Research Article - Interventional Cardiology (2021)
Randomized trial of complete vs. culprit-only revascularization in STEMI patients with cardiogenic shock and non-CTO multi-vessel disease
- Corresponding Author:
- Mohamed Atef Hamza
Department of Cardiology,
Ain-Shams University,
Cairo,
Egypt,
E-mail: matef77@hotmail.com
Received date: September 29, 2021; Accepted date: October 13, 2021;Published date: October 20, 2021
Abstract
Background: Clinical trials favor complete revascularization of non-culprit lesions in patients with STEMI and multivessel disease (MVD) over culprit only, however management of patients complicated by cardiogenic shock is still debatable.
Objectives: To study whether complete revascularization approach is better than culprit-only treatment STEMI patients with cardiogenic shock when excluding Chronic Total Occlusions (CTOs).
Methods: One hundred STEMI patients with cardiogenic shock and MVD were randomized to either culprit-only treatment (n=50) or complete revascularization (n=50) in the same sitting of Primary Percutaneous Coronary Intervention (PPCI). The primary endpoint was the incidence of Major Adverse Cardiac Events (MACE) at 6 months.
Results: Complete revascularization significantly reduced the rates of total MACE (38% vs. 66%; RR 0.58, 95% CI 0.38-0.86, p=0.005), all-cause mortality (32% vs. 52%; RR 0.62, 95% CI 0.38-0.99, p=0.033), with improvement in ejection fraction (44.2% vs. 33.0%, p=0.034), and lower rates of urgent revascularization (2% vs. 18%, p=0.008) when compared to culprit-only. There was no significant difference in the safety endpoints of stroke, contrast-induced nephropathy, major or minor bleeding between the groups.
Conclusion: In STEMI patients with cardiogenic shock and MVD, complete revascularization reduced the risk of mortality and total MACE when compared with culprit vessel only PCI, when excluding CTO lesions.
Keywords
Complete revascularization • Culprit STEMI • Primary PCI • Cardiogenic shock
Introduction
According to clinical trials and guidelines recommendations, Primary Percutaneous Coronary Intervention (PCI) is the preferred modality of management STEMI [1-3], and up to 30%-40% of those patients were found to have Multivessel Disease (MVD) [4,5]. Data from randomized trials and meta-analyses favors complete revascularization than culprit-only treatment [6-11].
STEMI patient complicated by cardiogenic shock tend to have worse long-term outcomes compared to those with less extensive coronary disease [5,12]. and management of this group is still debatable. Guidelines previously recommended total revascularization during the index procedure in STEMI patients with cardiogenic shock [13]. However, following the results of the CULPRIT-SHOCK (Culprit Lesion Only PCI Versus Multivessel PCI in Cardiogenic Shock) trial [14], total revascularization is no longer recommended [15]. One potential limitation of this study is Chronic Total Occlusions (CTO) revascularization in the acute setting, which consumed more time, more radiation and more contrast which could be more harmful to the patient causing worse outcomes.
This study was done to assess safety and efficacy of total revascularization in STEMI patients with cardiogenic shock and multi-vessel disease with exclusion of CTO lesions.
Methodology
Study design and patient selection
In this prospective multi-centric study, we randomized 100 STEMI patients complicated by cardiogenic shock presenting within 12 h of symptom and planned for primary PCI in the period between August 2018 and July 2020. Patients were randomly assigned to either group using a block randomization, we enrolled patients during this specified recruitment period, a sample size calculation was not performed.
After the confirmation of STEMI complicated by cardiogenic shock with Systolic Blood Pressure (SBP)<90 mmHg and clinical evidence of end organ damage, patients were randomized to either complete revascularization or culprit lesion only treatment after excluding CTO lesions. According to guidelines recommendations [1], all patients received oral anti-platelets (aspirin 300 mg plus ticagrelor 180 mg or clopidogrel 600 mg loading dose followed by regular maintenance dose). PPCI was undertaken in five different centers by experienced operators and could include aspiration thrombectomy, intra-aortic balloon counter-pulsation or positive inotropes or Glycoprotein IIb/IIIa inhibitor (GPI) unless clinically contraindicated and according to operators’ decision. Previous Coronary Artery Bypass Grafting (CABG) or left main stenosis >50% were excluded from the study. All patients were treated by drug eluting stents, with PCI of the Infarct Related Artery (IRA) first, and then complete revascularization was performed at the same sitting after angiographic assessment.
Laboratory investigations were done including cardiac biomarkers; Troponin T on admission and after 6 hours, CK-MB levels every 6 hours till normalization, serum creatinine level, CBC on admission and upon discharge, echocardiography was reviewed by experienced, blinded operators during hospitalization and at 6 months to assess left ventricular ejection fraction using Simpson’s method.
Before inclusion, full informed written consent was obtained from each participant and the study protocol was reviewed and approved by our local institutional human research committee (Ain shams university hospitals). Moreover, the protection of the privacy of the participants was ensured, as well as, the confidentiality of the research data.
Clinical outcomes and end points
All patients were followed-up after discharge by out-patient clinic visit or by phone calls. The primary endpoint was the rates of Major Adverse Cardiac Events (MACE) defined as all-cause mortality, recurrent MI, stroke and target vessel revascularization by PCI or Coronary Artery Bypass Grafting (CABG) at 6 m.
The secondary endpoints included safety outcomes, major bleeding associated with a drop in hemoglobin of ≥ 5 g/dL or according to Thrombolysis In Myocardial Infarction (TIMI) bleeding criteria) [16], minor bleeding or puncture site related complications, and Contrast-Induced Nephropathy (CIN); defined as increase of at least 0.5 mg/dL (44.2 lmol/L), or a relative increase of at least 25% in serum creatinine recorded after PCI, in comparison with baseline value [17].
Statistical analysis
The collected data was coded, tabulated, and statistically analyzed using IBM SPSS statistics (Statistical Package for Social Sciences) software version 22.0, IBM Corp., Chicago, USA, 2013.
Descriptive statistics was done for quantitative data as minimum and maximum of the range as well as mean ± SD (standard deviation) for quantitative normally distributed data, while it was done for qualitative data as number and percentage. Inferential analyses were done for quantitative variables using Kolmogorov- Smirnov (K-S) test for normality testing, independent t-test in cases of two independent groups with normally distributed data. In qualitative data, inferential analyses for independent variables were done using Chi square test for differences between proportions and Fisher’s Exact test for variables with small expected numbers. Kaplan-Meier curves and log-rank test were constructed for the time to the first event of the composite outcome. The level of significance was taken at p value<0.050 is significant, otherwise is non-significant.
Results
Study population
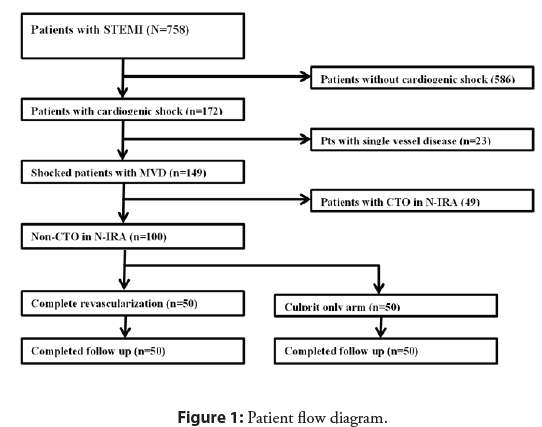
In this study, we enrolled 100 STEMI patients with cardiogenic shock and numerically randomized them to either complete revascularization (n=50) or culprit only treatment (n=50) (Figure 1). Baseline demographic data, history and cardiovascular risk factors are presented in Table 1.
| Complete revascularization (n=50) | Culprit only (n=50) | p | |
|---|---|---|---|
| Age | 58.7 ± 10.0 | 59.6 ± 7.2 | 0.574 |
| Male (%) | 40 (80%) | 37 (74%) | 0.476 |
| Smoking | 36 (72.0%) | 33 (66.0%) | 0.517 |
| Hypertension | 25 (50.0%) | 27 (54.0%) | 0.689 |
| DM | 25 (50.0%) | 25 (50.0%) | 1 |
| Hypercholesterolemia | 8 (16.0%) | 9 (18.0%) | 0.79 |
| Family history | 6 (12.0%) | 5 (10.0%) | 0.749 |
| CKD | 3 (6.0%) | 8 (16.0%) | 0.11 |
| PAD | 2 (4.0%) | 2 (4.0%) | 1 |
| Previous ACS | 6 (12.0%) | 5 (10.0%) | 0.749 |
| prior PCI | 5 (10.0%) | 4 (8.0%) | 1 |
| SBP | 57.4 ± 11.6 | 55.6 ± 10.5 | 0.418 |
| DBP | 31.2 ± 9.8 | 31.0 ± 7.4 | 0.908 |
| Creatinine (mg/dL) | 1.15 ± 0.40 | 1.25 ± 0.33 | 0.164 |
| Creatinine clearance (mL/min) | 73.8 ± 22.9 | 67.5 ± 20.2 | 0.147 |
| Peak CK-MB level | 280.5 ± 136.4 | 307.5 ± 131.2 | 0.317 |
Abbreviations: DM: Diabetes Mellitus; CKD: Chronic Kidney Disease; PAD: Peripheral Arterial Disease; ACS: Acute Coronary Syndrome; PCI: Percutaneous Coronary Intervention; SBP and DBP: Systolic and Diastolic Blood Pressure
Table 1: Baseline demographic data in the study groups.
| Complete revascularization (n=50) | Culprit only (n=50) | p | |
|---|---|---|---|
| Infarct related artery | |||
| LAD | 34 (68%) | 31 (62%) | 0.529 |
| RCA | 11 (22%) | 12 (24%) | |
| LCx | 3 (6%) | 4 (8%) | |
| Others (OM or Diagonal) | 2 (4%) | 3 (6%) | |
| Pain to balloon (hr) | 8.6 ± 7.3 | 9.4 ± 7.7 | 0.569 |
| Pain to balloon (hr) | 8.6 ± 7.3 | 9.4 ± 7.7 | 0.569 |
| Fluoroscopy time (min) | 23.2 ± 7.3 | 16.6 ± 3.4 | 0.001 |
| Stent implanted/patient | 2.4 ± 0.8 | 1.5 ± 1.1 | 0.001 |
| Thrombus aspiration | 6 (12) | 10 (20) | 0.275 |
| Inotropes | 41 (82.0%) | 42 (84.0%) | 0.79 |
| IABP | 1 (2) | 0 | 0 |
| Pre-dilatation | 34 (68) | 27 (54) | 0.151 |
| No-reflow | 13 (26) | 15 (30) | 0.656 |
| Contrast amount (ml) | 185.0 ± 98.6 | 105.4 ± 43.7 | 0.001 |
| TIMI III post procedural | 36 (72) | 32 (64) | 0.682 |
| GP IIb/ IIIa inhibitors | 17 (34) | 20 (40) | 0.534 |
| Radial approach | 23 (46) | 21 (42) | 0.76 |
Abbreviations: LAD: Left Anterior Descending artery; RCA: Right Coronary Artery; LCX: left circumflex artery; GP: glycoprotein IIb/IIIa
Table 2: Baseline angiographic and procedural parameters.
All patients received drug eluting stents, baseline angiographic and procedural parameters including the Infarct Related Artery (IRA), pain-to-balloon time use of thrombus aspiration or glycoprotein IIb/IIIa receptor inhibitors were similar in each group, however total amount of contrast and fluoroscopy time was higher in the total revascularization group (Table 2). All N-IRAs were stented successfully with satisfactory end results. There were no differences in the rate of use of Dual Antiplatelet Therapy (DAPT), β-blockers or statins upon discharge.
End points
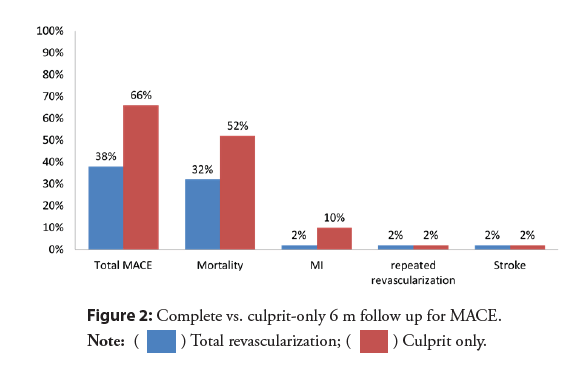
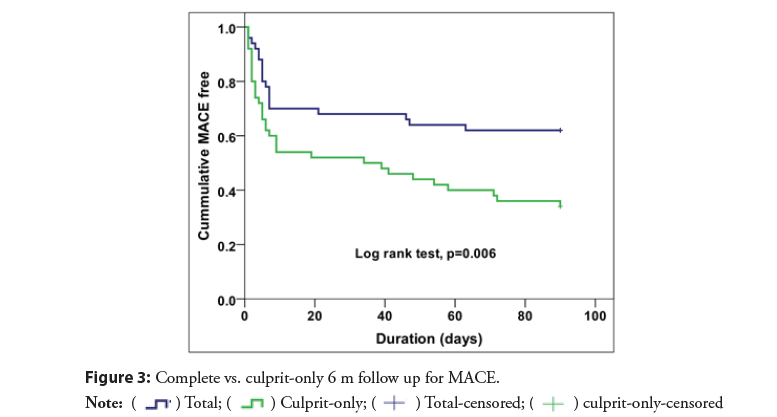
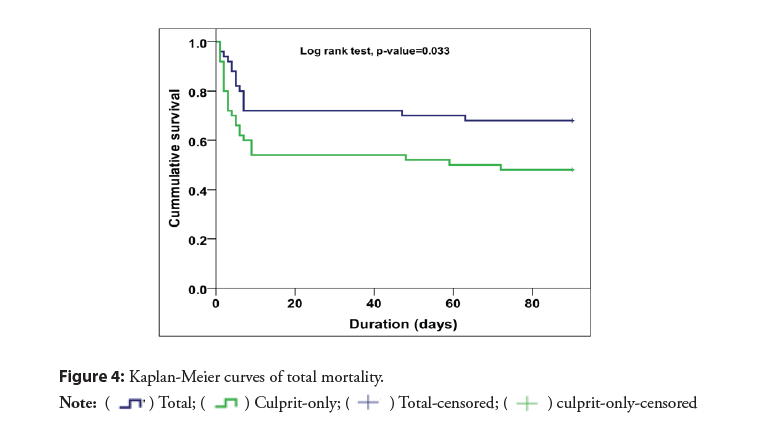
The rates of MACE were significantly lower in the complete revascularization group than in the culprit only (38% vs. 66%; RR 0.58, 95% CI 0.38-0.86, p=0.005) (Figure 2). With early divergence of the Kaplan-Meier curves (Figure 3) with separation continued up to 6 m during the study period. There was significant difference in mortality (32% vs. 52%; RR 0.62, 95% CI 0.38- 0.99, p=0.033) Most of deaths occurred within the in-hospital initial stay in both arms depicting that the initial period is the most critical as regards the mortality (Figure 4).
Recurrent MI and urgent revascularization were lower in the total revascularization arm (2% vs. 10%, p=0.04), and (2% vs. 18% RR 0.11, 95% CI 0.01-0.84; p=0.008) respectively. There was a significantly higher fluoroscopy time and total amount of dye in the total revascularization group vs. culprit only group (Table 3). The total revascularization at the index procedure showed better improvement in the ejection fraction during follow up in a statistically significant pattern from (33.2 ± 7.8 vs. 33.1 ± 9.1) post-procedural to (44.2% vs. 33.0%) at 6-month (p=0.034) (Table 3).
| Complete revascularization (n=50) | Culprit only (n=50) | p | |
|---|---|---|---|
| Total MACE | 19 (38) | 33 (66) | 0.005 |
| Mortality | 16 (32) | 26 (52) | 0.033 |
| MI | 1 (2.0%) | 5 (10.0%) | 0.04 |
| Stroke | 1 (2.0%) | 1 (2.0%) | 1 |
| Target vessel revascularization | 1 (2) | 1 (2) | 1 |
| Urgent revascularization | 1 (2.0%) | 9 (18.0%) | 0.008 |
| Ejection fraction post procedure | 33.20% | 33.10% | 0.819 |
| Ejection fraction at 6 m | 44.20% | 33.00% | 0.034 |
| Mechanical complication | 6 (12.0%) | 4 (8.0%) | 0.505 |
| HF hospitalization | 3 (6.0%) | 5 (10.0%) | 0.715 |
| Severe MR | 3 (6.0%) | 3 (6.0%) | 1 |
| Contained rupture | 0 (0.0%) | 1 (2.0%) | 1 |
| Minor bleeding | 1 (2.0%) | 2 (4.0%) | 1 |
| Major bleeding | 0 | 0 | 0 |
| CIN | 14 (28.0%) | 9 (18.0%) | 0.235 |
| Renal replacement therapy | 3 (6.0%) | 3 (6.0%) | 1 |
Abbreviations: MACE: Major Adverse Cardiac Events; MI: Myocardial Infarction; HF: Heart Failure; MR: Mitral Regurgitation; CIN: Contrast Induced Nephropathy
Table 3: Procedural details and end points.
CIN occurred in 14 patients in complete revascularization group and 9 patients in the culprit-only group and were managed successfully, three patients in each group required hemodialysis. During the follow up period, there were no differences in procedure related complications, stroke, or major bleeding between the two groups, only 3 cases experienced puncture site hematoma (1 case from the culprit-only group and 2 cases from the complete revascularization group) and were treated successfully (Table 3).
Discussion
In this randomized trial, we compared culprit-lesion only PCI with immediate multivessel PCI in STEMI patients with cardiogenic shock and multivessel coronary artery disease excluding CTO lesion. We demonstrated that total revascularization resulted in a significantly lower MACE rate at 6 months follow up than when only the IRA was treated, with no difference in the rates of CIN or renal replacement therapy.
The milestone SHOCK trial [18] reported that a strategy of early complete revascularization resulted in 6-year higher survival rates compared with initial medical stabilization in patients with acute myocardial infarction complicated by cardiogenic shock. However, in the CULPRIT-SHOCK trial [13] which have been recently published, patients with cardiogenic shock and MVD at index angiography were randomized to receive either culprit vessel revascularization (during index procedure with possible staged revascularization) or single-stage MV PCI. The use of intraaortic balloon pump and mechanical support device use was left at operator’s discretion. The 30-day risk of a composite of death or severe renal failure leading to renal replacement therapy was lower among those who initially underwent PCI of the culprit lesion-only than among those who underwent immediate MV PCI, but no difference in mortality between both strategies at 1 year [19]. Following these results, the guidelines noted that routine revascularization of non-IRA lesions is not recommended in cardiogenic shock during primary PCI. However, one potential limitation of this study is advocating attempts at CTO revascularization in the acute setting, which is usually performed only with evidence of ischemia and viability demonstrated in the CTO territory and consuming more contrast and more radiation causing more incidence of CIN and more post procedural complications. Another strong criticism is the fact that >50% were NSTEMI.
The primary end point of the CULPRIT-SHOCK, of a composite of death or renal-replacement therapy was significantly lower in the culprit-lesion-only PCI group when compared to multi-vessel PCI group (45.9% vs. 55.4%, p=0.01). This can probably be attributed to higher amount of dye as a result of the complicated nature of intervention among chronically occluded vessels resulting in a higher incidence of complications.
The KAMIR-NIH (Korea Acute Myocardial Infarction Registry- National Institutes of Health) registry [20] is a prospective registry that included 659 patients with STEMI, multivessel disease and cardiogenic shock. The study concluded that at 30 days follow up, the cardiac mortality was much lower in the complete revascularization group with significant statistical difference (14.3% vs. 26.1%, p=0.004). At 1 year follow up, the all-cause mortality was lower in multivessel PCI arm (21.3% vs. 31.7%, p=0.001). However, the study did not provide data about the procedure time, total radiation dose, amount of contrast dye used and occurrence of contrast-induced nephropathy. Retrospective sub-analysis of KAMIR registry evaluating 510 STEMI patients with cardiogenic shock and evidence of MV CAD at angiography revealed reduction of early mortality with complete revascularization. Similarly, a prospective observational study of 266 STEMI patients with cardiogenic shock showed improved 6-month survival associated with single-stage MV PCI strategy [21].
Our study showed that a complete revascularization strategy among STEMI patients with cardiogenic shock and multivessel disease excluding CTO might be of benefit in reducing future adverse events including mortality. It is important to note that the finding of lower mortality could be attributed to lack of adequate power and should be considered hypothesis generating. Accordingly, a larger randomized trial focusing on STEMI patients only and excluding CTO lesions might be considered.
Conclusion
In STEMI patients with cardiogenic shock and MVD, complete revascularization reduced the risk of mortality and total MACE when compared with culprit vessel only PCI, when excluding CTO lesions.
Impact on daily practice
This study is trying to address the management of a special group of STEMI patient with high risk of mortality and morbidity to find the best approach to minimize the rates of complications, improving survival with good safety profile.
Limitations
The current study is a relatively small, relatively short follow up period. However it investigated a debatable regimen of management of a special group of STEMI patients which are at higher risk of MACE.
Funding
The authors did not receive any specific funding for this work.
Conflict of Interest
All authors declare that they have no conflict of interest.
References
- Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 39(2): 119-177 (2018).
- Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: A quantitative review of 23 randomised trials. Lancet. 361(9351): 13-20 (2003).
- Huynh T, Perron S, O'Loughlin J, et al. Comparison of primary percutaneous coronary intervention and fibrinolytic therapy in ST-segment-elevation myocardial infarction: Bayesian hierarchical meta-analyses of randomized controlled trials and observational studies. Circulation. 119(24): 3101-3109 (2009).
- Muller DW, Topol EJ, Ellis SG, et al. Multivessel coronary artery disease: A key predictor of short-term prognosis after reperfusion therapy for acute myocardial infarction. Thrombolysis and Angioplasty in Myocardial Infarction (TAMI) study group. Am Heart J. 121: 1042-9 (1991).
- Toma M, Buller CE, Westerhout CM, et al. Non-culprit coronary artery percutaneous coronary intervention during acute ST-segment elevation myocardial infarction: Insights from the APEX-AMI trial. Eur Heart J. 31(14): 1701-1707 (2010).
- Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 369(12): 1115-1123 (2013).
- Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: The CvLPRIT trial. J Am Coll Cardiol. 65(10): 963-972 (2015).
- Engstrøm T, Kelbæk H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3—PRIMULTI): An open-label, randomised controlled trial. Lancet. 386(9994): 665-671 (2015).
- Elgendy IY, Huo T, Mahmoud A, et al. Complete versus culprit-only revascularization in patients with multi-vessel disease undergoing primary percutaneous coronary intervention: A meta-analysis of randomized trials. Int J Cardiol. 186: 98-103 (2015).
- Bangalore S, Toklu B, Wetterslev J. Complete versus culprit-only revascularization for ST-segment-elevation myocardial infarction and multivessel disease: A meta-analysis and trial sequential analysis of randomized trials. Circ Cardiovasc Interv. 8(4): e002142 (2015).
- Hamza M, Mahmoud N, Elgendy IY. A Randomized trial of complete versus culprit-only revascularization during primary percutaneous coronary intervention in diabetic patients with Acute ST Elevation myocardial infarction and multi vessel disease. J Interv Cardiol. 29(3): 241-247 (2016).
- Sorajja P, Gersh BJ, Cox DA, et al. Impact of multivessel disease on reperfusion success and clinical outcomes in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Eur Heart J. 28(14): 17090-1716 (2007).
- Thiele H, Desch S, de Waha S, Acute myocardial infarction in patients with ST-segment elevation myocardial infarction: ESC guidelines 2017. Herz. 42(8): 728-738 (2017).
- Navarese EP, De Servi S, Politi A, et al. Impact of primary PCI volume on hospital mortality in STEMI patients: Does time-to-presentation matter? J Thromb Thrombolysis. 32(2): 223-231 (2011).
- Sousa-Uva M, Neumann FJ, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur J Cardiothorac Surg. 55(1): 4-90 (2019).
- Makris K, Spanou L. Acute Kidney Injury: Definition, pathophysiology and clinical phenotypes. Clin Biochem Rev. 37(2): 85-98 (2016).
- Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation. 123(23): 2736-2747 (2011).
- Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med. 341(9): 625-634 (1999).
- Thiele H, Akin I, Sandri M, et al. One-year outcomes after PCI strategies in cardiogenic shock. N Engl J Med. 379(18):1699-1710 (2018).
- Park HW, Kang MG, Kim K, et al. Long-term prognosis and clinical characteristics of patients with newly diagnosed diabetes mellitus detected after first acute myocardial infarction: From KAMIR-NIH Registry. Korean Circ J. 48(2): 134-147 (2018).
- Lee JM, Rhee TM, Hahn JY, et al. Multivessel percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction with cardiogenic shock. J Am Coll Cardiol. 71(8): 844-856 (2018).