Review Article - Interventional Cardiology (2009) Volume 1, Issue 2
Recanalization of infrapopliteal occlusions
- Corresponding Author:
- William D Adair
Department of Radiology, Leicester Royal Infirmary
Infirmary Square, Leicester, LE1 5WW, UK
Tel: +44 116 254 1414
E-mail: william.adair@uhl-tr.nhs.uk
Abstract
Keywords
angioplasty, drug-eluting stent, limb salvage, magnetic resonance angiography, stents, vascular patency
Clinical context
Patients with peripheral arterial disease (PAD) have generalized atherosclerosis. Hence, they have a high incidence of coronary artery disease, the severity of which correlates well with their overall prognosis [1]. Compared with those who have no evidence of PAD, individuals with severe and symptomatic large vessel disease have a 15‑fold increase in cardiovascular and coronary heart disease mortality [2]. Many are also diabetic – a condition that alone also gives a threeto four-fold increased risk of PAD. Elderly individuals will also have other risk factors such as hypertension and hyperlipidemia.
In other words, these patients already have a poor prognosis. For those with intermittent claudication, the risk of progression to critical limb ischemia (CLI) and amputation is less than 1% per year [3]. For patients with chronic CLI a mortality rate of 20% in the first year following presentation is the norm. CLI is defined as rest pain, tissue loss (ulcer) or gangrene, attributable to objectively proven arterial occlusive disease [4], and by convention is chronic once greater than 2 weeks in duration.
Clinical presentation & patterns of disease
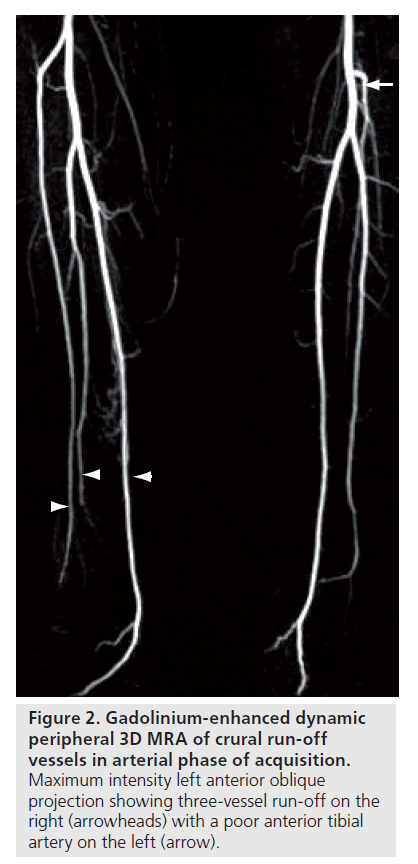
Patients presenting with symptoms and signs of CLI are clinically classified by Fontaine stage III and IV, and Rutherford categories 4, 5 and 6 (Table 1) [4,5]. There is multilevel occlusive arterial disease, including femoral popliteal disease. In particular, in those with diabetes, a pattern of above-knee sparing is characteristic, with popliteal trifurcation or distal disease. These arteries are prone to calcification and noncompliance [4,6]. In more than 85% of patients disease is either occlusive or involves multiple stenoses [7].
This then is representative of the group of patients with chronic crural vessel occlusions, and the subject of this article. Presentation may be quite late in the disease process. This perhaps reflects the body’s ability to form extensive collateral circulation below the knee, so that only one crural vessel may be sufficient for distal perfusion [3,8].
Investigations
Prior to a definitive procedure, radiological delineation of the extent of PAD is highly desirable. Although digital subtraction angiography (DSA) may still be considered the gold standard for this purpose, arterial duplex ultrasound is a cost-effective, widely available alternative carrying negligible risk. It has reasonable sensitivity to plan surgical or endovascular therapy [9] and has the added advantage of blood velocity quantification to establish the hemodynamic significance of lesions. It is, however, operator-dependent. Inflow from iliac arteries may be obscured by overlying bowel gas, and sensitivity falls in the distal calcified crural vessels.
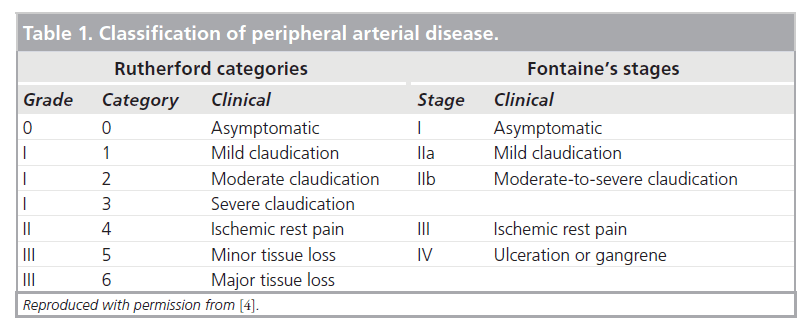
For these particular problems multidetector computed tomography (MDCT) or magnetic resonance imaging (MRI) is invaluable. In a mixed population of patients with claudication and CLI, MDCT has been shown to have an overall accuracy of 94%. However, accuracy falls to 89% in the calf compared with DSA (Figure 1) [10]. Future evolution of scanner hardware, and postprocessing software, is likely to lead to improved performance. Significantly, MDCT may result in radiation exposure 3.9- times lower than DSA when applied in the aorta and distal territories [11].
Figure 1: Anterior projection 3D volume rendered computed tomography peripheral arteriogram showing three-vessel run-off bilaterally. The contrast enhancement in the right limb is less concentrated due to inflow disease (not shown).
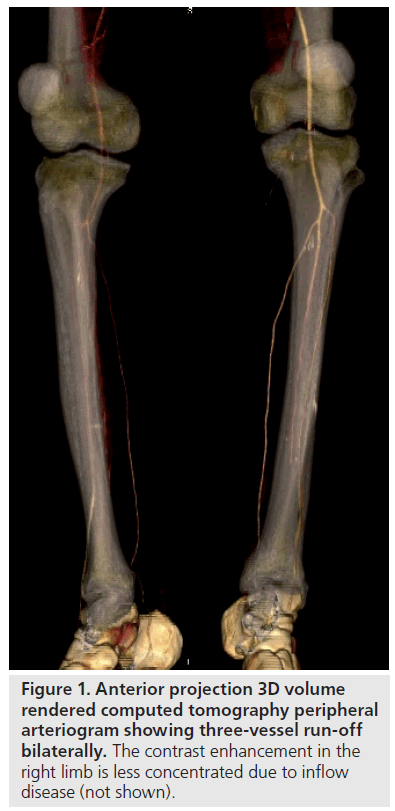
Magentic resonance imaging is more sensitive to contrast enhancement than MDCT (Figure 2). Despite having low spatial resolution, peripheral MR gadolinium contrast-enhanced dynamic angiography (MRA) can achieve sensitivities of 97% compared with DSA [12]. Although arterial calcification cannot be directly assessed, there is evidence that MRA can perform significantly better than DSA in the evaluation of peripheral run-off [13], even in diabetics [14]. Arterial stents produce susceptibility artifacts on MRI and it is therefore not possible to confirm their patency. For the same reason, imaging near prosthetic joints is also not fruitful. Of course, a proportion of patients have contraindications to MRI, such as a pacemaker.
Treatment options
▪ Conservative & medical management
All patients with PAD should maintain rigorous control of risk factors such as hypertension, cholesterol and diabetes [3], and should modify their lifestyle. This is to reduce overall morbidity and mortality as well as to optimize the results of revascularization. Up to 50% of patients with claudication will improve with these measures when combined with smoking cessation, a formal exercise program and antiplatelet treatment to reduce systemic vascular events.
Despite this, deterioration will continue in 25%, with approximately 500 to 1000 new cases of CLI per year per 1 million European or North American populations [4]. At this stage, either surgical or endovascular revascularization is indicated for limb salvage. Unfortunately, up to half of patients with severe limb ischemia are either not fit for revascularization, have unfavorable anatomy or lack a suitable vein graft. The definitive surgical option is then limited to amputation [15]. However, conservative measures including analgesia, local ulcer care and aggressive management of local infection continue to be important.
Treatment with parenteral prostanoids may benefit a minority at this stage [4]. Anticoagulants and vasoactive drugs (e.g., pentoxifylline) are not of proven benefit, whilst hyperbaric oxygen therapy and spinal cord stimulation have a limited role in medical treatment [4].
Considerable interest exists in the use of bone marrow-derived cells, which are rich in endothelial progenitors, and these are the subject of ongoing trials. In one trial, clinically significant improvement in limb salvage and Rutherford category were achieved within 6 months [16]. Simultaneous cytokine therapy may be synergistic [17]. Matrix metalloproteinases are also known to play a role in the complex pathogenesis of PAD, as well as aneurismal disease, and selective targeting of these may provide a further approach to treatment in the future [18].
▪ Surgical management
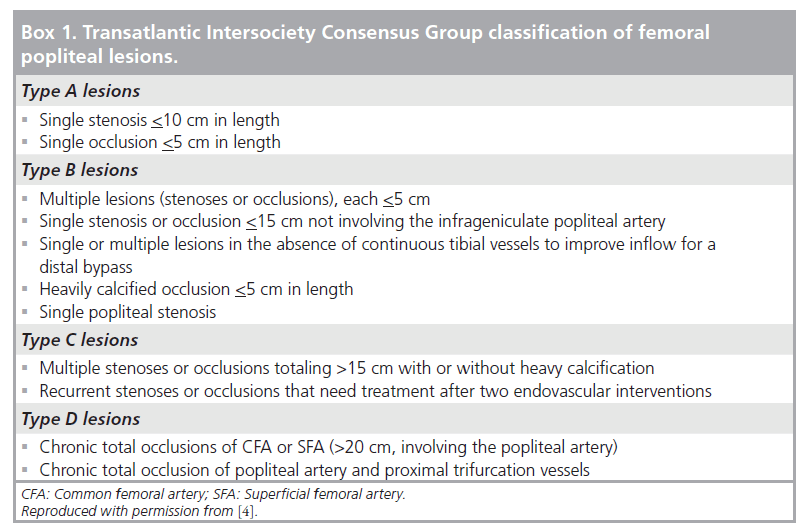
The Transatlantic Intersociety Consensus Group (TASC II) document provides a surgical and endovascular management framework for PAD, with revascularization recommendations based on anatomical distribution and extent of lesions (Box 1) [4]. When conservative measures fail in CLI, and the patient is medically fit, then surgical or endovascular revascularization is indicated. This may be combined with salvage procedures to preserve as much of the foot as possible. If revascularization is unsuccessful then secondary amputation may be required. Alternatively, if revascularization is not technically feasible, primary amputation may be undertaken.
Surgical revascularization to below-knee vessels is preferably performed using reversed or in situ saphenous vein graft. Patency rates of 73% at 2 years are achievable when anastomosing to crural vessels [19], and limb salvage of 87% at 3 years is reported [20]. Patency with synthetic grafts is somewhat worse, particularly the more distal the anastomosis, and range between 30 and 70% at 2 years, with similar limb salvage rates [21].
The Bypass versus Angioplasty in Severe Ischemia of the Leg (BASIL) randomized, controlled trial was instigated to compare the outcome of a bypass surgery-first versus an angioplasty- first strategy in patients with CLI [15]. Approximately 30% of those patients presenting with CLI were eligible for randomization into the trial [22]. The participants had multilevel infrainguinal disease. A total of 192 patients underwent surgery whilst 203 had attempted angioplasty, and overall, approximately two-thirds of interventions were distal to the superficial femoral artery (SFA).
In the short term, hospital costs and morbidity were greater in the surgical group. There was higher immediate failure (20% technical failure) and reintervention rate within 12 months in the angioplasty group. In the medium term, amputation-free survival, all-cause mortality and health-related quality of life scores were similar in the two groups.
The results suggested that angioplasty should be attempted first in patients not expected to live beyond 2 years, particularly if they were at higher risk for surgery, and this broadly concurs with recommendations of the TASC [4].
▪ Endovascular management
There are no randomized, controlled trials specifically comparing bypass and angioplasty in the crural vessels in claudicants [4]. In patients with mild-to-moderate intermittent claudication the exact role of angioplasty in iliac and femoral disease is still not clear [4,23]. Generally, 20% of claudicants have multisegment involvement including the below-knee vessels [24], and it is exceptionally rare to find isolated below-knee atherosclerotic disease in this group [7]. Because of the low occurrence of disease, lack of evidence and attendant risks, endovascular treatment of run-off vessels in claudicants is not recommended, although some would advocate or suggest treatment in severe claudication [4,7,8]. Although controversial, crural angioplasty may also be useful to maintain adequate run-off – and hence patency – following femoral popliteal angioplasty or stenting, or femoral distal surgical bypass [5,8,25].
▪ Below-knee angioplasty
Below-knee occlusions include TASC D femoral popliteal lesions for which surgery is traditionally the treatment of choice [4]. As such, it is inappropriate to perform below-knee angioplasty in this group unless the same strict selection criteria as for surgery are applied, even given the lower complication rate of angioplasty [7]. As stated above, no randomized, controlled trials exist, but numerous studies with small numbers of patients are reported.
In these series, technical success rates of crural angioplasty for stenotic or short occlusive disease vary between 86 and 97%. Lower rates (88%) for occlusions reflect an increased risk of vessel rupture or unsuccessful recanalization in these lesions [7,8,26]. Vessel recoil and osteal lesions can also present technical difficulties.
With regular Doppler ultrasound surveillance, 1-year patency rates of up to 79% can be achieved [8]. Minimal reintervention is required to assist patency. More significant perhaps, from the patient’s perspective, is clinical success (cessation of pain or symptoms, healing of distal lesions or amputation sites) varying between 50 and 88% in early follow-up [7,15,26]. Limb salvage rates of between 56 and 86% at 2 years are attainable [6,7].
The ability to restore straight-line flow to the foot is crucial for clinical success [26], and this is an important concept to grasp in infrainguinal angioplasty.
Disease in CLI tends to be diffuse and this lends itself less well to transluminal angioplasty. In addition, the recanalization of occlusions more than a few centimeters in length is difficult, leading to an overall worse outcome [7].
Subintimal angioplasty (SIA) has proved an effective method for dealing with TASC C and D lesions in the femoral popliteal territory [27], as well as below the knee [28]. Hence, it can be used in most patients with CLI as an alternative to surgical bypass [25]. Technical success is as high as 90% [28–30], even when treating lesions longer than 30 cm in length [25]. Failures may occur due to an inability to cross the lesion (or more often failure to re-enter the native lumen distally), or vessel perforation.
Although clinical and imaging patency at 12 months is approximately 50%, limb salvage rates of 92% are achievable [25]. Here illustrates one of the idiosyncrasies of SIA, with late patency rates considerably lower than limb salvage rates. Collaterals are thought to be an important reason for this, as these are often preserved in SIA, whilst sacrificed in surgical procedures [25]. It has also been suggested that once the initial ischemic lesion has healed then the metabolic demands of the limb decrease and so dependence on a patent vessel is less important for a good clinical result [5,29]. It may be that the opening of further collaterals during the procedure, even with only temporary or partial patency of the angioplastied segment, contributes to symptom reduction [29].
Increasing the number of run-off vessels (into the pedal arch) results in improved patency at 1 year, whilst treating longer occlusions will reduce patency. Both of these factors are of statistical significance [25].
Hence, whilst serving as a temporary bypass, SIA may provide limb salvage rates of 80 to 90%, similar to that of surgical reconstruction. Importantly, this may be achieved with minimal morbidity and mortality, and without prejudicing subsequent surgery or later angioplasty if required.
Technique
Angioplasty for crural vessel disease has become commonplace over the last decade or two, with the advent of low-profile pushable balloon catheters and steerable hydrophilic guidewires. Imaging advances such as digital road mapping and solid-state detector fluoroscopy, as well as pharmacological aids such as effective vasodilators, have facilitated this.
Nevertheless, the recanalization of infrapopliteal occlusions remains a challenge. Anatomically, the lesions are remote from the point of arterial access, and have a tendency to be calcified and multisegmental. In combination, these factors put high mechanical demands on wires, catheters and angioplasty balloons.
An antegrade puncture in the ipsilateral common femoral artery is highly desirable for maximum control of the guidewire and catheter. This may of course be difficult in the obese patient with a large abdominal apron where guided puncture using ultrasound may give suboptimal image quality due to the depth of the femoral vessels from the probe. In any event, it is important to puncture the common femoral artery over the lower half of the femoral head to avoid the morbidity and mortality associated with a ‘high’ puncture [101].
It should be remembered at all times that the crural vessels are fragile and easily perforated. They are also prone to spasm, and liberal use of antispasmodic agents is advisable. The a‑antagonist tolazoline may be employed to prevent spasm, whilst intraprocedural intra-arterial glyceryl trinitrate in 50–100 μg aliquots is useful to treat spasm if and when it occurs. All patients should be adequately heparinized. A total of 3000 units intra-arterially is sufficient for most cases, although some would advocate larger doses of between 5000 and 7000 units [29,31]. We do not use periprocedural glycoprotein IIb/IIIa platelet inhibition, or postprocedure anticoagulation, which may contribute to puncture sites or other complications [29].
The use of the highest quality digital subtraction fluoroscopy with road-mapping facility and digital zoom is a prerequisite. Whilst being mindful of radiation dose, the judicious use of magnified imaging is often very useful when working in these small vessels.
Uncomplicated crural angioplasty is unlikely to require a large sheath size, and we routinely use a 5‑F compatible system, although 4‑F systems are available. Procedural failure may not relate to the ability of the wire to cross the lesion, but rather the inability to subsequently advance the balloon catheter through a diseased and occluded calcified artery. Hence, we favor using an 0.035-inch hydrophilic wire with a short curved tip, and a 5‑F over-the-wire balloon catheter system, which provides sufficient sturdy support and resilience for this purpose. Balloons with a diameter of 2.5–3 mm are usually ideal.
There are a number of good 0.018- and 0.014‑inch systems that are very effective for crural and pedal vascular stenotic disease. These include 2-mm balloons for pedal arteries using a 3.5‑F system. Apart from requiring an atraumatic visible tip, it is important to choose the stiffest possible wire and catheter combination when using these smaller systems. Low-profile balloons are available in generous lengths that allow rapid treatment of long segments of disease with a single inflation. We have found them to be particularly useful in the diabetic patient with diffuse, calcified, run-off stenoses and occlusions. In these arteries, breaking back into the true lumen (from subintimal passage) may be problematic in the presence of calcification, and hence it is better to remain intraluminal as much as possible. Such systems are ideal for this purpose. However, their use is often limited in anything more than short occlusions. Where disease occurs at the origin of the anterior tibial artery, or at the bifurcation of the tibioperoneal trunk, a kissing balloon technique may be used to avoid inadvertent occlusion of the osteum adjacent to the site of angioplasty [7,31]. Again, smaller balloon systems are easier to manage in this way.
Utmost care is needed, first when negotiating stenoses to avoid converting these into occlusions, and second to avoid perforating the vessel. In the worst cases the procedure may then have to be abandoned. Alternatively, coil occlusion may be possible, or the dissection of a subintimal track from above may circumvent the arterial defect.
Standard curved 0.035-inch hydrophilic guide wires are also suitable for tight stenotic disease and short occlusions. When treating multiple occlusions or tight stenoses at various levels, some would advocate successive balloon dilatation of lesions from cranial to caudal. This improves catheter manipulation, prevents thrombi forming around the catheter where it occludes a tight proximal stenosis and improves the inflow [31]. We would argue the opposite – that unless severe flow-limiting distal disease can be crossed and opened to improve outflow then a proximal angioplasty is unlikely to stay patent – particularly with a proximal SIA channel that initially relies on rapid flow for patency [25].
Longer occlusions frequently require the use of subintimal dissection to cross. The presence of significant collaterals proximal and distal to the anticipated subintimal entry and re-entry points should be noted. Dissection past these collaterals should ideally be avoided, as they will provide continued distal perfusion should recanalization be unsuccessful.
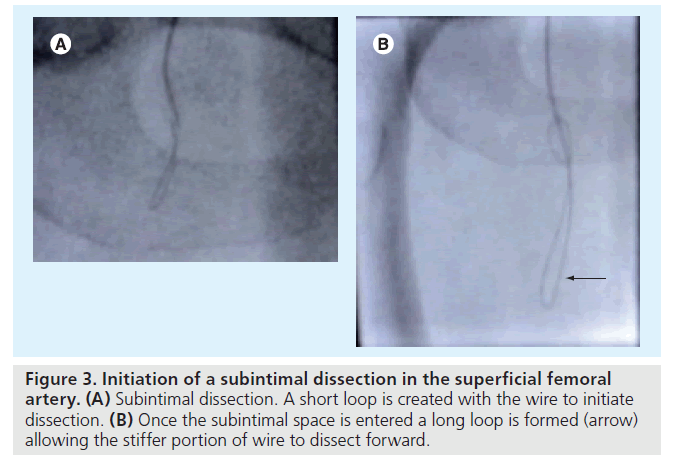
The technique is well described elsewhere [28], but briefly, and with particular reference to crural disease it is revised here. Using a 0.035‑inch hydrophilic wire, the tip is looped at the level of the occlusion and this loop is then advanced to dissect into the media. By increasing the size of the loop, the stiff portion of the wire can then be advanced through the subintimal plane (media) whilst following behind with the balloon catheter (Figure 3A & 3B). As already mentioned, this can be the most challenging part of the procedure from a mechanical point of view. One should be mindful of the possibility of the sheath or catheter retracting out of the arterial puncture and dissecting within the subcutaneous tissues beneath the skin incision if continued pushing of the catheter at the puncture site does not result in simultaneous catheter-tip advancement. Exchanging for a stiff hydrophilic guidewire is helpful in this situation. As a last resort, gentle inflations of the balloon within the subintimal track, distal to the entry point of dissection (which should itself not be dilated until successful distal recanalization), helps to advance the catheter through difficult long occlusions.
Normally, the arterial lumen can be re-entered at or immediately below the point of reconstitution of the artery. Sometimes this occurs somewhat more distally. Re-entry of the guidewire at this point is achieved by shortening the length of the loop to use the soft portion of the wire. Alternatively, a 1.5-mm J tip half-stiff hydrophilic wire, which is ideal for use in these small vessels, is very effective for this purpose. Entry into the true lumen is often felt as a characteristic ‘give’ on the wire, and confirmed by free flow of contrast injected via the catheter once advanced into the true lumen. Re-entry into the lumen may be difficult or impossible if the artery is calcified. Recently, catheters have been marketed for achieving re-entry into the true lumen from the subintimal space (e.g., Outback®, Cordis, NJ, USA). Although effective in more proximal peripheral territories these currently have no role in the crural vessels, due to their bulk.
Another option is to dissect the occlusion distally. SFA occlusions (particularly flush occlusions) can be successfully crossed and treated from a popliteal artery puncture. Similarly, the dorsalis pedis, for instance, can be punctured under ultrasound guidance and a retrograde dissection channel created to communicate with an existing antegrade dissection channel [32].
The subintimal channel is then rapidly angioplastied proximally to include the point of subintimal entry. A handheld inflation syringe is ideal for this purpose, and this allows rapid inflation and deflation over a few seconds, up to pressures of approximately 12 atmospheres, with tactile feedback.
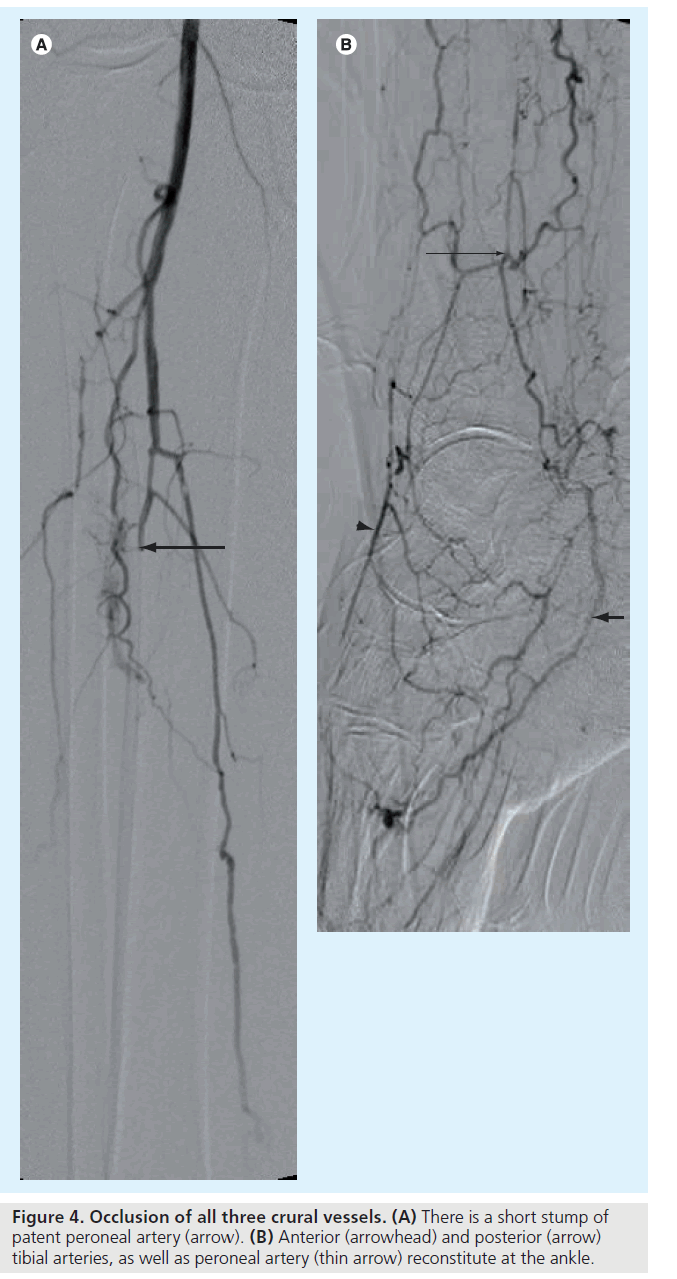
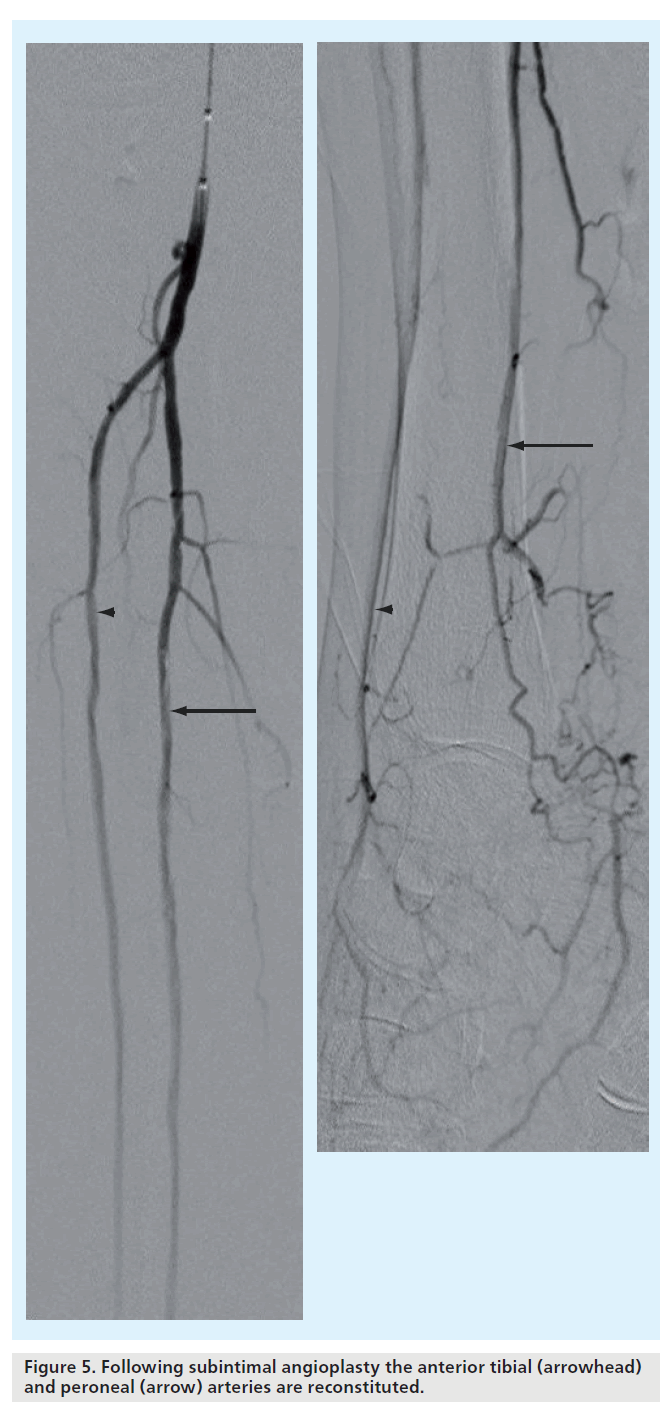
Many authors describe technical success by estimating residual stenoses (e.g., <30 or <50%), but in this technique good, rapid flow following angioplasty is far more important than appearance. Approximately 6 to 8 ml of contrast agent clearing the vessel lumen in 2 to 3 s is considered brisk and ideal (Figure 4 & 5) [29].
Adjuncts to angioplasty
As stated, arterial recoil in crural vessels is a particular problem and is difficult to treat. In the iliacs, SFA and popliteal artery, the situation can be salvaged with a suitable stent. Widespread use of stenting is not practiced in the crural vessels, which are of narrow caliber with relatively slow flow that may induce thrombosis. Intimal hyperplasia is another concern [33]. Primary stenting is not recommended [102]. However, stenting may have a role in bail-out following severe recoil or flow-limiting dissection [31]. It should be remembered that stenting may preclude or complicate repeat endovascular or subsequent surgical treatment.
Atheroma and thrombus load in patients with CLI is often considerable. This may be addressed by debulking with an excimer laser, a cool-tip ultraviolet laser that selectively ablates plaque and thrombus. Unlike mechanical atherectomy devices, it is not prerequisite to cross an occlusion in order to treat it. Debulking allows occlusions to be treated without subintimal dissection. Subsequent angioplasty may then be performed with lower balloon pressures and barotrauma [34]. The Laser Atherectomy for Critical Ischaemia trials demonstrated limb salvage at 6 months in up to 90% of patients with CLI [35]. These patients, who were unfit for surgery, had disease at or below the knee. Several studies with small patient numbers have shown similar results. However, careful case selection is required, as the mid-term results are not so impressive, with 12‑month limb salvage rates of 55% in one study [36].
Vibrational angioplasty is another technique in which a guidewire does not need to be placed through an occlusion first. This uses a device, attached to the back end of the catheter, which oscillates the guidewire at a variable frequency, facilitating its progress through the lesion. Limited evidence suggests that this has a high technical and clinical success rate, but its overall role has yet to be defined [37].
Cutting balloon angioplasty (CBA) has a recognized role in the treatment of neointimal hyperplasia affecting hemodialysis fistulae and graft anastomoses. Its role in peripheral CLI is more limited. No randomized, controlled trials against conventional angioplasty below the knee have been reported. Despite this, a high degree of technical success has been demonstrated. In most TASC A and B lesions, primary patency rates of 43% at 2 years below the knee are achievable [38]. Limb salvage of 89.5% at 1‑year follow-up has also been reported in popliteal and infrapopliteal lesions with Rutherford categories of 4 or higher [39]. Like laser, CBA allows treatment of recalcitrant lesions with less barotrauma than conventional angioplasty, thereby reducing the inflammatory response and subsequent restenosis. Perforation rates are low as long as oversizing of the balloon is avoided and care is taken during inflation and deflation to allow the embedded blades to gently score the atheroma.
Finally, mechanical atherectomy devices such as the SilverHawk® (FoxHollow, CA, USA) may be used in crural vessels of at least 2 mm in diameter. It is important to ensure that the wire crosses stenoses and occlusions intraluminally, as subintimal dissection may lead to perforation. Although long-term follow-up is limited, 6‑month patency rates in this territory of 94% are possible [40]. The device excises, packs and temporarily stores plaque debris within the catheter. Even so, distal embolization is still a concern, and some would advocate placement of a filter wire, which may be problematic in small crural vessels [41]. Overall, the high cost of these devices warrants further studies into their role [42,43].
Complications
▪ Hematoma
Up to 10% of patients will experience a symptomatic hematoma following angioplasty [26,31,44]. This may be significant (requiring blood transfusion or surgical repair) in 1% overall. Large or multiple punctures, systemic anticoagulation or bleeding diatheses will increase the risk of hematoma. Small pseudoaneurysms may be effectively treated by percutaneous thrombin injection under ultrasound guidance [45].
▪ Perforation
The earliest indication of perforation is usually the patient complaining of pain. Small guidewire- induced perforations in the crural vessels are usually self-limiting. Rarely, coiling is required. If a perforation occurs in a subintimal channel then re-establishing a new subintimal channel proximally will sometimes bypass the offending segment.
▪ Embolization/thrombosis
This can be reduced by adequate heparinization and use of vasodilators. In general, occlusions and tight stenoses should be dilated as soon as possible to re-establish good f low. Distal emboli can be aspirated with a suitable thrombectomy catheter [46], or mechanical thrombectomy devices are available for this purpose (e.g., Rotarex®, Straub, Switzerland). Alternatively, small aliquots of thrombolytic may be used [31].
▪ Dissection
Flow-limiting dissections occurring following angioplasty may initially be plastered against the vessel wall with gentle balloon inflation over the area in question for several minutes. Sometimes this is sufficient to remodel the lesion, but often stent placement is necessary to re-establish patency.
Future perspective
Improvements in balloon design have already increased the accessibility of crural vessels and this will no doubt continue in the future. High pushability (longitudinal shaft stiffness), with good trackability and a low profile are all desirable properties. Following the cardiac experience, we anticipate the continued development of stent technology for below-knee indications. Self-expanding nitinol stents with minimal strut profiles are ideally suited to this. They are highly flexible to resist fracture and subsequent occlusion, whilst limiting lumen loss as much as possible [33].
Recent studies of small numbers of patients with CLI suggest sirolimus-eluting balloonmounted stents in the crural vessels retain a significant advantage over bare-metal (steel) stents at 6‑month follow-up in terms of restenosis (16 vs 76%) and the need for target lesion revascularization [47]. This is important, as the options for endovascular reintervention are markedly limited once reocclusion occurs. Clinical benefit and long-term follow-up are still not clear. However, a similar study with paclitaxel-eluting stents perhaps gives an indication of this: results at 1 year are less impressive, with angiographic primary patency of only 30% [48]. Patients with renal failure, or initial occlusions, had worse outcomes. The place of dual antiplatelet therapy with aspirin and clopidogrel to limit this late occlusion is also not clear [33]. Despite the high risk of restenosis and loss of patency after stenting, limb salvage may still be excellent [49].
Bioabsorbable stents are a more recent alternative to drug-eluting stents. By reducing prolonged foreign body reaction, intimal hyperplasia may be reduced, especially when combined with drug elution [50]. Whilst maintaining their structural integrity over the initial essential few weeks, subsequent absorption reduces embolic complications, as well as allowing future endovascular intervention and MRI imaging.
In summary, randomized, controlled studies will be needed to establish the cost–effectiveness and overall role of below-knee arterial stenting compared with angioplasty [33].
In conclusion, the technical and clinical success of infrapopliteal recanalization has increased over recent decades. This is in a challenging patient population with high morbidity and mortality who already start at a poor baseline in terms of vascular disease. This has been driven by increasingly sophisticated imaging advances, in combination with developments in catheter, wire and stent technology. With meticulous technique and attention to detail, good results can be achieved.
Executive summary
Clinical context
▪ Patients with peripheral arterial disease have systemic cardiovascular disease and when presenting with critical limb ischemia, a mortality rate of 20% in the first year.
Clinical presentation & patterns of disease
▪ In patients with advanced Fontaine stages and Rutherford categories, disease is frequently multilevel and occlusive. When mainly affecting the crural vessels, presentation may be relatively late due to extensive collateral circulation, which tends to occur in this territory.
Investigations
▪ Multidetector computed tomography and contrast-enhanced dynamic magnetic resonance angiography are both sensitive and specific alternatives to catheter angiography in the imaging work-up of patients with suspected infrainguinal and crural arterial disease.
Treatment options
▪ Rigorous, conservative measures to control risk factors, including lifestyle modification and pharmacological agents, are an essential prerequisite to further endovascular or vascular intervention.
▪ For those who go on to develop critical limb ischemia, surgical options are limited. Limb salvage is indicated if the patient is fit. Angioplasty is a good first option for those with limited life expectancy (<2 years), especially if they are a high surgical risk. It is unlikely that this will prejudice future surgical options. Crural angioplasty is generally reserved for these patients rather than claudicants.
▪ Infrapopliteal disease may be technically difficult to treat by endovascular means due to its remote location from the puncture site, and a tendency to calcification, spasm and recoil in these arteries. Technical and clinical success is high in stenotic disease, but less so in occlusive disease.
▪ Extensive occlusive disease may be best dealt with by subintimal angioplasty. Once mastered, the technique has good technical success with high limb salvage rates. As with transluminal angioplasty, it is important to establish straight-line flow to the foot. Complication rates with angioplasty and subintimal angioplasty are generally low.
Technique
▪ Technical success requires meticulous attention to technique. Familiarity with the use of low-profile pushable balloons and supportive wires on 0.014- to 0.035-inch systems is essential.
▪ Stents currently have a limited role in crural recanalization, but may be useful in bale-out situations.
Future perspective
▪ Advances in balloon catheter and wire combinations promise continued improvement in technical success.
▪ The indications for stenting may widen with the development of drug-eluting and bioabsorbable stents specifically designed for this vascular territory.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
- Welten G, Schoutern O, Chonchol M et al.: Prognosis of patients with peripheral arterial disease. J. Cardiovasc. Surg. (Torino) 50, 109–121 (2009).
- Criqui M, Langer R, Fronek A et al.: Mortality over a period of 10 years in patients with peripheral arterial disease. N. Engl. J. Med. 326, 381–386 (1992).
- Burns P, Gough S, Bradbury AW: Management of peripheral arterial disease in primary care. Br. Med. J. 326, 584–588 (2003).
- Norgren L, Hiatt WR, Dormandy JA et al.: Inter-society consensus on peripheral arterial disease (TASC II). Eur. J. Vasc. Endovasc. Surg. 33(Suppl. 1), S1–S75 (2007).
- Tsetis D, Belli A-M: The role of infrapopliteal angioplasty. Br. J. Radiol. 77, 1007–1015 (2004).
- Fraser SCA, Al-Kutoubi MA, Wolfe JHN: Percutaneous transluminal angioplasty of the infrapopliteal vessels: the evidence. Radiology 200, 33–43 (1996).
- Schwarten DE, Cutcliff WB: Arterial occlusive disease below the knee: treatment with percutaneous transluminal angioplasty performed with low-profile catheters and steerable guide wires. Radiology 169, 71–74 (1998).
- Horvarth W, Oertl M, Haidinger D: Percutaneous transluminal angioplasty of crural arteries. Radiology 177, 565–569 (1990).
- Collins R, Cranny G, Burch J et al.: A systematic review of duplex ultrasound, magnetic resonance angiography and computed tomography angiography for the diagnosis and assessment of symptomatic, lower limb peripheral arterial disease. Health Technol. Assess. 11, 1–184 (2007).
- Romano M, Mainenti PP, Imbriaco M: Multidetector row CT angiography of the abdominal aorta and lower extremities in patients with peripheral arterial occlusive disease: diagnostic accuracy and interobserver agreement. Eur. J. Rad. 50, 303–308 (2004).
- Rubin GD, Schmidt AJ, Logan LJ, Sofilos MC: Multi-dectector row CT angiography of lower extremity arterial inflow and runoff: initial experience. Radiology 221, 146–158 (2001).
- Visser K, Hunink MGM: Peripheral arterial disease: gadolinium enhanced MR angiography versus color-guided duplex US – a meta analysis. Radiology 216, 67–77 (2000).
- Owen RS, Carpenter JP, Baum RA, Perloff LK, Cope C: Magnetic resonance imaging of angiographically occult runoff vessls in peripheral arterial occlusive disease. N. Engl. J. Med. 326, 1577–1581 (1992).
- Kreitner K-F, Kalden P, Neufand A et al.: Diabetes and peripheral arterial occlusive disease: prospective comparison of contrastenhanced three-dimensional MR angiography with conventional digital subtraction angiography. Am. J. Roentgenol. 174, 171–179 (2000).
- Basil Trial Participants: Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL): multicentre, randomised controlled trial. Lancet 366, 1925–1934 (2005).
- Amann B, Luedemann C, Ratei R et al.: Autologous bone marrow cell transplantation increases leg perfusion and reduces amputations in patients with advanced critical limb ischemia due to peripheral artery disease. Cell Transplant. 18, 371–380 (2009).
- Kuhlmann MT, Klocke R, Nikol S: Therapeutic angiogenesis for peripheral artery disease: cytokine therapy. Vasa 36, 253–260 (2007).
- Hobeika MJ, Edlin RS, Muhs BE: Matrix metalloproteinases in critical limb ischaemia. J. Surg. Res. 149, 148–154 (2008).
- Veterans Administration Cooperative Study Group 141: Comparative evaluation of prosthetic, reversed, and in situ vein bypass grafts in distal popliteal and tibial–peroneal revascularization. Arch. Surg. 123, 434–438 (1998).
- Harris PL, Veith FJ, Shanik GD et al.: Prospective randomized comparison of in situ and reversed infrapopliteal vein grafts. Br. J. Surg. 80, 173–176 (1993).
- Galaria II, Surowiec SM, Tanski WJ et al.: Popliteal to distal bypass: identifying risk factors associated with limb loss and graft failure. Vasc. Endovasc. Surg. 39, 393–400 (2005).
- Goy J-J, Urban P: Life and limb: bypass versus angioplasty in the ischaemic limb. Lancet 366, 1905–1906 (2005).
- Cao P, De Rango P: Endovascular treatment of peripheral artery disease (PAD): so old yet so far from evidence! Eur. J. Vasc. Endovasc. Surg. 37, 501–503 (2009).
- McDaniel M, Cronenwett J: Basic data related to the natural history of intermittent claudication. Ann. Vasc. Surg. 3, 273–277 (1989).
- Lazaris AM, Salas C, Tsiamis AC et al.: Factors affecting patency of subintimal infrainguinal angioplasty in patients with critical lower limb ischaemia. Eur. J. Vasc. Endovasc. Surg. 32, 668–674 (2006).
- Bakal CW, Sprayregen S, Scheinbaum K, Cynamon J, Veith FJ: Percutaneous transluminal angioplasty of the infrapopliteal arteries: results in 53 patients. Am. J. Roentgenol. 154, 171–174 (1990).
- London NJM, Varty K, Sayers RD, Thompson MM, Bell PRF, Bolia A: Percutaneous transluminal angioplasty for lower limb critical ischaemia. Br. J. Surg. 82, 1232–1235 (1995).
- Nydahl S, Hartshorne T, Bell PRF, Bolia A, London NJM: Subintimal angioplasty of infrapopliteal occulsions in critically ischaemic limbs. Eur. J. Vasc. Endovasc. Surg. 14, 212–216 (1997).
- Spinosa DJ, Leung DA, Matsumoto AH et al.: Percutaneous intentional extraluminal recanalization in patients with chronic critical limb ischaemia. Radiology 232, 499–507 (2004).
- Met R, van Lienden KP, Koelemay MJW, Bipat S, Legemate DA, Reekers JA: Subintimal angioplasty for peripheral arterial occlusive disease: a systematic review. Cardiovasc. Intervent. Radiol. 31, 687–697 (2008).
- Rastogi MS, Stavropoulos SW: Infrapopliteal angioplasty. Tech. Vasc. Interv. Radiol. 7, 33–39 (2004).
- Spinosa DJ, Harthun NL, Bissonette EA et al.: Subintimal arterial flossing with antegraderetrograde intervention (SAFARI) for subintimal recanalization to treat chronic critical limb ischemia. J. Vasc. Interv. Radiol. 16, 37–44 (2005).
- Bosiers M, Deloose K, Moreialvar R, Verbist J, Peeters P: Current status of infrapopliteal artery stenting in patients with critical limb ischemia. J. Vasc. Bras. 7, 248–255 (2008).
- Biamino G: The excimer laser: science fiction fantasy or practical tool. J. Endovasc. Ther. 11(Suppl. II), 207–222 (2004).
- Laird JR, Zeller T, Gray BH et al.: Limb salvage following laser assisted angioplasty for critical limb ischaemia: results of the LACI multicenter trial. J. Endovasc. Ther. 13, 1–11 (2006).
- Stoner MC, deFreitas DJ, Phade SV: Mid-term results with laser atherectomy in the treatment of infrainguinal occlusive disease. J. Vasc. Surg. 46, 289–295 (2007).
- Tsetis DK, Michalis LK, Rees MR: Vibrational angioplasty in the treatment of chronic infrapopliteal arterial occlusions: preliminary experience. J. Endovasc. Ther. 9, 889–895 (2002).
- Canaud L, Alric P, Berthet J-P: Infrainguinal cutting balloon angioplasty in de novo arterial lesions. J. Vasc. Surg. 48, 1182–1188 (2008).
- Ansel GM, Sample NS, Botti CF 3rd Jr et al.: Cutting balloon angioplasty of the popliteal and infrapopliteal vessels for symptomatic limb ischaemia. Catheter Cardiovasc. Interv. 61, 1–4 (2004).
- Zeller T, Rastan A, Schwarzwalder U et al.: Midterm results after atherectomy-assisted angioplasty of below-knee arteries with use of the Silverhawk device. J. Vasc. Interv. Radiol. 15, 1391–1397 (2004).
- Suri R, Wholey MH, Postoak D et al.: Distal embolic protection during femoropopliteal atherectomy. Catheter Cardiovasc. Interv. 67, 417–422 (2006).
- Zeller T, Sixt S, Schwarzwalder U: Two-year results after directional atherectomy of infrapopliteal arteries with the SilverHawk device. J. Endovasc. Ther. 14, 232–240 (2007).
- Chung S, Sharafuddin M, Chigurupati R, Hoballah JJ: Midterm patency following atherectomy for infrainguinal occlusive disease: a word of caution. Ann. Vasc. Surg. 22, 358–365 (2008).
- Belli A-M, Cumberland DC, Knox AM, Procter AE, Welsh CL: The complcation rate of percutaneous peripheral balloon angioplasty. Clin. Rad. 41, 380–383 (1990).
- Khoury M, Rebecca A, Greene K et al.: Duplex scanning-guided thrombin injection for the treatment of iatrogenic pseudoaneurysms. J. Vasc. Surg. 35, 517–521 (2002).
- Sniderman KW, Bodner L, Saddekni S, Srur M, Sos TA: Percutaneous embolectomy by transcatheter aspiration. Radiology 150, 357–361 (1984).
- Falkoswski A, Poncyljusz W, Wilk G, Szczerbo-Trojanowska M: The evaluation of primary stenting of siroliumus-eluting versus bare-metal stents in the treatment of atherosclerotic lesions of crural arteries. Eur. Radiol. 19, 966–974 (2009).
- Siablis D, Karnabatidis D, Katsanos K, Diamantopoulos A, Christeas N, Kagadis GC: Infrapopliteal application of paclitaxel-eluting stents for critical limb ischemia: midterm angiographic and clinical results. J. Vasc. Interv. Radiol. 18, 1351–1361 (2007).
- Siablis D, Karnabatidis D, Katsanos K et al.: Sirolimus-eluting versus bare stents after suboptimal infrapopliteal angioplasty for critical limb ischemia: enduring 1‑year angiographic and clinical benefit. J. Endovasc. Ther. 14, 241–250 (2007).
- Griffiths H, Peeters P, Verbist J et al.: Future devices: bioabsorbable stents. Br. J. Cardiol. (Acute Interv. Cardiol.) 11, AIC80–AIC84 (2004).
▪ Randomized, controlled trial of angioplasty versus surgery in critical limb ischemia.
▪ Seminal paper on technique and results in subintimal angioplasty.
▪ Recent systematic review on the evidence for subintimal angioplasty.
▪ Good summary of current and future roles of stenting in the crural vessels.
Websites
101 National confidential enquiry into patient outcome and death. Interventional Vascular Radiology and Interventional Neurovascular Radiology (2000). www.ncepod.org.uk/2000ir.htm (Accessed June 2009).
102 Tsetis D, Belli A-M: Quality improvement guidelines for stenting in infrainguinal aterial disease. www.cirse.org/files/03_qig.pdf (Accessed September 2009).