Case Report - Interventional Cardiology (2020) Volume 12, Issue 4
Razer percutaneous coronary intervention (Rotational and Laser atherectomy) for an undilatable coronary lesion with a under deployed stent
Vivek N Kodoth1*, Mark Spence2, Paul W Johnston21Department of Cardiology, The Royal Bournemouth Hospital, Bournemouth, United Kingdom
2The Belfast Health Care Trust, Belfast, Northern Ireland, United Kingdom
- *Corresponding Author:
- Vivek N Kodoth
Department of Cardiology
The Royal Bournemouth Hospital
Bournemouth, United Kingdom
E-mail: vkodoth@nhs.net
Received date: July 07, 2020; Accepted date: July 22, 2020; Published date: July 30, 2020
Abstract
An under expanded stent in a heavily calci€ed lesion is a technical challenge and a therapeutic dilemma. Under expanded stents are associated with instent restenosis, acute and sub-acute thrombosis and residual angina. Excimer laser (ELCA) has been successfully used in such cases and there are isolated reports of the use rotational atherectomy (RA). There are only few published reports where both these modalities were used together. We report a case in which ELCA with RA (Razer), was used successfully for percutaneous coronary intervention (PCI) of an undilatable coronary lesion with an under deployed stent.
Keywords
Coronary Artery; Ischaemia; PCI; IVUS
Case Presentation
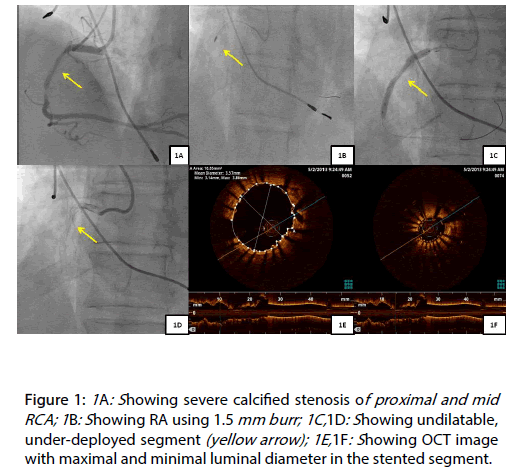
A 65 year old, patient underwent PCI of calcified proximal and mid right coronary artery (RCA) lesions (Figure 1A) [1,2]. Debulking was performed by RA using a 1.5 mm burr (Figure 1B), the lesions were predicated and Resolute Integrity 3.0 mm x 38 mm and 4.0 mm x 15 mm (Medtronic Inc., Minneapolis, USA) stents deployed in an overlapping fashion. At this stage, we noticed that the overlapped segment had not expanded adequately (Figures 1C and 1D) [3]. The unexpanded segment of the stent was post dilated, multiple times with 3.5 mm NC balloon to high pressures (34 atmps) without success. The procedure was stopped at this stage [3,4].
Figure 1: 1A: Showing severe calcified stenosis of proximal and mid RCA; 1B: Showing RA using 1.5 mm burr; 1C,1D: Showing undilatable, under-deployed segment (yellow arrow); 1E,1F: Showing OCT image with maximal and minimal luminal diameter in the stented segment.
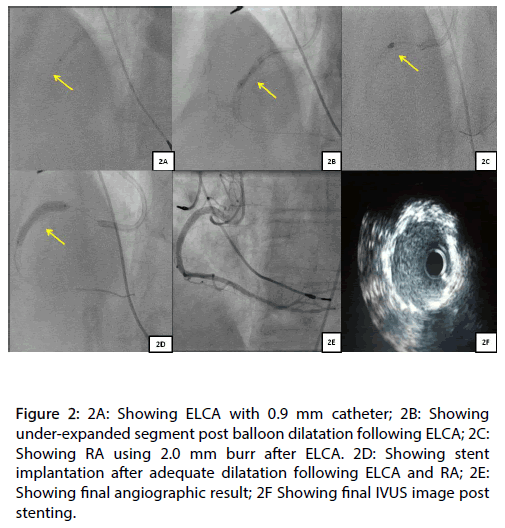
The patient was readmitted electively after one week for PCI with ELCA of the under deployed stented segment. An optical coherence tomography (OCT) demonstrated that the maximal and minimal luminal diameter in the stented segment was 3.57 mm and 1.52 mm, respectively (Figures 1E and 1F). We used 0.9 mm, ECLA catheter with saline flush (X-80, Vitresse, Spectranetics/Advanced Interventional Systems) to modify the under deployed stented segment. After 5, 8 and 12 passes of laser atherectomy catheter (Figure 2A), the lesion was dilated with a 3.0 mm NC balloon, followed by 2.5 mm and 3.5 mm OPN NC balloon to 30 atmps, respectively. All of above attempts did not have any significant impact on the stenosed segment (Figure 2B).
Figure 2: 2A: Showing ELCA with 0.9 mm catheter; 2B: Showing under-expanded segment post balloon dilatation following ELCA; 2C: Showing RA using 2.0 mm burr after ELCA. 2D: Showing stent implantation after adequate dilatation following ELCA and RA; 2E: Showing final angiographic result; 2F Showing final IVUS image post stenting.
At this stage, we decided to perform RA. Initially we used a 2.0 mm burr. However, the 2.0 mm burr would not cross the distal end of the calcified segment (Figure 2C). Eventually, a 1.5 mm burr successfully crossed the stenosis segment. After multiple passes, the 1.5 mm burr was upsized to 2.0 mm burr. A total of 9 minutes of RA was performed. Following RA the stenosed segment was adequately dilated using a 3.0 mm and 3.5 mm NC balloon (Figure 2D). After predilatation the stenosed segment was stented with a Resolute Integrity 4.0 x 30 mm stent and post dilated with a 4.0 mm NC balloon (Figure 2E). No stent constraint was demonstrated following post dilation angiographically or by intravascular ultrasound (IVUS) (Figure 2F).
Discussion
Debulking to facilitate dilation of calcified lesions prior to stent implantation can be achieved usually by using RA, ELCA, intravascular lithotripsy (IVL) or cutting balloon. Deployment of a stent prior to recognising that the lesion is non-dilatable leads to aunexpandable, under-deployed stent. Despite the use of high pressure post dilatation these segments can be highly resistant to balloon dilatation. ELCA has been used in under-deployed stent in an attempt to vaporize the atherosclerotic plaque by the accousto mechanical effect of the rapidly exploding bubble and to facilitate expansion of the under-deployed stented segment [3]. ELCA consists of a thin, flexible fiber-optic catheter connected to an external laser-generating source, with the tip of the catheter system emitting pulses of laser energy [1].
The absorption of the laser energy by blood and saline leads to fast vapour bubble expansion and implosion intra luminally, leading to the fragmentation of superficial fibrocalcific deposits, explosive dilation and vessel expansion [5]. The use of saline infusion at the time of laser pulse delivery has been shown to reduce vessel wall injury due to acoustic damage [6]. ELCA increased lumen by both atheroablation and forced vessel expansion facilitating dilatation of under-deployed stent [7]. However, in this case we could not achieve stent dilatation with ELCA with saline infusion. There are case reports of the use of contrast and high energy ELCA to facilitate expansion of under-deployed stent [5]. The use of contrast leads to larger sized bubbles and may lead to significant vessel injury. Moreover, the stent was deployed one week back and endothelialisation would not have been completed.
There are isolated published reports of the use of RA to abrade the stent and underlying calcification with subsequent drug eluting stent implantation (stentablation) [3,5]. RA leads to differential cutting of the inelastic tissue (calcium, stents) with deflection away from normal elastic tissue. The lumen improvement after RA is due to tissue ablation. Particulate debris of calcium and metal from RA of a deployed stent is generally under 5 to 15 micrometer and is cleared by the reticuloendothelial system. In this case we used ELCA and RA leading to atheroblation, fibrocalcific fragmentation, vessel expansion followed by tissue and stent ablation [3,7]. After ELCA and RA we successfully deployed a drug eluting stent. The procedure was performed without features of ischaemia, dissection, no re-flow or haemodynamic compromised achieving excellent final angiographic and IVUS results.
Conclusion
ELCA with RA (Razer therapy) can be used safely and effectively to facilitate PCI in aunexpandable, underdeployed stent in a calcified segment.
Disclosure
Dr. Vivek N Kodoth, Dr. Mark Spence and Dr. Paul W. Johnston have no conflict of interest in relation to this manuscript.
References
- Rawlins J, Din JN, Talwar S, et al. Coronary intervention with the excimer laser: review of the technology and outcome data. IntervCardiol. 11(1): 27-32 (2016).
- Fernandez JP, Hobson AR, McKenzie DB, et al. Treatment of calcific coronary stenosis with the use of excimer laser coronary atherectomy and rotational atherectomy. IntervCardiol. 2(6): 801-806 (2010).
- Kodoth V, Rana O, Sambu N, et al. Establishing interventional technologies to treat under expanded stents: a role for excimer laser coronary atherectomy. Pak Heart J. 51(01): 82-85 (2018).
- Akin I, Pohlmann S, Christoph A. et al. A different way of coronary lesion preparation: stentablation and rotastenting. Clin Med Insights Cardiol.6: 53–56 (2012).
- Merchant Q, Siddiqui NUR, Rehmat A. Comparison of enteral versus intravenous potassium supplementation in hypokalemia in postcardiac surgery pediatric cardiac intensive care patients: prospective open label randomized control trial (EIPS). BMJ Open.; 4(9): e005124 (2014).
- Deckelbaum LI, Natarajan K, Bittl JA, et al. Effect of intracoronary saline infusion on dissection during excimer laser coronary angioplasty: a randomised trial. J Am CollCardiol.26(5): 1264-1269 (1995).
- Mintz GS, Kovach JA, Javier SP, et al. Mechanisms of lumen enlargement after excimer laser coronary angioplasty an intravascular ultrasound study. Circulation. 92(12): 3408-14 (1995).