can predict adverse outcomes after carotid artery stenting. J. Vasc. Surg. 47, 81–87 (2008).
- Macdonald S, Lee R, Williams R, Stansby G; Delphi Carotid Stenting Consensus Panel: Towards safer carotid artery stenting: a scoring system for anatomic suitability. Stroke 40(5), 1698– 1703 (2009).
- Vogel TR, Dombrovskiy VY, Haser PB, Graham AM: Carotid artery stenting: impact of practitioner specialty and volume on outcomes and resource utilization. J. Vasc. Surg. 49(5), 1166–1171 (2009).
- Barr JD, Connors JJ, Sacks D et al.: Quality improvement guidelines for the performance of cervical carotid angioplasty and stent placement. J. Vasc. Interv. Radiol. 14, S321–S335 (2003).
- Vogel TR, Dombrovskiy VY, Haser PB, Graham AM: Carotid artery stenting: impact of practitioner specialty and volume on outcomes and resource utilization. J. Vasc. Surg. 49, 1166–1171 (2009).
- Ates M, Sahin S, Konuralp C et al.: Evaluation of risk factors associated with femoral pseudoaneurysms after cardiac catheterization. J. Vasc. Surg. 43, 520–524 (2006).
- Kent KC, McArdle CR, Kennedy B, Baim DS, Anninos E, Skillman JJ: A prospective study of the clinical outcome of femoral pseudoaneurysms and arteriovenous fistulas induced by arterial puncture. J. Vasc. Surg. 17, 125–131 (1993).
- Kumins NH, Landau DS, Montalvo J et al.: Expanded indications for the treatment of postcatheterization femoral pseudoaneurysms with ultrasound-guided compression. Am. J. Surg. 176, 131–136 (1998).
- Liau CS, Ho FM, Chen MF, Lee YT: Treatment of iatrogenic femoral artery pseudoaneurysm with percutaneous thrombin injection. J. Vasc. Surg. 26, 18–23 (1997).
- Messina LM, Brothers TE, Wakefield TW et al.: Clinical characteristics and surgical management of vascular complications in patients undergoing cardiac catheterization: interventional versus diagnostic procedures. J. Vasc. Surg. 13, 593–600 (1991).
- Ricci MA, Trevisani GT, Pilcher DB: Vascular complications of cardiac catheterization. Am. J. Surg. 167, 375–378 (1994).
- Verzini F, Cao P, De Rango P et al.: Appropriateness of learning curve for carotid artery stenting: an analysis of periprocedural complications. J. Vasc. Surg. 44, 1205–1211 (2006).
- Faggioli GL, Ferri M, Freyrie A et al.: Aortic arch anomalies are associated with increased risk of neurological events in carotid stent procedures. Eur. J. Vasc. Endovasc. Surg. 33, 436–441 (2007).
- Eskandari MK: Preventable complications of carotid stenting. Perspect. Vasc. Surg. Endovasc. Ther. 20, 17–25 (2008).
- Kastrup A, Gröschel K, Krapf H, Brehm BR, Dichgans J, Schulz JB: Early outcome of carotid angioplasty and stenting with and without cerebral protection devices: a systematic review of the literature. Stroke 34, 813–819 (2003).
- Reimers B, Schlüter M, Castriota F et al.: Routine use of cerebral protection during carotid artery stenting: results of a multicenter registry of 753 patients. Am. J. Med. 116, 217–222 (2004).
- Cremonesi A, Manetti R, Setacci F, Setacci C, Castriota F: Protected carotid stenting: clinical advantages and complications of embolic protection devices in 442 consecutive patients. Stroke 34, 1936–1941 (2003).
- Al-Mubarak N, Roubin GS, Vitek JJ, Iyer SS, New G, Leon MB: Effect of the distal-balloon protection system on microembolization during carotid stenting. Circulation 104, 1999–2002 (2001).
- Vos JA, van den Berg JC, Ernst SM et al.: Carotid angioplasty and stent placement: comparison of transcranial Doppler US data and clinical outcome with and without filtering cerebral protection devices in 509 patients. Radiology 234, 493–499 (2005).
- Ohki T, Marin ML, Lyon RT et al.: Ex vivo human carotid artery bifurcation stenting: correlation of lesion characteristics with embolic potential. J. Vasc. Surg. 27, 463–471 (1998).
- Biasi GM, Froio A, Diethrich EB et al.: Carotid plaque echolucency increases the risk of stroke in carotid stenting: the Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) study. Circulation 110, 756–762 (2004).
- Narins CR, Illig KA: Patient selection for carotid stenting versus endarterectomy: a systematic review. J. Vasc. Surg. 44, 661–672 (2006).
- Iyer V, de Donato G, Deloose K et al.: The type of embolic protection does not influence the outcome in carotid artery stenting. J. Vasc. Surg. 46, 251–256 (2007).
- Bosiers M, de Donato G, Deloose K et al.: Does free cell area influence the outcome in carotid artery stenting? Eur. J. Vasc. Endovasc. Surg. 33, 135–141 (2007).
- Schillinger M, Gschwendtner M, Reimers B et al.: Does carotid stent cell design matter? Stroke 39, 905–909 (2008).
▪▪ First manuscript assessing the advantage of a revascularization procedure over medical therapy.
▪▪ Pivotal trial comparing carotid artery stenting (CAS) with an embolic protection device with carotid endarterectomy (CEA).
▪▪ Pivotal trial comparing CAS or carotid artery angioplasty with CEA.
▪▪ Large, randomized trial comparing CAS with CEA in symptomatic patients.
▪ First systematic assessment of the risk:benefit ratio of CAS versus CEA.
▪ First systematic assessment of the risk:benefit ratio of protected versus unprotected CAS.