Special Report - Imaging in Medicine (2010) Volume 2, Issue 4
Real-time fMRI-based brain computer interfacing for neurofeedback therapy and compensation of lost motor functions
Rainer Goebel†1,2*, Anna Zilverstand1,2* & Bettina Sorger1,2,3*1Department of Cognitive Neuroscience, Maastricht University, Maastricht, The Netherlands
2Maastricht Brain Imaging Centre (M-BIC), Maastricht, The Netherlands
3Coma Science Group, Cyclotron Research Centre, University of Liège, Liège, Belgium
- Corresponding Author:
- Rainer Goebel
Netherlands Institute for Neuroscience
an institute of the Royal Netherlands Academy of Arts & Sciences (KNAW)
Amsterdam, The Netherlands
Tel: +31 343 884 014
Fax: +31 433 884 125
E-mail: r.goebel@maastrichtuniversity.nl
Abstract
Real-time functional MRI (rt-fMRI) allows for brain–computer interfaces based on hemodynamic brain signals and opens up various novel clinical applications. For example, rt-fMRI-based neurofeedback has been suggested as a novel tool for the treatment of neurological and psychopathological disorders. In contrast to conventional offline applications, neurofeedback requires the analysis of functional MRI signals online in order to provide participants with information about their brain activation during an ongoing MRI scan. Recent research supports the idea that an improvement of symptoms of diseases can be achieved if patients are trained with rt-fMRI-based neurofeedback to change their brain activation patterns. rt-fMRI also enables online ‘brain-reading‘ applications that can be exploited to develop alternative communication and control devices for patients with severe motor impairments (e.g., ‘locked-in’ patients). Although other methods, especially electroencephalography-based brain–computer interfacing, have been successfully used in this context, rt-fMRI-based methods may enable robust communication and control in cases where traditional approaches do not provide satisfactory results.
Keywords
brain–computer interface ▪ clinical neuroscience ▪ communication ▪ consciousness ▪ device control ▪ ‘locked-in’ syndrome ▪ motor disability ▪ neurofeedback ▪ real-time functional MRI ▪ self-modulation ▪ self-regulation
Since its invention in the early 1990s, functional MRI (fMRI) has rapidly assumed a leading role among the techniques used to localize brain activation. fMRI is a noninvasive, functional neuroimaging technique that enables the measurement of brain activation in humans and animals in vivo [1]. Generally, neural activation across the whole brain, including deep brain structures, can be measured simultaneously with relatively high spatial resolution in the range of a few cubic millimeters (the smallest resolved unit is called a volume element or voxel). As the functional measurements are continuously repeated (e.g., every 2 s), a time course of brain activation can be recorded while a participant is actively engaged in a cognitive, emotional, sensory or motor task. So far, fMRI is not known to be associated with any health risks, allowing for repeated and frequent scanning of the same participant.
Neuronal activity requires energy and oxygen, which is supplied via capillaries in the immediate vicinity of the neurons. When a particular population of neurons becomes active, several subsequent changes in the vascular system take place. At first, a transient local decrease in the blood oxygenation level is caused by the oxygen uptake of the neurons that is sometimes observable as an ‘initial dip’ in the fMRI response. After a short period of approximately 3 s the increased local neuronal activity also leads to a strong increase in local blood flow. This response of the vascular system to the increased energy demand is called the hemodynamic response. The hemodynamic response not only compensates quickly for the slightly increased oxygen extraction rate but it is so strong that it results in a substantial local oversupply of oxygenated hemoglobin. As oxygenated and deoxygenated hemoglobin have different magnetic properties (susceptibility), the fMRI signal obtained from the particular brain region alters accordingly. This phenomenon was discovered by Ogawa et al. and was labeled as the blood oxygenation level-dependent (BOLD) effect [2]. As a result of its functional principles (measuring the neurovascular response rather than neural activity itself), fMRI presents limited spatial and temporal resolution. Furthermore, it only provides an indirect measure of neural events. However, several studies that combined intracortical recordings and fMRI have demonstrated a tight coupling between neuronal activity, as reflected in local field potentials, and the corresponding BOLD signal [3–6].
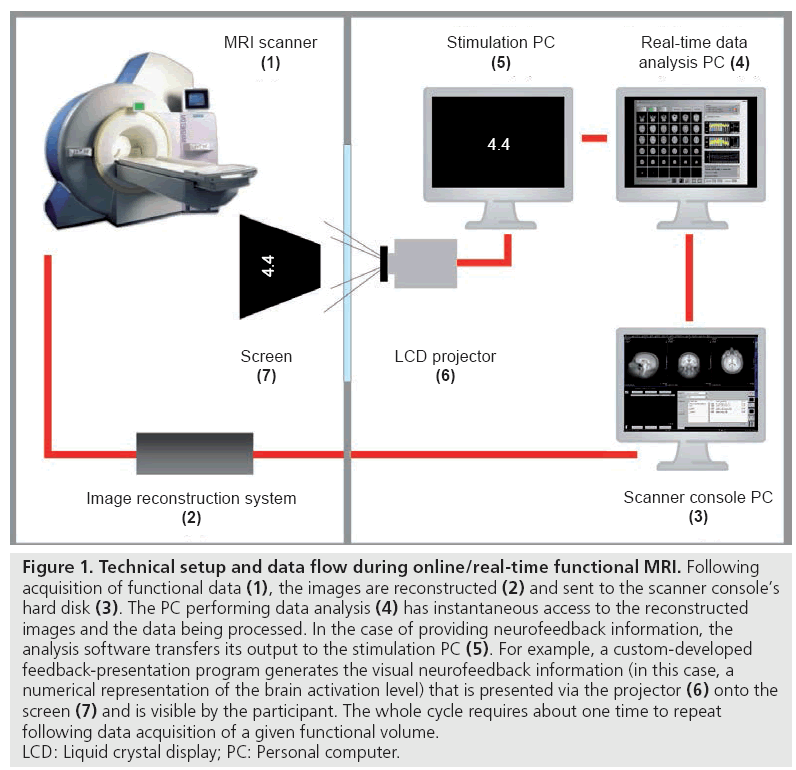
In conventional/traditional fMRI studies, the data obtained are processed offline. Thus, results become available long after the MRI scanning session has ended [7]. However, since the mid-1990s, several research groups have been working on the development of real-time (rt)-fMRI techniques that allow for online data processing and analysis during MRI scanning, immediately after acquisition of a functional image. This became possible through recent technical advances (e.g., in computational power, data acquisition and analysis techniques) that have considerably increased the general data processing speed (for reviews of rt-fMRI methods see [7,8]). After its initial introduction by Cox and colleagues [9], rt-fMRI has substantially improved [10]. Current rt-fMRI procedures include most state-of-the-art data preprocessing and analysis steps of its classical offline counterpart [7]. Figure 1 illustrates the general technical setup and information flow in rt-fMRI experiments.
Figure 1: Technical setup and data flow during online/real-time functional MRI. Following acquisition of functional data (1), the images are reconstructed (2) and sent to the scanner console’s hard disk (3). The PC performing data analysis (4) has instantaneous access to the reconstructed images and the data being processed. In the case of providing neurofeedback information, the analysis software transfers its output to the stimulation PC (5). For example, a custom-developed feedback-presentation program generates the visual neurofeedback information (in this case, a numerical representation of the brain activation level) that is presented via the projector (6) onto the screen (7) and is visible by the participant. The whole cycle requires about one time to repeat following data acquisition of a given functional volume. LCD: Liquid crystal display; PC: Personal computer.
A variety of potential rt-fMRI applications have been proposed [7,8], including assurance of data quality and subject compliance during data acquisition, educational purposes, adaptive fMRI experiments, and brain–computer interfacing, which is the specific topic of this article. Generally, a brain–computer interface (BCI) is used to transform brain activation into specific computer signals, such as commands for external devices. rt-fMRI-based BCIs are currently used in two major ways: one stream of research focuses on the possibility to provide participants with rt-fMRI-based neurofeedback (i.e., information reflecting brain activation in one or more brain regions with the aim to learn to voluntarily modulate it). The second direction deals with the development of alternative communication and control means for patients with severe motor disabilities.
rt-fMRI-based brain–computer interfacing for neurofeedback therapy
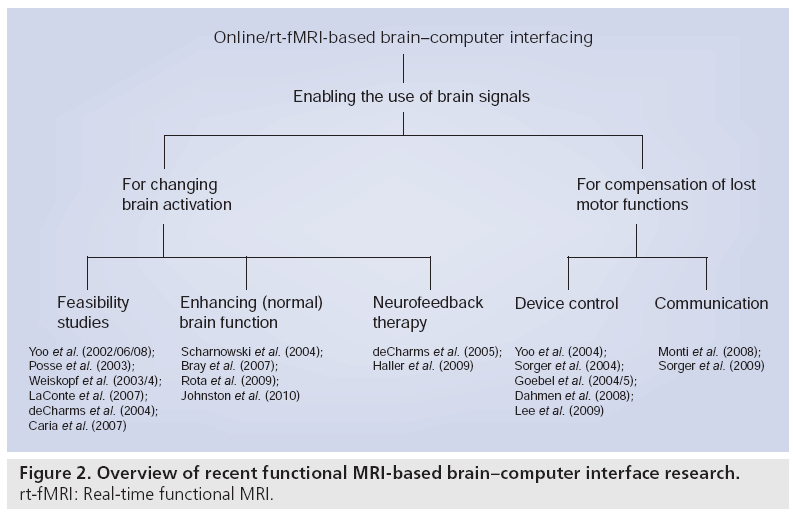
A research area that has emerged in recent years is training and treatment through rt-fMRIbased neurofeedback. Learning based on the highly spatially resolved hemodynamic signal may give way to new neurofeedback applications, complementing older methods as, for example, electroencephalography-based neurofeedback, which use more global brain signals [11]. In the past 10 years, research on rt-fMRI-based neurofeedback has moved from feasibility studies that mainly focused on a ‘proof of concept’ [12–19] towards studies investigating whether normal brain functions can be enhanced in healthy participants [20–23] and towards neurofeedback therapy studies with patients (Figure 2) [24,25]. These first studies showed that participants were able to achieve control over their brain activation [12,14–18], that rt-fMRI-based neurofeedback was a crucial aspect of learning [15–18,20,21,24], and that participants were able to increase their brain activation linearly throughout the training [14,15,17,18,20–22,24,25]. This was remarkable as the total duration of the pure training time of all neurofeedback studies was rather short (6–30 min). In the feasibility studies, the participants were instructed to use simple mental imagery strategies (with the content depending on the target region) as a starting point in the training, and to ‘fine-tune’ this strategy based on the neurofeedback that they received. In the beginning, the neurofeedback target region was defined based on anatomical criteria [14]. In order to optimize the feedback signal, individual target regions were later defined functionally using a task similar to the initial active training strategy.
The success of these feasibility studies led to a second generation of studies with the goal to modify cognition and behavior through neurofeedback- based learning. First studies suggested that participants were able to react faster in a finger sequence task and perform better in a word memory task after they learned to upregulate activation in the supplementary motor area [19,23]. In a second study it was demonstrated that learning to control the activation of finger and toe representations in motor and somatosensory areas could speed up reaction times in a hand and toe button press task [20]. In addition, it was demonstrated that participants improved their accuracy in a prosodic language task by learning to upregulate activation in the right inferior frontal gyrus [21]. A recent study investigated whether learning to control amygdala and insula activation through rt-fMRIbased neurofeedback can influence the scores of healthy participants on a mood-rating scale [22]. This research may be an important first step towards an rt-fMRI-based neurofeedback treatment for mood disorders. It has long been known that the hyperactivation of the amygdala, as well as the deregulation of brain circuits involved in emotion processing play a crucial role in depression [26]. Amygdala hyperactivation has also been linked to some behavioral patterns observed in depressed patients, for example excessive rumination or the intrusion of emotional memories [26].
Importantly, since pathological brain activation patterns and dysfunctional behavioral patterns are linked, ‘changing the brain’ in the right direction may change cognition and behavior accordingly. A technique such as rt-fMRI-based neurofeedback that gives patients direct access to underlying pathological brain activation could, thus, be a novel and immediate route in therapy. The most intriguing question is whether it is possible to learn with rt-fMRI-based neurofeedback to substantially influence the complex cognitive processes involved in neurological and psychopathological disorders. If this would be possible, rt-fMRI-based neurofeedback could enable new forms of therapies for patients.
A study that was recently conducted with tinnitus patients demonstrated that rt-fMRI-based neurofeedback learning may directly impact the patients’ symptoms. While an rt-fMRI-based neurofeedback study with healthy participants had already demonstrated that it is possible to control activation in auditory brain regions during auditory stimulation [16], the study with tinnitus patients demonstrated that this is also possible when tinnitus is the source of a hyperactivation within auditory brain regions [25]. Importantly, a mild decrease of symptoms could be achieved in some patients. Interestingly, in the above mentioned studies, slightly more complex mental strategies were applied than the ones used in the feasibility studies (which mainly implemented mental imagery). In the study with healthy participants, attention manipulation was suggested to control activation in auditory regions during auditory stimulation [16]. In the study with tinnitus patients, participants were advised to use their daily coping strategies [25].
The best-known rt-fMRI-based neurofeedback study with patients is the study by deCharms et al. [24]. To investigate the effects of rt-fMRI-based neurofeedback on pain symptoms, this study included healthy participants as well as refractory chronic pain patients and used several control groups. In healthy participants, the pain was provoked using a hot thermode. All participants were instructed to modulate the activation level in the anterior cingulate cortex through the use of attention-related strategies, reappraisal and emotion-control techniques. This study led to an interesting observation: while patients primarily learned to increase their brain activation during the training, which was accompanied by an increased pain level, a decrease in pain perception was reported immediately after the training [10]. A 40–60% reduction of pain symptoms was achieved in the experimental patient group receiving the neurofeedback, while the pain reduction was significantly lower (10–20%) in the patient control group. Providing patients with the experience that they can cope with an increased level of their symptoms during rt-fMRI-based neurofeedback may be a very potent therapeutic mechanism. However, it should be noted that the authors themselves reported the possibility that the results of the neurofeedback patient group may be also interpreted as “a trained, controllable form of the typically unconscious placebo effect” [24].
rt-fMRI-based brain–computer interfacing for compensation of lost motor functions
Certain medical conditions (e.g., stroke and progressive neurological diseases) can lead to a central nervous system-induced severe motor paralysis. The extreme case of such motor disability, when a patient suffers from a virtually complete motor de-efferentiation, leading to quadriplegia and speech anarthria, has been characterized as the so-called ‘locked-in’ syndrome [27]. The resulting inability to naturally communicate or to control the environment implies severe ethical and practical problems for affected patients, their relatives and carers. Therefore, developing motor-independent communication and control means is of prime importance. One remaining possibility is the employment of BCIs; when the patient’s sensory and cognitive functions and associated brain activation are preserved, so that she/he can intentionally generate distinguishable neuronal responses, a specific coding scheme can be implemented allowing the patient to convey basic thoughts and needs, or to control, for example, electromechanical hardware to a limited extent.
One major goal in this context is to increase the number of different commands that can be generated by the BCI user, measured by the applied brain imaging method, and ‘interpreted’ (decoded) by the BCI system as this would increase communication/control flexibility and efficiency. Since human brain functions can be spatially localized and fMRI provides relatively high spatial resolution, this method provides a great opportunity to increase the degrees of freedom in BCI applications. Separate commands can be encoded by employing different cognitive brain functions. Since different cognitive states evoke spatially distinct brain activation patterns that can be disentangled by fMRI analysis techniques, the encoder’s original intention can be derived.
This possibility has already been tested in several studies with healthy participants. In a pioneering study, participants were asked to perform four different mental tasks (right hand motor imagery, left hand motor imagery, mental calculation and inner speech) that evoke differential brain activation at four distinct brain locations and were interpreted as four predetermined BCI commands (right, left, up and down) [28]. This allowed the participants to virtually navigate through a simple 2D maze by solely using repeatedly performed mental processes. Each movement command was based on the average of three trial repetitions and took 2.15 min. While this study demonstrated feasibility of using rt-fMRI as the basis for BCI, the transferred information rate was very low (~1 bit/min). Recently, the same research group demonstrated that it is also possible to control 2D movements of a robotic arm by using the same principles [29]. A similar approach was followed by Monti and colleagues in an online fMRI study [30]. Participants were asked autobiographical questions that they were supposed to answer with ‘yes’ or ‘no’ by generating two different mental states (motor imagery and spatial navigation). Based on five trial repetitions of the particular mental task, the experimenters were able to infer each participant’s answer correctly.
Note, however, that only relatively few mental tasks seem to be suited to code separate BCI commands using fMRI signals. So far, only four classes of mental tasks have been successfully explored and employed for this purpose: motor imagery, spatial navigation, mental calculation and inner speech [28–33]. Thus, additional approaches for increasing the degrees of freedoms for coding separate information units are desired. Therefore, our research group has tested whether it might be feasible to hemodynamically encode different commands through using discrete BOLD signal levels. By implementing rt-fMRI-based neurofeedback training, participants succeeded to reach three different target levels within one fMRI session [34]. In a follow-up study, extending the training to four fMRI sessions, up to four levels could be accomplished [35]. However, a reliable differentiation was only possible when data were averaged across many trials, which called the practicality of this approach into question.
Recently, our research group has concentrated on the possibility to encode a particular BCI command based on a single mental process rather than several trial repetitions, which were implemented in all aforementioned studies. It had been previously demonstrated by Posse and colleagues that a single internal event can principally be assessed online [13,36]. In one study, we were able to demonstrate that participants can play an analogue of the computer game ‘pong’ simply by adjusting their single-trial brain activation level [37,38]. In another study [39], participants became able to answer multiple-choice questions (with four answer options) by means of singletrial BOLD responses that were intentionally generated by performing a certain mental task within a particular time window. It is worth noting that the procedure took less than 1 min per answer and did not involve any pretraining as required for most electroencephalography-based communication approaches. Furthermore, by further exploiting spatio-temporal BOLD characteristics, we developed a procedure that allows for selecting, hemodynamically encoding and offline decoding any letter of the alphabet based on a single cognitive event [40]. Currently, we are working on setting up an rt-fMRI-based BCI that allows for reliable letter decoding online, thus, enabling participants to communicate any given word during an ongoing MRI session [41].
Brain–computer interfaces based on hemodynamic (vs neuroelectric) brain signals may constitute an important alternative communication means, for example for patient populations so far not benefiting from electroencephalographybased approaches (e.g., complete locked-in syndrome patients) [42].
Future perspective
rt-fMRI-based neurofeedback therapy
While it is clear that research on treatment of patients with rt-fMRI-based neurofeedback is still in its early development, the first results convincingly show that this technique may offer fascinating new possibilities. Readjusting the balance in a deregulated brain system as performed in the study with tinnitus patients seems to be one possibility [25]. Another idea is that we may simply change relevant brain systems permanently by using them over and over again in a certain, productive way [43]. This rehearsal of new behaviors may become more effective if patients can be guided with rt-fMRI-based neurofeedback in this process. Another promising route to therapy may be that patients can learn to mimic desired brain states, or mimic the brain states of others [10]. This implies, of course, that we need to be able to define desirable brain states through fundamental research in cognitive and effective neuroscience. Yet another approach to rt-fMRI-based neurofeedback therapy may be to offer patients the possibility to work in a more goal-oriented fashion during therapy. If we would know the neural correlates of therapeutic success in its final stage, we could give patients more freedom in finding their own way towards this goal. This may eventually lead to the discovery of new coping mechanisms. In general, it can be concluded that rt-fMRI-based neurofeedback can be used to teach patients how to evoke certain mental states through evoking desirable brain states. However, more cognitive and affective neuroscience research will be necessary to define which brain states correlate to desirable mental states.
Since this approach involves a transfer of desirable neural activation states to other participants, further research of advanced brain normalization schemes, such as cortex-based alignment [44,45], is important in order to better relate homologue brain regions across participants’ brains. Before regular clinical use, fMRI-based neurofeedback training needs to be evaluated in larger clinical trials, including appropriate control groups, with the goal to assess its effectiveness alongside conventional treatment approaches. These trial studies will reveal for which diseases and/or for which patients rt-fMRI-based neurofeedback will be the most appropriate treatment method. Besides the aforementioned disorders (treatment of pain, mood disorders and tinnitus), we expect that several psychiatric conditions will benefit from rt-fMRI-based neurofeedback, including particular symptoms of schizophrenia (e.g., reducing the strength of auditory hallucinations), anxiety (e.g., reducing fear during confrontation with anxiety provoking cues), addiction (e.g., reducing craving), autism (e.g., reducing fear for facial expressions) and reduction of antisocial behavior [46]. For the treatment of some disorders it may turn out that the functional coupling between brain regions is more relevant than information about the mean activation level in small regions or larger networks. Thus, in the future it might be promising to modulate functional connectivity through rt-fMRI-based neurofeedback.
rt-fMRI-based communication & control devices
The success of the first rt-fMRI-based communication tools is based on the rather robust taskrelated BOLD signal changes, whole-brain coverage and high spatial resolution of fMRI. These properties have also been recently exploited to diagnose the level of awareness in patients with disorders of consciousness [32,33]. Advances in fMRI data analysis, such as better algorithms for the decoding of mental states, will further strengthen the role of fMRI for the detection of preserved consciousness in vegetative state patients and for developing robust communication tools for patients with severe motor impairments.
The assignment or ‘classification’ of activity patterns evoked by mental tasks to specific computer commands or choice selections has previously been performed mostly using signals from prelocalized regions of interest. An interesting new approach currently under investigation [47] constitutes the use of multivoxel pattern classification algorithms that have been used extensively for offline ‘brain reading’ in recent years [48]. Multivoxel pattern classifiers are adaptive and learn to associate distributed activation patterns with performed mental tasks. Since multivoxel pattern classification algorithms are ‘learning machines’ and more sensitive than univariate (or regions of interest based) analyses [49,50], they may lead to BCIs that adapt to the mental states evoked by participants, especially when they are changing over time. In light of these attractive properties, multivariate pattern classifiers are likely to become an integral component of rt-fMRI-based brain reading systems in the future. However, it is worth noting that generalization to novel brain states requires classifiers trained with a sufficient number of examples before they can assign subsequent brain states to one of the learned mental tasks.
The previous discussion indicates that progress in rt-fMRI-based neurofeedback and communication/ external control applications depends not only on technical and methodological advances but also on progress in cognitive and affective neuroscience. Improved knowledge with regard to relevant brain structures and their connectivity will be helpful for deciding what brain regions or networks to select in order to optimally achieve certain therapeutic effects – ideally more efficiently than when using traditional (e.g., behavioral) treatments. On the other hand, rt-fMRI-based neurofeedback may also serve as an innovative ‘introspective’ tool to learn about the functions subserved by specific brain regions. Since participants fine-tune their initially chosen mental tasks to improve voluntary modulation of signals in a targeted brain region, researchers can learn about the function of these brain structures by asking participants what they were mentally doing.
From stationary rt-fMRI-based to other mobile hemodynamics-based devices
While rt-fMRI offers exciting developments for neurofeedback and communication/control devices, fMRI is an immobile and expensive technology and it would be economically challenging to allow large patient populations to benefit from these innovative tools. However, there is a related optical technology – functional near-infrared spectroscopy (fNIRS) – that measures a signal similar to fMRI [51,52]. Several studies have already indicated that fNIRS might become a promising additional tool for BCI purposes [53–55]. It will be important in future years to further investigate the suitability of fNIRS for neurofeedback and communication/control of external devices since this technique could be used at the patient’s bedside.
While fNIRS is portable and much less expensive than fMRI, it only allows the measurement of activation in brain regions that are close to the surface of the head. fNIRS will, therefore, be better suited for replacing fMRIbased communication tools than fMRI-based neurofeedback tools since the latter usually require feedback from rather deep cortical (e.g., cingulate cortex and insula) and sub-cortical brain regions (e.g., amygdala).
Financial & competing interests disclosure
R Goebel is CEO and Chief Software Developer of Brain Innovation, a company that develops neuroimaging software, including ‘Turbo-BrainVoyager’, a software for real-time functional MRI data analysis that has been used in several published functional MRI neurofeedback studies. All authors gratefully acknowledge the support of the BrainGain Smart Mix Programme of The Netherlands Ministry of Economic Affairs and The Netherlands Ministry of Education, Culture and Science. B Sorger was also partially funded by The Netherlands Organization for Scientific Research (NWO, Rubicon program, project number: 446–09–010). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
• • of considerable interest
- Bandettini PA, Birn RM, Donahue KM: Functional MRI: background, methodology, limits, and implementation. In: Handbook of Psychophysiology. Cacioppo JT, Tassinary LG, Berntson GG (Eds). University Press, Cambridge, UK 978–1014 (2000).
- Ogawa S, Lee TM, Kay AR, Tank DW: Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl Acad. Sci. USA 87(24), 9868–9872 (1990).
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A: Neurophysiological investigation of the basis of the fMRI signal. Nature 412(6843), 150–157 (2001).
- Logothetis NK: The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 357(1424), 1003–1037 (2002).
- Logothetis NK: The underpinnings of the BOLD functional magnetic resonance imaging signal. J. Neurosci. 23(10), 3963–3971 (2003).
- Shmuel A, Augath M, Oeltermann A, Logothetis NK: Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat. Neurosci. 9(4), 569–577 (2006).
- Weiskopf N, Sitaram R, Josephs O et al.: Real-time functional magnetic resonance imaging: methods and applications. Magn. Reson. Imaging 25(6), 989–1003 (2007).
- Bagarinao E, Nakai T, Tanaka Y: Real-time functional MRI: development and emerging applications. Magn. Reson. Med. Sci. 5(3), 157–165 (2006).
- Cox RW, Jesmanowicz A, Hyde JS: Real-time functional magnetic resonance imaging. Magn. Reson. Med. 33(2), 230–236 (1995).
- deCharms RC: Applications of real-time fMRI. Nat. Rev. Neurosci. 9(9), 720–729 (2008).
- Schneider F, Backes V, Mathiak K: Brain imaging: on the way toward a therapeutic discipline. Eur. Arch. Psychiatry Clin. Neurosci. 259(Suppl. 2), S143–S147 (2009).
- Yoo SS, Jolesz FA: Functional MRI for neurofeedback: feasibility study on a hand motor task. Neuroreport 13(11), 1377–1381 (2002).
- Posse S, Fitzgerald D, Gao K et al.: Real-time fMRI of temporolimbic regions detects amygdala activation during single-trial self-induced sadness. Neuroimage 18(3), 760–768 (2003).
- Weiskopf N, Veit R, Erb M et al.: Physiological self-regulation of regional brain activity using real-time functional magnetic resonance imaging (fMRI): methodology and exemplary data. Neuroimage 19(3), 577–586 (2003).
- deCharms RC, Christoff K, Glover GH, Pauly JM, Whitfield S, Gabrieli JD: Learned regulation of spatially localized brain activation using real-time fMRI. Neuroimage 21(1), 436–443 (2004).
- Yoo SS, O’Leary HM, Fairneny T et al.: Increasing cortical activity in auditory areas through neurofeedback functional magnetic resonance imaging. Neuroreport 17(12), 1273–1278 (2006).
- Caria A, Veit R, Sitaram R et al.: Regulation of anterior insular cortex activity using real-time fMRI. Neuroimage 35(3), 1238–1246 (2007).
- Yoo S, Lee J, O’Leary H, Panych LP, Jolesz FA: Neurofeedback fMRI-mediated learning and consolidation of regional brain activation during motor imagery. Int. J. Imaging Syst. Technol. 18, 69–78 (2008).
- Weiskopf N, Scharnowski F, Veit R, Goebel R, Birbaumer N, Mathiak K: Self-regulation of local brain activity using real-time functional magnetic resonance imaging (fMRI). J. Physiol. Paris 98(4–6), 357–373 (2004).
- Bray S, Shimojo S, O’Doherty JP: Direct instrumental conditioning of neural activity using functional magnetic resonance imaging-derived reward feedback. J. Neurosci. 27(28), 7498–7507 (2007).
- Rota G, Sitaram R, Veit R et al.: Self-regulation of regional cortical activity using real-time fMRI: the right inferior frontal gyrus and linguistic processing. Hum. Brain Mapp. 30(5), 1605–1614 (2009).
- Johnston SJ, Boehm SG, Healy D, Goebel R, Linden DE: Neurofeedback: a promising tool for the self-regulation of emotion networks. Neuroimage 49(1), 1066–1072 (2010).
- Scharnowski F, Weiskopf N, Mathiak K et al.: Self-regulation of the BOLD signal of supplementary motor area (SMA) and parahippocampal place area (PPA): fMRI-neurofeedback and its behavioural consequences. Presented at: 10th Annual Meeting of the Organization for Human Brain Mapping. Budapest, Hungary, 13–17 June 2004.
- deCharms RC, Maeda F, Glover GH et al.: Control over brain activation and pain learned by using real-time functional MRI. Proc. Natl Acad. Sci. USA 102(51), 18626–18631 (2005).
- Haller S, Birbaumer N, Veit R: Real-time fMRI feedback training may improve chronic tinnitus. Eur. Radiol. 20(3), 696–703 (2009).
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K: Depression: perspectives from affective neuroscience. Annu. Rev. Psychol. 53, 545–574 (2002).
- Plum F, Posner JB: The Diagnosis of Stupor and Coma. Davis FA (Ed.). Oxford Univeristy Press PA, USA (1966).
- Yoo SS, Fairneny T, Chen NK et al.: Brain–computer interface using fMRI: spatial navigation by thoughts. Neuroreport 15(10), 1591–1595 (2004).
- Lee JH, Ryu J, Jolesz FA, Cho ZH, Yoo SS: Brain-machine interface via real-time fMRI: Preliminary study on thought-controlled robotic arm. Neurosci. Lett. 450(1), 1–6 (2009).
- Monti MM, Coleman MR, Owen AM: ‘Brain Reading’ with real-time fMRI: communication via detection of brain states in the absence of motor response. Presented at: 14th Annual Meeting of the Organization for Human Brain Mapping. Melbourne, Australia, 15–19 June 2008.
- Boly M, Coleman MR, Davis MH et al.: When thoughts become action: an fMRI paradigm to study volitional brain activity in non-communicative brain injured patients. Neuroimage 36(3), 979–992 (2007).
- Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD: Detecting awareness in the vegetative state. Science 313(5792), 1402 (2006).
- Monti MM, Vanhaudenhuyse A, Coleman MR et al.: Willful modulation of brain activity in disorders of consciousness. N. Engl. J. Med. 362(7), 579–589 (2010).
- Sorger B, Bareither I, Weiskopf N, Rodriguez EF, Birbaumer N, Goebel R: Voluntary modulation of regional brain activity to different target levels based on real-time fMRI neurofeedback. Presented at: 34th Annual Meeting of the Society for Neuroscience. CA, USA, 23–27 October 2004.
- Dahmen B, Sorger B, Sinke CBA, Goebel R: When the brain takes BOLD ’steps’: controlling differential brain activation levels via real-time fMRI-based neurofeedback training. Presented at: 14th Annual Meeting of the Organization for Human Brain Mapping. Melbourne, Australia, 15–19 June 2008.
- Posse S, Binkofski F, Schneider F et al.: A new approach to measure single-event related brain activity using real-time fMRI: feasibility of sensory, motor, and higher cognitive tasks. Hum. Brain Mapp. 12(1), 25–41 (2001).
- Goebel R, Sorger B, Kaiser J, Birbaumer N, Weiskopf N: BOLD brain pong: Self regulation of local brain activity during synchronously scanned, interacting subjects. Presented at: 34th Annual Meeting of the Society for Neuroscience. CA, USA, 23–27 October 2004.
- Goebel R, Sorger B, Birbaumer N, Weiskopf N: Learning to play BOLD brain pong: from individual neurofeedback training to brain– brain interactions. Presented at: 11th Annual Meeting of the Organization for Human Brain Mapping. ON, Canada, 10–14 June 2005.
- Sorger B, Dahmen B, Reithler J et al.: Another kind of ‘BOLD response’: answering multiple-choice questions via online decoded single-trial brain signals. Prog. Brain Res. 177, 275–292 (2009).
- Sorger B, Dahmen B, Reithler J, Goebel R: BOLD communication: when the brain speaks for itself. Presented at: 13th Annual Meeting of the Organization for Human Brain Mapping. IL, USA, 10–14 June 2007.
- Sorger B, Reithler J, Dahmen B, Goebel R: ‘Taking up a dialogue’ with the brain: automated letter decoding from single-trial BOLD responses in real-time. Presented at: 15th Annual Meeting of the Organization for Human Brain Mapping. CA, USA, 18–23 June 2009.
- Birbaumer N, Cohen LG: Brain–computer interfaces: communication and restoration of movement in paralysis. J. Physiol. 579(Pt 3), 621–636 (2007).
- deCharms RC: Reading and controlling human brain activation using real-time functional magnetic resonance imaging. Trends Cogn. Sci. 11(11), 473–481 (2007).
- Fischl B, Sereno MI, Tootell RB, Dale AM: High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp. 8(4), 272–284 (1999).
- Goebel R, Esposito F, Formisano E: Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: from single-subject to cortically aligned group general linear model analysis and selforganizing group independent component analysis. Hum. Brain Mapp. 27(5), 392–401 (2006).
- Birbaumer N: Operant conditioning of the emotional brain in criminal psychopaths. Presented at: 8th Dutch Endo-Neuro-Psycho Meeting. Doorwerth, The Netherlands, 5 June 2009.
- LaConte SM, Peltier SJ, Hu XP: Real-time fMRI using brain-state classification. Hum. Brain Mapp. 28(10), 1033–1044 (2007).
- Haynes JD, Rees G: Decoding mental states from brain activity in humans. Nat. Rev. Neurosci. 7(7), 523–534 (2006).
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P: Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science 293(5539), 2425–2430 (2001).
- Kamitani Y, Tong F: Decoding the visual and subjective contents of the human brain. Nat. Neurosci. 8(5), 679–685 (2005).
- Irani F, Platek SM, Bunce S, Ruocco AC, Chute D: Functional near infrared spectroscopy (fNIRS): an emerging neuroimaging technology with important applications for the study of brain disorders. Clin. Neuropsychol. 21(1), 9–37 (2007).
- Villringer A, Chance B: Non-invasive optical spectroscopy and imaging of human brain function. Trends Neurosci. 20(10), 435–442 (1997).
- Naito M, Michioka Y, Ozawa K, Ito Y, Kiguchi M, Kanazawa T: A communication means for totally locked-in ALS patients based on changes in cerebral blood volume measured with near-infrared light. IEICE Trans. Inf. Syst. E90–D7, 1028–1037 (2007).
- Coyle S, Ward T, Markham C, McDarby G: On the suitability of near-infrared (NIR) systems for next-generation brain–computer interfaces. Physiol. Meas. 25(4), 815–822 (2004).
- Sitaram R, Zhang H, Guan C et al.: Temporal classification of multichannel near-infrared spectroscopy signals of motor imagery for developing a brain–computer interface. Neuroimage 34(4), 1416–1427 (2007).
• First real-time functional MRI (rt-fMRI) neurofeedback study. In contrast to previous applications, feedback was provided for each time point.
• • The most relevant rt-fMRI-based neurofeedback study with patients to date since it demonstrated that rt-fMRI-based neurofeedback is effective in reducing pain perception in patients, and compared the findings with several control groups.
• Demonstrated that fMRI can be used to detect consciousness in patients originally diagnosed with vegetative state by exploiting the robustness and whole-brain high-resolution coverage of fMRI.
• • First research article to introduce a robust possibility to motor-independently communicate basic thoughts and needs based on single-trial fMRI signals. It is of particular note that the fMRI signals could be decoded online (e.g., during the MRI scanning session).