Research Article - International Journal of Clinical Rheumatology (2019) Volume 14, Issue 5
Procollagen III amino terminal propeptide (PIIINP): A marker of MTX induced liver fibrosis in rheumatoid arthritis patients?
- Corresponding Author:
- Noran O El-Azizi
Department of Internal Medicine & Rheumatology Department
Ain Shams University, Cairo, Egypt
E-mail: nowara2005@yahoo.com
noran.elazizi@med.asu.edu.eg
Abstract
Background: to evaluate the reliability of serum Procollagen III aminoterminal peptide (PIIINP) in the screening for hepatic fibrosis induced by long term Methotrexate Therapy (MTX) in Rheumatoid Arthritis (RA) patients.
Methods and Findings: The study included 90 RA patients divided into 60 on MTX therapy (group I) and 30 on other medications (group II). All subjected to history taking, rheumatological examination and lab investigations including CBC, ESR, CRP, AST, ALT, RF and PIINP levels measurement, DAS score and Fibrosis-4 (FIB-4) score were calculated. Results showed significant lower disease duration, HCT, platelet count and higher MCV, AST, ALT, FIB-4 score, PIIINP level and more patients’ complaint of right hypochondrial pain in MTX intake group (p<0.05). ROC curve for PIIINP in group I showed that the cutoff level of >170 can detect MTX induced hepatic fibrosis with (51.52%) sensitivity and (92.59%) specificity, 89.5% PPV and 61% NPV. Correlation between PIIINP and all studied variables showed a significant positive correlation between PIIINP level and disease duration, MTX intake duration, MTX current and cumulative doses, MCV, AST, ALT, AST/ALT, FIB-4 score and significant negative correlation with folic acid intake, platelet count (P<0.05). A positive correlation found between cumulative MTX dose and FIB-4 score (P<0.001).
Conclusion: PIIINP can be used as screening noninvasive marker for MTX induced hepatic fibrosis in RA patients; it is positively correlated with AST/ALT ratio, FIB-4 score and cumulative MTX dose. Folic acid is protective against liver fibrosis in RA patients on MTX therapy.
Keywords
rheumatoid arthritis • MTX • PIIINP • liver fibrosis
Introduction
RA is a systemic autoimmune disease characterized by inflammatory synovitis and progressive joint destruction, which are associated with severe disability and increased mortality [1]. The management of RA rests on several principles; non-pharmacological measures such as physical, occupational and psychological therapeutic approaches, and drug treatment, which comprises non-steroidal antiinflammatory drugs, glucocorticoids, Disease Modifying Anti Rheumatic Drugs (DMARDs), biological therapy all together may lead to therapeutic success [2].
MTX is an antifolate and antimetabolite; thereby inhibiting synthesis of purines and pyrimidines and decreasing DNA and RNA synthesis that is used extensively in the therapy of leukemia, lymphoma and several solid organ tumors. It also has potent activity against psoriasis and has immunomodulatory activity against inflammatory arthritis [3]. MTX is now the most popular anchor cornerstone drug for the treatment of RA worldwide. Low-dose, weekly MTX (10 to 25 mg/week) used as either monotherapy or in combination with other drugs as other DMARDs, biologic therapy or targeted biological therapies. It has a superior efficacy profile comparable with other drugs [4]. MTX is well tolerated; gastrointestinal toxicity is the most common toxicity with rarely bone marrow, lung, or liver toxicity [5].
Long term therapy with MTX has been associated with development of fatty liver and hepatic fibrosis and in rare instances portal hypertension and symptomatic cirrhosis. Symptoms are usually absent until cirrhosis is present and liver tests are typically normal or minimally and transiently elevated. Routine monitoring of patients with regular liver biopsies done at 1 to 2 years’ intervals or with cumulative MTX doses of 1 to 10 grams demonstrates that approximately 30% of patients develop mildto- moderate histological abnormalities and 2 to 20% of patients develop some degree of hepatic fibrosis [6].
Liver biopsy is the standard method for diagnosing liver fibrosis, but it may be associated with significant morbidity and mortality of up to 0.33% thereby limiting its use. So, the noninvasive markers of hepatic fibrosis such as serial platelet counts, serum PIIINP, serum bile acids, hepatic ultrasound and advanced imaging techniques may be more efficient in screening for fibrosis in patients on long term MTX [3].
The excess production of Extra Cellular Matrix (ECM) in Hepatic Stellate Cells (HSCs) and Disse’s space leads to the process of liver fibrosis [7]. The largest component of the ECM in liver is collagen type III [8]. During the synthesis of type III collagen, the N-terminal propeptide of procollagen type III (PIIINP) is detached from procollagen type III, so the fibrogenesis results in release of ECM fragments into the blood. So, the level of PIIINP in serum can be a direct indicator of collagen synthesis and its deposition in the extracellular space [9,10]. Increased amount of PIIINP may indicate the transformation of normal liver tissue into fibrous tissue [8].
Aim of the work
The aim of the study is to evaluate the reliability of serum PIIINP in screening for hepatic fibrosis induced by long term MTX therapy in rheumatoid arthritis patients.
Patients and methods
Patients
This study is a randomized cross-sectional case study, conducted on 90 RA patients diagnosed according to the ACR/EULAR classification criteria of RA [11] attending the outpatient clinics and inpatient of Rheumatology Department of Ain Shams University Hospitals. Patients having HCV or any other risk factors for fatty liver disease, including obesity, diabetes and concurrent administration of other potentially hepatotoxic agents were excluded. Subjects enrolled in the study were divided into two groups; group I included 60 RA patients who were on MTX therapy, Group II include 30 RA patients on other medications.
Clinical assessment
All patients were subjected to full history taking; rheumatological examination and laboratory investigations after informed written consent approved by the Research Ethics Committee of the Faculty of Medicine, Ain Shams University were obtained.
Laboratory investigations
The following laboratory investigations were done for all participants: complete blood count (CBC) performed on automated cell counter performed on coulter® LH 750 cell counter, serum CRP done on the dimension® clinical chemistry system with (cut off value 3.0 mg/L), erythrocyte sedimentation rate (ESR) according to Westergren's method, Alanine aminotransferase (ALT) and Aspartate aminotransferase (AST) done on Beckman coulter AU 480 system , FIB- 4 Score was calculated according to the following formula: (Age x AST) / (Platelet Count x (square root of ALT)) (12), RF by latex agglutination done by AVITEX RF latex kit (cut off value 8.0 IU/ml), and Serum PIIINP assayed by quantitative sandwich ELISA kit for detection of Serum PIIINP (Bioassay Technology Laboratory, Shanghai, China ). It was used according to manufacturer’s instructions.
Statistical analysis
Data were analyzed using Statistical Program for Social Science (SPSS) version 24.0. Quantitative data were expressed as mean ± Standard Deviation (SD). Qualitative data were expressed as frequency and percentage. Independentsamples t-test of significance was used when comparing between two means. Mann Whitney Test (U test) was used to assess the statistical significance of the difference of a non-parametric variable between two study groups. Chisquare test was used when comparing between qualitative data. ROC Curve was used to detect cutoff value, sensitivity, specificity, Positive Predictive Value (PPV) and Negative Predictive Value (NPV). Fisher’s exact test was used to examine the relationship between two qualitative variables when the expected count is less than 5 in more than 20% of cells. Correlation analysis (using Pearson's method) to assess the strength of association between two quantitative variables. Spearman’s correlation coefficient measures the strength and direction of association between two nonparametric ranked variables. Regression analysis is also used to understand which among the independent variables are related to the dependent variable, and to explore the forms of these relationships. P <0.05 was considered significant.
Results
This study is a ranadmized case study conducted on 90 RA patients divided into two groups. Group I included 60 (66.7%) RA patients treated with MTX therapy, 52 (86.7%) females and 8 (13.3%) males. Their ages ranged from 27 to 70 years with the mean ± SD was 53.3 ± 9.38. Their disease duration was 10.72 ± 3.59 years. Group II included 30 (33.3%) RA patients who were on other medications for RA were 26 (86.7%) females and 4 (13.3%) males. Their ages ranged from 32 to 66 years with the mean ± SD was 53.73 ± 9.92. Their disease duration was 12.9 ± 2.96 years.
In group I the duration of MTX intake is 1.88 ± 0.88 years, with current dose 8.67 ± 1.92 mg/ week, the calculated MTX cumulative dose was 819 ± 520.7 mg. only 42/60 (70%) of patients received folic acid, 15 (25%) patients on hydroxychloroquine and 18 (30%) patients on steroid in a dose ranging from (5-10 mg). In group II 23 (76.7%) patients were on leflunomide, 12 (40%) patients on hydroxychloroquine and 9 (30%) patients on steroids in dose ranging from (5-10 mg).
There was no statistically significant difference between groups as regard age, sex, hydroxychloroquine intake and dose, prednisolone intake and dose, number of tender joints, number of swollen joints, number of arthritic joints, ESR, CRP, DAS28 by ESR, DAS28 by CRP, hemoglobin, hematocrit and total leucocytic count. But there was statistically significant lower disease duration, HCT, platelet count and higher MCV, AST, ALT, FIB-4 score and PIIINP level in MTX intake group (p<0.05) (Table 1).
| Variables | Group | Fisher's Exact test/T-test/Mann-Whitney test | ||
|---|---|---|---|---|
| MTX intake n (60) | No MTX intake n (30) | |||
| mean ± SD (Max-Min)/no. (%) | mean ± SD (Max-Min)/no. (%) | P-Value | ||
| Sex | Male | 8 (13.33%) | 4 (13.33%) | 1 |
| Female | 52 (86.67%) | |||
| Age by years | 53.3 ± 9.38 | 53.73 ± 9.92 | 0.84(T) | |
| Smoking | 8 (13.33%) | 3 (10%) | 0.746 | |
| Disease Duration | 10.72 ± 3.59 | 12.9 ± 2.96 | 0.005(T) | |
| Hydroxychloroquine | 15 (25%) | 12 (40%) | 0.143 | |
| Hydroxychloroquine dose | 200 mg | 10 (66.67%) | 9 (75%) | 0.696(F) |
| 400 mg | 5 (33.33%) | 3 (25%) | ||
| Prednisolone | 18 (30%) | 9 (30%) | 1 | |
| Prednisolone dose | 5 mg | 13 (72.22%) | 7 (77.78%) | 1.00(F) |
| 10 mg | 5 (27.78%) | 2 (22.22%) | ||
| No of Arthritic Joints | 3 (2 - 4) | 4 (2 - 6) | 0.172(M) | |
| No of swollen Joints | 3 (2 - 5) | 4 (2 - 6) | 0.216(M) | |
| No of tender joints scale | 4 (2 - 5) | 5 (2 - 7) | 0.151(M) | |
| Global health assessment score 0 (best)-100 (worst) | 20 (10 - 30) | 20 (10 - 40) | 0.836(M) | |
| ESR (mm/1sthr) | 18.5 (9.5 - 31) | 18 (8 - 28) | 0.468(M) | |
| DAS28 by ESR | 3.89 ± 1.01 | 4.06 ± 1.3 | 0.491 | |
| Ds activity by DAS28 by ESR | Remission | 6 (10%) | 4 (13.33%) | 0.843(F) |
| Low | 16 (26.67%) | 7 (23.33%) | ||
| Moderate | 31 (51.67%) | 14 (46.67%) | ||
| High | 7 (11.67%) | 5 (16.67%) | ||
| CRP (mg/l) | 10.5 (5 - 18) | 8.5 (3 - 12.7) | 0.07(M) | |
| DAS28 by CRP | 3.69 ± 0.82 | 3.76 ± 1.06 | 0.718 | |
| Ds activity by DAS28 by CRP | Remission | 6 (10%) | 4 (13.33%) | 0.505(F) |
| Low | 15 (25%) | 8 (26.67%) | ||
| Moderate | 37 (61.67%) | 15 (50%) | ||
| High | 2 (3.33%) | 3 (10%) | ||
| RF (IU/ml) | 16 (16 - 32) | 16 (8 - 16) | 0.01(M) | |
| Hemoglobin(g/dl) | 10.89 ± 0.91 | 10.79 ± 0.8 | 0.612 | |
| HCT (%) | 34.23 ± 2.45 | 35.56 ± 3.18 | 0.05 | |
| MCV (fl) | 84.12 ± 3.42 | 82.57 ± 2.71 | 0.034 | |
| TLC (x103/mm3) | 12.79 ± 1.33 | 12.94 ± 1.18 | 0.599 | |
| Platelets (x103/mm3) | 179.25 ± 34.84 | 211.57 ± 65.37 | 0.016 | |
| AST (IU/L) | 26.5 (18 - 45) | 17.5 (14 - 25) | <0.001(M) | |
| ALT (IU/L) | 33.08 ± 12.58 | 23.23 ± 9.78 | <0.001 | |
| AST/ALT ratio | 0.91 ± 0.23 | 0.84 ± 0.16 | 0.116 | |
| FIB-4 score | 1.5 (1.1 - 2.05) | 1 (0.8 - 1.2) | <0.001(M) | |
| PIIINP (ng/ml) | 120 (50 - 200) | 25 (20 - 50) | <0.001(M) | |
ESR: Erythrocyte Sedimentation Rate; TLC: Total Leucocytic Count; RF: Rheumatoid Factor; HCT: Hematocrit; MCV: Mean Corpuscular Volume; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; FIB-4: Fibrosis-4 score; PIIINP: Procollagen III aminoterminal peptide; (F): Fisher’s exact test of significance; (M): Mann-Whitney test of significance; (T): Student t-test of significance.
Table 1. Demographic data, drug history, disease characteristics, disease activity and laboratory investigations between 2 studied groups.
There was no statistically significant difference between the 2 studied groups as regard hepatic disease characteristics; dyspepsia and lower limb edema or hepatic U/S finding as hepatomegaly, but there was a statistically significant more patients’ complaint of right hypochondrial pain in MTX intake group (p<0.05) (Table 2).
| Variables | Group | Chi-Square test | |
|---|---|---|---|
| MTX intake | No MTX intake | P-Value | |
| No (60) | No (30) | ||
| No (%) | No (%) | ||
| Rt. hypochondrial pain | 21 (35%) | 4 (13.33%) | 0.031 |
| Dyspepsia | 25 (41.67%) | 10 (33.33%) | 0.445 |
| L.L. oedema | 14 (23.33%) | 3 (10%) | 0.128 |
| Hepatomegaly in U/S | 10 (16.67%) | 2 (6.67%) | 0.324(F) |
Table 2. Comparison between the 2 groups as hepatic disease characteristics & hepatic ultrasound finding.
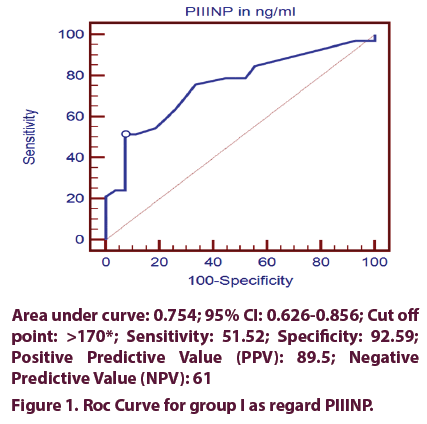
Using ROC curve for PIIINP in group I (MTX intake group) showed that the cutoff level of > 170 detect MTX induced hepatic fibrosis with 51.52% sensitivity, 92.59% specificity, 89.5% PPV and 61% NPV (Figure 1).
Correlation between PIIINP and all studied variables showed that there were significant positive correlation between PIIINP level and disease duration, MTX intake duration, MTX current & cumulative doses, MCV, AST, ALT, AST/ALT, FIB-4 score, and significant negative correlation PIIINP level and folic acid intake, platelet count (P<0.05) (Table 3).
| Variables | r2 | Pearson/Spearman(S) Correlation P-Value |
|---|---|---|
| Age by years | -0.246 | 0.058 |
| Disease Duration | -0.412 | 0.001 |
| Hydroxychloroquine dose | 0.037 | 0.896 |
| Prednisolone dose | -0.315 | 0.203 |
| MTX duration | 0.66 | <0.001 |
| MTX current dose | 0.66 | <0.001 |
| MTX cumulative dose | 0.805 | <0.001 |
| Folic acid | -0.795(S) | <0.001 |
| No of Arthritic Joints | 0.079(S) | 0.549 |
| No of swollen Joints | 0.091(S) | 0.489 |
| No of tender joints scale | 0.058(S) | 0.664 |
| Global health assessment score 0(best)-100(worst) | 0.138 | 0.292 |
| ESR (mm/1sthr) | -0.031 | 0.814 |
| DAS28 by ESR | 0.062 | 0.637 |
| CRP (mg/l) | -0.005 | 0.969 |
| DAS28 by CRP | 0.05 | 0.702 |
| RF (IU/ml) | 0.073 | 0.594 |
| Hemoglobin(g/dl) | 0.168 | 0.2 |
| HCT (%) | 0.144 | 0.271 |
| MCV (fl) | 0.307 | 0.017 |
| TLC(x103/mm3) | 0.18 | 0.168 |
| Platelets(x103/mm3) | -0.349 | 0.006 |
| AST (mg/dl) | 0.694 | <0.001 |
| ALT (mg/dl) | 0.677 | <0.001 |
| AST/ALT ratio | 0.352 | 0.006 |
| FIB-4 score | 0.703 | <0.001 |
ESR: Erythrocyte Sedimentation Rate; TLC: Total Leucocytic Count; RF: Rheumatoid Factor; HCT: Hematocrit; MCV: Mean Corpuscular Volume; ALT: Alanine aminotransferase; AST Aspartate aminotransferase; FIB-4: Fibrosis-4 score; PIIINP: Procollagen III aminoterminal peptide.
Table 3. Correlation study between PIIINP and all studied variables in group I.
There was a highly significantly positive correlation between cumulative MTX dose and FIB-4 score and PIIINP level (P<0.001) (Table 4). Regression analysis showed that AST, ALT. AST/ALT ratio, FIB-4 score variables were independent predictive variables for increasing PIIINP level (Table 5).
| FIB-4 score | PIIINP in ng/ml | ||
|---|---|---|---|
| MTX cumulative dose | Pearson Correlation | 0.748 | 0.805 |
| P-Value | <0.001 | <0.001 |
Table 4. Correlation between Cumulative MTX dose and FIB-4 score & PIIINP level.
| Variable | r2 | F | P-Value |
|---|---|---|---|
| MTX dose | 0.444 | 46.236 | <0.001 |
| MTX duration | 0.432 | 44.164 | <0.001 |
| MTX cumulative dose | 0.647 | 106.447 | <0.001 |
| AST | 0.482 | 81.895 | <0.001 |
| ALT | 0.439 | 68.727 | <0.001 |
| AST/ALT ratio | 0.125 | 12.522 | 0.001 |
| FIB-4 Score | 0.466 | 76.661 | <0.001 |
ALT: Alanine aminotransferase; AST Aspartate aminotransferase; FIB-4: Fibrosis-4 score
Table 5. Regression analysis between MTX dose, duration, cumulative dose, AST, ALT, AST/ALT ratio and FIB-4 Score variables and PIIINP level.
Discussion
Rheumatoid arthritis is a multifactorial chronic, progressive inflammatory autoimmune disease associated with articular, extra-articular and systemic effects. It has been reported that RA affects 1% of the adult population of developed regions. Although some patients have mild self-limited disease, many experience joint destruction, severe physical disability and multiple co-morbidities [13]. Mortality rates are more than twice as high in patients with RA as in the general population [14]. Although the exact cause of RA remains unknown, recent findings suggest a genetic basis for disease development. Pro-disease factors include smoking (especially in individuals with HLADR01/04) and other pulmonary exposures such as silica dust, vitamin D deficiency and obesity. Various immune modulators (cytokines and effector cells) and signaling pathways are involved in the pathophysiology of RA [15].
Therapy with DMARDs should be started as soon as the diagnosis of RA is made. MTX is classified as a small molecule Disease Modifying Anti- Rheumatic Drug (DMARD) [16,17]. MTX can cause transient elevated liver enzymes in 10-43% of RA patients that can resolve with temporary drug discontinuation. Recommendations to monitor and diagnose liver damages induced by MTX in inflammatory disorders vary according to the disease. Liver biopsy, which is considered as the gold standard for fibrosis assessment, is invasive, painful, can expose the patient to life-threatening complications and is poorly accepted. Therefore, there is a need for accurate non-invasive methods and more recent approaches include routine biochemical and hematological tests as well as surrogate serum fibrosis markers [18,19]. As an alternative to liver biopsy, noninvasive serological markers of liver fibrosis are now in use, such as PIIINP. The British Association of Dermatologists’ guidelines recommend the use of PIIINP in adults prior to initiating MTX treatment and at 3-monthly intervals throughout the treatment period [20]. The aim of the present work is to evaluate the reliability of PIIINP in screening for hepatic fibrosis induced by long term MTX therapy in rheumatoid arthritis patients.
In the present study, there were significantly higher MCV, AST, ALT, FIB-4 score and PIIINP level in MTX intake group (group I) compared to patients on other medications (group II) (p<0.05). As regard MCV, this result was agreed with the Weinblatt and Fraser [21] study who found that sustained elevation in the MCV may be a predictor of impending hematologic toxicity due to folate depletion RA on MTX therapy. And as regard AST, ALT, FIB score our data was in concordance with Miyata et al. [22] who found that AST, ALT and FIB-4 score were significantly increased with increasing the cumulative dose of MTX in RA patients, also he concluded that FIB- 4 score is simple to calculate and a valuable marker to diagnose liver disease in RA patients on longterm MTX therapy.
We also found that the serum level of PIIINP was significantly higher in RA patients on MTX therapy compared to RA patients on other medications by using ELISA technique, this was also confirmed by the ROC curve which showed that high degree of both sensitivity and specificity in group I (at >170 PIIINP). This result was in accordance with the studies carried out by Nøjgaard et al. [23]; Rosenberg et al. [24]; Chalmers et al. [25]; Bath et al. [26]. They established that an algorithm combining serum markers for liver fibrosis including PIIINP can be used in a wide range of chronic liver diseases to identify patients who have no or little fibrosis, distinguishing them from those with clinically significant hepatic fibrosis. They have concluded that the risk of patients developing hepatic fibrosis is negligible if the PIIINP remains consistently normal; whereas if serial PIIINP measurements are persistently elevated there is a significant risk that hepatic fibrosis is present. Contradictory to this Lynch et al. [27] stated that Serum PIIINP levels may be a less reliable marker of liver fibrosis in these patients because elevated levels may be related to active joint disease. Also, Gressner et al. [28] emphasized that PIIINP is not a liverspecific biomarker, since elevations are known in lung fibrosis, acromegaly, rheumatoid diseases, chronic pancreatitis, and others.
The present study assessed the sensitivity and specificity of a single serum PIIINP measurement in patients on MTX therapy at 51.52%, 92.59% respectively which goes in accordance with Maybury et al. [29] who assessed the sensitivity and specificity of a single PIIINP measurement and reported rates of 74%, 77% respectively.
Comparison of the studied groups as regard platelet count showed a statistically significant lower platelet count in patients on MTX therapy which goes in accordance with Paul et al. [30] who stated that MTX remarkably accelerates ROS-mediated oxidative stress in platelets triggering apoptotic events via JNK-mediated mitochondrial damage.
All the previous data confirmed by the presence of significant positive correlation between PIIINP level and disease duration, MTX intake duration, MTX current dose & cumulative dose, MCV, AST, ALT, AST/ALT, FIB-4 score, and also there was a highly significantly positive correlation between cumulative MTX dose and FIB-4 score and PIIINP level. As regard disease duration this result was agreed with Eberhardt et al. [31] who stated that PIIINP level markedly higher than in patients with RA with longer duration. As regard MTX duration and dose our results agreed with Maurice et al. [32], who recommend the measuring of PIIINP serially especially in patients on low dose long term MTX instead of liver biopsy for early detection of liver fibrosis. As regard AST, ALT, AST/ALT, and FIB-4 score, Castera [33], found that PIIINP correlated with aminotransferase levels in acute hepatitis which reflects the degree of fibrosis. These data also go in accordance with Parsian et al. [34], who stated that an increase in serum laminin and PIIINP concentrations above the predictive value is associated with liver fibrosis. In addition, the values of the AST/ALT ratio, Age platelet index, AST to platelet ratio index (APRI), FIB-4 and Fibro Q score and serum laminin and PIIINP levels for discrimination between patients with liver fibrosis and healthy individuals are in the same order. But there was negative correlation between PIIINP level and folic acid that prove the protective role of folic acid in patients on MTX therapy, but we didn’t find other studies study this data. But many studies found that folic acid use reduces toxic effects and improves continuation of MTX therapy and compliance without reducing the MTX efficacy [35]. Regression analysis showed that the FIB-4 score was independent predictor for increasing PIIINP levels and this mostly in concordance with Cheah et al [36], who found a good performance of FIB-4 score in the prediction of mild to moderate fibrosis in Non- Alcoholic Fatty Liver Disease (NAFLD).
Conclusion
PIIINP can be used as screening noninvasive marker for hepatic fibrosis induced by long term MTX therapy in rheumatoid arthritis patients and it is positively correlated with AST/ALT ratio, FIB-4 score and cumulative MTX dose. Folic acid is protective against liver fibrosis in RA patients on MTX therapy.
Conflict of interest
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-forprofit sectors..
References
- Peter CT, keystone EC, van der Haijde D et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N. Engl. J. Med. 376(7), 652–662 (2017).
- Felson DT, Smolen JS, Wells G et al. American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis. Rheum. 63(3), 573–586 (2011).
- Singh JA, Furst DE, Bharat A et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease‐modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis. Care. Res. 64(5), 625–639 (2012).
- Weinblatt ME. Methotrexate in Rheumatoid Arthritis: A Quarter Century of Development. Trans. Am. Clin. Climatol. Assoc. 124, 16–25 (2013).
- Kaltsonoudis E, Papagoras C, Drosos AA. Current and Future Role of Methotrexate in the Therapeutic Armamentarium for Rheumatoid Arthritis. Int. J. Clin. Rheumatol. 7(2), 179–189 (2012).
- Ng LC, Wong SM, Lee YY et al. A retrospective review of methotrexate- induced hepatotoxicity among patients with psoriasis in a tertiary dermatology center in Malaysia. Int. J. Dermatol. 52(1), 102–105 (2013).
- Liu X, Wu H, Byrne M et al. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. P. Natl. Acad. Sci. USA. 94(5), 1852–1856 (1997).
- Sarah D, Berry SD, Vasan S et al. Pollagen type III N-terminal peptide (P3NP) and lean mass: a cross-sectional study. J. Fraility. Aging. 2, 129–134 (2013).
- Baranova A, Lal P, Birerdinc A et al. Non-Invasive markers for hepatic fibrosis. BMC. Gastroenterol. 11(1), 91–96 (2011).
- Cross TJ, Calvaruso V, Maimone S et al. Prospective comparison of Fibroscan, King’s score and liver biopsy for the assessment of cirrhosis in chronic hepatitis C infection. J. Viral. Hepat. 17(8), 546–554 (2010).
- Aletaha D, Neogi T, Silman AJ et al. Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis. Rheum. 62(9), 2569–2581 (2010).
- Sterling RK, Lissen E, Clumeck N et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 43(6), 1317–1325 (2006).
- Gabriel SE, Michaud K. Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis. Res. Ther. 11(3), 229 (2009).
- Van den Hoek J, Boshuizen HC, Roorda LD et al. Mortality in patients with rheumatoid arthritis: a 15-year prospective cohort study. Rheumatol. Int. 37(4), 487–493 (2017).
- Ernest C. Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology. 51(Suppl 5), v3–v11 (2012).
- McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet. 389(10086), 2328–2337 (2017).
- Yu MB, Firek A, Langridge WH. Predicting methotrexate resistance in rheumatoid arthritis patients. Inflammopharmacology. 26(3), 699–708 (2018).
- Albrecht K, Müller-Ladner U. Side effects and management of side effects of methotrexate in rheumatoid arthritis. Clin. Exp. Rheumatol. 28, S95–S101 (2010).
- Laharie D, Seneschal J, Schaeverbeke T et al. Assessment of liver fibrosis with transient elastography and FibroTest in patients treated with methotrexate for chronic inflammatory diseases: a case-control study. J. Hepatol. 53(6), 1035–1040 (2010).
- Martyn-Simmons CL, Rosenberg, WMC, Cross R et al. Validity of noninvasive markers of methotrexate‐induced hepatotoxicity: a retrospective cohort study. Br. J. Dermatol. 171(2), 267–273 (2014).
- Weinblatt ME, Fraser P. Elevated mean corpuscular volume as a predictor of hematologic toxicity due to methotrexate therapy. Arthritis. Rheum. 32(12), 1592–1596 (1989).
- Miyata M, Kuroda M, Unakami M et al. Validation of the fibrosis-4 (FIB-4) index in the diagnosis of liver disease of rheumatoid arthritis patients treated with methotrexate. Mod. Rheumatol. 31, 1–7 (2018).
- Nøjgaard C, Johansen JS, Christensen E et al. Serum levels of YKL-40 and PIIINP as prognostic markers in patients with alcoholic liver disease. J. Hepatol. 39(2), 179–186 (2003).
- Roseberg WM, Voelker M, Thiel R et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 127(6), 1704–1713 (2004).
- Chalmers RJG, Kirby B, Smith A et al. Replacement of routine liver biopsy by pollagen III aminopeptide for monitoring patients with psoriasis receiving long‐term methotrexate: a multicentre audit and health economic analysis. Br. J. Dermatol. 152(3), 444–450 (2005).
- Bath RK, Brar N, Faripour AF et al. A review of methotrexate‐associated hepatotoxicity. J. Dig. Dis. 15(10): 517–524 (2014).
- Lynch M, Higgins E, McCormick PA et al. The use of transient elastography and FibroTest for monitoring hepatotoxicity in patients receiving methotrexate for psoriasis. JAMA. Dermatol. 150(8), 856–862 (2014).
- Gressner OA, Weiskirchen R, Gressner AM. Biomarkers of liver fibrosis: clinical translation of molecular pathogenesis or based on liver-dependent malfunction tests. Clin. Chim. Acta. 381(2), 107–113 (2007).
- Maybury CM, Samarasekera E, Douiri A et al. Diagnostic accuracy of noninvasive markers of liver fibrosis in patients with psoriasis taking methotrexate: a systematic review and meta‐analysis. Br. J. Dermatol. 170(6), 1237–1247 (2014).
- Paul M, Hemshekhar M, Thushara RM et al. Methotrexate promotes platelet apoptosis via JNK-mediated mitochondrial damage: alleviation by N-acetylcysteine and N-acetylcysteine amide. PloS one. 10(6), e0127558 (2015).
- Eberhardt K, Thorbjorn Jensen L, Horslev-Petersen K et al. Serum aminoterminal type III pollagen peptide in early rheumatoid arthritis: relation to disease activity and progression of joint damage. Clin. Exp. Rheumatol. 8(4), 335–340 (1990).
- Maurice PD1, Maddox AJ, Green CA et al. Monitoring patients on methotrexate: hepatic fibrosis not seen in patients with normal serum assays of aminoterminal peptide of type III pollagen. Br. J. Dermatol. 152(3), 451–458 (2005).
- Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology. 142(6), 1293–302 (2012).
- Parsian H, Nouri M, Rahimipour A et al. Comparison of five liver fibrosis indexes with serum levels of laminin and N terminal peptide of pollagen type III in chronic hepatitis patients. Liver. Biopsy. Intech. (2011).
- Whittle SL, Hughes RA. Folate supplementation and methotrexate treatment in rheumatoid arthritis: a review. Rheumatology. 43(3), 267–271 (2004).
- Cheah MCC , McCullough AJ , Goh GBB. Current Modalities of Fibrosis Assessment in Non-alcoholic Fatty Liver Disease. J. Clin. Trans. Hepatol. 5(3), 261–271 (2017).