Research Article - Diabetes Management (2024) Volume 14, Issue 1
Protecting allogeneic pancreatic islet grafts by engineered veto
- Corresponding Author:
- Uwe Staerz
Department of Research and Development, Greffex Laboratory, Colorado, USA
Email: ustaerz@greffex.com
Received: 01-Feb-2024, Manuscript No. FMDM-24-126464; Editor assigned: 05-Feb-2024, PreQC No. FMDM-24-126464 (PQ); Reviewed: 19-Feb-2024, QC No. FMDM-24-126464; Revised: 26-Feb-2024, Manuscript No. FMDM-24-126464 (R); Published: 04-Mar-2024, DOI: 10.37532/1758-1907.2024.14(2).567-576.
Abstract
In today’s clinical practice, general immune suppression regimens are used to prevent transplant rejection. Though highly effective, they impair a patient’s protection against infectious challenges. Strategies are being sought that prevent graft rejection without inhibiting beneficial immune functions. One such approach is based on the classical veto effect that employs donor-derived CD8+ T cells to inhibit cellular immune responses. Yet, allogeneic grafts may only be partially protected by classical veto as CD8+ T cells may fail to remove organ-specific alloreactive T cells. To induce transplant specific immune unresponsiveness, if not tolerance, it may be necessary to endow grafted tissues with veto. Adenoviral vectors were designed that expressed the CD8 α-chain as transgene and thus conferred the immune inhibitory veto function to cells of grafted tissues. In the present model, adenoviral vectors protected transduced allogeneic pancreatic islets from rejection in fully immune competent recipients. These studies demonstrated that a tissue-engineering approach could be used to create universally acceptable transplants.
Keywords
■ CD8+ T cells ■ islet graft ■ veto vector ■ pancreas
Introduction
Survival of allogeneic grafts is presently achieved by the administration of cocktails of immunesuppressive drugs. Although systemic treatment regimens efficiently protect transplants from rejection, they impair the patients’ defenses against infectious agents [1-3]. Improved transplantation protocols would induce specific rather than general immune suppression. Classical veto effect has been suggested as one such approach, in which donor-derived CD8+ T cells selectively inhibit cellular immune responses to antigens presented on their surface [4,5]. CD8+ T cells delete T cells from the peripheral repertoire in an antigen-specific Major Histocompatibility Complex (MHC)- restricted manner [6,7]. While activated CD8+ T cells represent the most potent veto population, other Bone Marrow-derived (BM) cells exert veto as well [7,8]. Classical veto results from the unidirectional recognition of veto cells by responding T cells [7,9]. The immunesuppressive function of the veto-ing T cell is independent of its recognition. It is linked to the surface expression of the CD8 α-chain (CD8). Veto function is lost when CD8 is removed from the cell surface, yet reconstituted by the surface expression of CD8 [10,11].
Veto is best explained by a ‘co-triggering’ hypothesis. T cells that recognize a given cell are ‘veto-ed,’ and thus commit suicide if they receive concurrent signals through the T Cell Receptor (TCR) complex and the α3-domain of the Major Histocompatibility Complex (MHC) class I molecules [12,13]. Thus, the exquisite specificity exhibited by veto is based on the engagement of the TCR. It has been argued that the immune inhibitory activity of classical veto cells could be expanded by the release of TGF- β1 [14,15]. However, CD8 α-chain ‘pseudo-variable,’ once anchored on the cell surface through a hybrid antibody (hAb), efficiently triggers veto [16,17]. Different animal models show that infusion of donor-derived classical veto cells prolongs survival of fully allogeneic grafts [18-20]. Nevertheless, it has been assumed that owing to its unique specificity, veto induced by the injection of classical veto cells such as activated CD8+ T cells cannot provide complete immune unresponsiveness to non-T cell grafts. That is to say, allo-reactive T cells with tissue-specificity for the transplanted tissue may not be inhibited. It may therefore be necessary to transform grafts into ‘veto’-ing tissues.
In initial feasibility studies, we established that the transfer of CD8 with the help of hybrid antibodies (hAbs) imparted veto to cells of diverse phenotypes [16]. Hybrid antibodies are cleared from the cell surface quite rapidly. As the immune system takes several days to raise alloreactive T cell responses in vivo, hAbs were not suitable for organ transplantation. We explored adenoviral gene transfer vectors to efficiently infect both dividing and non-dividing cells of most phenotypes and induce expression of a transgene promptly [21]. Since adenoviral vectors rarely integrate into the host cell genome, they mediate transient gene expression. For our application, this feature may be advantageous. In case a CD8-expressing Veto Vector (VV) were to infect an endogenous Antigen Presenting Cell (APC), resulting aberrant immune suppressions would self-terminate rapidly.
Here, we describe how VVs can be used to transform cells into immune inhibitory veto cells. We show how they imprint highly specific yet stable unresponsiveness to allogeneic pancreatic islets in fully immune-competent animals. Thus, we present a veto-based tissue-engineering strategy that allows the production of universally acceptable transplants.
Materials and Methods
■ Animals
Male and female mice (C57Bl/6, Balb/c and B10.BR) 8 to 24 weeks of age were purchased from Charles River Laboratory (Boston, MA).
■ Expression vectors
pCMV-mCD8 carries the mouse CD8 α-chain driven by the Cytomegalovirus (CMV) immediate early promotor/enhancer (Invitrogen). The Adenoviral expression vectors, mAdCD8 and hAdCD8, were of human Adenovirus type 5 and deleted for the E1 and E3 regions (Qbiogen). Expression of the mouse and human CD8 α-chain transgenes was controlled by a CMV immediate early promotor/enhancer.
■ Mixed lymphocyte cultures
Spleen and lymph node cells were harvested, and single cell suspensions were prepared. Erythrocytes were lysed with Tris-buffered ammonium chloride (Sigma-Aldrich) and resuspended in tissue culture medium. For the induction of Cytotoxic T Lymphocytes (CTLs), unfractionated responder spleen cells (4 × 106 cells/well) were stimulated with irradiated (3,000 rad) spleen cells (2 × 106 cells/well) in flatbottomed 24-well plates (Becton-Dickinson). The cultures were incubated for four days at 37ºC and 7% CO2 in a tissue culture incubator (Forma Scientific). Blasting T cells were counted and tested for their ability to specific target cells. CTLs were incubated with 1 × 104 [51Cr]-NaCr2O4-labeled EL4 (C57Bl/6-derived lymphoma) target cells for four hours in roundbottomed microtiter plates (Becton-Dickinson, total volume 0.2 ml tissue culture medium). Varied effector cell numbers were added as indicated in the text. The amount of 51Cr released into the supernatant was measured on a Gamma 4000 (Beckman-Coulter). Inhibitor cell populations were added to some cultures at stimulator-to-inhibitor ratios of 3-to-1. They consisted of murine fibroblasts (MC57T) or lymphoma cells (EL4) (both C57Bl/6-derived), mock-infected, stably transfected with pCMVmCD8, or transduced with mAdCD8 or hAdCD8. Wells containing target cells, but no effector cells were used to determine nonspecific release, and wells containing target cells in the presence of 1% Triton X-100 (Sigma-Aldrich) were used to determine total release. Percent specific release was calculated as: [(cpm released in the presence of effector cells)-(cpm of nonspecific release)]/[(cpm of total release)-(cpm of nonspecific release)] × 100. For measuring responses of CD4+ T cells, T cells were enriched on Nylon wool columns. Thereafter, CD8+ T cells were removed by chromatography through anti-CD8+ affinity columns (Mitenyi). More than 90% of the remaining cells represented CD4+ T cells (data not shown). CD4+ responder T cells (5 × 105 cells/well) were cultured in triplicate with irradiated (3,000 rad) spleen cells (5 × 105 cells/ well) in flat-bottomed 96-well plates (Becton- Dickinson, total volume 0.2 ml of tissue culture medium). The cultures were incubated for four days at 37ºC and 7% CO2. Supernatant from each culture was taken to determine interleukin levels. Blast cells were counted and then stained for the presence of CD4+ and CD8+ T cells. Iscove’s Modified Dulbecco’s Medium (IMDM, Sigma-Aldrich) was used as tissue culture medium, supplemented with Hepes, glutamine, hydroxypyruvate, 2-mercaptoethanol, nonessential amino acids, penicillin, gentamycin (Sigma) and 10% fetal bovine serum (Intergen).
■ Flow cytometry
Cells suspended in staining buffer (Phosphate Buffer Saline(PBS)/5% fetal bovine serum) were incubated with both phycoerythin-coupled anti- CD4 and fluorescein-isothiocyanate-coupled CD8 (Becton-Dickinson) in 96-well roundbottomed plates (Becton-Dickinson). The unbound antibodies were washed away and the extent of antibodies binding to the cell surface was analyzed on a fluorescence- activated cell sorter (Coulter Epics).
■ Cell infusion and tissue transplantation
Balb/c mice were injected i.v. with 1 × 106 or 5 × 106 cells stably transfected with pCMVmCD8. Pancreatic islets were harvested from C57Bl/6 and Balb/c mice and transplanted into Balb/c mice, which had developed stable diabetes mellitus induced by the i.v. injection of streptozotocin (Sigma-Aldrich). The preparation of pancreatic islets has been detailed by others. After donor mice were euthanized, the peritoneal cavity was opened, and the pancreatic duct identified and cannulated. Digestion was initiated by an infusion of 4 ml of collagenase type 5, dissolved in Hank’s balanced salt solution (Sigma-Aldrich). Digestion continued after the pancreas was placed into a 50 ml tube (Becton- Dickinson) containing collagenase solution (37ºC). With vigorous shaking, the tissue digestion continued for about 20 min, at which point the material was strained to remove large tissue fragments. The released pancreatic islets were spun down, re-suspended and purified utilizing a Histopaque (Sigma-Aldrich) gradient. Researcher-determined islet equivalents were subsequently hand-selected under a surgical microscope (Stereomaster, Fisher).
The purified pancreatic islets were incubated in (Roswell Park Memorial Institute) RPMI 1640/ BSA (Bovine Serum Albumin) (Sigma-Aldrich in a 15 ml tube (Becton-Dickinson, 500 islets per tube) for 18 hours at 37ºC and 7% CO2. Some pancreatic islets were transduced with mAdCD8 or Ad(empty). The vectors were added at an approximate Multiplicity of Infection (MOI) of 3-to-1 (plaque forming units to pancreatic islet surface cells). Upon completion of infection, free virus was washed away, and the pancreatic islets were re-incubated for 24 hours.
Pancreatic islets were stacked by centrifugation into teflon tubing. They were placed into small pouches that had been created under the capsule of the left kidney of recipient mice. C57Bl/6 pancreatic islets (800 islet equivalents) untreated (14 animals), transduced with Ad (empty) (8 animals) or with mAdCD8 (18 animals), were transplanted into Balb/c recipients. Of the latter group, 9 animals were observed for about 180 days and 9 animals for about 1 year.
In another trial, 450 C57Bl/6 pancreatic islet equivalents untreated (7 animals) or transduced with mAdCD8 (11 animals) were transplanted Balb/c mice. These animals were studied for 200 days. In some mice, transplanted pancreatic islet implants were removed by nephrectomy of the left kidney. In all grafted mice, blood glucose levels were determined twice weekly as a measure of pancreatic islet activity by evaluating tail vein blood using a glucometer (Therasense).
Full-thickness dorsal skin was harvested from C57Bl/6 and B10.BR mice and all fatty tissue was carefully removed. Skin segments (approximately 1 cm × 1 cm in size) had already been removed from the dorsal skin of recipient mice. They were replaced by donor skin of the same size. Nine Balb/c mice that had carried mAdCD8-transduced C57Bl/6 for at least 6 months were transplanted, as well as 9 untreated Balb/c control animals. Viability of the skin transplant was determined visually.
■ Histology
Kidneys harvested from pancreatic islet transplanted mice were fixed with 10% neutralized formalin (Sigma-Aldrich), paraffin embedded, sectioned (Vibratom, Technical Products International), and stained with H&E (Sigma-Aldrich).
■ Interleukin assays
Supernatants harvested from mixed lymphocyte cultures established from three mice of each group were tested using commercial assay kits (e.Bioscience, R&D Systems, Bender MD Systems) for the presence of interferon-γ, IL- 2, IL-4, and IL-10. The amount of cytokines released was calculated with the help of a standard curve of known lymphokine concentrations.
Results
■ Veto vector design
To develop a tissue-engineering strategy to prevent the rejection of grafts, we first constructed a plasmid-based expression vector. This vector carried the mouse CD8 α-chain as a transgene under the control of a CMV immediate early promotor/enhancers (pCMV-mCD8). For organ transplantation studies, adenoviral VVs were used that were replication deficient due to the deletion of the E1 region (ΔE1) [22]. A deletion of the E3 region avoided the down-regulation of MHC class I molecules on transduced cells [23], because veto depends on the engagement of MHC class I molecules by the TCR [12]. Both the mouse and the human CD8 α-chains were incorporated into adenoviral VVs (mAdCD8 and hAdCD8). Two adenoviral control vectors were produced, in which CD8 was exchanged for a β-galactosidase gene (AdLacZ) or completely deleted (Ad(empty)).
■ Ability of VVs to inhibit T cell responses
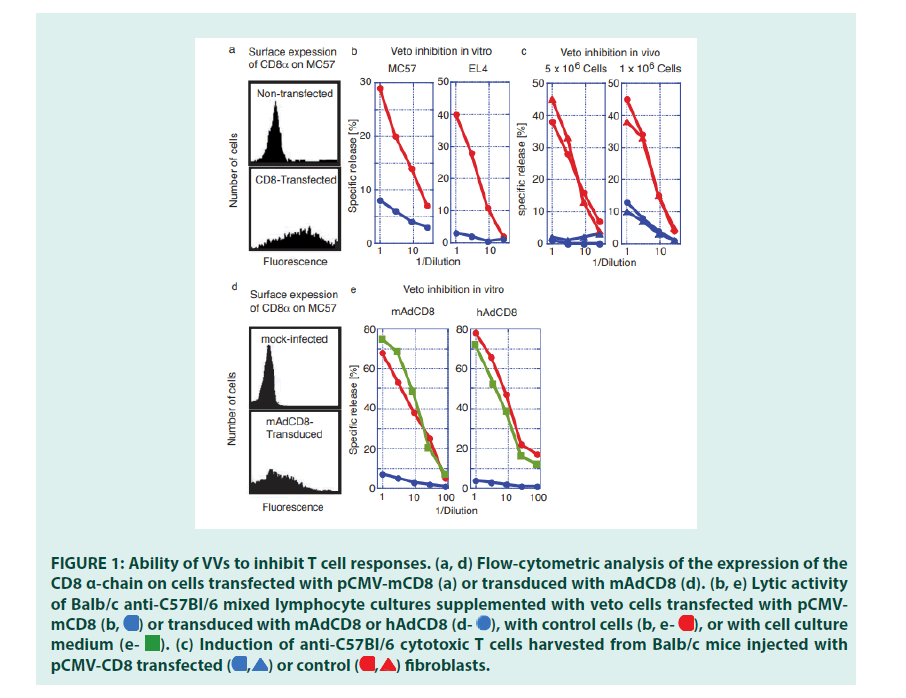
Initial studies established that CD8 expression transformed cells of different phenotypes into veto cells (FIGURE 1). C57Bl/6-(H-2b)- derived fibroblasts (MC57) and lymphoma cells (EL4) were transfected with the plasmidbased VV, pCMVmCD8 (MC-CD8 and EL-CD8), and selected for stable surface expression of CD8 (FIGURE 1a). They were tested for their ability to inhibit the induction of T lymphocytes in vitro. Mixed Lymphocyte Cultures (MLC), Balb/c anti-C57Bl/6 (H- 2d anti-H-2b), were set up in the presence of CD8-expressing fibroblasts or lymphoma cells. Whereas Cytotoxic T Lymphocytes (CTL) were activated in the presence of non-transfected fibroblasts or lymphoma cells, the addition of CD8-expressing cells drastically reduced their responses (FIGURE 1b). Having established their immune-suppressive potential , we probed whether CD8+ MC57 could inhibit allogeneic immune response in vivo.
Balb/c (H-2d) mice were injected i.v. with 5 × 106 or 1 × 106 MC-CD8 cells. After three weeks, spleen cells were harvested, challenged in vitro with C57Bl/6 stimulator cells, and tested for the presence of specific CTLs. These studies revealed that the injection of MC57 control cells did not affect the induction of CTLs. In contrast, infusion of MC-CD8 severely limited (1 × 106) or deleted (5 × 106) the activity of H-2b-specific CTLs retrieved from these mice (FIGURE 1c). These findings proved that cells other than BMderived cells acquired veto when transfected with a plasmid-based VV.
Plasmids are ill-suited to deliver transgenes to tissues; a more efficient gene transfer vehicle was needed. We therefore evaluated the inhibitory function of the adenoviral VVs, mAdCD8 and hAdCD8, which induced the expression of the CD8 α-chain on approximately 50% of MC57 (FIGURE 1d). We examined their inhibitory potential in Balb/c anti-C57Bl/6 MLCs, to which control MC57 cells, mAdCD8-, or hAdCD8-transduced counterparts were added. As exemplified in FIGURE 1e, MLCs supplied with control cells harbored CTL activity similar to those seen in unchallenged cultures. In contrast, the addition of MC57 cells expressing either the mouse or the human CD8 α-chain suppressed the induction of CTLs (FIGURE 1e). These results firmly demonstrated that adenoviral VVs conferred veto.
Figure 1: Ability of VVs to inhibit T cell responses. (a, d) Flow-cytometric analysis of the expression of the CD8 α-chain on cells transfected with pCMV-mCD8 (a) or transduced with mAdCD8 (d). (b, e) Lytic activity of Balb/c anti-C57Bl/6 mixed lymphocyte cultures supplemented with veto cells transfected with pCMVmCD8 or transduced with mAdCD8 or hAdCD8
or transduced with mAdCD8 or hAdCD8 with control cells
with control cells or with cell culture medium
or with cell culture medium (c) Induction of anti-C57Bl/6 cytotoxic T cells harvested from Balb/c mice injected with pCMV-CD8 transfected
(c) Induction of anti-C57Bl/6 cytotoxic T cells harvested from Balb/c mice injected with pCMV-CD8 transfected
■ Transduction of pancreatic islets with adenoviral VVs
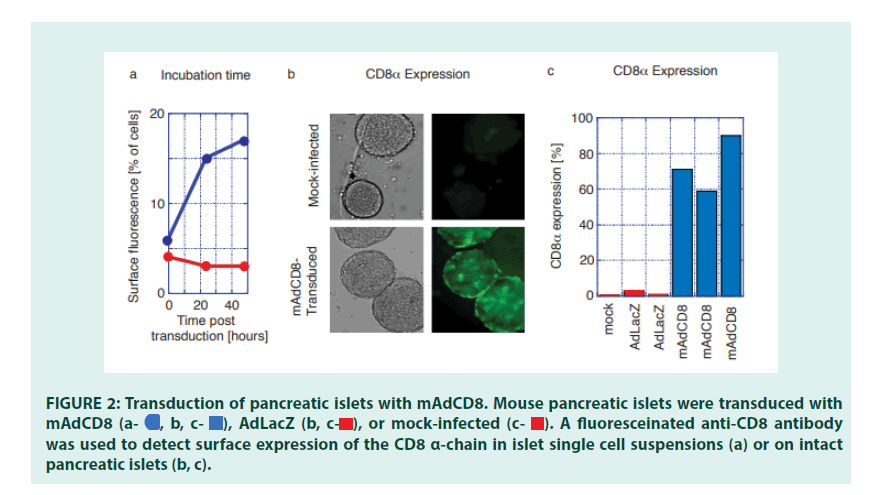
Having established the efficacy of VVs in transforming defined population of cells, we examined whether veto could be applied to a more complex tissue, such as pancreatic islets. Pancreatic islets were purified from C57Bl/6 mice using conventional enzyme digestion methods [24]. To optimize transduction protocols for the Adenoviral VV mAdCD8, both infection time and the Multiplicity of Infection (MOI) were varied. We observed that an extended (18-hr) virus exposure time pared with a relatively low multiplicity of infection resulted in high transduction efficiencies without affecting islet function (data not shown). Under these conditions, expression of CD8 was primarily seen on surface cells of the pancreatic islets (FIGURES 2a and 2b). On average 70% of all pancreatic islets showed evidence of visible CD8 expression (FIGURE 2c). CD8 could not be detected on non-infected islets, or on islets transduced with the control vector AdLacZ (FIGURES 2a, 2b and 2c).
Figure 2: Transduction of pancreatic islets with mAdCD8. Mouse pancreatic islets were transduced with mAdCD8  was used to detect surface expression of the CD8 α-chain in islet single cell suspensions (a) or on intact pancreatic islets (b, c).
was used to detect surface expression of the CD8 α-chain in islet single cell suspensions (a) or on intact pancreatic islets (b, c).
■ Protection of VV transduced pancreatic islets from rejection
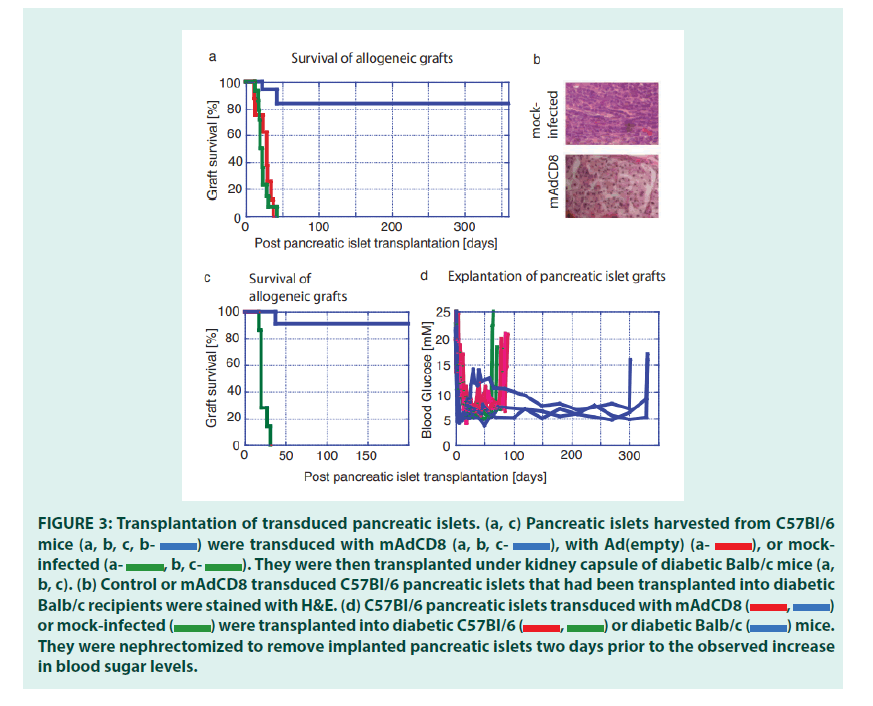
Having optimized VV transduction protocols, we studied whether VVs would interfere with the rejection of allogeneic pancreatic islets (FIGURE 3). We chose C57Bl/6 mice as organ donors and Balb/c mice as recipients. These strains are disparate across both MHC class I and MHC class II regions and numerous minor histocompatibility antigens. Therefore, rejection of these allogeneic pancreatic islets should be effective and broad, and as others had shown, mediated by both CD4+ and CD8+ T lymphocytes [25]. Pancreatic islets harvested from C57Bl/6 were either transduced with both mAdCD8 and Ad(empty) or mock-infected then transplanted under the kidney capsule of Balb/c mice suffering from chemically induced diabetes mellitus. The recipients did not receive any adjuvant immune inhibition and thus remained fully immune competent. In the first set of studies, we increased the number of islets to approximately 800 islet equivalents to compensate for any possible detrimental consequences of the in vitro transduction and to provide more substantial doses of veto cells. As seen in FIGURE 3a, Balb/c recipients rejected all mock-infected and Ad(empty)- transduced control islets in 23 ± 7 and 28 ± 10 days, respectively. The failing grafts were characterized by cellular infiltration (FIGURE 3b). In contrast, 83% of mAdCD8-transduced C57Bl/6 islets survived permanently. In these mice the allogeneic pancreatic islets showed no evidence of cellular invasion (as shown in FIGURE 3b). Immune protection was tested more stringently in another study that used smaller numbers of C57Bl/6 pancreatic islets (450 islet equivalents). As before, mock-infected pancreatic islets were lost swiftly (22 ± 5 days) whereas 91% of mAdCD8- transduced were protected indefinitely from rejection (FIGURE 3c).
Figure 3: Transplantation of transduced pancreatic islets. (a, c) Pancreatic islets harvested from C57Bl/6 mice were transduced with mAdCD8
were transduced with mAdCD8 with Ad(empty)
with Ad(empty) or mock- infected
or mock- infected They were then transplanted under kidney capsule of diabetic Balb/c mice (a, b, c). (b) Control or mAdCD8 transduced C57Bl/6 pancreatic islets that had been transplanted into diabetic or mock-infected
They were then transplanted under kidney capsule of diabetic Balb/c mice (a, b, c). (b) Control or mAdCD8 transduced C57Bl/6 pancreatic islets that had been transplanted into diabetic or mock-infected were transplanted into diabetic C57Bl/6
were transplanted into diabetic C57Bl/6 or diabetic Balb/c
or diabetic Balb/c mice. They were nephrectomized to remove implanted pancreatic islets two days prior to the observed increase in blood sugar levels.
mice. They were nephrectomized to remove implanted pancreatic islets two days prior to the observed increase in blood sugar levels.
Since control pancreatic islets were destroyed consistently, it was unlikely that a recovery of endogenous insulin production was responsible for the observed normalization of the blood glucose levels. Yet, to formally exclude this possibility, implanted islets were removed by nephrectomy. In mice that had received syngeneic pancreatic islets (C57Bl/6 into C57Bl/6) either VV-transduced or mock-infected, hyperglycemia returned swiftly (FIGURE 3d). The same held true for Balb/c mice that had carried allogeneic islets under the protection of mAdCD8. Even animals that had maintained allogeneic islets for one year developed diabetes within two days. Therefore, we concluded that pancreatic islet grafts had been solely responsible for the permanent diabetes cures.
■ Immune status of transplanted animals
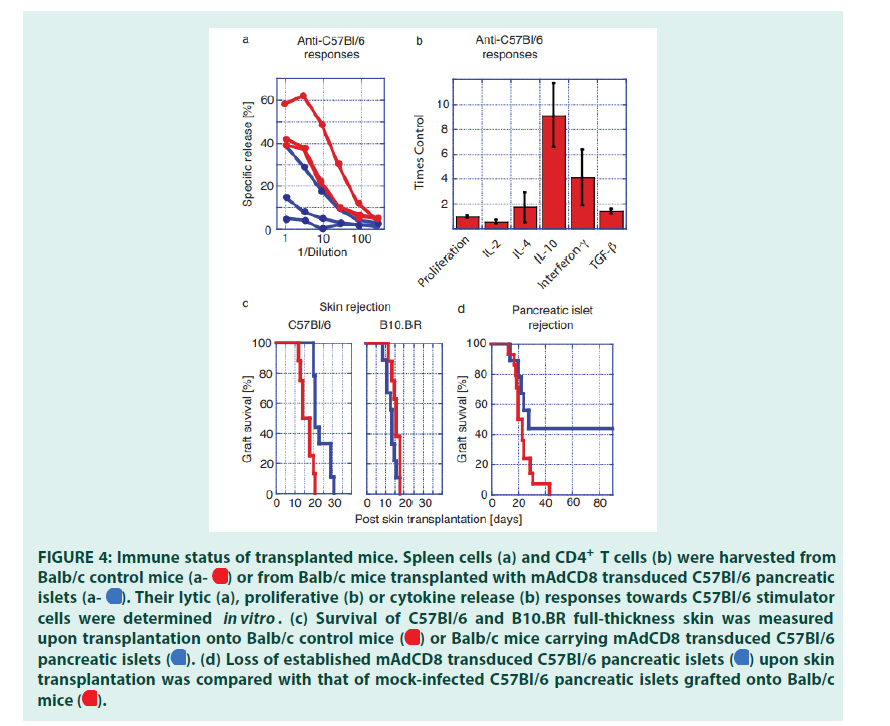
Balb/c mice that had maintained mAdCD8- transduced C57Bl/6 pancreatic islets for at least 6 months were used to investigate how graft acceptance had affected alloreactive responses of both CD8+ and CD4+ T cells. To evaluate the activity of alloreactive CTLs, spleen cells were challenged in vitro with C57Bl/6 stimulator cells. Balb/c control mice that had not received an islet transplant were tested in parallel. These studies revealed that some of the transplant recipients no longer provided donor-specific CD8+ CTLs that could be activated in vitro (FIGURE 4a). Others retained donor-specific CTLs that nevertheless did not interfere with the presence of the C57Bl/6- derived pancreatic islets.
Peripheral CD4+ T cells harvested from transplant carriers and control mice were stimulated with Balb/c spleen cells. T cells harvested from control mice and organ carriers proliferated to the same extent. Differences in the release of Inter Leukins (IL) were seen between transplanted and control mice (FIGURE 4b). A reduction in IL-2 production may indicate a partial inhibition of the activity of alloreactive CD4+ T helper-1 cells [26,27]. IL-4 secretion as a marker of T helper-2 cells did not show significant differences. The syntheses of IL-10, Interferon-γ (IFN) and TGF-β1 were enhanced in transplanted mice (FIGURE 1b). This change in the interleukin profile suggested that the presence of the allogeneic graft had expanded the activity of regulatory T cells [28-32].
We then studied whether the observed immune protection could be broken with a strong immune challenge. Long-term carriers of C57Bl/6 pancreatic islets and Balb/c control mice were transplanted with patches of full-thickness dorsal skins harvested from C57Bl/6 and ‘third-party’ B10.BR donors. We discovered that the rejection of donor-type C57Bl/6 skin was significantly delayed when grafted onto pancreatic islet carriers (FIGURE 4c). In contrast, B10.BR skins were destroyed swiftly by control mice and longterm carriers of allogeneic islets. This indicated that third-party immune responses had not been affected by the presence of veto- engineered tissue. Thus, VV-transduced grafts had inhibited immune responses in an MHC- haplotype specific manner. Approximately half of all skin-transplanted mice lost their pancreatic islet graft within 30 days (FIGURE 4d). In these animals allo-reactive T cells had not been completely deleted as they could be activated by a stringent immune challenge. In these mice veto had induced permanent immune unresponsiveness rather than stable tolerance. In a second cohort pancreatic islets remained healthy even though donor-type skin had been r (FIGURE 4d) ejected . In these mice, a stable immune tolerance had been imprinted that encompassed broadly reactive and tissue- specific, i.e. islet-specific, alloreactive T cells, Protecting allogeneic pancreatic islet grafts by engineered veto and that could not be broken by a most potent challenge afforded by full-thickness skin. Taken together these findings demonstrated that as we had predicted veto-induced immune tolerance was indeed tissue-specific [33-35].
Figure 4 : Immune status of transplanted mice. Spleen cells (a) and CD4+ T cells (b) were harvested from Balb/c control mice or from Balb/c mice transplanted with mAdCD8 transduced C57Bl/6 pancreatic islets
or from Balb/c mice transplanted with mAdCD8 transduced C57Bl/6 pancreatic islets  Their lytic (a), proliferative (b) or cytokine release (b) responses towards C57Bl/6 stimulator cells were determined in vitro. (c) Survival of C57Bl/6 and B10.BR full-thickness skin was measured upon transplantation onto Balb/c control mice
Their lytic (a), proliferative (b) or cytokine release (b) responses towards C57Bl/6 stimulator cells were determined in vitro. (c) Survival of C57Bl/6 and B10.BR full-thickness skin was measured upon transplantation onto Balb/c control mice or Balb/c mice carrying mAdCD8 transduced C57Bl/6 pancreatic islets
or Balb/c mice carrying mAdCD8 transduced C57Bl/6 pancreatic islets  (d) Loss of established mAdCD8 transduced C57Bl/6 pancreatic islets
(d) Loss of established mAdCD8 transduced C57Bl/6 pancreatic islets  upon skin transplantation was compared with that of mock-infected C57Bl/6 pancreatic islets grafted onto Balb/ mice
upon skin transplantation was compared with that of mock-infected C57Bl/6 pancreatic islets grafted onto Balb/ mice 
Discussion
Even though classical veto had been shown to induce specific immune unresponsiveness, it has not been vigorously pursued for clinical use. Classical veto requires the enrichment and in the case of CD8+ T cells, the in vitro stimulation of donor derived T cells [36]. Infusion of activated donor T cells is not without peril: A Graftversus- Host Disease (GvHD) is set off unless alloreactive T cells have been removed from the veto cell preparation [37]. Although GvHD may be avoided by the use of other BM-derived veto cells, classical veto may nonetheless fail to imprint complete transplantation tolerance. Due to the exquisite specificity of veto, only T cells reactive with antigens presented on veto cells are deleted. Allo-reactive T cells that see grafts in a tissue- specific fashion remain, and may become instrumental in rejecting the transplant [33-35]. Our studies supported this hypothesis. Even though veto engineered pancreatic islets avoided rejection by the recipient’s immune system, they failed to completely eliminate skin-specific alloreactive T cells. As a consequence, skin patches harvested from the same donor were not fully protected (FIGURE 4).
Earlier we had developed hAbs that anchored CD8 α-chain homodimers onto the cell surface [16] and had demonstrated that surface expression of CD8 did transform cells of different phenotypes into specifically immune inhibitory cells [16,17]. These results led us to examine whether complex tissues could be similarly engineered for CD8 mediated veto. Extended CD8 expression was achieved by a switch from the hAb approach to genetic delivery systems. We first used fibroblasts stably transfected with a plasmid-based VV to establish immune inhibitory potential a VV in vitro and in vivo. We switched to a more efficient adenoviral VV for the ultimate transplantation studies. Adenoviral vectors infect most cells, even non-proliferating ones, with high efficiency [21] and thus are well suited to deliver CD8 to a complex tissue. Their transience in expression though detrimental to conventional gene therapy applications, may indeed be beneficial for immune suppression.
In the improbable case that host APCs were infected, any aberrant inhibition of the immune system would be self-terminating. In gene therapy, the use of conventional adenoviral gene therapy vectors is also hampered by their strong immunogenicity [38]. In our transplantation studies, syngeneic pancreatic islets transduced with adenoviral VV were maintained permanently without any evidence of rejection (FIGURE 3d). This observation suggested that incorporation of veto into adenoviral vectors lessened their immunogenicity. Indeed, we found in other studies that i.v. or i.m. injection of free adenoviral VVs only raised weak, if any immune responses in mice (data not shown). VV-transduced pancreatic islets successfully evaded immune rejection in fully immune competent allogeneic recipients. These islets were maintained indefinitely in the absence of any adjuvant immune-suppressive therapy.
At the one-year mark, we surveyed grafted islets for the presence of CD8. The results were highly variable, as we found evidence of CD8 expression in only some islets (data not shown). Therefore, the long-term survival of the allogeneic tissue may not depend on the continued presence of CD8. Yet, there seem to be timing and dose effects on the activity of alloreactive T cells. 5 × 106 CD8 expressing fibroblasts functionally deleted CTLs in vivo. 1 × 106 resulted in only a partial inhibition of allo-reactive CTLs. In our pancreatic islet transplantations, the number of CD8+ cells transferred to the recipient had to be even lower (FIGURE 1c). Yet, CD8 expression might have been maintained longer due the slow turnover of pancreatic islet cells.
The small size of the ‘veto-engineered’ tissue might explain the incomplete elimination of allo- reactive T cells seen in some of the animals (FIGURES 4a and 4b). Nevertheless, an extended presence of CD8 may have completely suppressed T cell responses in other animals. Our immune response studies suggested that immune regulatory T cells had been induced in mice that maintained pancreatic islets over longer periods of time. In these animals, the permanent protection of veto-engineered pancreatic islets might have been due to the combination of two immune inhibitory effects: The veto deletion mechanism, complemented by the activity of regulatory T cells [30,32,39]. Even so, specificity of the veto effect was maintained long term.
Our animal studies established the overall feasibility of a CD8- mediated genetic veto engineering strategy. It transformed complex tissues into grafts acceptable to immune competent recipients. Other genetic approaches have been investigated to reduce the immunogenicity of grafts. Since the Fas ligand (FasL) was detected in sites of immune privilege, it had been postulated that it would similarly protect other tissues from immune destruction, as transplants carrying FasL would induce the apoptosis of Fas expressing T cells [40]. Transduction studies with the FasL revealed that inflammatory processes were also induced, which resulted in the accelerated rather than delayed destruction of grafted pancreatic islets [41,42]. Additionally, FasL-expressing tissues might inhibit T cells in a non-specific manner, as they may act as a sink for activated T cells, irrespective of their specificity. In another approach, tissues were engineered to secrete immune inhibitory compounds. In the case of CTLAIg, when given systemically or released locally by transduced tissues, it was effective within an immune- suppressive combination therapy [43,44]. TGF-β1 was also successfully used, transforming dendritic cells into highly immune suppressive cells [45]. However, a release of TGF-β1 to modify the immunogenicity of other organs would be problematic. For instance TGF-β1 induces a fibrosis of the liver [46].
Conclusion
In contrast to a release of soluble factors, veto is intrinsically specific, since it relies on the engagement of TCR on engineered tissues. T cells that do not react with the engineered graft will not be affected. We expect that that unique activity of veto can be harnessed for transplantations of many tissues and organs. In preliminary experiments, we have successfully transplanted liver and heart tissues under veto protection (data not shown). We will undertake large animal trials to investigate how adenoviral VV can be optimized for clinical use, by itself or complemented with other immune-suppressive therapies. In addition, we will engineer the VVs as fully deleted adenoviral vectors so avoid the expression of adenoviral genes in the transduced tissues.
References
- Fishman JA. Infection in Renal Transplant Recipients. Semin Nephrol. 27(4):445-461 (2007).
[Crossref] [Google Scholar] [Pubmed]
- Rubin RH. The Direct and Indirect Effects of Infection in Liver Transplantation: Pathogenesis, Impact, and Clinical Management. Curr Clin Top Infect Dis. 22:125-154 (2002).
[Google Scholar] [Pubmed]
- Snydman DR. Epidemiology of Infections After Solid-Organ Transplantation. Clin Infect Dis. 33(Supplement-1):S5-S8 (2001).
[Crossref] [Google Scholar] [Pubmed]
- Rammensee HG, Nagy ZA, Klein J, et al. Suppression of Cell‐Mediated Lymphocytotoxicity against Minor Histocompatibility Antigens Mediated by Lyt‐1+ Lyt− 2+ T Cells of Stimulator‐Strain Origin. Eur J Immunol. 12(11):930-934 (1982).
[Crossref] [Google Scholar] [Pubmed]
- Claesson MH, Miller RG. Functional Heterogeneity in Allospecific Cytotoxic T Lymphocyte Clones. I. CTL Clones Express Strong Anti-Self Suppressive Activity. J Exp Med. 160(6):1702-1716 (1984).
[Crossref] [Google Scholar] [Pubmed]
- Fink PJ, Weissman IL, Bevan MJ, et al. Haplotype-Specific Suppression of Cytotoxic T Cell Induction by Antigen Inappropriately Presented on T Cells. J Exp Med. 157(1):141-154 (1983).
[Crossref] [Google Scholar] [Pubmed]
- Rammensee HG. Veto Function in vitro and in vivo. Int Rev Immunol. 4(2):175-191 (1989).
[Crossref] [Google Scholar] [Pubmed]
- Muraoka SH, Miller RG. Cells in Murine Fetal Liver and in Lymphoid Colonies Grown from Fetal Liver Can Suppress Generation of Cytotoxic T Lymphocytes Directed Against Their Self Antigens. J Immunol. 131(1):45-49 (1983).
[Crossref] [Google Scholar] [Pubmed]
- Fink PJ, Rammensee HG, Benedetto JD, et al. Studies on the Mechanism of Suppression of Primary Cytotoxic Responses by Cloned Cytotoxic T Lymphocytes. J Immunol. 133(4):1769-1774 (1984).
[Crossref] [Google Scholar] [Pubmed]
- Hambor JE, Tykocinski ML, Kaplan DR, et al. Functional Consequences of Anti-Sense RNA-Mediated Inhibition of CD8 Surface Expression in A Human T Cell Clone. J Exp Med. 168(4):1237-1245 (1988).
[Crossref] [Google Scholar] [Pubmed]
- Hambor JE, Kaplan DR, Tykocinski ML, et al. CD8 Functions as an Inhibitory Ligand in Mediating the Immunoregulatory Activity of CD8+ Cells. J Immunol. 145(6):1646-1652 (1990).
[Crossref] [Google Scholar] [Pubmed]
- Sambhara SR, Miller RG. Programmed Cell Death of T Cells Signaled by the T Cell Receptor and the α3 Domain of Class I MH. Science. 252(5011):1424-1427 (1991).
[Crossref] [Google Scholar] [Pubmed]
- Hiruma K, Nakamura H, Henkart PA, et al. Clonal Deletion of Postthymic T Cells: Veto Cells Kill Precursor Cytotoxic T Lymphocytes. J Exp Med. 175(3):863-868 (1992).
[Crossref] [Google Scholar] [Pubmed]
- Verbanac KM, Carver FM, Haisch CE, et al. A Role for Transforming Growth Factor-Beta in the Veto Mechanism in Transplant Tolerance. Transplantation. 57(6):893-839 (1994).
[Crossref] [Google Scholar] [Pubmed]
- Asiedu C, Meng Y, Wang W, et al. Immunoregulatory Role of Cd8 α in the Veto Effect: Transforming Growth Factor-B1 Activation 1. Transplantation. 67(3):372-380 (1999).
[Crossref] [Google Scholar] [Pubmed]
- Qi Y, Berg R, Singleton MA, et al. Hybrid Antibody Mediated Veto of Cytotoxic T Lymphocyte Responses. J Exp Med 183(5):1973-1980 (1996).
[Crossref] [Google Scholar] [Pubmed]
- Staerz UD, Lee DS, Qi Y, et al. Induction of Specific Immune Tolerance with Hybrid Antibodies. Immunol Today. 21(4):172-176 (2000).
[Crossref] [Google Scholar] [Pubmed]
- Thomas JM, Carver FM, Cunningham P, et al. Veto Cells Induce Long-Term Kidney Allograft Tolerance in Primates without Chronic Immunosuppression. Transplant Proc. 23(1): 11-13 (1991).
[Google Scholar] [Pubmed]
- Bachar-Lustig E, Reich-Zeliger S, Reisner Y, et al. Anti-Third-Party Veto CTLs Overcome Rejection of Hematopoietic Allografts: Synergism with Rapamycin and BM Cell Dose. Blood. 102(6):1943-1950 (2003).
[Crossref] [Google Scholar] [Pubmed]
- Takahashi T, Maki T. Prolongation of Mouse Skin Allograft Survival by Cloned Veto Suppressor Cells. Transplant Proc. 23(1):192-193 (1991).
- Campos SK, Barry MA. Current Advances and Future Challenges in Adenoviral Vector Biology and Targeting. Curr Gene Ther. 7(3):189-204 (2007).
[Crossref] [Google Scholar] [Pubmed]
- Rich DP, Couture LA, Cardoza LM, et al. Development and Analysis of Recombinant Adenoviruses for Gene Therapy of Cystic Fibrosis. Hum Gene Ther. 4(4):461-476 (1993).
[Crossref] [Google Scholar] [Pubmed]
- Tanaka Y, Tevethia SS. Differential Effect of Adenovirus 2 E3/19K Glycoprotein on the Expression of H-2Kb And H-2Db Class I Antigens and H-2Kb-And H-2Db-Restricted SV40-Specific CTL-Mediated Lysis. Virology. 165(2):357-366 (1988).
[Crossref] [Google Scholar] [Pubmed]
- Gill RG, Rosenberg AS, Lafferty KJ, et al. Characterization of Primary T Cell Subsets Mediating Rejection of Pancreatic Islet Grafts. J Immunol 143(7):2176-2178 (1989).
[Crossref] [Google Scholar] [Pubmed]
- Makhlouf L, Yamada A, Ito T, et al. Allorecognition and Effector Pathways of Islet Allograft Rejection in Normal Versus Nonobese Diabetic Mice. J Am Soc Nephrol. 14(8):2168-2175 (2003).
[Crossref] [Google Scholar] [Pubmed]
- TR M. Definition According to Profiles of Lymphokine Activities and Secreted Proteins. J Immunol. 136:2348-2357 (1986).
[Google Scholar] [Pubmed]
- Papp IL, Wieder KJ, Sablinski TO, et al. Evidence for Functional Heterogeneity of Rat CD4+ T Cells in vivo. Differential Expression of IL-2 And IL-4 Mrna in Recipients of Cardiac Allografts. J Immunol. 148(5):1308-1314 (1992).
[Crossref] [Google Scholar] [Pubmed]
- Miller A, Lider O, Roberts AB, et al. Suppressor T Cells Generated by Oral Tolerization to Myelin Basic Protein Suppress Both in vitro and in vivo Immune Responses by the Release of Transforming Growth Factor Beta After Antigen-Specific Triggering. Proc Natl Acad Sci USA. 89(1):421-425 (1992).
[Crossref] [Google Scholar] [Pubmed]
- Spadafora-Ferreira M, Fonseca JA, Granja C, et al. Predominant IL-10 Production in Indirect Alloreactivity is Not Associated with Rejection. Clin Immunol.101(3):315-27 (2001).
[Crossref] [Google Scholar] [Pubmed]
- Wood KJ, Luo S, Akl A, et al. Regulatory T Cells: Potential in Organ Transplantation. Transplantation. 77(1):S6-S8 (2004).
[Crossref] [Google Scholar] [Pubmed]
- Kingsley CI, Karim M, Bushell AR, et al. CD25+ CD4+ Regulatory T Cells Prevent Graft Rejection: CTLA-4- and IL-10-Dependent Immunoregulation of Alloresponses. J Immunol. 168(3):1080-1086 (2002).
[Crossref] [Google Scholar] [Pubmed]
- Wood KJ, Feng G, Wei B, et al. Interferon Gamma: Friend or Foe?. Transplantation. 84(1):S4-S5 (2007).
[Crossref] [Google Scholar] [Pubmed]
- Marrack P, Kappler J. T Cells Can Distinguish Between Allogeneic Major Histocompatibility Complex Products on Different Cell Types. Nature. 332(6167):840-843 (1988).
[Crossref] [Google Scholar] [Pubmed]
- Heath WR, Sherman LA. Cell‐Type‐Specific Recognition of Allogeneic Cells by Alloreactive Cytotoxic T Cells: A Consequence of Peptide‐Dependent Allorecognition. Eur J Immunol. 21(1):153-159 (1991).
[Crossref] [Google Scholar] [Pubmed]
- Poindexter NJ, Naziruddin B, Mccourt DW, et al. Isolation of A Kidney-Specific Peptide Recognized by Alloreactive HLA-A3-Restricted Human CTL. J Immunol. 154(8):3880-3887 (1995).
[Crossref] [Google Scholar] [Pubmed]
- Reich-Zeliger S, Bachar-Lustig E, Gan J, et al. Tolerance Induction by Veto CTLs in the TCR Transgenic 2C Mouse Model. I. Relative Reactivity of Different Veto Cells. J Immunol. 173(11):6654-6659 (2004).
[Crossref] [Google Scholar] [Pubmed]
- Reich-Zeliger S, Bachar-Lustig E, Bar-Ilan A, et al. Tolerance Induction in Presensitized Bone Marrow Recipients by Veto Ctls: Effective Deletion of Host Anti-Donor Memory Effector Cells. J Immunol. 179(10):6389-6394 (2007).
[Crossref] [Google Scholar] [Pubmed]
- Schagen FH, Ossevoort M, Toes RE, et al. Immune Responses Against Adenoviral Vectors and Their Transgene Products: A Review of Strategies for Evasion. Crit Rev Oncol Hematol. 50(1):51-70 (2004).
[Crossref] [Google Scholar] [Pubmed]
- Onodera K, Hancock WW, Graser E, et al. Type 2 Helper T Cell-Type Cytokines and the Development of Infectious Tolerance in Rat Cardiac Allograft Recipients. J Immunol. 158(4):1572-1581 (1997).
[Crossref] [Google Scholar] [Pubmed]
- Bellgrau D, Gold D, Selawry H, et al. A Role for CD95 Ligand in Preventing Graft Rejection. Nature. 377(6550):630-632 (1995).
[Crossref] [Google Scholar] [Pubmed]
- Allison J, Georgiou HM, Strasser A, et al. Transgenic Expression of CD95 Ligand on Islet Β Cells Induces A Granulocytic Infiltration but Does Not Confer Immune Privilege upon Islet Allografts. Proc Natl Acad Sci USA. 94(8):3943-3947 (1997).
[Crossref] [Google Scholar] [Pubmed]
- Kang SM, Schneider DB, Lin Z, et al. Fas Ligand Expression in Islets of Langerhans Does Not Confer Immune Privilege and Instead Targets Them for Rapid Destruction. Nat Med. 3(7):738-743 (1997).
[Crossref] [Google Scholar] [Pubmed]
- Kirk AD, Harlan DM, Armstrong NN, et al. CTLA4-Ig and Anti-CD40 Ligand Prevent Renal Allograft Rejection in Primates. Proc Natl Acad Sci. 94(16):8789-8794 (1997).
[Crossref] [Google Scholar] [Pubmed]
- Li ZL, Xue WJ, Tian PX, et al. Prolongation of Islet Allograft Survival by Coexpression of CTLA4Ig and CD40LIg in Mice. Transplant Proc. 39(10):3436-3437 (2007).
[Crossref] [Google Scholar] [Pubmed]
- Asiedu C, Dong SS, Pereboev A, et al. Rhesus Monocyte-Derived Dendritic Cells Modified to Over-Express TGF-Β1 Exhibit Potent Veto Activity1. Transplantation. 74(5):629-637 (2002).
[Crossref] [Google Scholar] [Pubmed]
- Kanzler S, Lohse AW, Keil A, et al. TGF-Β1 in Liver Fibrosis: An Inducible Transgenic Mouse Model to Study Liver Fibrogenesis. Am J Physiol. 276(4):G1059-G1068 (1999).
[Crossref] [Google Scholar] [Pubmed]