Research Paper - Neuropsychiatry (2019) Volume 9, Issue 5
Psychophysiological Modifications of Nurse Students in Hospital Clinical Simulations
- Corresponding Author:
- Vicente Javier Clemente Suárez
Facultad de Ciencias de la Actividad Física y el Deporte. Universidad Europea. Calle Tajo s/n. Madrid
email: vctxente@yahoo.es
Abstract
The aim of this study was to analyze the psychophysiological responses of nursing students during a clinical simulation, as well as their psychological profile in relation to their coping style in stressful situations and its impact on academic performance. We analyzed several variables in three students: i) heart rate variability (HRV) at 5 moments: pre-session, beginning part, middle part, last part of the session and post-session; ii) cortical arousal by Critical Flicker Fusion Threshold; iii) brain oscillations by means of electroencephalography; iv) perceived stress before and after starting the practice; and v) the objective and subjective evaluation of academic performance. We expected to find psychophysiological markers indicating a habituation to the situation. However, the results based on HRV and the parameters of related indexes failed to indicate the expected autonomic adaptation during the practice, showing a high sympathetic autonomic nervous system modulation. This non-habituation outcome might be related to the students’ high scores in the perceived stress scale associated with non-adaptive coping styles. In conclusion, a nursing clinical simulation scenario produced an anticipatory anxiety response in the students, which prevented them from achieving a habituation response, decreasing the Critical Flicker Fusion Threshold, and increasing anterior and decreasing temporal electroencephalogram activity.
Keywords
Autonomic modulation, Electroencephalographic activity, Personality, Psychology
Introduction
The implementation of hospital clinical simulations has been highlighted as essential in health sciences higher studies learning processes [1,2] specifically in nursing degrees, where students are subjected to repeated evaluation scenarios in hospital clinical simulation environments [3]. These scenarios are designed by practitioners and professors to provide the students with the necessary skills for their future professional practice, integrating theoretical and practical knowledge. In this context, students must be able to apply previous theoretical knowledge in a realistic and stressful environment [4]. The evidence of the negative effects of the stress response in uncontrolled environments has been studied in various fields, showing how repeated exposure to this context facilitates the process of habituation in students, and therefore, the improvement of the teaching-learning process [5-7]. The exposure to these uncontrolled, unpredictable and stressful scenarios can produce different effects in the participants’ psychophysiological responses, increasing sympathetic nervous system modulation, decreasing cortical arousal, and negatively affecting memory and perceived exertion [8-10]. Additionally, brain oscillations, measured by means of electroencephalography (EEG), have been linked to different cognitive and emotional states such as attention [11-13] or anxiety [14-16], but they have never been recorded in realistic stressful scenarios like clinical simulations.
The way stressful situations are dealt with is also determined by the personal psychological profile. Nursing students must face crisis situations involving high emotional intensity, physical and emotional exhaustion, and intense feelings on the part of their patients, such as the anger, frustration or despair [17,18]. Therefore, the student’s coping strategies and psychological profile are important variables to take into account in order to predict how students will face these situations during the training process and in their professional practice [19,20]. It is known that some factors of the individual personality will define the coping style when dealing with stressors and might present a negative impact on a student’s academic performance in psychological profiles associated with poor stress management, interfering with skills such as cognitive flexibility, resilience or collaborative work [21,22].
The objective of the present study was to analyze the psychophysiological response of nursing degree students during a hospital clinical simulation and its relationship with their performance. The initial hypotheses were: i) students would present a habituation response at the end of the scenario compared to the beginning, reducing the sympathetic nervous system modulation, and ii) we would find a difference in frontal EEG oscillatory activity when comparing the recordings at the beginning and the end of the scenario due to the cognitive demands of the clinical simulation.
Materials and Methods
Participants
Three nursing students participated voluntarily in the study. Their ages ranged between 21 and 28 years old (M=24.3; SD=3.51). The procedure was conducted in accordance with the Helsinki Declaration (as revised in Brazil, 2013) and had been approved by the University’s Ethics Committee. The data were collected anonymously. Prior to participation, all participants were informed about the experimental procedures, indicating their right to withdraw from the study at any time, and they provided written informed consent. All students had the same experience in coping with clinical practices, so the acquisition of competencies was done under the same conditions.
Design and procedure
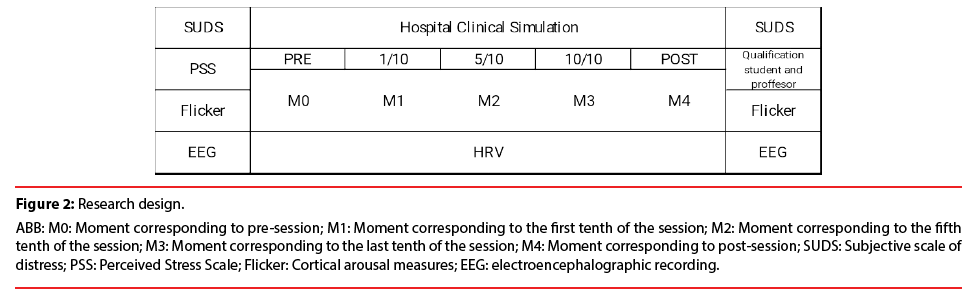
Prior to starting the hospital clinical care simulation, participants completed the perceived stress scale (PSS) questionnaire; and then we recorded autonomic modulation by heart rate variability (HRV), cortical arousal by indirect measures (Critical Flicker Fusion Threshold) and brain oscillations by means of electroencephalography (EEG). EEG recordings consisted of two resting state sessions: 3 min. of eyes closed, and 3 min. of eyes opened while looking at a fixed point at 1 m distance. After that, the student’s subjective perception of distress strait was requested. Then, the professor guided the participant through the simulation scenario and instructed her on the characteristics of the scenario that she would encounter. The student had 15 min. to solve the unknown demands of the scenario, which consisted in a simulated patient with decreased O2 saturation and an obstructive respiratory pathology. Once the simulation was finished, the researcher asked the participant for her subjective perception of distress strait and her perceived evaluation of her performance in the simulation. The objective evaluation of the simulation as judged by three experienced professors was also taken into account. Then, the cortical arousal measurements and the EEG recordings were also conducted in the same manner as prior to the scenario (Figure 1). The HRV was recorded during the entire simulation. The scenario was divided in 5 analysis moments M0, (pre-session interval); M1 (interval corresponding to the first tenth of the hospital clinical situation); M2 (interval corresponding to the fifth tenth of the hospital clinical situation); M3 (interval corresponding to the final tenth of the hospital clinical situation) M4 (post-session interval) (Figure 2).
Measurements and instruments
Autonomic modulation was measured by the analysis of HRV. Variation in the time interval between beats (R-R interval) was used as a measure of autonomic modulation. It was recorded with a Polar V800 heart rate monitor (Polar, Kempele, Finland) consistent with previous research [23]. The R-R series were analyzed using the Kubios HRV software (version 2.0, Biosignal Analysis and Medical Imaging Group, University of Kuopio, Finland), developed in accordance with the recommendations of the existing scientific literature [24]. We analyzed the following HRV parameters: LF: The low-frequency band in normalized units (low-frequency, LFn); HF: The high-frequency band in normalized units (high-frequency, HFn); LF/HF ratio: The ratio of Low Frequency to High Frequency; PNN50: The number of successive intervals which differ by more than 50 ms expressed as a percentage of the total; RMSSD: Square root of the mean of the sum of the squared differences between adjacent normal R-R intervals (RMSSD); SD1: Sensitivity of the short-term variability of the non-linear HRV spectrum; and SD2: Long-term variability of the non-linear HRV spectrum.
Cortical arousal was measured through the Critical Flicker Fusion Threshold (CFFT). Subjects were seated in front of a viewing chamber (Lafayette Instrument Flicker Fusion Control Unit Model 12021), which was constructed to control extraneous factors that might distort CFFT values. Two light-emitting diodes (58 cd/m2) were presented simultaneously in the viewing chamber, one for the left eye and one for the right eye. The stimuli were separated by 2.75 cm (center to center) with a stimulusto- eye distance of 15 cm and a viewing angle of 1.9°. The inside of the viewing chamber is painted flat black to minimize reflection. The flicker frequency increment (2 Hz/sec) increased from 20 to 100 Hz until the participant perceived fusion. After a fovea binocular fixation, participants were required to respond by pressing a button upon identifying the visual fusion thresholds. Prior to the experiment, they performed as many practice trials as needed to become familiar with the exigencies of the CFF test. Then, five trials were performed. The final CFFT value was calculated as the average values of the five trials. Increases in this value indicate a central nervous system fatigue and a reduction in the efficiency of information processing systems and a decrease would show an increase in cortical arousal and information processing [25].
We recorded electroencephalographic activity using a cap with 32 active electrodes (actiCAP, Brain Products) referenced to AFz. The recording was written at a sampling rate of 500 Hz. The signal was band-pass filtered online between 0.01 and 249 Hz. We applied an Independent Component Analysis to remove artifacts provoked by blinks and deleted segments of data pertaining to other artifacts. We band-pass filtered the signal offline between 1 and 50 Hz and determined the power spectrum of the pre-processed EEG signal by using Fast Fourier Transform as integrated in Fieldtrip software [26]. The spectrum was averaged in epochs of 2000 ms and divided into 5 frequency bands: delta (1-4 Hz), theta (4-8 Hz), alpha (8-12), beta (13-30 Hz), and gamma (30-50 Hz). We averaged the channels creating four cortical areas: frontal/anterior (FP1, FP2, F7, F3, Fz, F4, F8), central (FC5, FC1, FC2, FC6, C3, Cz, C4, CP1, CP2), temporal (FT9, T7, TP9, F10, T8, TP10, CP5, CP6) and posterior/occipital (P7, P3, Pz, P4, P8, O1, Oz, O2).
The student’s perceived evaluation indicated the individual’s self-perception of the performance given in the practice on a scale of 0 (poorest performance) to 10 (best performance). The professor’s evaluation was based on the practice objective determined and presented to the student prior to the practice and was on a scale of 0 (poorest performance) to 10 (best performance).
The subjective perception of distress strait was analyzed on a scale of subjective distress units (SUDS), showing scores of between 0 and 100 [27].
The Perceived Stress Scale (PSS) assessed the level of perceived stress over a one-month period. It was composed of 14 items that were answered on a five-point Likert scale, where 0=Never and 4=Very often. An example item was: “In the last month, how often have you felt that had everything under control?” High scores are related to a higher perception of stress [28].
Data analysis
The statistical analysis was carried out using the SPSS 23.0 statistical program. Descriptives were analyzed for each variable (M, SD) and a Friedman test was carried out to evaluate the differences between the different analysis moments. The Effect Size (ES) was tested by Cohen’s D [ES=(Post-test mean-Pre-test mean)/Pre-test SD]. The significance level was 0.05.
Results
We found a moderate effect size in the decrease Ratio HF/LF between M1 vs. M2, in the increase in the variables RMSSD, SD1 and SD2 in M1 vs. M3, in the decrease in the variables RMSSD, SD1 and SD2 in M1 vs. M4 and in the increase in LF and Ratio HF/LF in M0 vs. M4 (Table 1).
| Session | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PRE | 1/10 | 5/10 | 10/10 | POST | Effect size | ||||||
| M0 | M1 | M2 | M3 | M4 | Chi2 | Sig. | M1 vs. M2 | M1 vs. M3 | M1 vs. M4 | M0 vs. M4 | |
| LF (nu) | 60.38 ± 24.77 | 73.17 ± 19.54 | 71.89 ± 10.99 | 72.57 ± 13.08 | 71.00 ± 22.41 | 4.000 | 0.406 | -0.13 | -0.03 | -0.11 | 0.42 |
| HF (nu) | 39.49 ± 24.68 | 26.79 ± 19.51 | 27.99 ± 10.90 | 27.27 ± 12.92 | 28.97 ± 22.40 | 4.000 | 0.406 | 0.06 | 0.02 | 0.11 | -0.42 |
| Ratio LF/HF (nu) | 2.42 ± 2.30 | 4.36 ± 3.66 | 3.06 ± 1.93 | 3.59 ± 2.96 | 4.49 ± 4.41 | 4.000 | 0.406 | -0.35 | -0.21 | 0.03 | 0.90 |
| PNN50 (No.) | 17.15 ± 17.07 | 16.56 ± 24.87 | 7.93 ± 6.17 | 20.30 ± 20.41 | 13.47 ± 20.46 | 1.153 | 0.886 | -0.34 | 0.15 | -0.12 | -0.21 |
| RMSSD (ms) | 49.34 ± 38.00 | 40.86 ± 30.17 | 32.00 ± 21.85 | 53.89 ± 52.84 | 30.82 ± 26.74 | 2.133 | 0.711 | -0.29 | 0.43 | -0.33 | -0.48 |
| SD1 (ms) | 34.92 ± 26.89 | 28.99 ± 21.41 | 22.68 ± 15.50 | 38.20 ± 37.45 | 21.81 ± 18.92 | 2.133 | 0.711 | -0.29 | 0.43 | -0.33 | -0.48 |
| SD2 (ms) | 106.09 ± 69.66 | 84.32 ± 40.56 | 92.02 ± 53.89 | 99.34 ± 58.24 | 95.76 ± 63.55 | 0.800 | 0.938 | 0.18 | 0.37 | 0.28 | -0.14 |
M: Moment; HRV: Heart Rate Variability; Low-frequency LFn: The Low-Frequency Band in Normalized Units; High-Frequency, HFn: The High-Frequency Band in Normalized Units; LF/HF ratio: Low Frequency to High Frequency ratio; PNN50: The Proportion of NN50 Divided by Total Number of NNs; RMSSD:Square Root of the Mean of the Sum of the Squared Differences between Adjacent Normal R-R Intervals; n.u: Normalized Unit; SD1: Standard Deviations of the Scattergram 1; SD2: Standard Deviations of the Scattergram 2
Table 1: Differences in the measurements throughout the hospital clinical simulation.
The SUDS increased from 51.6 ± 11.6 to 56.6 ± 10.9 after the clinical simulation, and the academic performance of students was 7.17 ± 0.29 points out of 10. There is no correlation between the variables analyzed and the academic performance of the students. CFFT presented a decrease at the end of the simulation (32.63 ± 1.65 to 32.29 ± 2.38; ES: -1.11 ± 2.70). In addition, the students presented high scores on the PSS: 38.0 ± 1.0.
EEG activity showed a high effect size in prepost differences in the eyes-open condition in the following frequency bands: alpha, beta and gamma at anterior locations; delta and gamma at central locations; and posterior alpha (Table 2). Whereas in the eyes-closed condition, we found a high effect size in pre-post differences in beta and gamma at anterior locations; delta and alpha at central locations; delta, theta, alpha, beta and gamma at temporal locations; and delta and theta at posterior areas (Table 3).
| PRE | POST | Z | P | Effect size | |
|---|---|---|---|---|---|
| Anterior Deltha (Hz.) | 1425.5 ± 1148.59 | 614.2 ± 60.09 | -1.069 | 0.285 | -0,71 |
| Anterior Theta (Hz.) | 615.0 ± 468.17 | 710.1 ± 189.52 | -1.604 | 0.109 | 0.20 |
| Anterior Alpha (Hz.) | 292.1 ± 205.58 | 654.0 ± 629.01 | -.535 | 0.593 | 1.76 |
| Anterior Beta (Hz.) | 238.3 ± 22.27 | 832.7 ± 22.27 | -.535 | 0.593 | 26.69 |
| Anterior Gamma (Hz.) | 108.1 ± 57.02 | 812.7 ± 1253.23 | -1.069 | 0.285 | 12.36 |
| Central Deltha (Hz.) | 657.9 ± 126.66 | 510.6 ± 217.3 | -1.604 | 0.109 | -1.14 |
| Central Theta (Hz.) | 548.3 ± 165.63 | 431.0 ± 201.7 | -1.604 | 0.109 | -0.71 |
| Central Alpha (Hz.) | 317.3 ± 115.4 | 265.4 ± 158.7 | -.535 | 0.593 | -0.45 |
| Central Beta (Hz.) | 150.3 ± 60.8 | 177.3 ± 67.2 | -.535 | 0.593 | 0.44 |
| Central Gamma (Hz.) | 45.1 ± 22.08 | 83.6 ± 55.8 | -1.069 | 0.285 | 1.74 |
| Temporal Deltha (Hz.) | 3887.9 ± 3543.6 | 1493.8 ± 701.5 | -1.604 | 0.109 | -0.68 |
| Temporal Theta (Hz.) | 3887.9 ± 3543.6 | 1493.8 ± 701.5 | -1.604 | 0.109 | -0.68 |
| Temporal Alpha (Hz.) | 3887.9 ± 3543.6 | 1493.8 ± 701.5 | -1.604 | 0.109 | -0.68 |
| Temporal Beta (Hz.) | 3887.9 ± 3543.6 | 1493.8 ± 701.5 | -1.604 | 0.109 | -0.68 |
| Temporal Gamma (Hz.) | 3887.9 ± 3543.6 | 1493.8 ± 701.5 | -1.604 | 0.109 | -0.68 |
| Posterior Deltha (Hz.) | 1268.1 ± 935.4 | 1723.4 ± 982.2 | -1.604 | 0.109 | 0.49 |
| Posterior Theta (Hz.) | 1317.3 ± 285.3 | 1231.0 ± 421.1 | .000 | 1.000 | -0.30 |
| Posterior Alpha (Hz.) | 788.5 ± 143.0 | 623.3 ± 231.7 | -1.069 | 0.285 | -1.16 |
| Posterior Beta (Hz.) | 392.4 ± 135.8 | 445.0 ± 132.8 | -.535 | 0.593 | 0.39 |
| Posterior Gamma (Hz.) | 86.0 ± 11.6 | 126.4 ± 50.0 | -1.609 | 0.285 | 3.48 |
Table 2: Electroencephalogram activity (power spectrum in µv2) before and after the simulation, with open eyes.
| PRE | POST | Z | P | Effect size | |
|---|---|---|---|---|---|
| Anterior Deltha (Hz.) | 1892.0 ± 1186.9 | 1292.2 ± 1003.7 | .000 | 1.000 | -0.51 |
| Anterior Theta (Hz.) | 672.4 ± 148.7 | 720.7 ± 272.1 | -.535 | 0.593 | 0.33 |
| Anterior Alpha (Hz.) | 669.4 ± 190.1 | 668.9 ± 465.5 | .000 | 1.000 | 0.00 |
| Anterior Beta (Hz.) | 274.6 ± 41.1 | 503.8 ± 267.9 | -1.604 | 0.109 | 5.57 |
| Anterior Gamma (Hz.) | 161.7 ± 90.7 | 573.6 ± 599.2 | -1.604 | 0.109 | 4.54 |
| Central Deltha (Hz.) | 935.6 ± 434.0 | 1369.4 ± 1542.5 | .000 | 1.000 | 1.00 |
| Central Theta (Hz.) | 652.7 ± 439.2 | 954.9 ± 784.2 | -1.604 | 0.109 | 0.69 |
| Central Alpha (Hz.) | 1736.9 ± 219.7 | 1404.7 ± 1690.6 | .000 | 1.000 | -1.51 |
| Central Beta (Hz.) | 316.6 ± 56.5 | 304.8 ± 92.2 | 0.000 | 1.000 | -0.21 |
| Central Gamma (Hz.) | 84.7 ± 35.9 | 110.1 ± 96.4 | -1.604 | 0.109 | 0.71 |
| Temporal Deltha (Hz.) | 2508.7 ± 161.6 | 1245.4 ± 1028.6 | -1.604 | 0.109 | -7.81 |
| Temporal Theta (Hz.) | 2508.7 ± 161.6 | 1245.4 ± 1028.6 | -1.604 | 0.109 | -7.81 |
| Temporal Alpha (Hz.) | 2508.7 ± 161.6 | 1245.4 ± 1028.6 | -1.604 | 0.109 | -7.81 |
| Temporal Beta (Hz.) | 2508.7 ± 161.6 | 1245.4 ± 1028.6 | -1.604 | 0.109 | -7.81 |
| Temporal Gamma (Hz.) | 2508.7 ± 161.6 | 1245.4 ± 1028.6 | -1.604 | 0.109 | -7.81 |
| Posterior Deltha (Hz.) | 1984.1 ± 499.3 | 1010.8 ± 767.9 | -1.604 | 0.109 | -1.95 |
| Posterior Theta (Hz.) | 1463.4 ± 450.6 | 1955.1 ± 1126.4 | -.535 | 0.593 | 1.09 |
| Posterior Alpha (Hz.) | 6625.4 ± 4601.0 | 5289.4 ± 3736.5 | -.535 | 0.593 | -0.29 |
| Posterior Beta (Hz.) | 1027.7 ± 429.4 | 993.2 ± 328.2 | 0.000 | 1.000 | -0.08 |
| Posterior Gamma (Hz.) | 139.4 ± 18.9 | 134.4 ± 68.3 | 0.000 | 1.000 | -0.26 |
Table 3: Modification in electroencephalogram activity before and after the simulation, with closed eyes.
Discussion
The objective of this study was to analyze the psychophysiological response of nursing degree students during a hospital clinical simulation and its relationship to their performance. The initial hypotheses were i) that students would present an habituation response, reducing the sympathetic nervous system modulation; and ii) that we would find a difference in frontal EEG oscillatory activity when comparing the recordings at the beginning and the end of the scenario due to the cognitive demands of the clinical simulation. The first hypothesis was not fulfilled; however, we found the expected differences in EEG activity.
We found a high anxiety anticipatory response as the autonomic response measurements at the beginning of the simulation scenario (M0 and M1) indicated: the variables PNN50, RMSSD and SD1 were low, whereas LF values were high [29]. The high response of the sympathetic system has been studied in recent years in environments that demand optimal cognitive performance, such as academic and military contexts, showing that it is largely determined by the subject’s ability to assess the demands of the environment and select appropriate coping tools. In addition, a high anxiety response can interfere with the skills needed to achieve the expected performance [30-33].
Regarding the habituation process during the clinical simulation, no significant modifications were found in the HRV measures. We found a moderate increase effect size in LF and a moderate decrease effect size in HF, in M0 vs. M4. We also recorded a moderate decrease in the variables RMSSD, SD1 and SD2 when M1 and M2, and M1 and M4 were compared. These results indicate that the habituation process was not achieved, as they do not show the expected decrease in sympathetic modulation. This is consistent with the increased subjective perception of stress indicated by the students. Lack of experience in the professional clinical setting or certain personality factors associated with a high sympathetic response may explain why students did not habituate. These factors have been examined in previous studies with military professionals and in university academic environments showing how a high stress response could impact on the performance of cortical higher functions such as information processing, essential in coping with clinical simulation scenarios like the one our participants faced [3,4,9,27].
We found high scores on the PSS that are associated with the wrong choice of coping strategies when facing unknown and disturbing events [28]. It is known that passive forms of coping such as avoidance, external support or negative focus are associated with high levels of stress. Conversely, strategies such as a focus on problem solving or positive re-evaluation are associated with low levels of stress and psychological well-being [34]. This could explain why the students did not acquire habituation because they perceived that the demands of the environment were greater than their tools for dealing with them, which did not allow the decrease of sympathetic activity even after the end of the simulation. In addition, the high level of anxiety presented by the students was consistent with the central nervous system fatigue obtained in the decreased CFFT values, a response also observed in other high stress contexts such as parachuting, ultra-endurance races and combat [35-37].
After the clinical simulation, we found increased activity of the anterior cortical area in alpha, beta and gamma EEG waves. This could be related to the high demands of the simulation. This highly demanding context requires a large use of functional memory, executive functions, attention, language production, social abilities and rational judgment [38]. The nature of the simulation, its unpredictability and the large number of stimuli, together demand high activation of the right frontal lobe, as well as the orbitofrontal lobe, since the student is not able to inhibit irrelevant stimuli [39]. The increased alpha waves in this region could be related to a high anxiety strait [14], but the different response in temporal, parietal and occipital regions lead us to question this idea. Future studies should explore this aspect. Specifically, regarding memory demands, we found a decrease in all EEG waves (large ES) in the temporal lobe with closed eyes, highlighting the large cognitive demands of these simulation practices and the importance of this scenario in the educational process of biomedical studies [40]. This result was consonant with the decrease in CFFT, a result that shows how this system could be an important tool to monitor cortical demands in practice in a simple, faster and cheaper way than using EEG, as other researchers have also postulated in sport contexts [41], but future studies should validate this.
Limitations of the Study
The most important limitation of this study is the small sample analyzed. This limitation relates to the difficulty of EEG evaluations in real environments, such as clinical scenarios, due to both the logistics and the difficulty in finding students willing to participate in the study. Also, the analysis of stress hormones such as alpha amylase and cortisol would improve the level of accuracy of the organic stress response measurement. As a future research line, we propose increasing the sample and replicating the study with students of other health science degrees such as medicine, odontology and/or psychology.
Practical Applications
The evaluation of the psychological profile, specifically the construct related to stress coping, would provide teachers with valuable information to improve their educational relationship with students, as well as incorporate stress coping training tools into these scenarios. In addition, the use of HRV and CFFT would provide students and teachers with an effective tool to monitor autonomous and cortical responses in the learning process, allowing them to know the psychophysiological responses of students and giving them the opportunity to adapt the learning process to their specific profiles.
Conclusion
A nursing clinical simulation scenario produced an anticipatory anxiety response in the students, which prevented them from achieving a habituation response, decreasing Critical Flicker Fusion Threshold, and increasing anterior and decreasing temporal EEG activity.
Acknowledgments
This research was carried out under the David A. Wilson Award for Excellence in Teaching and Learning 2017, project number XOTRIO1712.
References
- Sobh AH, Izham M, Diab MI, et al. Qualitative evaluation of a cumulative exit-from-degree objective structured clinical examination (OSCE) in a Gulf context. Pharmacy. Edu 17(1), 73-80 (2017).
- Munroe B, Buckley T, Curtis K, et al. Designing and implementing full immersion simulation as a research tool. Aust. Emerg.Nur. J 19(2), 90-105 (2016).
- Bellido-Esteban A, Ruisoto-Palomera P, Beltrán-Velasco AI, et al. State of the art on the use of portable digital devices to assess stress in humans. J. Med. Syst 42(1), 100 (2018).
- Beltrán-Velasco AI, Bellido-Esteban A, Ruisoto-Palomera P, et al. Use of portable digital devices to analyze autonomic stress response in psychology objective structured clinical examination. J. Med. Sys 42(2), 35 (2018).
- Billman G. The effect of heart rate on the heart rate variability response to autonomic interventions. Front. Physiol 26(4), 222 (2013).
- McEwen BS. Protective and damaging effects of stress mediators: Central role of the brain. Dial. Clin. Neurosci 8(4), 367-381 (2006).
- Prinsloo GE, Rauch HG, Derman WE. A brief review and clinical application of heart rate variability biofeedback in sports, exercise, and rehabilitation medicine. Phys. Sportsmed 42(2), 88-99 (2014).
- Clemente-Suárez VJ, de la Vega R, Robles-Pérez JJ, et al. Experience modulates the psychophysiological response of airborne warfighters during a tactical combat parachute jump. Int. J. Psychophysiol 110(1), 212-216 (2016).
- Delgado-Moreno R, Robles-Pérez JJ, Clemente-Suárez VJ. Combat stress decreases memory of Warfighters in action. J. Med. Syst 41(8), 124 (2017).
- Chan R, Shum D, Toulopoulou T, et al.Assessment of executive functions: Review of instruments and identification of critical issues. Arch. Clin. Neuropsychol 23(2), 201-216 (2008).
- Van Diepen R, Miller L, Mazaheri A, et al. The role of alpha activity in spatial and feature-based attention. eNeuro 3(5), 204-216 (2016).
- Lee Y, Hsieh S. Classifying different emotional states by means of EEG-based functional connectivity patterns. PloSone 9(4), e95415(2014).
- Lord V, Opacka-Juffry J. Electroencephalography (EEG) Measures of neural connectivity in the assessment of brain responses to salient auditory stimuli in patients with disorders of consciousness. Front. Psychology 7(1), 397 (2016).
- Knyazev GG, Savostyanov AN, Levin EA. Alpha oscillations as a correlate of trait anxiety. Int. J. Psychophysiol 53(2), 147-160 (2004).
- Roohi-Azizi M, Azimi L, Heysieattalab S, et al. Changes of the brain's bioelectrical activity in cognition, consciousness, and some mental disorders. Med. J. Isla. Rep. Iran 31(1), 53 (2017).
- Fingelkurts A, Fingelkurts A. Altered structure of dynamic "Electroencephalogram oscillatory pattern" in major depression. Biolo.Psychiatry 77(12), 1050-1060 (2015).
- Gomez M, Dodino CN, Aponte C, et al. Burnout syndrome and its association to psychological profile and quality of life in nurses. Univ. Psychol 4(1), 63-75(2005).
- Mealer M, Jones J, Newman J, et al. The presence of resilience is associated with a healthier psychological profile in intensive care unit (ICU) nurses: Results of a national survey. Int. J. Nur. Stud 49(3), 292-299 (2012).
- DruryC, Francis K, Aoun S, et al. Compassion satisfaction, compassion fatigue, anxiety, depression and stress in registered nurses in Australia: phase 2 results. J. Nurs. Manag 22(4), 506-518 (2014).
- Hegney DG, Rees CS, Eley R, et al. The contribution of individual psychological resilience in determining the professional quality of life of Australian nurses. Front. Psychology 6(1613),1-10 (2015).
- Cheng C, Lau H, Chan M. Coping flexibility and psychological adjustment to stressful life changes: a meta-analytic review. Psycholo. Bull 140(6), 1582-1607 (2014).
- Korner A, Czajkowska Z, Albani C, et al. Efficient and valid assessment of personality traits: population norms of a brief version of the NEO Five-Factor Inventory (NEO-FFI). Arch. Psychiatry. Psychother 17(1), 21-32 (2015).
- Clemente-Suárez VJ, Fernandes RJ, Arroyo-Toledo JJ, et al. Autonomic adaptation after traditional and reverse swimming training periodizations. Acta. Physiolo. Hung 102(1), 105-113 (2015).
- Malik M, Bigger J, Camm A, et al. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Euro. Heart. J 17, 354-381 (1996).
- Clemente-Suárez VJ, Díaz-Manzano M. Evaluation of central fatigue by the critical flicker fusion threshold in cyclists. J. Me. Sys 43(3), 61 (2019).
- Oostenveld R, Fries P, Maris E, et al. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computat. Intell. Neurosci 2011(156869), 1-9 (2011).
- Clemente-Suárez VJ, Beltrán-Velasco AI, Bellido-Esteban A, et al. Autonomic adaption to clinical simulation in psychology students: Teaching applications. App. psychophysiol. Biofee. 43(3), 239-245 (2018).
- Cohen S, Kamarck T, Mermelstein RA. Global measure of perceived stress. J. Health. Social Behav 24(4), 385 (1983).
- Diaz-Manzano M, Fuentes JP, Fernandez-Lucas J, et al. Higher use of techniques studied and perdormance in melee combat produce a higher psychophysiological stress response. Stress. Health 34(5), 622-628 (2018).
- Clemente-Suárez VJ, Robles-Pérez JJ. Psycho-physiological response of soldiers in urban combat. An. de. Psicología 29(2), 598-603 (2013).
- Ferrey AE, Burleigh TJ,Fenske MJ. Stimulus-category competition, inhibition, and affective devaluation: A novel account of the uncanny valley. Front. Psychology 6(1), 249 (2015).
- Scult MA, Knodt AR, Radtke SR, et al. Prefrontal executive control rescues risk for anxiety associated with high threat and low reward brain function. Cereb. Cortex 29(1), 70-76 (2017).
- Clemente-Suárez VJ, Palomera P, Robles-Pérez J. Psychophysiological response to acute‐high‐stress combat situations in professional soldiers. Stress.Health 34(2), 247-252 (2018).
- Zhao F, Lei X, He W, et al. The moderating effect of self‐efficacy. Int. J. Nurs. Pract 21(1), 401-409 (2015).
- Clemente-Suarez VJ, Robles-Pérez JJ, Herrera-Mendoza K, et al. Psychophysiological response and fine motor skills in high-altitude parachute jumps. High. Alti. Med. Biol 18(4), 392-399(2017).
- Belinchon-deMiguel P, Clemente-Suárez VJ. Psychophysiological, body composition, biomechanical and autonomic modulation analysis procedures in an ultraendurance mountain race. J. Med. Syst 42(2), 32 (2018).
- Sánchez-Molina J, Robles-Pérez JJ, Clemente-Suárez VJ. Assessment of psychophysiological response and specific fine motor skills in combat units. J. Med. Syst 42(4), 67 (2018).
- Chayer C, Freedman M. Frontal lobe functions. Curr. Neurol. Neurosci. Rep 1(6), 547-552 (2001).
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychono. Bull.Rev 9(4), 637-671 (2002).
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science 253(5026), 1380-1386 (1991).
- Clemente-Suárez VJ. The application of cortical arousal assessment to control neuromuscular fatigue during strength training. J. Motor Behav 49(4), 429-434 (2017).