Research Article - International Journal of Clinical Rheumatology (2019) Volume 14, Issue 6
Prognostic role of plasma cell free DNA in a cohort of Egyptian systemic lupus erythematosus patients
- Corresponding Author:
- Noran O El-Azizi
Department of Internal Medicine & Rheumatology
Faculty of Medicine
Ain Shams University, Egypt
E-mail: noran.elazizi@med.asu.edu.eg
Abstract
Background: Cell death has an important event in lupus pathogenesis as it leads to release of antigens as nucleic acids for immune complex formation. DNA-antibody complexes in the circulation are one of the hallmarks of Systemic Lupus Erythematosus (SLE) that leads to the clinical manifestations of SLE. Fluctuation in circulating DNA level might be one of the driving factors behind flare-ups. The objective of this study is to estimate the prevalence of plasma circulating cell-free deoxyribonucleic acid (cf DNA) in SLE patients and evaluate it as a prognostic marker in SLE and its relation to drug therapy. Methods: A case control study conducted on 75 Egyptian SLE patients divided into 2 groups; 45 SLE patients on therapy and 30 SLE patients recently diagnosed (without therapy) and 25 matched healthy control group. All patients are subjected to detailed medical history and clinical examination. Disease activity was done using SLEDAI-2k, related laboratory investigations in addition to estimation of cf-DNA concentration by real-time PCR technique. Findings: On comparing the 2 SLE groups, there was significant increase in nephritis, neuropsychiatric manifestations, vasculitis, fever, SLEDAI -2k, ESR, ANA, Anti-DNA titre and cf-DNA concentration and significant decrease in TLC, Hb, PLT, C3 and C4 in SLE without therapy than those on therapy (P<0.001). On comparing the 3 studied groups there was significant increase in cf-DNA concentration in SLE patients without therapy than those on therapy than in the control group (p <0.001). There was a significant correlation between cf-DNA concentration and neuropsychiatric manifestations, nephritis, fever, ESR, lymphopenia, anemia, thrombocytopenia, ANA titre, Anti-DNA titre, C3, C4 levels and SLEDAI -2k in all SLE patients (p <0.001). Conclusion: Plasma level of cf-DNA is significantly increased in SLE patients especially before starting therapy, so cf-DNA can be used as a marker of disease activity and treatment follow-up.
Keywords
systemic lupus erythematosus • prognostic • autoimmune disease
Introduction
Systemic Lupus Erythematosus (SLE) is a prototypic autoimmune disease with a complex pathogenesis involving multiple genetic and environmental factors. The disease is characterized by enhanced autoantibody production, abnormalities in function of immune system leading to inflammatory manifestations in several organs [1]. The clinical course of lupus disease usually occurs in exacerbation and remission pattern. It may involve virtually any organ system and have a wide range of disease severity [2].
Cell death has been regarded as an important event in lupus pathogenesis as it leads to release of antigens as nucleic acids for immune complex formation. DNA-antibody complexes in the circulation are one of the hallmarks of SLE that leads to events such as complement activation, immune complex deposition, cytokine release and many other detrimental effects causing manifestations of SLE. Fluctuation in circulating DNA level might be one of the driving factors behind flare-ups of SLE [3].
Circulating cell-free deoxyribonucleic acid (cf- DNA), defined as extracellular DNA occurring in blood serum or plasma, present in only limited amounts in healthy individuals, since dying cells and remnants of dead cells are efficiently removed, mainly in the liver. Reactive oxygen species are implicated as a cause of damage to DNA, including breaking of single and double strands, releasing of free nucleobases, chemical changes of nucleobases, and modification of sugar moieties [4]. Circulating cf-DNA has been widely studied and is considered as a potential biomarker for the detection and monitoring of various human diseases such as stroke, myocardial infarction, sepsis, acute pancreatitis, as well as cancer [5].
For many years, free DNA research has been focused on examining the level of free DNA in autoimmune diseases like SLE, rheumatoid arthritis, systemic sclerosis and primary Sjogren’s syndrome as most of autoimmune disorders are associated with chronic inflammation that lead to increased rate of cell death events (apoptosis and necrosis) which are the main sources for circulating DNA in addition to active metabolic secretion of DNA from involved cells [6].
In SLE patient’s circulation, DNA-antibody binding and subsequent events such as immune complex deposition, complement activation, cytokines release, and many other detrimental effects can only take place if DNA or DNA fragments are present in the location due to excessive formation or defective clearance [7]. Vasculature pathology in SLE could be induced by DNA-anti DNA complex deposition. Also, the free mitochondrial DNA could promote endothelial dysfunction as well as other side effects on vascular system through the activation of toll like receptor 9 (LTR9). TLR9 are expressed on different cell types (e.g. T or B lymphocytes, mastocytes, epithelial and endothelial cells). They are localized intracellularly and recognize nonmethylated dinucleotides of viral, bacterial and mitochondrial DNA. Therefore, the fluctuation of cf DNA in the circulation might be one of the driving forces behind flare -ups of SLE. For these reasons investigators speculated that an increase in the concentration of free plasma DNA would result in an increase in disease activity [6]. So analyzing and quantitating cell-free plasma DNA could serve as a valuable, non-invasive, rapid, sensitive and accurate method for diagnosis and follow up of several diseases [8].
The aim of this work was to estimate the prevalence of plasma cf - DNA in SLE patients and evaluate it as a prognostic marker in SLE and its relation to drug therapy.
Patients and methods
This is a randomized case control study conducted on 75 Egyptian SLE patients diagnosed according to the Systemic Lupus International Collaborating Clinics (SLICC) classification criteria [9]. All patients were recruited from outpatient clinic and inpatient department of Rheumatology at Ain Shams University Hospitals. And 25 age and sex matched healthy subjects as a control group. The nature of the present study was explained to all participants. Consents from all participants approved from the Research Ethics Committee of the Faculty of Medicine, Ain Shams University were obtained. Patients with a history of stroke, cancer, myocardial infarction, hepatic, cardiac or renal impairment and diabetic patients were excluded from the study.
All patients were subjected to detailed medical history and clinical examination. Disease activity and damage were assessed using SLE disease activity index-2000 (SLEDAI- 2k) [10], Laboratory investigations included complete blood count (CBC) performed on 5 part differential automated cell counter Sysmex XN1000 Japan, serum C-reactive protein (CRP) level, serum creatinine, 24hrs urinary protein were performed on Beckman coulter AU 480 system (Beckman coulter, Inc. 250s. Kraemer Blvd. Brea, CA92821, USA), erythrocyte sedimentation rate (ESR) according to westergren's method, C3 and C4 level by nephelometry minineph Japan. ANA titer was done by indirect immunofluorescent assay on HEP2 substrate, DiaSorin, Italy with starting dilution 1/40. Anti-ds-DNA titre was done by indirect immunofluorescent assay on Crithedia Lucilia substrate, DiaSorin, Italy with starting dilution 1/10.
Detection of plasma cell free DNA (cf-DNA) by real time PCR
DNA was extracted from 400 ul plasma using QIA amp DNA Blood Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The final elution was performed in 50 ul of AE buffer of the QIA amp DNA blood mini kit, and the eluted DNA was stored at -20C until further processing. For analyzing cf-DNA, the RNase P housekeeping gene has been used with forward 5’ AGATTTGGACCTGCGAGCG 3’ and reverse 5’ GAGCGGCTGTCTCCACAA GT3’ primers and 5’FAM-TTCTGACCTGAA GGCTCTGCGCG-BHQ1-3’ as the probe has been applied. The PCR reaction was performed using the Stratagene Mx3005P Real-Time Thermal cycler (Corbett Research, Australia). The real time PCR was carried out in 20 μl of total reaction volume containing 7μl of DNA elusion, 11μl of Taq Man– Universal PCR Master Mix, 2 primers and 1 probe to detect amplification of the RNase P gene and 2μl of H2O. The reaction was processed by an initial denaturation step at 95°C for 10 min and 45 cycles of 1 min at 60°C and 15 sec. at 95°C. The system software uses the fluorescence measurements from each well made during the plate read, and then plots signal values. The positive reaction was detected by the accumulation of fluorescent signals proportionate to cf-DNA concentration.
Statistical analysis
Results were analyzed using SPSS 24. Quantitative data were expressed as mean± standard deviation (SD). Qualitative data were expressed as frequency and percentage. Chisquare test was used to compare qualitative variables. Independent t-test was used to compare two independent quantitative variables. ANOVA test was used to compare more than two groups. Spearman’s correlation co-efficient test was used to assess the relationship between variables in the same group. P <0.05 was considered significant.
Results
The 75 SLE patients were categorized retrospectively into 2 subgroups; 45 SLE patients who were on therapy (corticosteroids, hydroxychloroquine, cyclophosphamide, azathioprine & mycophenolate mofetil) 42 (93.3%) females and 3 (6.7%) males, their ages ranged from 20-55 years with mean ± SD age of 31.8 ± 3.6 years and 30 SLE patients who were recently diagnosed (without therapy) 29 (97%) females and 1(3%) males, their ages ranged from 22-57 years with mean ± SD age of 31.1 ± 4.2 years. And 25 age and gender matched healthy subjects were included as a control group. 23 (92%) females and 2 (8%) males, their ages ranged from 19-45 years with mean ± SD age of 31.4 ± 2.8 years. Clinical characteristics and laboratory findings of all SLE patients are shown in Table 1.
Table 1. Clinical characteristics and laboratory findings of all SLE patients.
| Variable Mean ± SD or n (%) | SLE patient (n=75) |
|---|---|
| SLEDAI-2k | 9.1 ± 6.1 |
| Mucocutaneous | 38 (50.6) |
| Arthritis | 41 (54.6) |
| Nephritis | 45 (60) |
| Serositis | 7 (9.3) |
| Neuropsychiatric | 21 (28) |
| Vasculitis | 16 (21.3) |
| Fever | 13 (17.3) |
| Laboratory investigations: | |
| ESR (mm/1sthr) | 40.7 ± 9.2 |
| CRP positive | 7 (9.3) |
| TLC (x103/mm3) | 6.5 ± 2.09 |
| Lymphocytes (x103/mm3) | 1.8 ± 1.2 |
| Hemoglobin (g/dl) | 10.1 ± 1.4 |
| Platelets (x103/mm3) | 173.3 ± 35.5 |
| Creatinine (mg/dl) | 0.9 ± 0.52 |
| ANA positive | 65 (86.7) |
| ANA with titer | 1/40 40 (54.1%) |
| 1/80 18 (24.3%) | |
| 1/160 6 (8.1%) | |
| Anti-DNA | 2.6 ±1.1 |
| C3 | 96.5 ±11.7 |
| C4 | 19.2 ±4.02 |
| cf-DNA (con) | 10.23 ±17.1 |
SLE: Systemic Lupus Erythematosus; LN: Lupus Nephritis; ESR: Erythrocyte Sedimentation Rate; TLC: Total Leucocytic Count; SLEDAI-2k: SLE Disease Activity Index-2000; cf-DNA (con): cell free DNA concentration
On comparing patients who received therapy and those who did not receive, there was significant increase in myositis, neuropsychiatric manifestations, nephritis, vasculitis, fever, ESR, proteinuria, ANA titre, Anti-DNA titre, SLEDAI -2k and cf-DNA concentration. While there was significant decrease in TLC, Hb, PLT, C3 and C4 and in SLE without therapy than those on therapy (P<0.05), but on the other hand no significant difference between the 2 groups as regard the presence of mucocutaneous manifestations, arthritis, serositis, lymphocyte count, change in CRP and serum creatinine levels (Table 2).
Table 2. Comparison of the disease characteristics, laboratory findings and SLEDAI-2k in systemic lupus erythematosus patients with therapy and without therapy.
| Variables | SLE patients (n=75) | ||
|---|---|---|---|
| Mean+SD (range)/n (%) | Cases with therapy (n=45) | Cases without therapy (n=30) | P |
| Sex F:M | 14:01 | 9:01 | 0.9 |
| Age | 31.8 + 3.6 | 31.1 + 4.2 | 0.7 |
| Mucocutaneous | 21 (47.7) | 17 (56.7) | 0.5 |
| Arthritis | 24 (54.5) | 17 (56.7) | 0.9 |
| Myositis | 9 (20.5) | 15 (50) | 0.008 |
| Serositis | 2 (4.5) | 5 (16.7) | 0.8 |
| Neuropsychiatric | 4 (9) | 17 (56.7) | <0.001 |
| Nephritis | 19 (43.2) | 26 (86.7) | <0.001 |
| Vasculitis | 6 (13.6) | 10 (33.3) | 0.04 |
| Fever | 2 (4.5) | 11 (36.6) | <0.001 |
| ESR (mm/1sthr) | 37.73 ± 7.02 | 45.07 ±10.42 | <0.001 |
| TLC (x103/mm3) | 6.82 ± 2.16 | 6.10 ± 1.94 | 0.001 |
| Lymphocytes (x103/mm3) | 1.93 ± 0.82 | 1.59 ± 1.69 | 0.4 |
| Hemoglobin (g/dl) | 10.8 ± 0.81 | 9.1 ± 1.43 | <0.001 |
| Platelets (x103/mm3) | 188.16 ± 33.21 | 151.53 ± 26.52 | <0.001 |
| CRP with titer | 3 (6.8) | 4 (13.3) | 0.2 |
| Creatinine (mg/dl) | 0.9 ± 0.31 | 0.9 ± 0.52 | 0.9 |
| Proteinuria (g/24hr) | 17 (38.6) | 20 (66.6) | 0.02 |
| ANA titer | 1/40 (87.9%) 1/80 (12.1%) 1/160 (0%) | 1/40 (33.3%) 1/80 (46.7%) 1/160 (20%) | <0.001 |
| Anti-DNA titre | 1/10 (8.9%) 1/20 (31.1%) 1/40 (31.1%) 1/80 (24.5%) 1/160 (4.4%) | 1/10 (6.7%) 1/20 (13.3%)1/40 (13.3%) 1/80 (13.3%)1/160 (20%)1/320 (33.4%) | <0.001 |
| C3 | 100.24 ± 12.01 | 90.9 ± 8.81 | <0.001 |
| C4 | 20.27 ± 3.76 | 17.57 ± 3.9 | <0.001 |
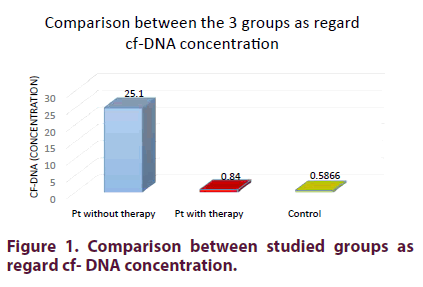
| cf-DNA concentration | 0.84 ± 0.16 | 25.1 ± 18.7 | <0.001 |
| SLEDAI-2k | 8.1 ± 2.7 | 20.9 ± 2.5 | <0.001 |
SLE: Systemic Lupus Erythematosus; ESR: Erythrocyte Sedimentation Rate; TLC: Total Leucocytic Count; SLEDAI-2k: SLE Disease Activity Index-2000; Bold values are significant at p<0.05.
On comparing the 3 studied groups (SLE patients who received and those who did not receive therapy with the control group) there was a highly significant increase in cf-DNA concentration in SLE patients without therapy than those on therapy than in the control group (p <0.001) (Figure 1).
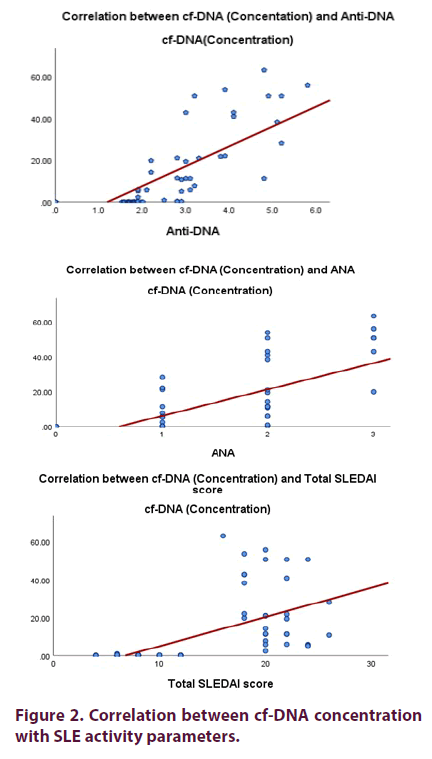
There is a significant positive correlation between cf-DNA concentration and neuropsychiatric manifestations, nephritis, fever, ESR, ANA titre, Anti-DNA titre, and SLEDAI -2k and there was a significant negative correlation between cf-DNA concentration with lymphocytes, Hb, PLT, C3 and C4 levels in all SLE patients (p <0.001) (Table 3 and Figure 2).
Table 3. Correlations between cf-DNA concentration with disease characteristics, activity and laboratory findings in all SLE patients.
| Variables | ||
|---|---|---|
| Mean + SD (range)/n (%) | cf –DNA (Concentration) r2 | P |
| Mucocutaneous | -0.022 | >0.005 |
| Arthritis | 0.073 | >0.005 |
| Myositis | 0.119 | >0.005 |
| Serositis | 0.069 | >0.005 |
| Neuropsychiatric | 0.346 | <0.001 |
| Nephritis | 0.274 | 0.007 |
| Vasculitis | 0.015 | >0.005 |
| Fever | 0.431 | <0.001 |
| ESR (mm/1sthr) | 0.772 | < 0.001 |
| TLC (x103/mm3) | -0.147 | >0.005 |
| Lymphocytes (x103/mm3) | -0.253 | 0.01 |
| Hemoglobin (g/dl) | -0.596 | < 0.001 |
| Platelets (x103/mm3) | -0.471 | < 0.001 |
| CRP with titer | 0.268 | >0.005 |
| ANA titer | 0.705 | < 0.001 |
| Anti-DNA titre | 0.772 | < 0.001 |
| C3 | -0.286 | < 0.001 |
| C4 | -0.361 | 0.002 |
| SLEDAI-2k | 0.606 | < 0.001 |
SLE: Systemic Lupus Erythematosus; ESR: Erythrocyte Sedimentation Rate; TLC: Total Leucocytic Count; SLEDAI-2k: SLE Disease Activity Index-2000; Bold values are significant at p<0.05
Discussion
SLE is a systemic autoimmune disease with multiorgan inflammation, shows variable manifestations with multifactorial etiology. Epidemiological studies on SLE showed marked gender, age, social, and regional variations, indicating hormonal, genetic and environmental disease triggers. Assessment of disease activity poses a challenging problem as the nature of this disease is associated with fluctuation levels of disease activity which may vary between patients and within the same patient over time [11].
The production of pathogenic autoantibodies directed against nucleic acids and their binding proteins reflecting a global loss of self-tolerance is characteristic for SLE pathogenesis. Aberrant immune response (innate and adaptive) plays a significant role in the pathogenesis of SLE, contributing both to tissue injury as well as to activation of autoreactive T and B cells. Autoantigenic nucleic acids and their binding proteins activate innate immune cells via Fc receptors mediated uptake of complexes, with nucleic acid component of these complexes in the case of autoreactive B cells upon endosomal trafficking engaging intracellular Toll Like Receptors (TLR) with subsequent innate and B cells activation [12]. Our target in this work was to estimate the prevalence of plasma cf - DNA in SLE patients and evaluate it as a prognostic marker in SLE and its relation to drug therapy.
In the present study, on comparing those who received and those who did not receive therapy, there was significant increase nephritis, neuropsychiatric manifestations, fever, proteinuria, ESR, ANA titre, Anti-DNA titre, plasma cf-DNA concentration and SLEDAI -2k and significant decrease in TLC, Hb, PLT, C3 and C4 levels in SLE without therapy than those on therapy, these results going on with studies done by Kurien and Scofield [13]who attributed these changes due to the effect of inflammatory cytokines produced in the pathogenesis of SLE leading to the previous data and confirming the effect of therapy (corticosteroids, hydroxychloroquine, cyclophosphamide, azathioprine & mycophenolate mofetil) as immunosuppressive therapy which inhibit the production of inflammatory cytokines and leading to improvement of symptoms and immunological markers. Also, these finding explained by study [14] which attributed these changes to be due to suppressive effect of inflammatory cytokines on hematopoiesis, the possibility of the presence of autoantibodies directed against blood cells and the possibility of associated splenomegaly and the presence of autoantibodies directed against different organs. Increasing the anti-DNA level more on the SLE without therapy is an evidence of its important role in the pathogenesis of inflammation occurred in SLE patients [15].
On other hand no significant difference between the 2 groups as regard the presence of mucocutaneous manifestations, arthritis, serositis and change in level of CRP. This is agreed with Pisetsky [16] study who explained that the CRP is hardly changed during SLE flare.
On comparing the 3 studied groups SLE patients who received and those who did not receive therapy with the control group there was a highly significant increase in the plasma cf-DNA concentration in SLE patients without therapy than those on therapy than in the control group (p <0.001). This data was agreed with Hendy et al. [7], who found that cf-DNA concentration was highly statistically significant increase in SLE patient’s pretreatment than post treatment than in control group. All these results agreed with other studies [17-20], this was attributed to ineffective clearance of apoptotic and necrotic cells, the release of DNA from neutrophils extracellular traps and its impaired degradation which occur in SLE pathogenesis [21]. In addition, the neutrophil extracellular traps were implicated in sterile inflammation and could leads to autoinflammatory conditions, vascular inflammation and atherogenesis [22]. This data also confirms the prognostic value cf-DNA concentration as it was decreased in the SLE patients on therapy as all the activity markers decreased in these patients, so this confirm the effect of therapy on decreasing the level of cf- DNA.
There is a significant correlation between cf- DNA concentration and neuropsychiatric manifestations, nephritis, fever, ESR, lymphopenia, anemia, thrombocytopenia ANA titre, Anti-DNA titre, C3, C4 levels and SLEDAI -2k in all SLE patients and this data refers that higher plasma cf DNA concentration was associated by higher disease activity. This could be explained by the possibility that the high amount of antibody bound nucleic acid could impede the detection of cf-DNA in the circulation either by the formation of complexes or by clearance from the circulation. On the other hand there was no significant correlation between cf-DNA concentration and mucocutaneous manifestations, arthritis, vasculitis, serositis and CRP level. This result agreed with Hendy et al. study [7] who also found a significant correlation between cf-DNA and the parameters of SLE disease activity. Also, in concordance with Abdelalet al. study [6] who also found significant correlation between cf-DNA levels and the disease variables in SLE patients including ESR, CRP, anti-ds-DNA titer, C3, C4 and SLEDAI. Also, this result agreed with Zhang et al. [17], who reported a significant higher cf- DNA concentration in SLE patients more in those with active lupus nephritis. In contrast other study done by Barteloni et al. [23] didn’t find any correlation between them. However, there were a conflict between the studies as use of complement activation products as markers of disease activity this may be attributed to methodological differences between studies [24].
Our findings and Abdelal study [6] support that the measurement of cf-DNA appear to be a useful marker of disease activity. Our findings and Hendy study [7] recommended cf-DNA as a possible marker of treatment follow-up.
Conclusion
Plasma level of cf-DNA is significantly increased in SLE patients especially before starting therapy, so cf-DNA can be used as a marker of disease activity and treatment follow-up.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-forprofit sectors.
Conflict of interest
None.
References
- Young FL, Tucker D, Rubinstein T et al. Molecular analysis of a germ line-encoded idiotypic marker of pathogenic human lupus autoantibodies. J. Immunol. 145(8), 2545–2553 (2009).
- Uzuelli JA, Dias-Junior CA, Izidoro-Toledo TC et al. Circulating cell-free DNA levels in plasma increase with severity in experimental acute pulmonary thromboembolism. Clin. Chim. Acta. 409(1-2), 112–116 (2009).
- Chen JA. Sensitive detection of plasma/serum DNA in patients with systemic lupus erythematosus. Autoimmunity. 40(4), 10–16 (2010).
- Su KY, Pisetsky DS. The role of extracellular DNA in autoimmunity in SLE. Scand. J. Immunol. 70(3), 110–115 (2009).
- Rainer TH, Wong LKS, Lam W et al. Prognostic use of circulating plasma nucleic acid concentrations in patients with acute stroke. Clin. Chem. 49(4), 562–569 (2003).
- Abdelal IT, Zakaria MA, Sharaf DM et al. Levels of plasma cell free DNA and its correlation with disease activity in rheumatoid arthritis and systemic lupus erythematosus patients. Egypt. Rheumatol. 38(4), 295–300 (2016).
- Hendy OM, Abdel-Motalib TA, Abdel-Shafie M et al. Circulating cell free DNA as a predictor of systemic lupus erythematosus severity and monitoring of therapy. Egypt. J. Med. Hum. Genet. 17(1), 79–85 (2016).
- Bala A, Chetia P, Dolai N et al. Cat’s whiskers flavonoid attenuated oxidative DNA damage and acute inflammation: its importance in lymphocytes of patients with rheumatoid arthritis. Inflammopharmacology. 22(1), 55–61 (2014).
- Petri M, Orbai AM, Alarcón GS et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis. Rheumatol. 64(8), 2677–2686 (2012).
- Gladman D, Ginzler E, Goldsmith C et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis. Rheum. 39(3), 363–369 (1996).
- Romero‐Diaz J, Isenberg D, Ramsey-Goldman R. Measures of adult systemic lupus erythematosus. Arthritis. Care. Res. 63(S11), S37–S46 (2011).
- Papayannopoulos V, Metzler KD, Hakkim A et al. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 191(3), 677–691 (2010).
- Kurien BT, Scofield RH. Lipid peroxidation in systemic lupus erythematosus. Indian. J. Exp. Biol. 44(5), 349–356 (2006).
- Kurien BT, Scofield RH. Autoantibody determination in the diagnosis of systemic lupus erythematosus. Scand. J. Immunol. 64(3), 227–235 (2006).
- Frese S, Diamond B. Structural modification of DNA- a therapeutic option in SLE? Nat. Rev. Rheumatol. 7(12), 733–738 (2011).
- Pisetsky DS. The immune response to cell death in SLE. Autoimm. Rev. 3(7-8), 500–504 (2004).
- Zhang S, Lu X, Shu X et al. Elevated plasma cfDNA may be associated with active lupus nephritis and partially attributed to abnormal regulation of neutrophil extracellular traps (NETs) in patients with systemic lupus erythematosus. Intern. Med. 53(24), 2763–2771 (2014).
- Breitbach S, Tug S, Simon P. Circulating cell-free DNA. Sports. Med. 42(7), 565–586 (2010).
- Suzan T, Suzan H, Julia M et al. Correlation between cell free DNA levels and medical evaluation of disease progression in systemic lupus erythematosus patients. Cell. Immunol. 292(1-2), 32–39 (2014).
- Atamaniuk J, Hsiao YY, Mustak M et al. Analysing cell-free plasma DNA and SLE disease activity. Eur. J. Clin. Invest. 41(6), 579–583 (2011).
- Atamaniuk J, Ruzicka K, Stuhlmeier KM et al. Cell-free plasma DNA: A marker for apoptosis during hemodialysis. Clin. Chem. 52(3), 523–526 (2006).
- Hakkim A, Fu¨ rnrohr BG, Amann K et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl. Acad. Sci. USA. 107(21), 9813–9818 (2010).
- Barteloni E, Ludovini V, Alunno A et al. Increased levels of circulating DNA in patients with systemic autoimmune diseases: A possible marker of disease activity in Sjogren’s syndrome. Lupus. 20(9), 928–935 (2011).
- GIllei Gabor, Edward T, Larissa L et al. Biomarkers in systemic lupus erythromatosus. Arthritis. Rheum. 50(7), 2048–2065 (2004).