Review Article - Imaging in Medicine (2012) Volume 4, Issue 5
Radiological assessment of white matter injury in very preterm infants
Francisca T de Bruïne*1 & Gerda van Wezel-Meijler21Department of Radiology, Leiden University Medical Center, PO Box 9600, 2300 RC Leiden, The Netherlands
2Department of Pediatrics, Division of Neonatology, University of Toronto, Toronto, Canada
- Corresponding Author:
- Francisca T de Bruïne
Department of Radiology
Leiden University Medical Center
PO Box 9600, 2300 RC Leiden, The Netherlands
Tel: +31 71 526 4376
Fax: +31 71 524 8256
E-mail: f.t.wiggers-de_bruine@lumc.nl
Abstract
Despite improvements in neonatal care, infants born very prematurely who survive the neonatal period are still at risk of neurodevelopmental disabilities. One of the main determinants for a poorer outcome seems to be damage to the cerebral white matter, which frequently occurs during the perinatal period. This article summarizes the radiological assessment of white matter injury in very preterm infants, striving to aid clinicians who provide parents and care takers with prognostic information on the development of their preterm born infants. As the expertise of radiologists in assessing neonatal brain MRI may vary widely amongst centers, we also strive to provide radiologists with information on imaging findings of white matter injury.
Keywords
cranial ultrasonography ▪ MRI ▪ prognostication ▪ very preterm infants ▪ white matter injury
In infants born very prematurely (gestational age <32 weeks), germinal matrix, intraventricular hemorrhage and white matter injury are frequently encountered [1–3], while cerebellar injury is increasingly recognized [4–6]. These injuries are associated with later cognitive and motor impairment [1,7–13].
White matter injury, also called periventricular leucomalacia, is one of the most frequently occurring forms of brain injury in infants born very prematurely.
Over the last few years, there has been a gradual change in incidence from cystic white matter injury to more diffuse white matter injury, where the majority of very preterm infants now show more subtle abnormalities of the developing white matter [3,7,14,15].
Diffuse white matter injury is generally held responsible for the high incidence of cognitive and behavioral disorders in very preterm born infants [1,2,8].
The two neuroimaging modalities generally used in the neonatal period are cranial ultrasonography (CUS) and MRI. CUS is safe, easily accessible, can be used on a serial basis and is reliable for the detection of most forms of neonatal brain injury [16]. MRI is a safe and valuable tool to assess development and pathology of the very preterm infant’s brain and gives detailed information on the exact location and extension of injury [9,17–20]. Advanced MR techniques, such as diffusion tensor imaging (DTI) or volumetric analyses, can detect axonal disturbances and volume loss resulting from diffuse white matter injury.
CT should only be used for specific limited indications in the neonate as it involves considerable radiation and generally will not provide more information than CUS and/or MRI [16].
The aim of this article is to describe, in detail, the role and limitations of both widely accepted neonatal neuroimaging modalities (CUS and MRI), with a specific focus on preterm white matter injury, the findings that can be encountered and the prognostic significance of these findings.
White matter injury
The main pathogenic mechanisms for white matter injury in the very preterm neonate are ischemia and infection. These often coexist and may lead to focal or diffuse white matter injury and/or hemorrhages in the perinatal period due to the vulnerability of the developing white matter, immature vasculature and impaired cerebrovascular autoregulation of the immature brain [2,21,22].
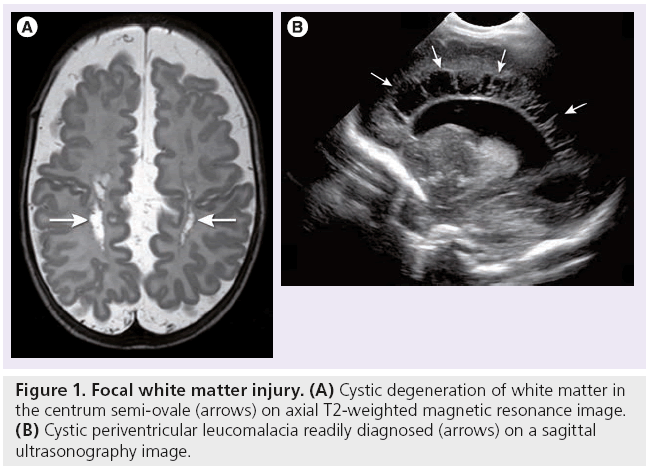
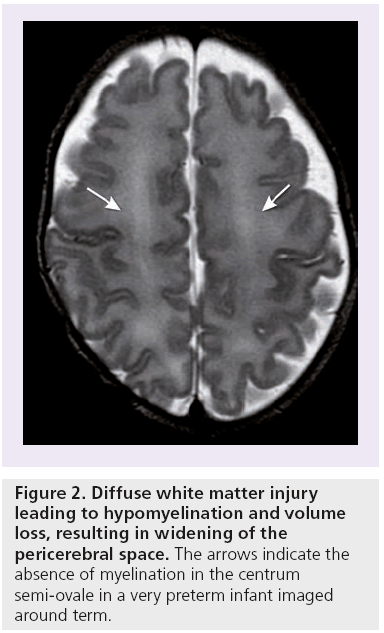
In focal white matter injury or cystic periventricular leucomalacia (Figure 1), there is localized necrosis with loss of cellular elements that evolves over several weeks into macroscopic cystic lesions, readily visualized by both CUS and MRI. More commonly, the necrosis is microscopic in size and evolves into glial scars over several weeks. This more diffuse white matter injury accounts for the vast majority of cases [2]. The glial scars are characterized by astrogliosis and microgliosis. Damage to and significant decrease in premyelinating oligodendrocytes occurs [2,21,23]. Subsequently, this leads to hypomyelination and cerebral white matter loss (Figure 2), resulting in decreased volumes of commissures, such as the corpus callosum [24]. The white matter injury will eventually also lead to gray matter loss and decreased volumes of the thalamus, basal ganglia, cerebral cortex and cerebellum as early as term equivalent age, as a result of neuronal and axonal loss and abnormal connectivity [2,7,23,25,26]. Diffuse noncystic white matter injury in itself is not readily depicted by neuroimaging. The resulting volume loss can be identified by measuring the ventricular dilatation, or by volumetric analysis of white and gray matter structures [27,28]. DTI studies have suggested axonal loss in the white matter of preterm infants at term equivalent age [2,26,29–33].
Figure 1: Focal white matter injury. (A) Cystic degeneration of white matter in the centrum semi-ovale (arrows) on axial T2-weighted magnetic resonance image. (B) Cystic periventricular leucomalacia readily diagnosed (arrows) on a sagittal ultrasonography image.
Imaging findings of white matter injury on CUS & MRI
■ Periventricular echodensities
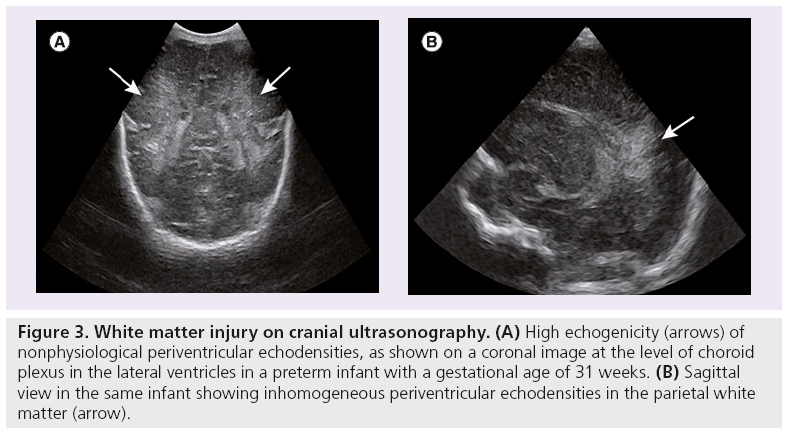
On CUS, nonphysiological periventricular echodensities (PVE) (Figure 3) of the white matter are thought to reflect white matter injury. Their appearance is classified as homogeneous or inhomogeneous, and the echogenicity staged as grade one or two [34]. The more inhomogeneous and echogenic the PVE and the longer their duration, the more likely it is that they present white matter injury. Presence of nonphysiological PVE on CUS is predictive of abnormal white matter on MRI at term. However, absence of PVE does not predict normal white matter on MRI, but does predict a favorable outcome [35]. Presence of nonphysiological PVE in itself is not associated with unfavorable short-term outcomes [35–37].
Figure 3: White matter injury on cranial ultrasonography. (A) High echogenicity (arrows) of nonphysiological periventricular echodensities, as shown on a coronal image at the level of choroid plexus in the lateral ventricles in a preterm infant with a gestational age of 31 weeks. (B) Sagittal view in the same infant showing inhomogeneous periventricular echodensities in the parietal white matter (arrow).
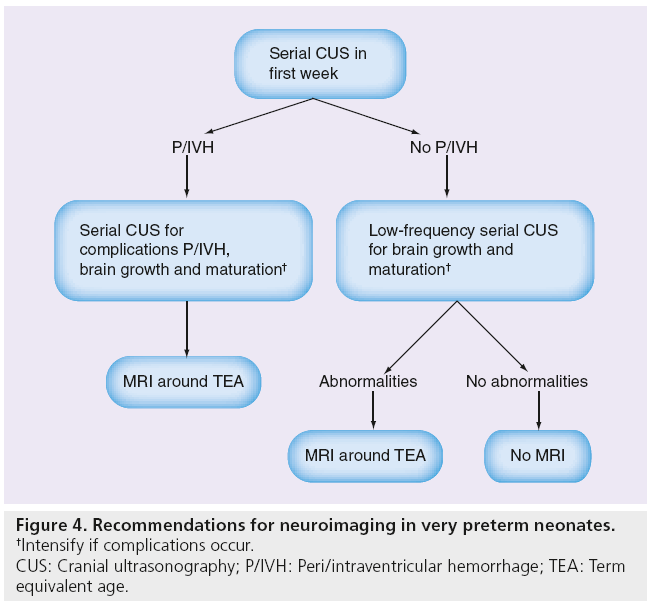
In a retrospective study, inhomogeneous PVE showed no association with punctate white matter lesions (PWMLs) on MRI [38]. The MRI or histological equivalent of inhomogeneous PVE remains unknown. The retrospective study by Leijser et al. showed that the performance of a MRI study before term equivalent age besides sequential CUS did not seem warranted in infants with mild-to-moderate abnormal white matter. Additional MRI only slightly increased the predictive value of CUS in severe white matter changes [38]. In our recent study on ultrasound detection of white matter injury and its practical implications, the authors provided recommendations for performing serial CUS in all very preterm neonates during the perinatal period and a MRI at term equivalent age in some (Figure 4) [35].
Figure 4: Recommendations for neuroimaging in very preterm neonates.
†Intensify if complications occur.
CUS: Cranial ultrasonography; P/IVH: Peri/intraventricular hemorrhage; TEA: Term
equivalent age.
■ Diffuse excessive high-signal intensity
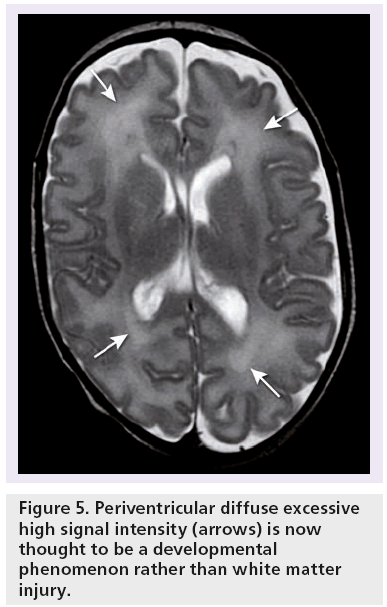
Diffuse excessive high-signal intensity (DEHSI) (Figure 5) on conventional T2-weighted (w) MRI has been described in the periventricular white matter in premature infants and is seen in the majority of these infants [39–41]. For a long time, it was thought to represent diffuse white matter injury on account of altered apparent diffusion coefficient (ADC) and fractional anisotropy (FA) values compared with normal term born neonates [31]. However, this has recently been questioned by several authors [41–43]. It is now assumed that DEHSI represents a developmental phenomenon rather than white matter injury because of its high incidence and the lack of association with short-term neurodevelopmental outcome [41,44,45]. So far, no histological equivalent of DEHSI has been found.
■ Punctate white matter lesion
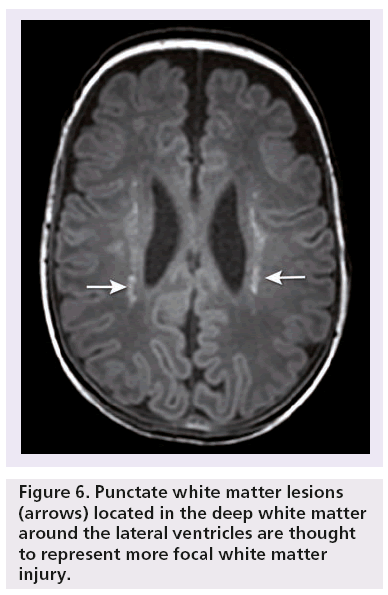
Focal small PWMLs (Figure 6) have been described as small areas with high signal intensity on T1-w MRI images and a less pronounced low-signal intensity on T2-w MRI images [46,47]. These lesions can be differentiated from small hemorrhages by using gradient echo MRI techniques, which are susceptible to hemorrhages, and blood break down products, such as hemosiderin [48].
PWMLs are thought to represent more focal white matter injury. There is no known histological correlate and the pathogenesis is not completely understood, although they may be the MR equivalent of astrogliosis [40]. Some PWMLs are hemorrhagic. If so, these lesions probably occur due to increased pressure in the medullary veins draining towards the ventricles and represent small hemorrhagic venous infarctions [48]. In the acute phase, some of these lesions show diffusion restriction on diffusion-weighted imaging (DWI) sequences compatible with small venous infarcts. In the perinatal period when these lesions occur, they can easily be missed on CUS.
Since PWMLs occur during the perinatal period and tend to fade and decrease in number over time, it is likely that the exact incidence of these lesions is underestimated at term equivalent age, the preferred age of MRI for most preterm infants to investigate the extent of white matter injury [40]. These focal PWMLs are associated with a poorer neurodevelopmental outcome [41,46].
Neuroimaging modalities used to depict white matter injury
■ Cranial ultrasonography
Serial CUS is very reliable for the detection of peri- and intra-ventricular hemorrhage and its complications (posthemorrhagic ventricular dilatation and periventricular hemorrhagic infarction) [3,49]. In addition, it is used to evaluate ventricular size, and the status of the basal ganglia and the white matter in very preterm neonates during the perinatal period [16]. Recent studies have shown that ultrasonography can reliably detect severe (cystic) white matter injury, but it is less reliable for the detection of mild or moderate white matter abnormalities [50,51]. Moreover, it has been shown that PVE of the white matter on ultrasonography can predict abnormal white matter on MRI at term equivalent age, but absence of PVE did not predict absence of white matter changes. Germinal matrix and intraventricular hemorrhages, on the other hand, were predictive of abnormal white matter on MRI and together with abnormal ventricular size or shape, were reasonably predictive of an unfavorable outcome [35].
Optimization of CUS to increase its accuracy and reliability has been extensively described by the authors’ group [16,49,52]. However, even while using optimal protocols and a modern ultrasound system operated by an experienced ultrasonographist, CUS seems to underestimate diffuse white matter injury. As 25–50% of very preterm infants with diffuse white matter injury develop cognitive problems [2], this may prompt the use of MRI around term equivalent age in these infants [35].
■ MRI
MRI is becoming more widely available and is increasingly important for neonatal brain imaging. It is safe and reliable, but poses challenges regarding patient preparation, safety and sequence optimization in neonates [19]. Compared with ultrasonography, it has the disadvantage of the necessity to transport the neonate from the neonatal intensive care unit to the radiology department. The development of MRI compatible incubators has largely overcome this disadvantage as patient preparation can now be performed in the neonatal intensive care unit and after transportation, the entire incubator can be placed into the MR scanner [9].
At the Leiden University Medical Center, all neonatal MRI examinations are performed using a 3T MRI system (Philips Medical Systems, Best, The Netherlands) according to a standard protocol for imaging the newborn infant’s brain [19]. The infants are sedated using chloral hydrate (55 mg/kg), lay supine and are swaddled during the scanning procedure. Ear protection consists of neonatal earmuffs (Natus Minimuffs®; Natus Medical Inc., CA, USA) covered by a headphone. All MRI examinations include a 3D T1-Turbo Field Echo sequence (TR 9.7 ms, TE 4.6 ms, FOV 180 mm, matrix size 192 × 152, flip angle 8°, TFE factor 128, slice thickness 1 mm), a T2-Turbo Spin Echo sequence (TR 6269 ms, TE 120 ms, FOV 180 mm, matrix size 336 × 234, TSE factor 18, slice thickness 2 mm), a T2* fast field echo sequence (TR 735 ms, TE 16 ms, FOV 230 mm, matrix size 256 × 163, f lip angle 18°, slice thickness 4 mm) and a DWI sequence (SE-EPI in three directions, b-value of 1000 s/mm2, TR 2406 ms, TE 64 ms, EPI factor: 37, FOV: 180 mm, matrix size: 6 × 69, slice thickness 4 mm).
■ Frequently used MRI techniques
Most MRI sequences are performed to assess the development or injury of the brain in preterm infants. Specifically, myelination can be assessed on T1-w and T2*-w MRI sequences. MRI can easily detect germinal matrix/intraventricular hemorrhages, periventricular hemorrhagic infarctions, cystic white matter lesions and PWML using T1-w, T2-w, T2*-w gradient echo and/or DWI sequences. White matter volume loss resulting in increased pericerebral spaces, ventricular dilatation and thinning of the corpus callosum can be reliably evaluated on T1-w and T2-w sequences. The gray matter volume loss resulting from white matter injury can be recognized as a less complicated gyral pattern and lower volumes of the basal ganglia and/or thalami.
MRI obtained at term equivalent age in preterm infants has prognostic significance, as parenchymal lesions, such as hemorrhages, changes consistent with white matter injury, infarctions, hypomyelination and reduction of white matter volumes have been shown to be predictive of cognitive and motor delay and cerebral palsy at 2 years of age [41,44,53]. The combination of these different parenchymal lesions adds up to predict an adverse outcome in most preterm infants with severe white matter lesions, but prognostication is less certain in infants with mild or moderate white matter lesions, which occurrs in the majority [54].
■ Advanced MRI techniques
DTI has been proposed as an additional tool in the assessment of white matter injury in preterm infants and may provide more adequate diagnostic and prognostic information in relation to neurological outcome than other MR techniques [55,56]. DTI describes the diffusion of water molecules in tissues and reflects the direction of the underlying microstructure. In DTI, diffusion is measured in at least six diffusional directions, while in DWI, diffusion is measured in only three perpendicular directions. Contrast is based on the Brownian motion of water molecules, which is influenced by various factors, including fiber orientation, integrity of cell membranes and degree of myelination. DTI can be used to assess cerebral development and connectivity by calculating diffusivity values [55,57].
The physical constant characterizing water molecule displacement is called the ADC or mean diffusivity. In very preterm infants, the ADC of the white matter is high due to the high water content of the immature brain. When the brain further matures, the ADC will decrease [55]. ADC values may be abnormal in infants with brain injury or abnormal brain development [58].
While the axons in the developing brain organize and myelinate, the displacement of water molecules, as described by the FA value, is most restricted in the perpendicular direction and least restricted parallel to the myelinating fibers. The maturation of white matter is accompanied by an increase in anisotropic diffusion and thus in FA.
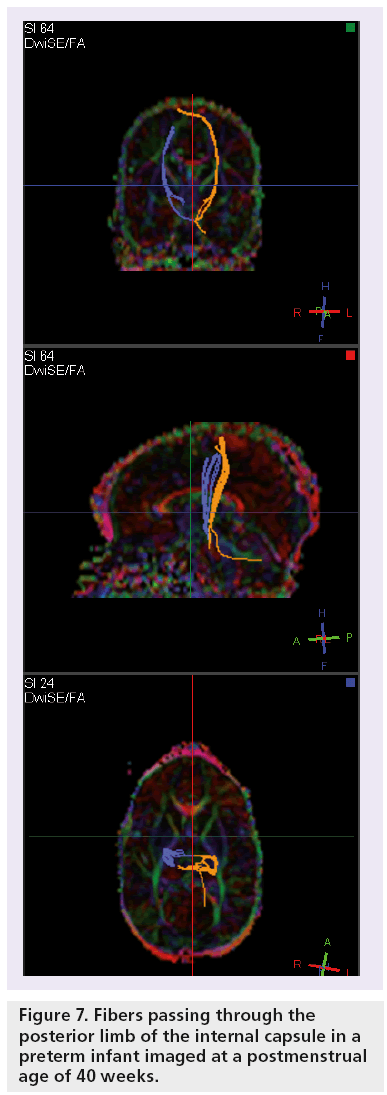
Fiber tractography offers insight into developing white matter by visualization of the white matter tracts (Figures 7 & 8) [55,59–63].
Diffusion parameters at term equivalent age have only scarcely been studied in relation to neurodevelopmental outcome and have shown an association between lower FA values in the posterior limb of the internal capsule and higher ADC values in the splenium of the corpus callosum at term, and motor delay around 2 years of age [64,65]. DTI values at term equivalent age may help further prognostication of neurodevelopmental outcome at 2 years. In combination with clinical parameters and white matter injury seen on T1-w and T2-w MRI, specificity further increases.
Over the last decade, numerous MRI techniques have been proposed to measure brain volumes in the very preterm infant as a measure of brain development and injury. Segmentation techniques for gray matter, unmyelinated and myelinated white matter, and cerebrospinal fluid have been developed [28,66]. However, in daily clinical practice, their use is not feasible and the relation to neurodevelopmental outcome has not been studied extensively. Linear measurements have been developed and validated in the preterm infants’ brain and can be applied manually to 2D and 3D data sets [67].
The utility of MR spectroscopy for risk-stratifying preterm infants in relation to longterm adverse outcome is not well established. There are difficulties concerning the use of this technique, such as age-related differences in metabolites, as measured by MR spectroscopy in the perinatal and early childhood period [68]. MR spectroscopy has not been found to be a good predictor of outcome in preterm infants at the age of 18–24 months [69].
Conclusion
Long-term clinical follow-up remains necessary to further evaluate the prognostic values of certain neuroimaging findings and quantitative values around term equivalent age, especially for cognitive neurodevelopmental outcome in very preterm neonates.
Advanced techniques, such as DTI, magnetization transfer imaging, functional resting state MRI and volumetric methods, are still under active investigation. Serial MRI and the application of these newer analysis techniques will provide insights into the trajectories of brain development and the impact of injury on the development [9].
Future perspective
Development of brain functions and the structural-functional correlates of brain injury remain difficult to evaluate in preterm infants. MRI at term equivalent age better depicts diffuse white matter injury in very preterm infants than ultrasonography. Combined grading of white matter injury and advanced (quantitative) MRI techniques, such as DTI, help to prognosticate adverse neurodevelopmental outcome [54]. However, most very preterm infants show mild-to-moderate diffuse white matter injury and in this group, prediction of outcome remains uncertain. Whole-brain statistical methods developed for neonatal DTI analysis, such as optimized tract-based spatial statistics [70] and atlas-based analysis [71], might have the potential to detect mild-to-moderate white matter injury related to the neurological outcome. Another quantitative MR technique to evaluate brain development and possibly brain injury in preterm infants is magnetization transfer imaging, which can be used to evaluate myelination [72]. Magnetization transfer is a MR imaging phenomenon based on the interaction between immobile protons in macromolecules and free water protons of tissue. A magnetization transfer ratio is obtained by calculating the percentage difference between two images, one with and one without an off-resonance radio frequency pulse [73]. Magnetization transfer ratio provides a reproducible measurement sensitive to myelination and thus an index to brain maturation [74].
Functional resting state MRI may be a new noninvasive technique to assist evaluating early life brain function and its recovery from injury [75,76]. This technique is based on data analysis applied to functional MRI, revealing patterns of interconnections between neural networks. Resting state networks have been identified in preterm infants [77–79]. Additional research is necessary to determine the clinical utility of resting- state functional connectivity analyses and the potential for the method to reveal the anatomical substrate for cognitive deficits in preterm infants who do not appear to have abnormalities on other imaging techniques [68].
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.

References
Papers of special note have been highlighted as:
• of interest
- Dyet LE, Kennea N, Counsell SJ et al. Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics 118(2), 536–548 (2006).
- Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 8(1), 110–124 (2009).
- Leijser LM, de Bruïne FT, Steggerda SJ, van der Grond J, Walther FJ, van Wezel-Meijler G. Brain imaging findings in very preterm infants throughout the neonatal period: part I. Incidences and evolution of lesions, comparison between ultrasound and MRI. Early Hum. Dev. 85(2), 101–109 (2009).
- Limperopoulos C, Robertson RL, Sullivan NR, Bassan H, du Plessis AJ. Cerebellar injury in term infants: clinical characteristics, magnetic resonance imaging findings, and outcome. Pediatr. Neurol. 41(1), 1–8 (2009).
- Steggerda SJ, Leijser LM, Wiggers-de Bruïne FT, van der Grond J, Walther FJ, van Wezel-Meijler G. Cerebellar injury in preterm infants: incidence and findings on US and MR images. Radiology 252(1), 190–199 (2009).
- 6 Tam EW, Ferriero DM, Xu D et al. Cerebellar development in the preterm neonate: effect of supratentorial brain injury. Pediatr. Res. 66(1), 102–106 (2009).
- 7 Miller SP, Ferriero DM. From selective vulnerability to connectivity: insights from newborn brain imaging. Trends Neurosci. 32(9), 496–505 (2009).
- Ment LR, Hirtz D, Huppi PS. Imaging biomarkers of outcome in the developing preterm brain. Lancet Neurol. 8(11), 1042–1055 (2009).
- Mathur AM, Neil JJ, Inder TE. Understanding brain injury and neurodevelopmental disabilities in the preterm infant: the evolving role of advanced magnetic resonance imaging. Semin. Perinatol. 34(1), 57–66 (2010).
- Spittle AJ, Cheong J, Doyle LW et al. Neonatal white matter abnormality predicts childhood motor impairment in very preterm children. Dev. Med. Child Neurol. 53(11), 1000–1006 (2011).
- Woodward LJ, Clark CA, Pritchard VE, Anderson PJ, Inder TE. Neonatal white matter abnormalities predict global executive function impairment in children born very preterm. Dev. Neuropsychol. 36(1), 22–41 (2011).
- Limperopoulos C, Bassan H, Gauvreau K et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics 120(3), 584–593 (2007).
- Tam EW, Miller SP, Studholme C et al. Differential effects of intraventricular hemorrhage and white matter injury on preterm cerebellar growth. J. Pediatr. 158(3), 366–371 (2011).
- Groenendaal F, Termote JU, van der Heide-Jalving, van Haastert I, de Vries LS. Complications affecting preterm neonates from 1991 to 2006: what have we gained? Acta Paediatr. 99(3), 354–358 (2010).
- van Haastert, I, Groenendaal F, Uiterwaal CS et al. Decreasing incidence and severity of cerebral palsy in prematurely born children. J. Pediatr. 159(1), 86–91.e1 (2011).
- van Wezel-Meijler G. Neonatal cranial ultrasonography (2nd Edition). Springer, Berlin, Germany (2012).
- Barkovich AJ, Maroldo TV. Magnetic resonance imaging of normal and abnormal brain development. Top. Magn. Reson. Imaging 5(2), 96–122 (1993).
- Counsell SJ, Rutherford MA, Cowan FM, Edwards AD. Magnetic resonance imaging of preterm brain injury. Arch. Dis. Child Fetal Neonatal Ed. 88(4), F269–F274 (2003).
- van Wezel-Meijler G, Leijser LM, de Bruïne FT, Steggerda SJ, van der Grond J, Walther FJ. Magnetic resonance imaging of the brain in newborn infants: practical aspects. Early Hum. Dev. 85(2), 85–92 (2009).
- Rutherford M, Biarge MM, Allsop J, Counsell S, Cowan F. MRI of perinatal brain injury. Pediatr. Radiol. 40(6), 819–833 (2010).
- Back SA. Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Ment. Retard. Dev. Disabil. Res. Rev. 12(2), 129–140 (2006).
- Glass HC, Bonifacio SL, Chau V et al. Recurrent postnatal infections are associated with progressive white matter injury in premature infants. Pediatrics 122(2), 299–305 (2008).
- Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch. Dis. Child Fetal Neonatal Ed. 93(2), F153-F161 (2008).
- Anderson NG, Laurent I, Cook N, Woodward L, Inder TE. Growth rate of corpus callosum in very premature infants. AJNR Am. J. Neuroradiol. 26(10), 2685–2690 (2005).
- Judas M, Rados M, Jovanov-Milosevic N, Hrabac P, Stern-Padovan R, Kostovic I. Structural, immunocytochemical, and mr imaging properties of periventricular crossroads of growing cortical pathways in preterm infants. AJNR Am. J. Neuroradiol. 26(10), 2671–2684 (2005).
- Counsell SJ, Dyet LE, Larkman DJ et al. Thalamo-cortical connectivity in children born preterm mapped using probabilistic magnetic resonance tractography. Neuroimage 34(3), 896–904 (2007).
- Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics 115(2), 286–294 (2005).
- Mewes AU, Huppi PS, Als H et al. Regional brain development in serial magnetic resonance imaging of low-risk preterm infants. Pediatrics 118(1), 23–33 (2006).
- Huppi PS, Murphy B, Maier SE et al. Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics 107(3), 455–460 (2001).
- Miller SP, Vigneron DB, Henry RG et al. Serial quantitative diffusion tensor MRI of the premature brain: development in newborns with and without injury. J. Magn. Reson. Imaging 16(6), 621–632 (2002).
- Counsell SJ, Shen Y, Boardman JP et al. Axial and radial diffusivity in preterm infants who have diffuse white matter changes on magnetic resonance imaging at termequivalent age. Pediatrics 117(2), 376–386 (2006).
- Counsell SJ, Edwards AD, Chew AT et al. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain 131(Pt 12), 3201–3208 (2008).
- Liu Y, Aeby A, Baleriaux D et al. White matter abnormalities are related to microstructural changes in preterm neonates at term-equivalent age: a diffusion tensor imaging and probabilistic tractography study. AJNR Am. J. Neuroradiol. 33(5), 839–845 (2012).
- van Wezel-Meijler G, van der Knaap MS, Sie LT et al. Magnetic resonance imaging of the brain in premature infants during the neonatal period. Normal phenomena and reflection of mild ultrasound abnormalities. Neuropediatrics 29(2), 89–96 (1998).
- van Wezel-Meijler G, de Bruïne FT, Steggerda SJ et al. Ultrasound detection of white matter injury in very preterm neonates: practical implications. Dev. Med. Child Neurol. 53(Suppl. 4), 29–34 (2011).
- Pisani F, Leali L, Moretti S, Turco E, Volante E, Bevilacqua G. Transient periventricular echodensities in preterms and neurodevelopmental outcome. J. Child Neurol. 21(3), 230–235 (2006).
- Resch B, Jammernegg A, Perl E, Riccabona M, Maurer U, Muller WD. Correlation of grading and duration of periventricular echodensities with neurodevelopmental outcome in preterm infants. Pediatr. Radiol. 36(8), 810–815 (2006).
- Leijser LM, Liauw L, Veen S, de Boer IP, Walther FJ, van Wezel-Meijler G. Comparing brain white matter on sequential cranial ultrasound and MRI in very preterm infants. Neuroradiology 50(9), 799–811 (2008).
- Maalouf EF, Duggan PJ, Rutherford MA et al. Magnetic resonance imaging of the brain in a cohort of extremely preterm infants. J. Pediatr. 135(3), 351–357 (1999).
- Rutherford MA, Supramaniam V, Ederies A et al. Magnetic resonance imaging of white matter diseases of prematurity. Neuroradiology 52(6), 505–521 (2010).
- de Bruïne FT, van den Berg-Huysmans AA, Leijser LM et al. Clinical implications of MR imaging findings in the white matter in very preterm infants: a 2-year follow-up study. Radiology 261(3), 899–906 (2011).
- Hart A, Whitby E, Wilkinson S, Alladi S, Paley M, Smith M. Neuro-developmental outcome at 18 months in premature infants with diffuse excessive high signal intensity on MR imaging of the brain. Pediatr. Radiol. 41(10), 1284–1292 (2011).
- Kidokoro H, Anderson PJ, Doyle LW, Neil JJ, Inder TE. High signal intensity on T2-weighted MR imaging at term-equivalent age in preterm infants does not predict 2-year neurodevelopmental outcomes. AJNR Am. J. Neuroradiol. 32(11), 2005–2010 (2011).
- Skiold B, Vollmer B, Bohm B et al. Neonatal magnetic resonance imaging and outcome at age 30 months in extremely preterm infants. J. Pediatr. 160(4), 559–566 (2012).
- Jeon TY, Kim JH, Yoo SY et al. Neurodevelopmental outcomes in preterm infants: comparison of infants with and without diffuse excessive high signal intensity on MR images at near-termequivalent age. Radiology 263(2), 518–526 (2012).
- Miller SP, Ferriero DM, Leonard C et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J. Pediatr. 147(5), 609–616 (2005).
- Cornette LG, Tanner SF, Ramenghi LA et al. Magnetic resonance imaging of the infant brain: anatomical characteristics and clinical significance of punctate lesions. Arch. Dis. Child Fetal Neonatal Ed. 86(3), F171–F177 (2002).
- Niwa T, De Vries LS, Benders MJ, Takahara T, Nikkels PG, Groenendaal F. Punctate white matter lesions in infants: new insights using susceptibility-weighted imaging. Neuroradiology 53(9), 669–679 (2011).
- van Wezel-Meijler G, Steggerda SJ, Leijser LM. Cranial ultrasonography in neonates: role and limitations. Semin. Perinatol. 34(1), 28–38 (2010).
- Leijser LM, de Bruïne FT, van der Grond J, Steggerda SJ, Walther FJ, van Wezel-Meijler G. Is sequential cranial ultrasound reliable for detection of white matter injury in very preterm infants? Neuroradiology 52(5), 397–406 (2010).
- Inder TE, Anderson NJ, Spencer C, Wells S, Volpe JJ. White matter injury in the premature infant: a comparison between serial cranial sonographic and MR findings at term. AJNR Am. J. Neuroradiol. 24(5), 805–809 (2003).
- Steggerda SJ, Leijser LM, Walther FJ, van Wezel-Meijler G. Neonatal cranial ultrasonography: how to optimize its performance. Early Hum. Dev. 85(2), 93–99 (2009).
- Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N. Engl. J. Med. 355(7), 685–694 (2006).
- Nongena P, Ederies A, Azzopardi DV, Edwards AD. Confidence in the prediction of neurodevelopmental outcome by cranial ultrasound and MRI in preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 95(6), F388–F390 (2010).
- Huppi PS, Dubois J. Diffusion tensor imaging of brain development. Semin. Fetal Neonatal Med. 11(6), 489–497 (2006).
- Dudink J, Larkman DJ, Kapellou O et al. High b-value diffusion tensor imaging of the neonatal brain at 3T. AJNR Am. J. Neuroradiol. 29(10), 1966–1972 (2008).
- Dubois J, Hertz-Pannier L, Dehaene- Lambertz G, Cointepas Y, Le Biahn D. Assessment of the early organization and maturation of infants’ cerebral white matter fiber bundles: a feasibility study using quantitative diffusion tensor imaging and tractography. Neuroimage 30(4), 1121–1132 (2006).
- Counsell SJ, Allsop JM, Harrison MC et al. Diffusion-weighted imaging of the brain in preterm infants with focal and diffuse white matter abnormality. Pediatrics 112(1 Pt 1), 1–7 (2003).
- Watts R, Liston C, Niogi S, Ulug AM. Fiber tracking using magnetic resonance diffusion tensor imaging and its applications to human brain development. Ment. Retard. Dev. Disabil. Res. Rev. 9(3), 168–177 (2003).
- Berman JI, Mukherjee P, Partridge SC et al. Quantitative diffusion tensor MRI fiber tractography of sensorimotor white matter development in premature infants. Neuroimage 27(4), 862–871 (2005).
- Partridge SC, Mukherjee P, Berman JI et al. Tractography-based quantitation of diffusion tensor imaging parameters in white matter tracts of preterm newborns. J. Magn. Reson. Imaging 22(4), 467–474 (2005).
- Dudink J, Lequin M, van PC et al. Fractional anisotropy in white matter tracts of very-low-birth-weight infants. Pediatr. Radiol. 37(12), 1216–1223 (2007).
- de Bruïne FT, van Wezel-Meijler G, Leijser LM et al. Tractography of developing white matter of the internal capsule and corpus callosum in very preterm infants. Eur. Radiol. 21(3), 538–547 (2011).
- Arzoumanian Y, Mirmiran M, Barnes PD et al. Diffusion tensor brain imaging findings at term-equivalent age may predict neurologic abnormalities in low birth weight preterm infants. AJNR Am. J. Neuroradiol. 24(8), 1646–1653 (2003).
- Rose J, Butler EE, Lamont LE, Barnes PD, Atlas SW, Stevenson DK. Neonatal brain structure on MRI and diffusion tensor imaging, sex, and neurodevelopment in very-low-birthweight preterm children. Dev. Med. Child Neurol. 51(7), 526–535 (2009).
- Anbeek P, Vincken KL, Groenendaal F, Koeman A, van Osch MJ, van der GJ. Probabilistic brain tissue segmentation in neonatal magnetic resonance imaging. Pediatr. Res. 63(2), 158–163 (2008).
- Nguyen The Tich S, Anderson PJ, Shimony JS, Hunt RW, Doyle LW, Inder TE. A novel quantitative simple brain metric using MR imaging for preterm infants. AJNR Am. J. Neuroradiol. 30(1), 125–131 (2009).
- Panigrahy A, Wisnowski JL, Furtado A, Lepore N, Paquette L, Bluml S. Neuroimaging biomarkers of preterm brain injury: toward developing the preterm connectome. Pediatr. Radiol. 42(Suppl. 1), 33–61 (2012).
- Augustine EM, Spielman DM, Barnes PD et al. Can magnetic resonance spectroscopy predict neurodevelopmental outcome in very low birth weight preterm infants? J. Perinatol 28(9), 611–618 (2008).
- Ball G, Counsell SJ, Anjari M et al. An optimised tract-based spatial statistics protocol for neonates: applications to prematurity and chronic lung disease. Neuroimage 53(1), 94–102 (2010).
- Oishi K, Mori S, Donohue PK et al. Multi-contrast human neonatal brain atlas: application to normal neonate development analysis. Neuroimage 56(1), 8–20 (2011).
- Nossin-Manor R, Chung AD, Whyte HE, Shroff MM, Taylor MJ, Sled JG. Deep gray matter maturation in very preterm neonates: regional variations and pathology-related age-dependent changes in magnetization transfer ratio. Radiology 263(2), 510–517 (2012).
- Grossman RI, Gomori JM, Ramer KN, Lexa FJ, Schnall MD. Magnetization transfer: theory and clinical applications in neuroradiology. Radiographics 14(2), 279–290 (1994).
- van Buchem MA, Steens SC, Vrooman HA et al. Global estimation of myelination in the developing brain on the basis of magnetization transfer imaging: a preliminary study. AJNR Am. J. Neuroradiol. 22(4), 762–766 (2001).
- Seghier ML, Lazeyras F, Huppi PS. Functional MRI of the newborn. Semin. Fetal Neonatal Med. 11(6), 479–488 (2006).
- Seghier ML, Huppi PS. The role of functional magnetic resonance imaging in the study of brain development, injury, and recovery in the newborn. Semin. Perinatol. 34(1), 79–86 (2010).
- Fransson P, Skiold B, Horsch S et al. Resting-state networks in the infant brain. Proc. Natl. Acad. Sci. USA 104(39), 15531–15536 (2007).
- Smyser CD, Inder TE, Shimony JS et al. Longitudinal analysis of neural network development in preterm infants. Cereb. Cortex 20(12), 2852–2862 (2010).
- Doria V, Beckmann CF, Arichi T et al. Emergence of resting state networks in the preterm human brain. Proc. Natl Acad. Sci. USA 107(46), 20015–20020 (2010).
• Review on encephalopathy of prematurity.
• Review on the effects of injury and the age of insult on brain development in neonates.
• Review on the current status of advanced MRI techniques.