Review Article - Interventional Cardiology (2024)
Pulmonary flow restrictors in congenital heart diseases: Procedural considerations, clinical outcomes, and future directions
- Corresponding Author:
- Raymond N. Haddad
Department of Pediatric Cardiology, Hotel Dieu de France University Medical Center, Saint Joseph University, Beirut, Lebanon,
E-mail: raymondhaddad@live.com
Received date: 17-Feb-2024, Manuscript No. FMIC-24-127761; Editor assigned: 19-Feb-2024, PreQC No. FMIC-24-127761 (PQ); Reviewed date: 07-Mar-2024, QC No. FMIC-24-127761; Revised date: 13-Mar-2024, Manuscript No. FMIC-24-127761 (R); Published date: 22-Mar-2024, DOI: 10.37532/1755-5310.2023. 16 (S21).550
Abstract
The percutaneous endovascular pulmonary arterial banding technique currently relies on approved Microvascular Plugs (MVPs) from Medtronic. These manually created flow restrictors, known as Pulmonary Flow Restrictors (PFRs), can be effortlessly inserted via small diagnostic catheters, offering a minimally invasive treatment avenue for newborns and infants afflicted with life-threatening congenital and acquired heart diseases. Building on the pioneering efforts of Schranz and colleagues, we, together with various international centers have initiated transcatheter programs aimed at managing pulmonary over circulation, harmonizing pulmonary and systemic circulation, or enhancing interventricular interaction. Strategies centered on customized PFRs, with or without additional duct stenting, have demonstrated efficacy in facilitating delayed Norwood surgery or comprehensive Stage II operations. Additionally, in neonates with hypoplastic left hearts, these strategies have proven useful in delaying biventricular repair attempts until later infancy. Ultimately, these PFRs are safely extracted during corrective or palliative surgeries at a later developmental stage, ensuring the patient’s transition beyond the neonatal period in terms of both size and age. The advantages of this innovative procedure are manifold. It potential to shorten the intensive care stay and ensure a smoother recovery process for the patient. Bypassing the need for a sternotomy for a surgical Pulmonary Artery (PA) band spares the infant from additional trauma. Furthermore, the mortality rate associated with PFRs appears to be lower compared to traditional PA band operations. Moreover, the Infrastructural needs for carrying out this procedure are notably less demanding compared to cardiac surgery, making it feasible for implementation worldwide. Additionally, when the time for subsequent open-heart surgery arrives, the surgical removal of PFRs is straightforward for surgeons. Therefore, the utilization of PFR-based treatment represents a potential change of opinion. To ensure its successful adoption and widespread acceptance, consistency, and standardization in practice across centers is imperative. This review provides our opinion alongside a comprehensive overview of PFRs within the area of Congenital Heart Disease (CHD), covering indications, procedural considerations, and outcomes associated with PFR implantation. Additionally, it delves into emerging trends and future directions in pulmonary flow regulation, including advancements in device design, optimization of patient selection criteria, and ongoing research efforts to enhance the efficacy and safety of PFR therapy.
Keywords
Microvascular plug • Pulmonary artery band • pulmonary flow regulator • Stage 1 Norwood procedure
Abbreviations
CHD: Congenital heart disease; MVP: Microvascular plug; PA: Pulmonary artery, PFR: Pulmonary flow restrictor; Qp/Qs: Pulmonary to systemic flow ratio
Introduction
Among the challenges faced by pediatric cardiologists, managing pulmonary over circulation in newborns and infants with Congenital Heart Disease (CHD) stands out as a critical endeavor, requiring precise intervention to safeguard pulmonary vascular health and optimize cardiac function. Heart failure symptoms resulting from pulmonary to systemic flow ratio (Qp/ Qs) mismatch can prove fatal or classify infants as inoperable if not promptly addressed. For critically ill newborns, particularly those with high-risk, ductal-dependent, or complex two-ventricle conditions coupled with comorbidities, conventional surgical interventions may pose significant challenges [1-3].
In such cases, pulmonary blood flow restriction serves as a potential association to a more definitive procedure. However, the existing surgical options may not always be well-tolerated by these patients. Historically, Pulmonary Artery (PA) banding has served as the conventional method for staging these patients toward definitive surgeries, whether full repair or palliative repair leading to single ventricle circulation. Mortality and morbidity rates associated with this staged approach remain significant [4,5].
Moreover, early neonatal open-heart surgeries face challenges stemming from the delicate hemodynamics of neonates, the pronounced inflammatory response to heart-lung bypass machines, and the complex intensive care required for this patient population. An emerging alternative involves the transcatheter placement of a fenestrated Microvascular Plug (MVP) in each PA to serve as an endoluminal Pulmonary Flow Restrictor (PFR), offering a potential avenue for addressing the complex needs of these individuals in the catheterization laboratory [6,7].
Literature Review
Microvascular Plug
The MVP is a self-expanding mechanical occlusion device that has obtained approval from both the US FDA and the CE marking [8]. Crafted with precision, it features a single-cage hexagonal framework constructed from a flexible laser-cut Nitinol wire. Its design incorporates an ovoid-shaped cylinder with tapered extremities converging towards the center axis. The device boasts a Polytetrafluoroethylene (PTFE) coating with an innovative asymmetrical parachute design, extending from the proximal tapering to the distal end of the tubular part, leaving the distal tapering uncovered. Each end is marked with a single radiopaque platinum marker, facilitating precise positioning during placement procedures. For ease of use, the plug is securely attached to either a 180 cm or 165 cm long highly flexible delivery wire. Detachment is achieved through mechanical anticlockwise torque. Additionally, a 4 cm plastic sleeve encases the delivery cable, streamlining the loading process. The Mitral Valve Prolapse (MVP) is available in four sizes, boasting un-constricted diameters of 5.3 mm, 6.5 mm, 9.2 mm, and 13 mm. Notably, the MVP-3Q and MVP-5Q models feature 6 and 8 covered segments respectively, while the MVP-7Q and MVP-9Q variants comprise ten covered segments.
Plug modification technique
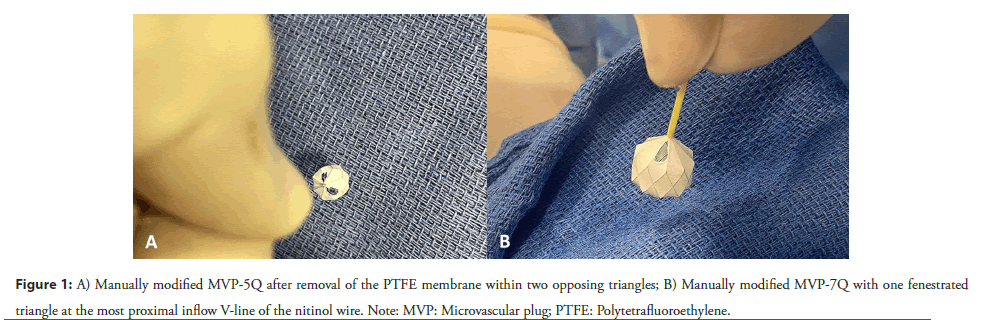
We have previously explained the careful technique involved in modifying the MVP [9]. This process is manually performed by the interventional pediatric cardiologist, who utilizes toothless forceps and a surgical scalpel to carefully create a small fenestration within a precise segment of the PTFE covering. Given the pliable and delicate nature of the device, it is important to avoid compelling the nitinol cage and exercise utmost care throughout the process to prevent any deformation of the plug. It’s imperative to ensure that the size of the fenestration remains within prescribed limits: not exceeding one triangle, equivalent to half of the diamond segment, in the MVP-Q7 and MVP-Q9 models, and two separate triangles in the MVP-Q5 (Figure 1). The fenestration is strategically positioned at the proximal end of the PTFE, adjacent to the device’s screw. We and others hypothesized and showed that positioning the fenestration at the inflow segment of the device would keep it open even in the event of device compression following implantation.
We now acknowledge that past attempts often fell short due to the fenestration being larger than necessary [10]. Thus, it is potential to keep the hole small and avoid using fenestration balloon dilation, as this maneuver can potentially weaken the device. In specific cases, a narrow, slit-like opening often proves highly effective for the intended purpose, especially considering that paraprosthetic leaks typically occur at varying rates with oversized devices. Subsequently, the modified plug is gently retracted into the provided small plastic sleeve, which also functions as an introducer within the delivery catheter or microcatheter, depending on size requirements.
Interventional procedure
The intervention is carried out under general anesthesia or sedation, with prophylactic antibiotics, systemic heparinization, and guidance through biplane fluoroscopy. Correction of anemia before the procedure is essential to ensure accurate hemodynamic assessment. The measurement of QP/QS is conducted with an inspired Fraction of Oxygen (FiO2) set at 21%. It is important to note that hemodynamics, including PFR characteristics, exhibit variations between deeply anesthetized or sedated patients, leading to markedly different PFR effects. Achieving balanced, gentle analgesic sedation in a spontaneously breathing baby represents the optimal physiological state. It is potential to recognize that procedures such as intubation, extubation, administration and cessation of anesthetics, and controlled ventilation may induce adverse effects on systemic blood flow by reducing pulmonary vascular resistance. This underscores significant concerns regarding the reliability of hemodynamic measurements during such procedures [11].
A 4F sheath serves as the conduit for accessing either the femoral or jugular vein. Following this, a 4F glide catheter is typically employed to capture angiographic images of both pulmonary arteries in 30 degree anterior oblique and lateral projections, thus elucidating their anatomical configuration. Emphasis is placed on discerning the branching patterns of the upper lobe branches within both the left and right PAs. Before proceeding with the procedure, it’s potential to carefully assess several factors, including the size of the vessels, the proximity of the pulmonary valve, and the origin of the arterial duct. One significant challenge we face is achieving a thorough balance in oversizing to ensure stable device implantation without excessively enlarging the devices, as the PTFE covering of the plug must fully open to prevent para-device leaks, thus optimizing the PFR outcome. For vessels measuring less than 4 mm, opting for an MVP-5Q could be considered acceptable. However, it’s essential to note that such vessels tend to be pulsatile, necessitating slight oversizing, potentially up to MVP-7Q if pulsatility is significant. Device oversizing, with appropriate adjustment to fenestration size, may reduce migration risk and provide a clinically appropriate balance between the resulting pressure gradient and Qp/Qs [12]. Vessels falling within the 4 mm-6 mm range typically merit an MVP-7Q, while those up to 8 mm in diameter may benefit from the MVP-9Q device. The risk of device migration distally is elevated in cases involving larger vessels characterized by dynamic motion, which mirrors the heightened pulmonary flow. However, these plugs offer distinct advantages, notably the exposed one-third distal portion, which mitigates the risk of occluding upper lobe branches. Additionally, a straightforward snaring technique can always be employed, if necessary, further enhancing procedural safety and efficacy
A 5 mm micro snare catheter proves sufficient for snaring these plugs. While the MVP-9Q plug is ideally deployed using a 5F glide catheter for optimal performance, it can still be delivered through a 4F catheter. However, this smaller catheter size may pose challenges for retrieval if needed. We have found the quick unsheathing technique to be the most reliable method of device deployment. This involves placing the device within the glide catheter in the intended area and then promptly unsheathing it to deploy it in one fluid motion, rather than partially or fully opening it distally and retracting it to the desired position. In any case, should the initial attempt fall short, the device can be recaptured within the glide catheter and redeployed as needed. The flexible Nitinol skeleton facilitates retracting and re-positioning the MVP until achieving satisfactory implantation. Consequently, caution is advised when repeatedly re-sheathing the device, as it could potentially cause small tears in the PTFE membrane.
Our preferred sequence involves deploying the device in the left PA first, followed by the right PA. This choice is informed by the fact that the origin of the right PA is generally closer to the pulmonary valve, making it more susceptible to accidental contact with wires post-deployment. By deploying the device in the left PA initially, and then in the right PA, we aim to minimize this risk. In cases where ductal stenting is performed concurrently, as part of a DIDI (Double Intention, Double Intervention) procedure, we adhere to a specific order. The left PFR is placed before ductal stenting, with subsequent flexibility for stenting the duct or deploying the right PFR. This sequencing is necessary due to the difficulty in engaging the left PA post-ductal stenting.
Moreover, we support for the initial use of the SwiftNINJA steerable microcatheter following baseline non-selective angiography. This approach facilitates precise cannulation of a challenging right PA in a single attempt. Subsequently, it allows for the PFR implantation without disturbing the newly implanted PFR in the left PA [13]. By utilizing this method, we mitigate the potential risk of inadvertently manipulating the left PFR, which may occur when employing standard pre-shaped hardware.
As part of our routine protocol, we conduct angiograms both post-device positioning and before device release. A 2D short-axis ultrasound is also performed to visualize the proximal screw of the MVP device at the level of the PA bifurcation and to measure the pressure gradient with continuous-wave Doppler tracing. If the device is found to be distal on ultrasound or fluoroscopy and or jailing the upper lobe branches during angiography, it suggests undersizing. In such instances, the device must be retracted and upsized to ensure optimal function and safety. In cases where the branch PA is short and the device needs elongation, particular attention is paid to safeguarding the pulmonary valve. Often, the main PA may be dilated, allowing accommodation of the protruding part of the device from the branch PA. This compatibility can be readily confirmed through pre-release ultrasound, which is essential for assessing the gradient across the PFR and the paraprosthetic leaks.
While changes in lung perfusion can be observed on angiography before device release, alterations in oxygen saturation, heart rate, and systemic blood pressure become evident only after deploying both PFRs. We maintain our threshold of accepting a minimum 10% drop in saturation levels post-procedure. Conversely, follow-up cardiac ultrasound may not always provide an accurate measurement of the gradient, as optimal angulation for obtaining correct continuous wave measurements may not be achievable, especially for the left PFR.
To mitigate thrombotic risks, we closely monitor the activated clotting time every 30 minutes, aiming to maintain it within the range of 200 to 300 seconds throughout the entire procedure. Additionally, we implement a standard protocol of continuous heparin infusion for 48 hours, overlapped by dual oral anti-platelet therapy, for optimal patient management. Encouragingly, we have not encountered any instances of thrombosis during follow-up or post-surgical resection.
Follow-up
The durability of PFRs has been evident under both normal and supraphysiologic conditions, showing minimal alteration in fenestration size. Midterm results showed that there was limited variability in the oxygen saturation and the maximum velocity of the continuous-wave Doppler tracing on both PA branches. However, in cases of smaller vessel sizes, there is a notable increase in pressure gradient. This is attributed to the diminished peridevice flow and a reduction in the effective fenestration size. The efficacy of the endoluminal PFRs should always be evaluated in terms of clinical signs, Doppler patterns, and most importantly the impact of cardiovascular co-medications on residual or intermittent overflow conditions.
Surgical device explantation
There are two distinct patient categories in our practice. The first group comprises patients who have had PFRs for less than 12 weeks, typically as a connection beyond the neonatal period for procedures such as Norwood, Taussig-Bing repair, or arterial trunk correction. The surgical device removal in this group is usually straightforward: the device is extracted from the PA branches using surgical forceps. This approach is viable because the adhesions of the PTFE to the PA tissue have not fully formed during this relatively brief period. We recognize the specific considerations regarding patients with single ventricle physiology. Therefore, our current practice typically involves connecting them to deferred Norwood operation rather than retaining the PFRs for 4-6 months until comprehensive stage II. This approach ensures optimal management and outcomes for this patient population, as has been highlighted by Dietmar Schranz in his latest editorial. The second group consists of patients undergoing delayed explantation of PFRs. In these cases, removal involves longitudinal opening of the main PA, and the possibility of requiring a patch exists. However, this concern is mitigated by the patients’ typically older age, reducing the associated risks.
There are confident that the removal of those plugs via surgery was straightforward, either by direct extraction under visual inspection, whether intact or in fragments. It is potential during the extraction process to firmly understand the proximal end of the MVP with forceps. The proximal portion, housing the PTFE membrane, can be readily detached from the distal segment and the vessel wall. Ensuring the covered portion is extracted in one piece is imperative. The uncovered portion of the device may become embedded in the endothelium, necessitating the removal of individual pieces as required.
While we encountered no need to snare any plug aside from during the deployment procedure, we anticipate that snaring the plug within the initial 3 weeks will be manageable without causing harm to the vessel. Although Khan et al. reported a success rate of 50% for MVP device removal using snares after 12 weeks, attempting removal 4 weeks’ post-implantation seems perilous, potentially resulting in PA damage and constriction.
There have observed potential outcomes in the mid-term followup of patients, whether pre- or post-full surgical revision. Notably, PAs grew adequately for age, and devices were easily removed without complications. There have been no documented cases of branch PA narrowing following explantation in either group. Additionally, none of the patients who underwent patching of the branch PAs required any further reintervention.
Future vision
This innovative procedure holds immense potential for the future, potentially revolutionizing neonatal surgery. It offers a potential solution not only for certain groups of neonates with dilated cardiomyopathy but also for palliative cases, enabling them to receive care at home [14-16]. Nevertheless, it’s potential to acknowledge that this procedure is not suitable for all neonates. Many neonatal surgeries already boast excellent outcomes, and incorporating a connecting procedure may not always be necessary [17]. Developing “best practices” for novel, patientspecific procedures necessitates collaboration among multiple centers. This collaboration is essential for accruing an adequate number of patients to draw definitive conclusions. As more cases are performed, we anticipate accumulating data that will enable us to compare the outcomes of patients treated via this bridging pathway with those undergoing routine neonatal surgery.
Another consideration is the size and modification of the PA flow restrictor device. We anticipate significant interest from research centers in designing a device tailored to larger branch PAs. Ideally, such a device would be deliverable via a 4F sheath, feature a controlled deployment system, be easily retrievable, possess a short length, and incorporate a central metal-reinforced hole with a PTFE cover on the side. Collaboration between clinicians and researchers will be essential in realizing these advancements.
Conclusion
The percutaneous treatment of newborns and infants with lifethreatening congenital and acquired heart diseases using PFRs has already shown potential feasibility and effectiveness. To ensure consistent outcomes, it is imperative to implement a standardized approach that incorporates custom-made materials within a comprehensive therapy protocol.
References
- Krishnamurthy G, Ratner V, Bacha E, et al. Neonatal cardiac care, a perspective. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu.16(1):21-31 (2013).
- Henmi S, Essa Y, Öztürk M, et al. Cardiovascular surgery in very low birth weight ( ≤ 1500 g) neonates. Eur J Cardiothorac Surg. 63(1):ezac552 (2022).
- Kalfa D, Krishnamurthy G, Duchon J, et al. Outcomes of cardiac surgery in patients weighing<2.5 kg: Affect of patient-dependent and -independent variables. J Thorac Cardiovasc Surg. 148(6):2499-2506 (2014).
- Horowitz MD, Sundgaard-Riise K, Ochsner JL, et al. Pulmonary artery banding: Analysis of a 25-year experience. Ann Thorac Surg. 48(3):444-450 (1989).
- Alsoufi B. Commentary: Pulmonary artery banding in infants with atrioventricular septal defect, valid strategy or backward move?. J Thorac Cardiovasc Surg. 159(4):1504-1506 (2020).
- Khan AH, Hoskoppal D, Kumar TKS, et al. Utility of the medtronic microvascular plug™ as a transcatheter implantable and explantable pulmonary artery flow restrictor in a swine model. Catheter Cardiovasc Interv. 93(7):1320-1328 (2019).
- Schranz D, Esmaeili A, Schrewe R, et al. Hypoplastic left heart stage I: No norwood, no hybrid. Circulation. 142(14):1402-1404 (2020).
- Haddad RN, Bonnet D, Malekzadeh-Milani S, et al. Embolization of vascular abnormalities in children with congenital heart diseases using medtronic micro vascular plugs. Heart Vessels. 37(7):1271-1282 (2022).
- Haddad RN, Bentham J, Adel Hassan A, et al. Outcomes of manually modified microvascular plugs to pulmonary flow restrictors in various congenital heart lesions. Front Cardiovasc Med. 10:1150579 (2023).
- Nageotte S, Shahanavaz S, Eghtesady P, et al. Total transcatheter stage 1: A word of caution. Pediatr Cardiol. 42(6):1410-1415 (2021).
- Schranz D. A developmentally based proposal for neonates with hypoplastic left heart: What a great progress! But please don't stop here! A broad result is emerging. JTCVS Open. 16:710-713 (2023).
- Kizilski SB, Recco DP, Sperotto F, et al. Transcatheter pulmonary artery banding in high-risk neonates: In-vitro study provoked by initial clinical experience. Cardiovasc Eng Technol. 14(5):640-654 (2023).
- Haddad RN, Adel Hassan A, Al Soufi M, et al. SwiftNINJA steerable microcatheter: A new kid on the block for selective catheterization of vascular and valvular congenital lesions. Front Cardiovasc Med. 10:1322787 (2023).
- Sperotto F, Lang N, Nathan M, et al. Transcatheter palliation with pulmonary artery flow restrictors in neonates with congenital heart disease: Feasibility, outcomes, and comparison with a historical hybrid stage 1 cohort. Circ Cardiovasc Interv. 16(12):e013383 (2023).
- Schranz D, Krause U, Kerst G, et al. Functional regeneration of dilated cardiomyopathy by transcatheter bilateral pulmonary artery banding: First-in-human case series. Eur Heart J Case Rep. 7(2):ytad052 (2023).
- Kurtz JD, Alsoufi B, Wilkens SJ, et al. Modified microvascular plug as a flow restrictor in hypoplastic left heart syndrome with dysplastic tricuspid and pulmonary valves. Pediatr Cardiol. 42(7):1653-1657 (2021).
- Wernovsky G, Ozturk M, Diddle JW, et al. Rapid bilateral pulmonary artery banding: A developmentally based proposal for the management of neonates with hypoplastic left heart. JTCVS Open. 14:398-406 (2023).