Review Article - Interventional Cardiology (2011) Volume 3, Issue 6
Real-time imaging for transcatheter closure of atrial septal defects
- Corresponding Author:
- Manabu Taniguchi
Division of Cardiac Intensive Care Unit,
Okayama University Hospital, Okayama 700–8558, Japan
Tel: +81 862 357 351
Fax: +81 862 357 353
E-mail: tmnb@md.okayama-u.ac.jp
Abstract
Keywords
atrial septal defect,imaging modality,intracardiac echocardiography,3D,transcatheter closure,transesophageal echocardiography
More than half a century has passed since the first surgical closure of an atrial septal defect (ASD) was performed with cardiopulmonary bypass [1], and it has been the standard treatment for all types of ASDs. The first transcatheter closure of an ASD was reported in 1976 [2]. With further development and improvement of the transcatheter technique and devices, these transcatheter techniques have become an alternative to surgical closure in most patients with secundum type of ASDs [3–5]. Various devices have been developed, however some of them are no longer used. The AMPLATZER® Septal Occluder (ASO) (AGA Medical, Plymouth, MN, USA) is one of the most frequently used devices. More than 200,000 devices have been implanted worldwide since 1996, and excellent outcome of the use of the device including cardiac remodeling and exercise capacity for both pediatric and adult patients, has been reported in short-term and long-term follow-up [5–8].
Remarkable progress has recently been made in noninvasive imaging modalities including echocardiography, computed tomography (CT) and cardiac magnetic resonance (CMR) imaging and they have been absolutely essential for structural heart intervention in such situations as selection of patients, guiding the procedure and post-procedural assessment. Although visualization of high quality images with these advanced imaging modalities is often hard to learn, images obtained by expert hand can facilitate an understanding of the complex morphology of structural heart disease and can contribute to procedural success. Therefore, interventionalists performing structural heart interventions should be familiar with these imaging modalities.
Morphologic variation of ASDs is common [9] and the shape of ASDs change dynamically during the cardiac cycle [10]. Thus, imaging modalities for evaluating ASDs are required to have sufficient temporal and spatial resolutions. Device portability and real-time imaging capability are also indispensable for guiding the procedure in the catheterization laboratory. From these standpoints, echocardiography has a central role in imaging for this treatment [11]. Therefore, in this article we discuss the role of imaging for transcatheter ASD closure using ASO focusing on echocardiography.
Patient selection for transcatheter ASD closure
Candidates for ASD closure have a hemodynamically significant atrial shunt or the presence of right ventricular volume overload and/or clinical symptoms of dyspnea, reduced exercise capacity, or paradoxical embolism [12]. Pulmonary vascular resistance <5 Wood units/m2 and peak pulmonary artery pressure ≤70% of systemic blood pressure are also important conditions for ASD closure [13]. In general, an ASD of more than 10 mm in diameter is considered to account for a significant left-to-right shunt, although ASD can enlarge with time independent of age at diagnosis and body surface area [14]. In addition to these indications, ASD morphology should be suited for transcatheter closure.
▪ Morphological indication
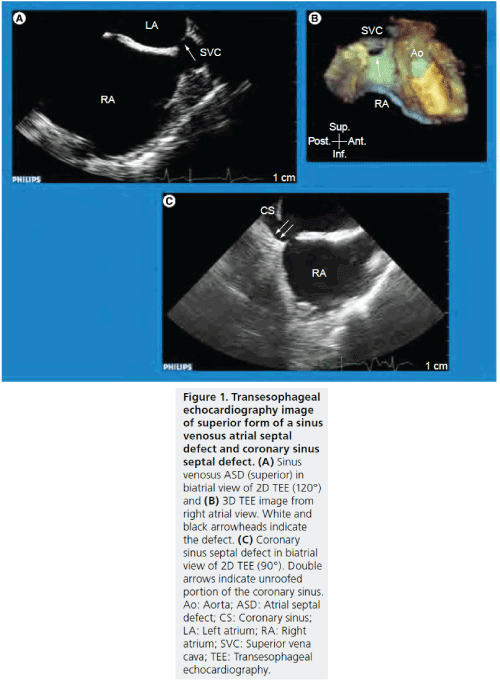
It is well known that morphological variations of ASD are frequent and that appropriate patient selection for transcatheter ASD closure is crucial for a successful procedure [4,9,15].ASDs are grouped into four major categories: ostium primum, ostium secundum, sinus venosus and coronary sinus septal defect (Figure 1). Ostium secundum is the most common type of ASD in which the defect involves the region of the fossa ovalis and this type is indicated for transcatheter ASD closure. Coronary sinus septal defect is a rare type, in which a communication occurs between the coronary sinus and the left atrium as a result of an unroofed coronary sinus. Ostium primum and sinus venosus are indicated for surgical repair. Although surgical repair is the standard treatment for a coronary sinus septal defect, there are some case reports in which transcatheter closure was successful [16,17].
Figure 1: Transesophageal echocardiography image of superior form of a sinus venosus atrial septal defect and coronary sinus septal defect. (A) Sinus venosus ASD (superior) in biatrial view of 2D TEE (120°) and (B) 3D TEE image from right atrial view. White and black arrowheads indicate the defect. (C) Coronary sinus septal defect in biatrial view of 2D TEE (90°). Double arrows indicate unroofed portion of the coronary sinus. Ao: Aorta; ASD: Atrial septal defect; CS: Coronary sinus; LA: Left atrium; RA: Right atrium; SVC: Superior vena cava; TEE: Transesophageal echocardiography.
In patients with ostium secundum, two crucial parameters, the maximal ASD diameter in order to select a device with the appropriate size and the surrounding rim dimensions to optimize the placement of the device, should be assessed to select patients for transcatheter ASD closure. The defect must have a maximal balloon sizing diameter of less than 38 mm. Most ASDs have an ellipsoidal shape and the shape varies during the cardiac cycle [18,19]. The major axis diameter of the defect measured in the phase of ventricular end systole is mandatory for selecting the optimal ASO size, especially in patients undergoing the procedure without balloon sizing [20]. Transcatheter closure of a large ASD with a maximal native diameter >25 mm remains challenging and alternative special techniques for deployment of the device are usually required [21]. With regards to the classification of surrounding rims, although there are some differences among studies [9,22,23], distances from the ASD to the aorta (superoanterior rim), superior vena cava (superoposterior rim), right upper pulmonary vein (posterior rim), inferior vena cava (inferoposterior rim), coronary sinus and atrioventricular valve (inferoanterior rim) are assessed. The definition of rim deficiency varies among different studies: <2mm [24], <3mm [25], <5mm [5,9, 15,21,23,26–28] and <7mm [29], but in many studies any rim was considered deficient if its length was less than 5mm. Deficiency of superoanterior rim is not an absolute contra-indication for the procedure with ASO.
▪ Role of echocardiography
2D and color Doppler transthoracic echocardiography (TTE) can demonstrate the presence of ASDs, chamber dilatation, estimated pulmonary artery pressure, shunt ratio and other coexisting heart disease with high sensitivity and specificity in real time. The use of tissue Doppler imaging can facilitate an understanding of cardiac diastolic function. Impaired cardiac function before ASD closure may lead to the development of congestive heart failure after ASD closure, especially in elderly patients [30,31].However, in terms of accurate assessment of ASD morphology, including measurements of maximal diameter and surrounding rims, 2D TTE sometimes has limited ability to clearly visualize ASDs in detail, especially in adult patients, and precise evaluation using transesophageal echocardiography (TEE) is therefore necessary in most ASD patients (Figure 2) [6,9,15].
Figure 2: Assessment of surrounding rims using 2D transesophageal echocardiography. Surrounding tissue rims and ASD diameter should be measured in the phase of ventricular end systole with multiple cross-sectional TEE plane. Distances from the ASD to the Ao (superoanterior rim; 2D TEE view at 0–30°), superior vena cava (superoposterior rim; 2D TEE view at 90–120°), right upper pulmonary vein (posterior rim; 2D TEE view at 110–120°), inferior vena cava (inferoposterior rim; 2D TEE view at 60–90°), coronary sinus (2D TEE view at 100–120°) and atrioventricular valve (inferoanterior rim; 2D TEE view at 135–150°) are assessed. Ao: Aorta; ASD: Atrial septal defect; CS: Coronary sinus; RUPV: Right upper pulmonary vein; TEE: Transesophageal echocardiography.
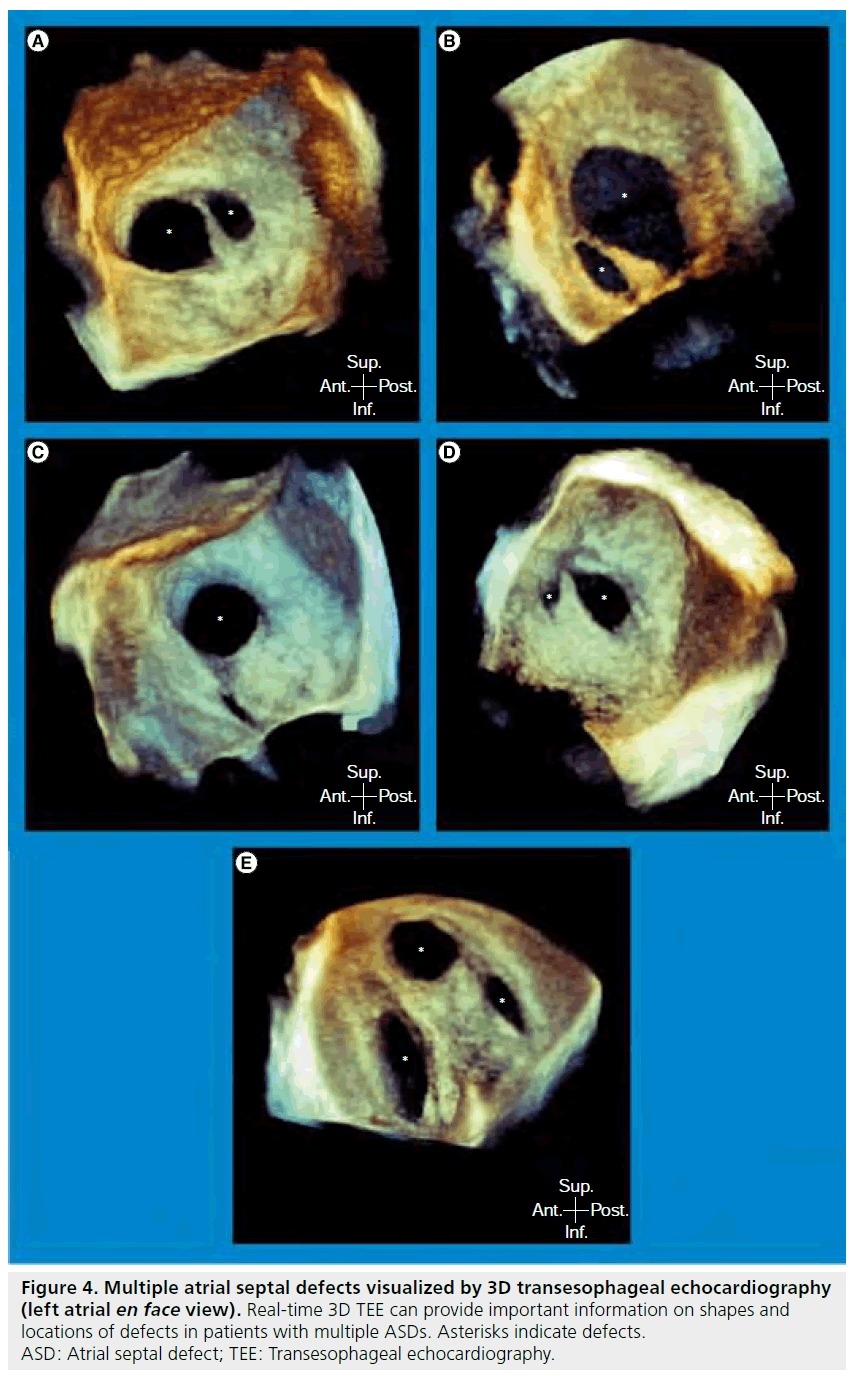
3D echocardiography provides better spatial visualization, and 3D TEE can delineate the 3D structure with high resolution images. Thus, 3D echocardiography offers the ability to improve display and our understanding of complex lesions such as valvular and congenital heart diseases [32–34]. In addition, although 3D echocardiography was initially based on reconstructed images from serial 2D images, which required cumbersome acquisition and time-consuming offline analysis, real-time 3D echocardiography, using a matrix array transducer has recently become available in TTE as well as in TEE [35]. 3D TTE is a promising modality to provide comprehensible en face image of ASD because of its noninvasiveness, low cost, portability and wide availability [36]. 3D TTE can also provide accurate information of ASD morphology, including location, size and surrounding rims, for transcatheter ASD closure both in children [37–39] and adults [38,40]. However, there are several limitations of 3D TTE at present including dependence on skill of the operator, restrictive echo window, especially in elderly patients, echo dropout in the region of the mid portion, which can lead to false diagnosis of a large defect [41] and lower temporal and spatial resolutions than those of 2D TTE. On the other hand, ASD morphology can be recognized with a high quality en face image using 3D TEE [10,18,19,27,42,43]. Realtime 3D TEE allows for evaluation of ASDs of various shapes, especially in patients with45 complex-shaped ASD, such as multiple ASDs (Figure 3 & 4) [44,45].
Figure 3: Various shapes of atrial septal defect visualized by 3D transesophageal echocardiography (left atrial en face view). (A) Sufficient rim, (B) deficient superoanterior rim, (C) deficient inferoposterior rim, (D) deficient superoanterior and inferoposterior rims. Arrowhead indicates the portion of rim deficiency. Ao: Aorta; RUPV: Right upper pulmonary vein
Figure 4: Multiple atrial septal defects visualized by 3D transesophageal echocardiography (left atrial en face view). Real-time 3D TEE can provide important information on shapes and locations of defects in patients with multiple ASDs. Asterisks indicate defects. ASD: Atrial septal defect; TEE: Transesophageal echocardiography.
▪ CMR & CT
Although echocardiography has a central role in patient selection for transcatheter ASD closure in clinical practice, CMR imaging and CT can provide complementary information with a large field of view and low-operator dependency.
CMR imaging has been regarded as the gold standard method for evaluation of right ventricular volumes and function, and CMR has demonstrated favorable right heart remodeling after transcatheter ASD closure [7]. Previous studies showed that CMR can provide an accurate assessment of shunt flow and morphology of an ASD both in pediatric [46,47] and adult patients [48–50] with high temporal and spatial resolutions. Regarding assessment of ASD morphology for transcatheter closure, Thompson et al. demonstrated in 44 adult patients that CMR could identify correctly the type of ASD in 95% of the patients compared with assessment with TEE or intracardiac echocardiography (ICE) [49]. In addition, both CMR and ICE measurements of the defect area correlated with deployed ASO size, especially in patients with ASD area <3 cm2 or extremely eccentric defects, CMR also correlated significantly with ASO size, although ICE did not [49]. In pediatric patients with ASD who have inconclusive TTE results, CMR can provide satisfactorily accurate defect size and rim distances compared with TEE measurements [47]. However, lengthy acquisition time is one disadvantage of using CMR, and general anesthesia or sedation may be required in pediatric patients.
With advancement of CT technology in recent years, electrocardiographic gated multidetector CT allows for evaluation of not only extra-cardiac anatomy, but also intra-cardiac anatomy, with high temporal and spatial resolutions, multiplanar reconstruction capabilities and wide field of view [51]. Quaife et al. demonstrated that ASD area measured by CT angiography with multiplanar reformation images, was strongly correlated with ICE measurements of balloon cross-sectional area calculated as a circle, although CT angiography appeared to slightly overestimate the defect size in larger ASD than with balloon sizing. Especially in patients with a large ASD (>15 mm) or inferior rim deficiency, CT angiography was superior to conventional TEE [24].Although the rapid acquisition time is a major advantage of CT angiography over echocardiography and CMR, ionizing radiation exposure is one of the major limitations of CT angiography, especially in pediatric patients [52]. In addition, use of contrast agent may induce renal failure, especially in elderly patients with impaired renal function.
Real-time imaging during transcatheter ASD closure in the catheterization laboratory
Although conventional transcatheter intervention as represented by percutaneous coronary intervention has been performed mainly under angiographic and fluoroscopic guidance, structural heart interventions including interventions for congenital and valvular heart disease, require more detailed anatomical information with high quality real time imaging during the procedure, because of the complex structure of disease and their complex procedures.
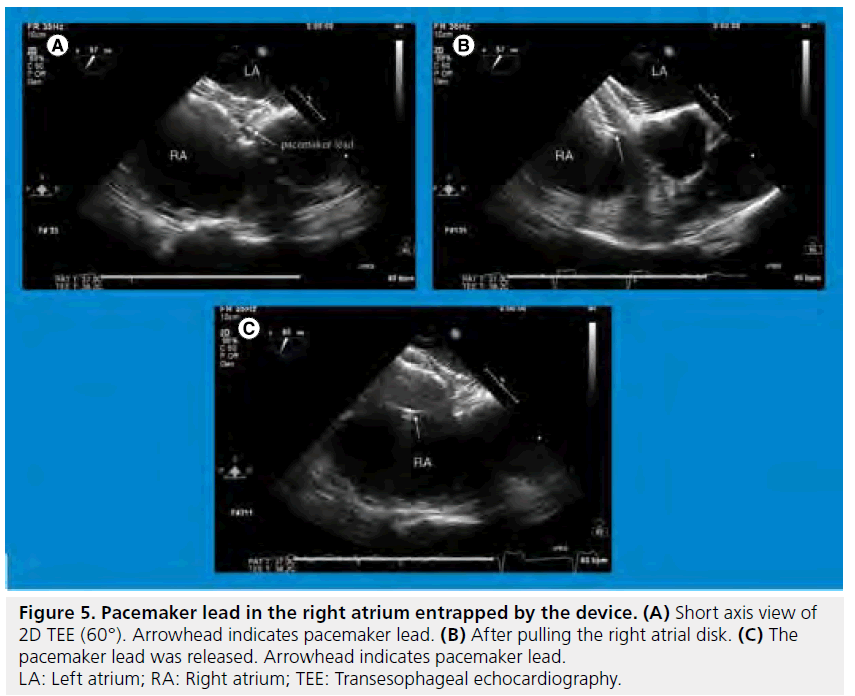
In transcatheter ASD closure, either TEE or ICE and fluoroscopy are more commonly used for guiding the procedure. There are several checkpoints in guidance of the procedure with echocardiography in the catheterization laboratory. Firstly, ASD morphology and other comorbid abnormalities such as valvular heart disease, ventricular function or pulmonary hypertension evaluated in the echocardiographic laboratory preprocedurally should be confirmed just prior to the procedure in the catheterization laboratory. A prominent Eustachian valve can interfere with the procedure with valve tissue becoming trapped on the delivery cable [53].In guidance of the procedure, checking the position of the tip of the guide wire and catheters; measurement of balloon sizing diameter with the stop flow method, using color Doppler if necessary; ensuring the position of the device after deployment and assessment of residual shunt and potential complications before and after releasing the device are required (Supplementary Figure 1). Although transcatheter ASD closure has been established as a reliable and safe procedure [4–6,54] and echocardiography contributes greatly to the procedural result, several complications caused by the procedure have been reported. The most frequent major complication is device embolization. A multicenter retrospective study of surgery for complications of transcatheter ASD closure over a 10 year period (1997–2007) revealed that early emergency operations were required due to device embolization (n = 22), thromboembolism, cerebral ischemia or stroke (n = 4), hemopericardium (n = 5), significant residual shunt (n = 6), early endocarditis (n = 1) and esophageal perforation (n = 1) [55]. In a survey of ASO company-designated proctors,the incidence of ASO embolization was 0.55% (21 embolizations in 3824 device placements). Most of the embolizations occurred because of an inadequate rim or undersized device [56]. In patients with a pacemaker lead in the right atrium, the device can entrap the lead accidentally (Figure 5) [57]. In addition, occurrence of complications was associated with hospital procedural volume [58]. When complications have required surgical management after the procedure, the patients can have a worse prognosis than for those patients who have undergone primary surgical ASD closure [55]. Therefore, meticulous preprocedural evaluation of the ASD and precise guidance of the procedure, including selection of optimal device size using high quality imaging should be mandatory.
Figure 5: Pacemaker lead in the right atrium entrapped by the device. (A) Short axis view of 2D TEE (60°). Arrowhead indicates pacemaker lead. (B) After pulling the right atrial disk. (C) The pacemaker lead was released. Arrowhead indicates pacemaker lead. LA: Left atrium; RA: Right atrium; TEE: Transesophageal echocardiography.
▪ Echocardiography as a tool for guiding the procedure
TEE
TEE can provide high resolution multiplanar images with high frequency capability from the outside of the heart. Echocardiography is mobile, but the size of its console for connecting the TEE probe has conventionally been large. TEE-guided intervention usually requires patients to be under general endotracheal anesthesia because of the length of the interventional procedure and the discomfort associated with TEE and therefore, there has usually not been enough room around the head of the patient due to the anesthetic equipment, C-arms and console of the echocardiography in the catheterization laboratory during TEEguided intervention. Thus, several portable consoles have recently become available and some TEE probes can be connected to these consoles (Figure 6 & Supplementary T able 1).
Figure 6: Portable consoles capable of connecting the transesophageal echocardiography probe. These portable consoles can contribute to making space around the head of the patient during TEE-guided intervention in the catheterization laboratory. Figures were provided by Philips Medical Systems, GE Healthcare and Siemens Healthcare and are used with permission. TEE: Transesophageal echocardiography.
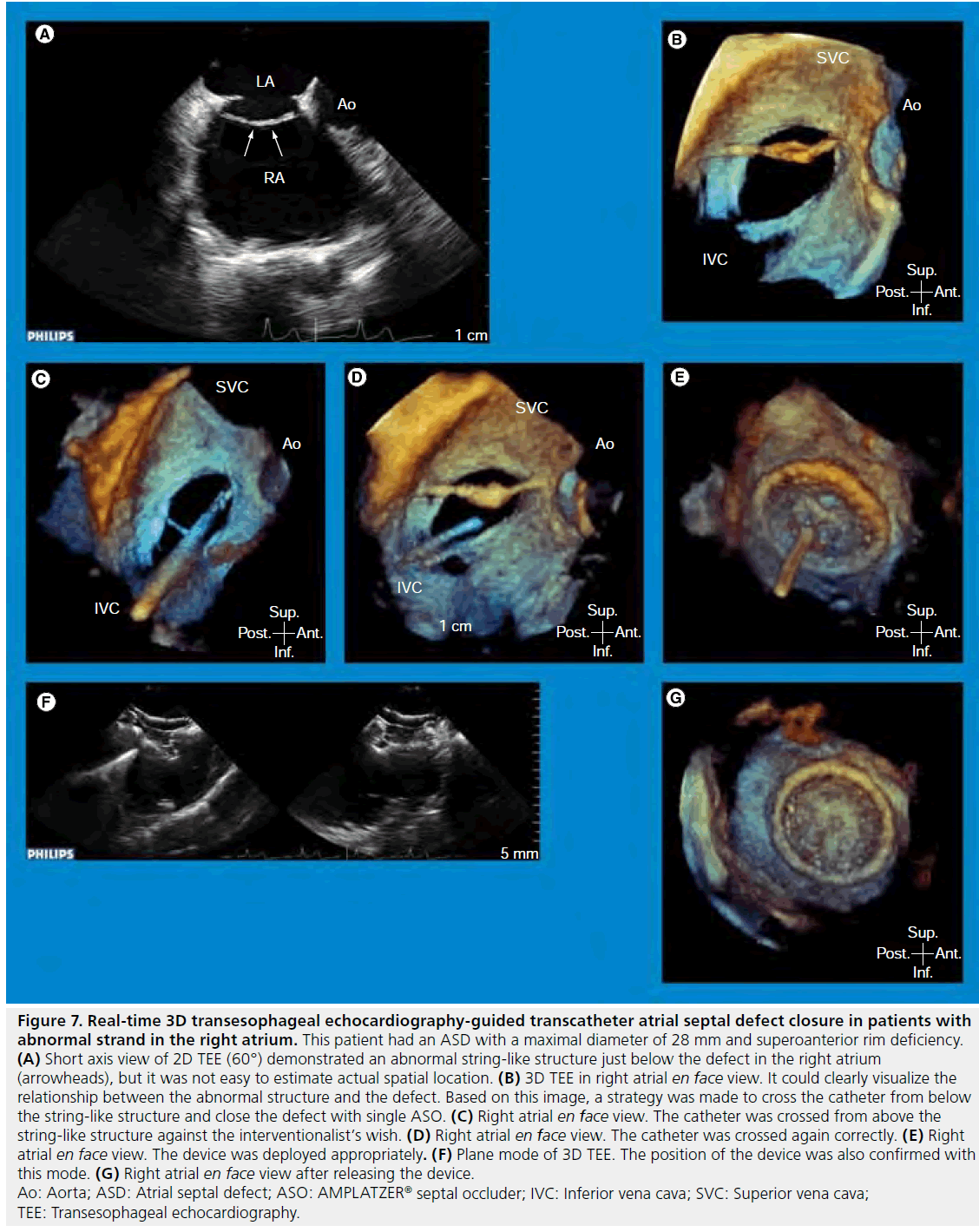
A matrix-array 3D TEE probe (X7-2t; Philips Medical Systems, Andover, MA, USA) in which 2500 elements are used, not only enables easy visualization by providing comprehensible en face 3D images but also provides high quality 2D TEE images with the same probe. Since temporal and spatial resolutions of 3D TEE images have been limited compared with those of 2D TEE images, the ability to switch between 2D and 3D modes freely is important for guiding the procedure in the catheterization laboratory. Comprehensible 3D TEE images contribute to a quick understanding of ASD morphology by interventionalists and echocardiographers. This is highlighted in patients with a complex-shaped ASD especially (Figure 7). Real-time 3D TEE was useful for understanding the complication in our case, with a torn atrial septum during the procedure and it allowed us to choose an appropriate therapeutic strategy [59]. For determining the maximal ASD diameter, correct measurement of which is critical for selecting the optimal device size in patients without balloon sizing or with inadequate balloon sizing diameter due to a large defect or inferoposterior rim deficiency, 3D TEE is more useful than 2D TEE or ICE [27,60,61].
Figure 7: Real-time 3D transesophageal echocardiography-guided transcatheter atrial septal defect closure in patients with abnormal strand in the right atrium. This patient had an ASD with a maximal diameter of 28 mm and superoanterior rim deficiency. (A) Short axis view of 2D TEE (60°) demonstrated an abnormal string-like structure just below the defect in the right atrium (arrowheads), but it was not easy to estimate actual spatial location. (B) 3D TEE in right atrial en face view. It could clearly visualize the relationship between the abnormal structure and the defect. Based on this image, a strategy was made to cross the catheter from below the string-like structure and close the defect with single ASO. (C) Right atrial en face view. The catheter was crossed from above the string-like structure against the interventionalist’s wish. (D) Right atrial en face view. The catheter was crossed again correctly. (E) Right atrial en face view. The device was deployed appropriately. (F) Plane mode of 3D TEE. The position of the device was also confirmed with this mode. (G) Right atrial en face view after releasing the device. Ao: Aorta; ASD: Atrial septal defect; ASO: AMPLATZER® septal occluder; IVC: Inferior vena cava; SVC: Superior vena cava; TEE: Transesophageal echocardiography.
The semi-invasive nature of the TEE procedure is one of the major limitations of TEE-guided interventions. Due to the recent advancement concerning the TEE probe, a miniature-sized multiplane micro TEE probe has also become available. Micro TEE (S8– 3; Philips Medical Systems, Andover, MA) is equipped with M-mode, 2D, color, pulse wave and continuous wave Doppler (Figure 8). Although this probe is obviously useful for small infants, the use of this probe for adult patients has the potential to reduce the patient’s discomfort during the procedure (Figure 9). Stec et al. reported the use of micro TEE probe for 12 patients undergoing atrial fibrillation ablation enable all patients to tolerate the procedure in the supine position without sedation for a mean of 54 ± 17 min [62]. Although image quality and rise in probe temperature should be improved in the future [62], a downsized TEE probe will be a promising tool during guidance of the procedure for both pediatric and adult patients.
Figure 8: Micro transesophageal echocardiography probe. Micro-TEE (S8-3; Philips Medical System, Andover, MA, USA) has a tip length of 18.5 mm, tip width of 7.5 mm and tip height of 5.5 mm. The shaft size is 5.2 mm, which is approximately one half of the shaft size for the standard TEE probe for adults. M-mode, 2D, color Doppler, pulse-wave wave Doppler and continuous wave Doppler are available. TEE: Transesophageal echocardiography.
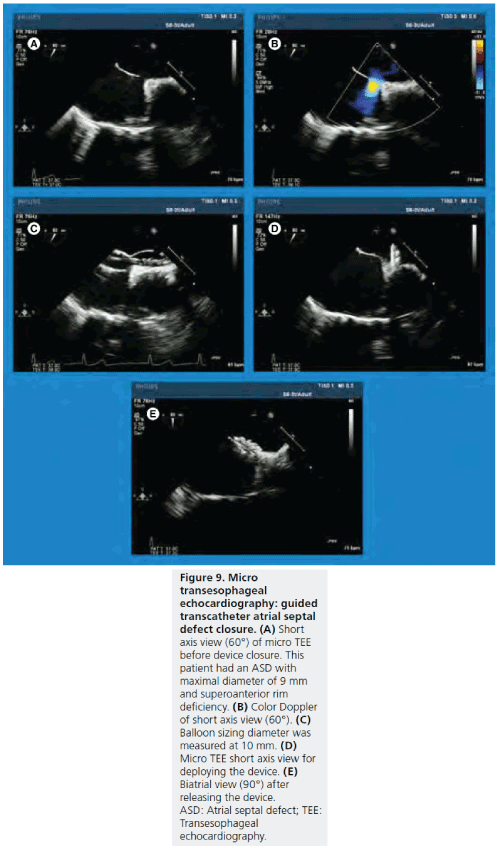
Figure 9: Micro transesophageal echocardiography probe. Micro-TEE (S8-3; Philips Medical System, Andover, MA, USA) has a tip length of 18.5 mm, tip width of 7.5 mm and tip height of 5.5 mm. The shaft size is 5.2 mm, which is approximately one half of the shaft size for the standard TEE probe for adults. M-mode, 2D, color Doppler, pulse-wave wave Doppler and continuous wave Doppler are available. TEE: Transesophageal echocardiography.
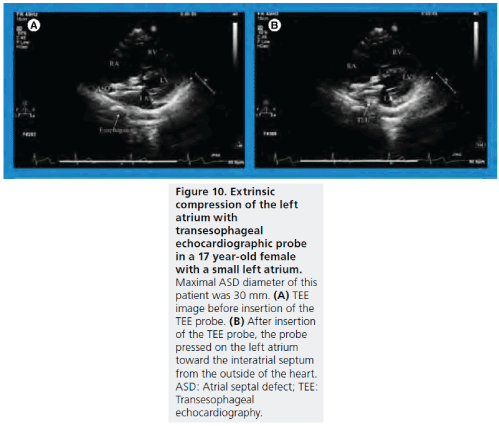
Another limitation of TEE is poor visualization of the inferoposterior rim if it is deficient. In some pediatric and young adult patients with a small left atrium, a large TEE probe may interfere with deployment of the device, due to the TEE probe pressing on the left atrium toward the inter-atrial septum from the outside of the heart (Figure 10) [63].
Figure 10: Micro transesophageal echocardiography probe. Micro-TEE (S8-3; Philips Medical System, Andover, MA, USA) has a tip length of 18.5 mm, tip width of 7.5 mm and tip height of 5.5 mm. The shaft size is 5.2 mm, which is approximately one half of the shaft size for the standard TEE probe for adults. M-mode, 2D, color Doppler, pulse-wave wave Doppler and continuous wave Doppler are available. TEE: Transesophageal echocardiography.
▪ ICE
ICE has recently undergone remarkable developments in image resolution, tissue penetration, catheter size and its manipulations. As a result, it has been used widely in clinical settings for monitoring and guiding in the field of electrophysiology and structural heart interventions [64]. Originally, transcatheter ASD closure was undertaken with TEE guidance; however, excellent ICE imaging, capability of manipulating the ICE catheter for interventionalists and visualization and interpretation of images with local anesthesia have led to the spread of the ICE-guided procedure [64–68].
The ICE-guided procedure does not require an anesthesiologist or, in some instances, even an echocardiographer. These advantages result in shorter fluoroscopic time and procedural time [66,68] and reduced hospital stay compared with the TEE-guided procedure. Phased-array ICE imaging during transcatheter ASD closure can provide equivalent information compared with TEE imaging including information on blood flow with color Doppler. In addition, a previous study demonstrated that success rate, complication rate and rate of complete defect closure during a 6‑months follow-up period were similar in patients who underwent ICEguided procedure and patients who underwent the TEE-guided procedure [69]. In addition, ICE is superior to TEE for visualization of the inferior interatrial septum (Figure 11). An expensive and nonreusable ICE catheter is one of the disadvantages of the ICE-guided procedure, although total costs for ICE and TEE were reported to be comparable because general anesthesia was not necessarily required [70]. Current limitations of ICE other than cost include single plane imaging, sheath size and incapability of 3D imaging (Supplementary T able 2).
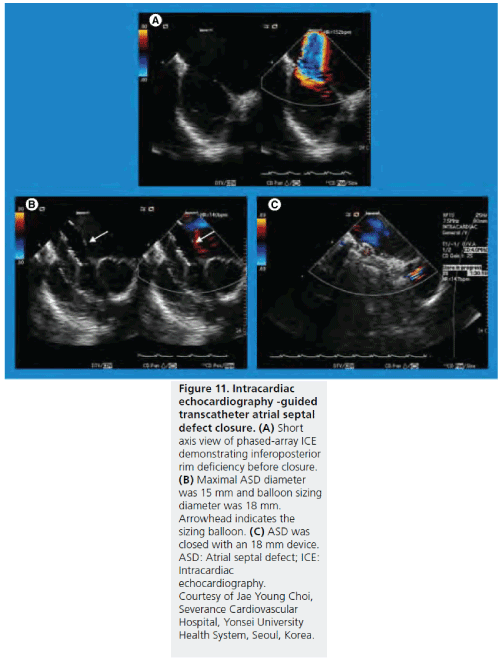
Figure 11: Intracardiac echocardiography -guided transcatheter atrial septal defect closure. (A) Short axis view of phased-array ICE demonstrating inferoposterior rim deficiency before closure. (B) Maximal ASD diameter was 15 mm and balloon sizing diameter was 18 mm. Arrowhead indicates the sizing balloon. (C) ASD was closed with an 18 mm device. ASD: Atrial septal defect; ICE: Intracardiac echocardiography. Courtesy of Jae Young Choi, Severance Cardiovascular Hospital, Yonsei University Health System, Seoul, Korea.
▪ TEE-guided procedure or ICE-guided procedure for ASD?
Each echocardiographic modality has some drawbacks and advantages, both modalities can be performed safely and can provide images of excellent quality for transcatheter ASD closure. Therefore, selection of modality depends on factors of the institution, patients and interventionalists (Box 1).
Future perspective
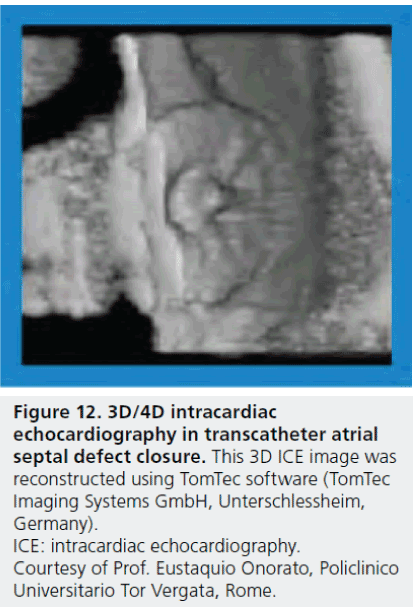
The development of real-time 3D TEE has made a great impact on structural heart interventions, especially in patients who have a complex structure and require a complicated procedure. One of the advantages of the 3D echocardiography-guided procedure, compared with the 2D procedure, is the unnecessity of mental spatial reconstruction of complex morphology of ASD in the catheterization laboratory. 3D ICE will be available in the near future (Figure 12) [71]. Although cost is an issue in a single-use 3D ICE catheter, this development will greatly contribute to the field of structural heart interventions.
Figure 12: 3D/4D intracardiac echocardiography in transcatheter atrial septal defect closure. This 3D ICE image was reconstructed using TomTec software (TomTec Imaging Systems GmbH, Unterschlessheim, Germany). ICE: intracardiac echocardiography. Courtesy of Prof. Eustaquio Onorato, Policlinico Universitario Tor Vergata, Rome.
Meanwhile, miniaturized TEE probe size may enable a transnasal approach in adult patients during the procedure, eliminating the necessity of general anesthesia [72,73]. Although the spatial resolution is still limited in the current available micro TEE probe and new release of the probe is expected, development of a miniaturized TEE probe will solve the problem of cost in the ICEguided procedure.
Conclusion
Transcatheter closure has become the standard treatment for an ASD and its safety and effectiveness are widely accepted in most cases of secundum ASD. However, morphologic variation of ASDs is common and imaging modalities with high diagnostic ability and patient-friendliness are therefore required.
Supplementary data
Supplementary data accompanies this paper and can be found at www.future-science.com/doi/ suppl/10.2217/ICA .11.73.
Acknowledgements
We wish to thank Dr. Eustaquio Onorato (Division of Cardiology, Policlinico Universitario Tor Vergata, Rome, Italy) and Dr. Jae Young Choi (Division of Pediatric Cardiology, Severance Cardiovascular Hospital, Yonsei University Health System, Seoul, Korea) for their help in providing ICE images.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Patient selection for transcatheter atrial septal defect closure
▪▪ Morphologic variation of atrial septal defect (ASD) is common and the shape of ASDs changes dynamically during the cardiac cycle.
▪▪ In general, ASD of more than 10 mm in diameter is considered to account for a significant left-to-right shunt.
▪▪ In terms of accurate assessment of ASD morphology, including measurements of maximal diameter and surrounding rims, transesophageal echocardiography (TEE) is usually necessary, especially in most adult patients.
▪▪ Real-time 3D TEE allows for evaluation of ASDs of various shapes, especially in patients with complex-shaped ASD, such as multiple ASDs.
▪▪ Cardiac MRI and computed tomography can provide complementary information with a large field of view and low-operator dependency.
▪▪ Either TEE or ICE and fluoroscopy are commonly used for guiding the procedure.
▪▪ Several portable consoles have become available recently and some TEE probes can be connected to these consoles.
▪▪ Comprehensible 3D TEE images contribute to a quick understanding of ASD morphology by interventionalists and echocardiographers, especially in patients with complex-shaped ASD.
▪▪ A downsized TEE probe will be a promising tool during guidance of the procedure for both pediatric and adult patients, although the spatial resolution of current available micro TEE probe is expected to evolve.
▪▪ Excellent intracardiac echocardiography (ICE) imaging, capability of manipulating the ICE catheter for interventionalists and visualization and interpretation of images with local anesthesia have led to the spread of the ICE-guided procedure.c
▪▪ Phased-array ICE imaging during transcatheter ASD closure can provide equivalent information compared with TEE imaging, including information on blood flow with color Doppler.
▪▪ Current limitations of ICE include cost, single plane imaging, sheath size and incapability of 3D imaging.
▪▪ Selection of TEE or ICE guiding as the tool depends on factors including the institution, patients and interventionalists.
Future perspective
▪▪ 3D ICE will greatly contribute to the field of structural heart interventions.
▪▪ Miniaturized TEE probe size may enable a transnasal approach in adult patients during the procedure, eliminating the requirement for general anesthesia.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Gibbon JH. Application of a mechanical heart and lung apparatus to cardiac surgery. Minn. Med. 37(3), 171–185 (1954).
- King TD, Thompson SL, Steiner C, Mills NL. Secundum atrial septal defect. Nonoperative closure during cardiac catheterization. JAMA 235(23), 2506–2509 (1976).
- Thanopoulos BD, Laskari CV, Tsaousis GS et al. Closure of atrial septal defects with theAmplatzer occlusion device: preliminary results. J. Am. Coll. Cardiol. 31(5), 1110–1116 (1998).
- Du ZD, Hijazi ZM, Kleinman CS et al. Amplatzer investigators: comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multicenter nonrandomized trial. J. Am. Coll. Cardiol. 39(11), 1836–1844 (2002).
- Wang JK, Tsai SK, Wu MH et al. Short- and intermediate-term results of transcatheter closure of atrial septal defect with the amplatzer septal occluder. Am. Heart J. 148(3), 511–7 (2004).
- Masura J, Gavora P, Padnar T. Long-term outcome of transcatheter secundum-type atrial septal defect closure using amplatzer septal occluders. J. Am. Coll. Cardiol. 45(4), 505–507 (2005).
- Schoen SP, Kittner T, Bohl S et al. Transcatheter closure of atrial septal defects improves right ventricular volume, mass, function, pulmonary pressure, and functional class: a magnetic resonance imaging study. Heart 92(6), 821–826 (2006).
- Giardini A, Donti A, Specchia S et al. Long-term impact of transcatheter atrial septal defect closure in adults on cardiac function and exercise capacity. Int. J. Cardiol. 124(2), 179–182 (2008).
- Butera G, Romagnoli E, Carminati M et al. Treatment of isolated secundum atrial septal defects: impact of age and defect morphology in 1013 consecutive patients. Am. Heart J. 156(4), 706–12 (2008).
- Handke M, Schäfer DM, Müller G et al. Dynamic changes of atrial septal defect area: new insights by three-dimensional volume- rendered echocardiography with high temporal resolution. Eur. J. Echocardiogr. 2(1), 46–51 (2001).
- Silvestry FE, Kerber RE, Brook MM et al. Echocardiography-guided interventions. J. Am. Soc. Echocardiogr. 22(3), 213–231(2009).
- Bannan A, Shen R, Silvestry FE, Herrmann HC. Characteristics of adult patients with atrial septal defects presenting with paradoxical embolism. Catheter Cardiovasc. Interv. 74(7), 1066–1069 (2009).
- Marie Valente A, Rhodes JF. Current indications and contraindications for transcatheter atrial septal defect and patent foramen ovale device closure. Am. Heart J. 153(Suppl. 4), 81–84 (2007).
- McMahon CJ, Feltes TF, Fraley JK et al. Natural history of growth of secundum atrial septal defects and implications for transcatheter closure. Heart 87(3), 256–259 (2002).
- Prokselj K, Kozelj M, Zadnik V et al. Echocardiographic characteristics of secundum-type atrial septal defects in adult patients: implications for percutaneous closure using amplatzer septal occluders. J. Am. Soc. Echocardiogr. 17(11), 1167–1172(2004).
- Torres A, Gersony WM, Hellenbrand W. Closure of unroofed coronary sinus with acovered stent in a symptomatic infant. Catheter Cardiovasc. Interv. 70(5), 745–748(2007).
- Kijima Y, Taniguchi M, Akagi T. Catheter closure of coronary sinus atrial septal defect using amplatzer septal occluder. Cardiol.Young doi:10.1017/S1047951111001077(2011) (Epub ahead of print).
- Maeno YV, Benson LN, McLaughlin PR, Boutin C. Dynamic morphology of the secundum atrial septal defect evaluated by three dimensional transoesophageal echocardiography. Heart 83(6), 673–677 (2000).
- Acar P, Saliba Z, Bonhoeffer P et al. Influence of atrial septal defect anatomy in patient selection and assessment of closure with the Cardio seal device; a three-dimensional transoesophageal echocardiographic reconstruction. Eur. Heart J. 21(7), 573–581 (2000).
- Wang JK, Tsai SK, Lin SM et al. Transcatheter closure of atrial septal defect without balloon sizing. Catheter Cardiovasc. Interv. 71(2), 214–221 (2008).
- Varma C, Benson LN, Silversides C et al. Outcomes and alternative techniques for device closure of the large secundum atrial septal defect. Catheter Cardiovasc. Interv. 61(1), 131–139 (2004).
- Chau AK, Leung MP, Yung T et al. Surgical validation and implications for transcatheter closure of quantitative echocardiographic evaluation of atrial septal defect. Am. J. Cardiol. 85(9), 1124–1130 (2000).
- Mazic U, Gavora P, Masura J. The role of transesophageal echocardiography in transcatheter closure of secundum atrial septal defects by the amplatzer septal occluder. Am. Heart J. 142(3), 482–488 (2001).
- Quaife RA, Chen MY, Kim M et al. Pre-procedural planning for percutaneous atrial septal defect closure: transesophageal echocardiography compared with cardiac computed tomographic angiography. Cardiovasc. Comput. Tomogr. 4(5),330–338 (2010).
- Mathewson JW, Bichell D, Rothman A, Ing Absent posteroinferior and anterosuperior atrial septal defect rims: factors affecting nonsurgical closure of large secundum defects using the amplatzer occluder. J. Am. Soc. Echocardiogr. 17(1), 62–69 (2004).
- Amin Z, Hijazi ZM, Bass JL, et al. Procedural results and acute complications in stenting native and recurrent coarctation of the aorta in patients over 4 years of age: a multi-institutional study. Catheter Cardiovasc. Interv. 63(4), 496–502 (2004).
- Taniguchi M, Akagi T, Watanabe N et al. Application of real-time three-dimensional transesophageal echocardiography using a matrix array probe for transcatheter closure of atrial septal defect. J. Am. Soc. Echocardiogr. 22(10), 1114–1120 (2009).
- Remadevi KS, Francis E, Kumar RK. Catheter closure of atrial septal defects with deficient inferior vena cava rim under transesophageal echo guidance. Catheter Cardiovasc. Interv. 73(1), 90–96 (2009).
- Butera G, De Rosa G, Chessa M et al. Transcatheter closure of atrial septal defect in young children: results and follow-up. Am. Coll. Cardiol. 42(2), 241–245(2003).
- Schubert S, Peters B, Abdul-Khaliq H et al. Left ventricular conditioning in the elderly patient to prevent congestive heart failure after transcatheter closure of atrial septal defect. Catheter Cardiovasc. Interv. 64(3), 333–337 (2005).
- Hörer J, Eicken A, Müller S et al. Risk factors for prolonged intensive care treatment following atrial septal defect closure in adults. Int. J. Cardiol. 125(1), 57–61 (2008).
- Pepi M, Tamborini G, Maltagliati A et al. Head-to-head comparison of two- and three-dimensional transthoracic and transesophageal echocardiography in the localization of mitral valve prolapse. J. Am. Coll. Cardiol. 48(12), 2524–2530 (2006).
- De Castro S, Caselli S, Papetti F et al. Feasibility and clinical impact of live three-dimensional echocardiography in the management of congenital heart disease. Echocardiography 23(7), 553–561 (2006).
- Baker GH, Shirali G, Ringewald JM et al. Usefulness of live three-dimensional transesophageal echocardiography in a congenital heart disease center. Am. J. Cardiol. 103(7), 1025–1028 (2009).
- Hung J, Lang R, Flachskampf F et al. 3D echocardiography: a review of the current status and future directions. J. Am. Soc.Echocardiogr. 20(3), 213–233 (2007).
- Marx GR, Fulton DR, Pandian NG et al. Delineation of site, relative size and dynamic geometry of atrial septal defects by real-time three-dimensional echocardiography. J. Am. Coll. Cardiol. 25(2), 482–490 (1995).
- Acar P, Dulac Y, Roux D et al. Comparison of transthoracic and transesophageal three-dimensional echocardiography for assessment of atrial septal defect diameter in children. Am. J. Cardiol. 91(4), 500–502 (2003)
- van den Bosch AE, Ten Harkel DJ, McGhie JS et al. Characterization of atrial septal defect assessed by real-time 3-dimensional echocardiography. J. Am. Soc. Echocardiogr. 19(6), 815–821 (2006).
- Chen FL, Hsiung MC, Hsieh KS et al. Real time three-dimensional transthoracic echocardiography for guiding Amplatzer Septal occluder device deployment in patients with atrial septal defect. Echocardiography 23(9), 763–770( 2006).
- Mehmood F, Vengala S, Nanda NC et al. Usefulness of live three-dimensional transthoracic echocardiography in the characterization of atrial septal defects in adults. Echocardiography 21(8), 707–713 (2004).
- Konstantinides S, Kasper W, Geibel A et al. Detection of left-to-right shunt in atrial septal defect by negative contrast echocardiography: a comparison of transthoracic and transesophageal approach. Am. Heart J. 126(4), 909–917 (1993).
- Saric M, Perk G, Purgess JR, Kronzon I. Imaging atrial septal defects by real-time three-dimensional transesophageal echocardiography: step-by-step approach. J. Am. Soc. Echocardiogr. 23(11), 1128–1135(2010).
- Roberson DA, Cui W, Patel D et al. Three-dimensional transesophageal echocardiography of atrial septal defect: a qualitative and quantitative anatomic study. Am. Soc. Echocardiogr. 24(6), 600–610(2011).
- Cao Q, Radtke W, Berger F et al. Transcatheter closure of multiple atrial septal defects. Initial results and value of two- and three-dimensional transoesophageal echocardiography. Eur. Heart J. 21(11), 941–947 (2000).
- Johri AM, Witzke C, Solis J et al. Real-time three-dimensional transesophageal echocardiography in patients with secundum atrial septal defects: outcomes following transcatheter closure. J. Am. Soc. Echocardiogr.24(4), 431–437 (2011).
- Beerbaum P, Körperich H, Barth P et al. Noninvasive quantification of left-to-right shunt in pediatric patients: phase-contrast cine magnetic resonance imaging compared with invasive oximetry. Circulation 103(20), 2476–2482 (2001).
- Beerbaum P, Körperich H, Esdorn H et al. Atrial septal defects in pediatric patients: noninvasive sizing with cardiovascular MR imaging. Radiology 228(2), 361–369 (2003).
- Holmvang G, Palacios IF, Vlahakes GJ et al. Imaging and sizing of atrial septal defects by magnetic resonance. Circulation 92(12), 3473–3480 (1995).
- Thomson LE, Crowley AL, Heitner JF et al. Direct en face imaging of secundum atrial septal defects by velocity-encoded cardiovascular magnetic resonance in patients evaluated for possible transcatheter closure. Circ. Cardiovasc. Imaging. 1(1), 31–40(2008).
- Teo KS, Disney PJ, Dundon BK et al. Assessment of atrial septal defects in adults comparing cardiovascular magnetic resonance with transoesophageal echocardiography. Cardiovasc. Magn. Reson. 12, 44 (2010).
- Rajiah P, Kanne JP. Computed tomography of septal defects. J. Cardiovasc. Comput. Tomogr.4(3), 155–163 (2010).
- Brody AS, Frush DP, Huda W et al. Radiation risk to children from computed tomography. Pediatrics 120(3), 677–682 (2007).
- Onorato E, Pera IG, Melzi G et al. Persistent redundant Eustachian valve interfering with Amplatzer PFO occluder placement: anatomico-clinical and technical implications. Catheter Cardiovasc. Interv. 55(4), 521–524 (2002).
- Majunke N, Bialkowski J, Wilson N et al. Closure of atrial septal defect with the Amplatzer septal occluder in adults. Am. J. Cardiol. 103(4), 550–554 (2009).
- Sarris GE, Kirvassilis G, Zavaropoulos P et al. Surgery for complications of trans-catheter closure of atrial septal defects: a multi-institutional study from the European Congenital Heart Surgeons Association. Eur. J. Cardiothorac. Surg. 37(6), 1285–1290(2010).
- Levis DS, Moore JW. Embolization and retrieval of the amplatzer septal occluder. Catheter Cardiovasc. Interv. 61(4), 543–547(2004).
- Meltser H, Hoyer MH, Kalaria VG. Entrapment of right atrial pacemaker lead by patent foramen ovale closure device: successful percutaneous salvage. Catheter Cardiovasc. Interv. 65(4), 593–596 (2005).
- Opotowsky AR, Landzberg MJ, Kimmel SE, Webb GD. Percutaneous closure of patent foramen ovale and atrial septal defect in adults: the impact of clinical variables and hospital procedure volume on in-hospital adverse events. Am. Heart J. 157(5), 867–874 (2009).
- Kijima Y, Taniguchi M, Akagi T et al. Torn atrial septum during transcatheter closure of atrial septal defect visualized by real-time three-dimensional transesophageal echocardiography. J. Am. Soc. Echocardiogr. 23(11), 1222 e5–e8 (2010).
- Abdel-Massih T, Dulac Y, Taktak A et al. Assessment of atrial septal defect size with 3D-transesophageal echocardiography: comparison with balloon method. Echocardiography 22(2), 121–127 (2005).
- Lodato JA, Cao QL, Weinert L et al. Feasibility of real-time three-dimensional transoesophageal echocardiography for guidance of percutaneous atrial septal defect closure. Eur. J. Echocardiogr. 10(4), 543–548 (2009).
- Stec S, Zaborska B, Sikora-Frac M et al. First experience with microprobe transoesophageal echocardiography in non-sedated adults undergoing atrial fibrillation ablation: feasibility study and comparison with intracardiac echocardiography. Europace 13(1), 51–56 (2011).
- Patel A, Cao QL, Koenig PR et al. Intracardiac echocardiography to guide closure of atrial septal defects in children less than 15 kilograms. Catheter Cardiovasc. Interv. 68(2), 287–291 (2006).
- Hijazi ZM, Shivkumar K, Sahn DJ. Intracardiac echocardiography during interventional and electrophysiological cardiac catheterization. Circulation 119(4), 587–96 (2009).
- Hijazi Z, Wang Z, Cao Q et al. Transcatheter closure of atrial septal defects and patent foramen ovale under intracardiac echocardiographic guidance: feasibility and comparison with transesophageal echocardiography. Catheter Cardiovasc. Interv.52(2), 194–199 (2001).
- Bartel T, Konorza T, Arjumand J et al. Intracardiac echocardiography is superior to conventional monitoring for guiding device closure of interatrial communications. Circulation 107(6), 795–797 (2003).
- Zanchetta M, Onorato E, Rigatelli G et al. Intracardiac echocardiography-guided transcatheter closure of secundum atrial septal defect: a new efficient device selection method. J. Am. Coll. Cardiol. 42(9), 1677–1682 (2003).
- Kim SS, Hijazi ZM, Lang RM, Knight BP. The use of intracardiac echocardiography and other intracardiac imaging tools to guide noncoronary cardiac interventions. J. Am. Coll. Cardiol. 53(23), 2117–2128 (2009).
- Boccalandro F, Baptista E, Muench A et al. Comparison of intracardiac echocardiography versus transesophageal echocardiography guidance for percutaneous transcatheter closure of atrial septal defect. Am. J. Cardiol. 93(4), 437–440 (2004).
- Alboliras ET, Hijazi ZM. Comparison of costs of intracardiac echocardiography and transesophageal echocardiography in monitoring percutaneous device closure of atrial septal defect in children and adults. Am. J. Cardiol. 94(5), 690–692 (2004).
- Light ED, Idriss SF, Wolf PD et al. Real-time three-dimensional intracardiac echocardiography. Ultrasound Med. Biol. 27(9), 1177–1183 (2001).
- Spencer KT, Goldman M, Cholley B et al. Multicenter experience using a new prototype transnasal transesophageal echocardiography probe. Echocardiography 16(8), 811–817 (1999).
- Greco C, Chiavari PA, Campolongo G et al. Transnasal transesophageal echocardiography: a new approach for the PFO occlusion in awake patients. Catheter Cardiovasc. Interv. 72(4), 538–541 (2008).
▪ The first report showing transcatheter closure of an atrial septal defect.
▪▪ The sole large cohort study comparing between transcatheter closure and surgical closure of atrial septal defectsin a multicenter registry.
▪ Current guideline of echocardiography for several structural heart interventions.