Mini Review - Clinical Investigation (2020) Volume 10, Issue 3
Rationale for a randomized, placebo-controlled, Phase 2 study of Rejuveinix (RJX) in COVID-19 patients with acute lung injury and hypoxemic respiratory failure
Submitted: 11 June 2020; Accepted: 18 June 2020; Published online: 24 June 2020
Abstract
New drug products that can effectively improve the ability of supportive care to reduce the risk of ARDS or its mortality rate in COVID-19 patients are urgently needed. Rejuveinix (RJX) is a formulation of physiologically compatible active ingredients, including several vitamins, such as ascorbic acid, cyanocobalamin, thiamine hydrochloride, riboflavin 5' phosphate, niacinamide, pyridoxine hydrochloride, calcium d-pantothenate that exhibited promising anti-ARDS activity in animal models of sepsis and ARDS as well as clinical studies in patients with sepsis, septic shock, and ARDS. RJX could favorably affect the patient journey in COVID-19 at multiple stages, including (i) prevention of progression of mild disease, (ii) faster resolution of ARDS, prevention of multi-organ failure, and reduction of case mortality during respiratory failure, and (iii) improved long-term healing after recovery. Here we are reporting the critical design elements for a two-cohort, two-part placebo-controlled, and double-blind Phase II study of RJX in COVID-19 patients.
Keywords
COVID-19 • SARS-CoV-2 • Acute respiratory distress syndrome • Rejuveinix
Introduction
COVID-19 patients are at risk of developing Acute Respiratory Distress Syndrome (ARDS) with a high fatality rate especially if they are older and have comorbidities [1-6]. Proinflammatory cytokines, especially Interleukin-6 (IL-6), contribute to the development, progression, and severity of ARDS as well as its complications [2,6]. Immunocompromised COVID-19 patients such as patients with cancer, AIDS, organ transplant recipients, as well as patients receiving immune-suppressive therapies for an inflammatory disorder (e.g. inflammatory bowel disease, autoimmune diseases) receiving chemotherapy are at an increased risk for developing fatal ARDS and multi-organ failure as a result of severe viral sepsis caused by SARS-CoV-2. Despite the implementation of the best available supportive care measures, the fatality rate for COVID-19 associated ARDS remains high [7]. Therefore, new drug products that can effectively improve the ability of supportive care to reduce the risk of ARDS or its mortality rate in COVID-19 patients are urgently needed.
Rejuveinix (RJX) [8] is a formulation of several vitamins, including ascorbic acid, cyanocobalamin, thiamine hydrochloride, riboflavin 5’ phosphate, niacinamide, pyridoxine hydrochloride, calcium d-pantothenate that exhibited promising anti-ARDS activity in animal models of sepsis and ARDS as well as clinical studies in patients with sepsis, septic shock and ARDS [9]. Notably, RJX exhibited potent and dose-dependent activity in the LPS-GalN model of ARDS in mice. RJX-treated mice had a significantly improved survival outcome after being challenged with an otherwise invariably fatal dose of LPS-GalN. RJX could favorably affect the patient journey in COVID-19 at multiple stages, including (i) prevention of progression of mild disease, (ii) faster resolution of ARDS, prevention of multiorgan failure, and reduction of case mortality during respiratory failure, and (iii) improved long-term healing after recovery.
The clinical tolerability of RJX was confirmed in a recently completed double-blind, placebo-controlled Phase 1 dose-escalation study in healthy volunteers (ClinicalTrials.gov Identifier: NCT03680105). It is our central working hypothesis that RJX will prevent the progression of the disease from mild to moderate or severe in hospitalized COVID-19 patients. We further postulate that RJX may shorten the time to resolution of the hypoxemic respiratory failure and reduce the case mortality rate when used in combination with standard of care in COVID-19 by eliminating the contribution of Interleukin-6 (IL-6) and Tumor Necrosis Factor-Alpha (TNF-α) to Cytokine Release Syndrome (CRS) and Acute Respiratory Distress Syndrome (ARDS) and thereby reducing acute lung injury and lung inflammation in COVID-19 patients with imminent or early-stage ARDS. These observations and considerations have provided the medical-scientific rationale for our clinical development strategy for RJX and a clinical study in COVID-19 patients.
Clinical Development Strategy for COVID-19 Patients
Based on the clinical safety profile and PK of RJX in healthy volunteers and the aforementioned medical-scientific rationale, we have designed our study in COVID-19 patients as a two-cohort, twopart study, placebo-controlled, double-blind Phase II study. The two cohorts in this study will be comprised of hospitalized COVID-19 patients allocated to one of the two cohorts based on their stage of disease according to the 8-point ordinal scale. Cohort 1 in the study will be hospitalized COVID-19 patients without hypoxemia who are either not receiving any oxygen therapy or are receiving supplemental oxygen via mask or nasal prongs (namely, clinical status score 4 or 5 on an 8-point ordinal scale). Cohort 1 patients will be required to have the following high risk characteristics: (i) Age ≥ 65 years AND type 2 diabetes, or (ii) abnormal blood tests with CRP >50 mg/L PLUS at least 1 of the following biomarkers: (a) D-dimer >1,000 ng/mL, (b) Ferritin >500 mcg/L, (c) High sensitivity cardiac troponin >2 × Upper Limit of Normal (ULN), (c) Lactate Dehydrogenase (LDH) >245 U/L. By comparison, Cohort 2 will be comprised of hospitalized COVID-19 patients with hypoxemia without ARDS who are receiving either NIPPV or high flow oxygen (namely, clinical status score 3 on 8-point ordinal scale).
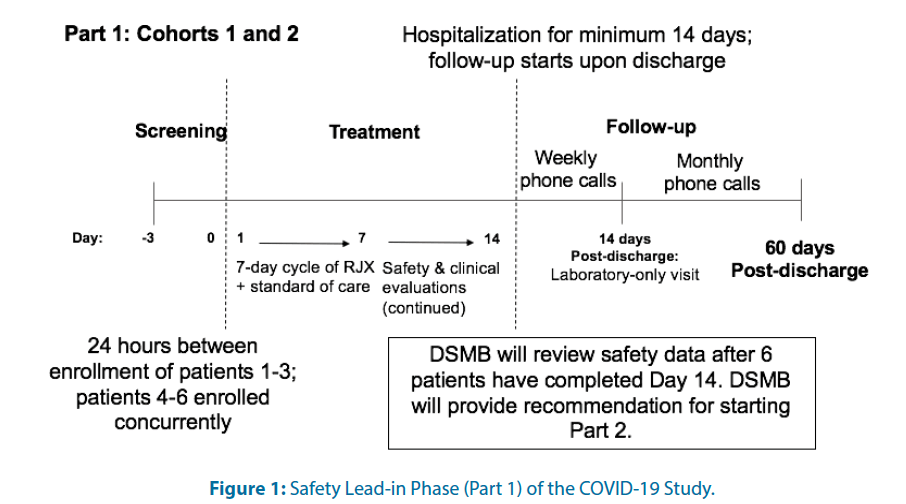
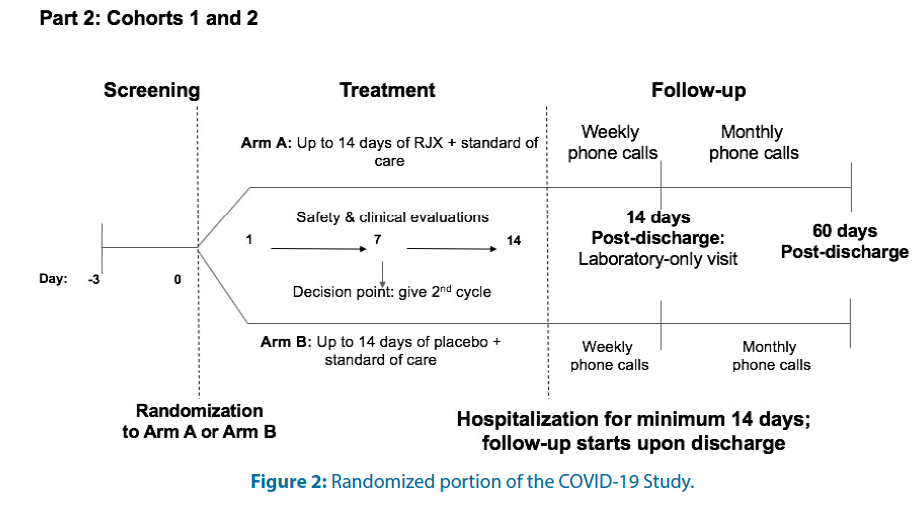
For each cohort, there will be an open-label Safety Lead-in (Part 1) Figure 1 and a placebo-controlled, randomized; double-blind portion (Part 2) Figure 2. In Part 1 and the active treatment arm of Part 2 for both cohorts, RJX will be administered as a 40 ± 10 min IV infusion daily for seven days.
The primary objective in Part 1 of this study is to evaluate the safety and tolerability of RJX when used in combination with standard of care in hospitalized COVID-19 patients who have certain high-risk features but no hypoxemia (Cohort 1) or patients who have hypoxemia without ARDS (Cohort 2). Patients in Part 1 are allowed to receive only one, 7-day cycle of RJX. Eligible patients will be entered in a staggered fashion with a minimum of 24 hours between the first 3 patients in each cohort. If a patient develops a DLT or dies within 24 hours of dosing, no new patient will be entered until the case has been fully reviewed by the DSMB. If a patient develops any Treatment-Emergent Adverse Event (TEAE) that is a Grade 3 or Grade 4 Adverse Event (AE) related or possibly related to RJX, the Medical Monitor and investigator will review the case and approve the enrollment of the next patient if medically appropriate. A safety follow up period will begin at Day 14, or when treatment is discontinued and will continue for approximately 60 days post-discharge. An independent Data Safety Monitoring Board (DSMB) will oversee subject safety during the study. Specific withholding and discontinuation criteria will be used for risk mitigation to maximize patient safety regarding the continuation of RJX for the full 7-day cycle for any patient who develops a Dose- Limiting Toxicity (DLT) or a Grade 3/4 AE that is related or possibly related to RJX. Treatment with RJX will be discontinued for any patient in Cohort 2 who progresses to moderate ARDS according to the Berlin criteria [10]. Patients will be monitored for AEs throughout the study. Treatment/standard of care will be discontinued upon resolution of the patient’s respiratory distress and discharge from the hospital. For patients who are discharged before Day 14, follow-up phone calls (3 × weekly) will be used until Day 14 to collect safety data. After Day 14, patients will move into the safety follow-up period as described above. Part 2 will be initiated if RJX is not associated with drug-related SAE in more than 1 of 6 patients during the 7 day treatment period in Part 1.
In Part 2, an Interactive Voice Response/ Interactive Web Response (IVRS/IWR)-based randomization procedure will be applied to assign patients to 1 of the 2 treatment groups. Dynamic randomization will be used to allow an increase in the recruitment target to prevent an underpowered trial. Patients will be stratified based on their age, prior therapy, and absolute lymphocyte count. The primary objective in Part 2 of this study is to evaluate the clinical activity of RJX when used in combination with standard of care in patients hospitalized with COVID-19 who have certain high-risk features but no hypoxemia (Cohort 1); patients who have hypoxemia without ARDS (Cohort 2). The primary endpoints of this study are: (i) For Part 1, Cohorts 1 and 2: Cumulative incidence of Dose-Limiting Toxicities (DLTs) and drug-related Serious Adverse Events (SAEs); (ii) For Part 2, Cohort 1: Time to progression to severe disease with status change on 8-point ordinal scale from a clinical status score of 4 or 5 to a clinical status score of 3, 2, or 1) and (iii) For Part 2, Cohort 2: Time to resolution of respiratory failure, with status change on 8-point ordinal scale from a clinical status score of 3 to a clinical status score of ≥ 4.
In both Part 1 and Part 2, patients will remain hospitalized for a minimum of 14 days and will subsequently move into the safety follow-up period of the study after discharge. After Day 14, patients will be followed with weekly follow-up phone calls for two weeks after discharge and then monthly phone calls for approximately 60 days postdischarge to document their survival status, interim hospitalization history, medical care since discharge from the hospital, and oxygen requirements. The follow-up period also includes one laboratory only visit, which will occur 14 days after discharge.
In Part 2, all patients and clinical staff will be blinded to the assigned treatment. Study treatments (RJX and placebo) will be labeled and coded in a manner that protects the blind. The investigator will be ultimately responsible for ensuring that the integrity of the blind is maintained throughout the study and will be required to document and notify the sponsor in the event of any breaking of the blind for any reason.
If an SAE occurs during the study that meets the criteria for expedited reporting (i.e., is a Suspected Unexpected Serious Adverse Reaction), the patient will be unblinded by the contract research organization.
Conclusion
In an emergency situation, the investigator may unblind the study patient’s treatment assignment. When possible, the medical monitor should be notified before unblinding. The investigator should inform the medical monitor of the unblinding if there is no notification before the unblinding. Unblinding procedures will be provided to each site.
References
- Uckun FM. Reducing the fatality rate of COVID-19 by applying clinical insights from immuno-oncology and lung transplantation. Front Pharmacol 11: 796 (2020).
- Uckun FM, Trieu V. Medical-scientific rationale for a randomized, placebo-controlled, phase study of trabedersen/ot-101 in COVID-19 patients with hypoxemic respiratory failure. Ann Pul Crit Care Med 3: 1-9 (2020).
- Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: Observations and hypotheses. Lancet 395: 1517-1520 (2020).
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:4 97-506 (2020).
- Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with Sars-Cov-2 in Singapore. JAMA 323: 1488-1494 (2020).
- Zumla A, Hui DS, Azhar EI, et al. Reducing mortality from 2019-nCoV: Host-directed therapies should be an option. Lancet 395: e35-e36 (2020).
- Uckun FM. Treating severe COVID-19 in cancer patients. Cancer Rep Rev 3: 1-2 (2020).
- Van Wyk H, Van Wyk M, Krebs T, et al. US Patent Application No. 9775798 2017; Composition and methods for the prevention and treatment of cardiovascular diseases - Continuation of U.S. patent application Ser. No. 14/550,677 as a divisional of U.S. patent application Ser. No. 13/055,691, which issued as U.S. Pat. No. 9,101,537. Publication 20170360696.
- Uckun FM, Erwin J, Sahin K, et al., Clinical impact potential of rejuivenix for prevention of fatal acute respiratory distress syndrome (ARDS) and multiorgan failure in covid-19 patients.Clinical Investigation Pre-proof (2020).
- Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med 38: 1573-1582 (2012).