Review Article - Interventional Cardiology (2012) Volume 4, Issue 6
Recent insights from scanning electron microscopic assessment of durable polymer-coated drug-eluting stents
- Corresponding Author:
- Clemens von Birgelen
Department of Cardiology
Thoraxcentrum Twente
Medisch Spectrum Twente
Haaksbergerstraat 55, 7513ER Enschede, The Netherlands
Tel: +31 53 487 2105
Fax: +31 53 487 2107
E-mail: c.vonbirgelen@mst.nl
Abstract
Keywords
bench-side testing, biodegradable polymer(s), coating irregularity(-ties), drug-eluting stent(s), durable polymer(s), in vitro, postdilatation (postdilation), scanning electron microscopy, stent surface, stent thrombosis
Drug-eluting stents (DES) were developed to improve invasive treatment of coronary artery disease by reducing the restenosis rate and the need for repeat revascularization procedures. This is achieved through antiproliferative drugs, which are carried and released from thin polymer- based coatings covering the metallic stent platforms. Since their introduction in 2002 [1,2], DES have become one of the most widely used medical devices [3–8].
Widespread utilization of DES entailed extensive clinical research, with the result that DES are one of the most examined medical devices in the clinical setting [9]. In addition, numerous laboratory examinations of DES, such as preclinical studies in animal models and postmortem studies, have been reported [10–12]. More technical bench-side assessment of the components of DES are generally performed by the developing parties and are occasionally accessible to the public [13].
Although there is no doubt that the results of large clinical trials are most significant for the evaluation of the safety and efficacy of medical implants, postmarketing bench-side research may provide additional insights that could help to interpret clinical performance. One may argue that locally reduced polymer thickness could theoretically promote restenosis, while a local increase in thickness of DES coating may locally delay the endothelialization process [14]. In addition, clinicians may consider data from bench-side examinations as complementary information to help guide their choice of device in some challenging lesions; in addition, insights from bench-top experiments may help to improve stent implantation techniques. Recently, a number of novel bench-side studies with scanning electron microscopy (SEM) addressed the surface morphology of polymerbased DES coatings [13–17]; in addition, some novel DES types have been introduced. This certainly justifies providing an updated overview of this particular field of research.
Surface of DES & rationale for morphologic assessment
The main function of a DES coating is to carry and deliver the antiproliferative drug to the vessel wall [9]. This is generally achieved by biodurable materials, which may also be called permanent polymers [18] or biodegradable polymeric layers [19], which need to be biocompatible and should maintain maximum physical integrity during challenging percutaneous coronary interventions as performed in daily practice [3–8]. The homogeneity and physical integrity of DES coatings may be important, as coating irregularities or defects might locally disturb drug-elution kinetics and elicit a neointimal response [20].
By use of SEM and other imaging techniques, surface irregularities of DES have been documented [15,20–22,101]. These irregularities could theoretically impair DES performance through different mechanisms. DES efficacy may be locally reduced as a result of decreased thickness or absence of the coating. In addition, the displacement of coating, with or without embolization of fragments of a relevant size, could lead to vascular or microvascular obstruction and myocardial necrosis. Another potential mechanism is that increased roughness of the DES surface may promote thrombus formation [22].
Initial observations of coating defects on dilated DES suggested that irregularities on DES coatings could be related to stent expansion and/or implantation maneuvers, and that coating defects may vary among DES types [101]. For that reason, a stepwise approach, ranging from the examination of unexpanded DES to the assessment of aggressively postdilated stents, was considered most appropriate to correlate coating irregularities on various DES types with interventional maneuvers.
Scanning electron microscopic assessment of DES coatings
SEM has a 3D character and the capacity to obtain highly magnified high-resolution images [24], which enables identification, classification and quantification of DES coating irregularities [20]. SEM uses a finely focused high-energy electron beam across the sample, causing the material to emit secondary electrons. These secondary electrons, and also the backscattered electrons, are of the greatest interest because these vary primarily as a result of differences in surface topography. The secondary electron emission results in SEM images at a resolution of approximately the size of the focused beam (the maximum resolution is 100,000-fold) [24].
▪ SEM & DES coating irregularities
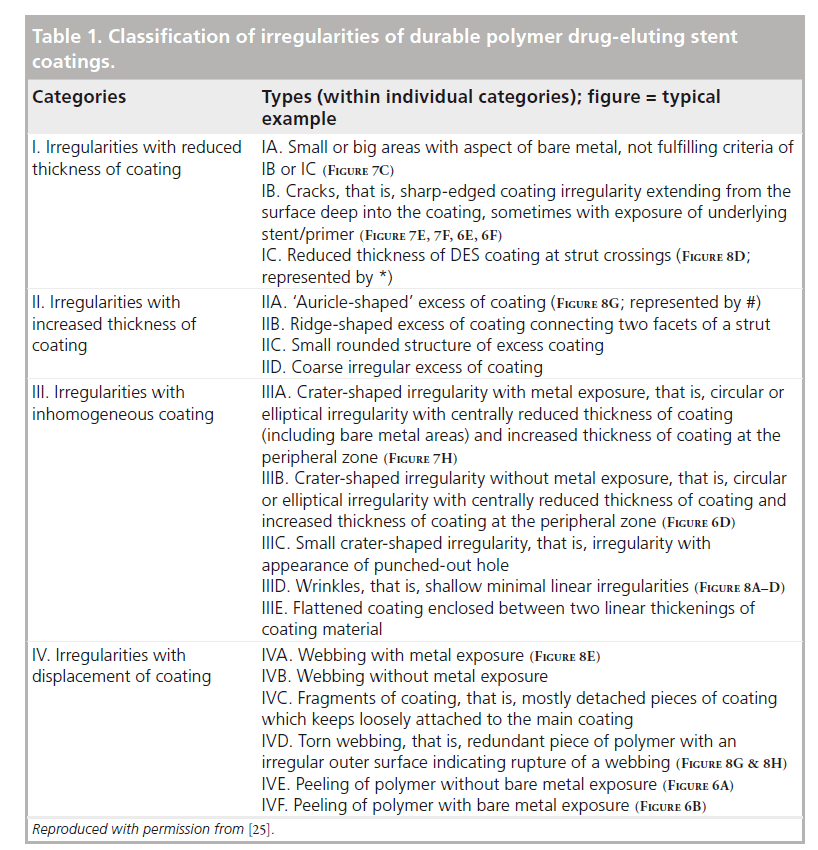
SEM assessment of DES revealed various coating irregularities with diverse morphologies and a wide range of severity and dimensions. For a better understanding of these irregularities, our group has developed a SEM-based classification of DES coating irregularities [14,20,25]. This classification has recently been followed by others [17]. Tab le 1 presents the most recent version of the SEMbased classification of DES coating irregularities, as introduced by our research group [14]. Based on the thickness and/or displacement of the polymer coating, we classified coating irregularities into four main categories: reduced coating thickness; increased coating thickness; inhomogeneous thickness of coating; and displaced coating.
▪ Reporting on the frequency of DES coating irregularities & defects
Semiquantitative approaches have been used to distinguish between minor marks and veryhigh- degree damage of DES coatings. Guérin et al., for instance, graded polymer coating damage as: no significant lesion (0%); low degree of damage (25%); moderate damage (50%); high degree of damage (75%); or complete destruction of the polymer coating (100%) [16]. This semiquantitative approach is easily applicable, but lacks a categorization of lesion types.
Our group expressed the incidence of coating irregularities as the number of coating irregularities per 60-fold magnification SEM field to facilitate meaningful comparisons of coating irregularities [20]. As the final magnification setting of the SEM changed slightly during focusing, correction factors were applied to adjust these slight differences in the size of the image field [20]. During our bench-side experiments, stents were partially postdilated with oversized balloons, which resulted in wider distances between stent struts (i.e., a lower strut density per image field) in the postdilated segments versus the nonpostdilated segments [26]. To permit meaningful comparisons between these stent regions, normalization of strut density was required. However, when comparing samples with a greater difference in strut density (e.g., unexpanded stents versus expanded stents), normalization would have carried a higher risk of distorting the results [25]. Therefore, in such instances we compared the incidence of coating irregularities per stent ring rather than applying the image-field, method outlined above [14]. However, different stent types may differ in the number of stent rings for the same length. This might limit the use of this method to compare different stent types. The use of normalization factors for the number of stents will facilitate comparisons between different stent types.
▪ DES coating morphology after gentle stent deployment
In this study, we performed SEM-based examinations of several durable polymer-coated DES, including a meticulous assessment of the incidence and dimensions of coating irregularities [20]. Four commercially available durable polymer-coated DES were examined following gentle deployment at 14 atm in a water bath: paclitaxel-eluting stents with styrene-b-isobutylene- b-styrene coating (Taxus Liberté™; Boston Scientific Corp., MA, USA); Zotarolimuseluting stents with phosphorylcholine coating (Endeavor Sprint™; Medtronic Cardiovascular, CA, USA); zotarolimus-eluting stents with Biolinx™ coating (Endeavor Resolute™; Medtronic Cardiovascular); and everolimuseluting stents with a fluoropolymer coating (Xience V™; Abbott Vascular, CA, USA) [20].
All four types of DES demonstrated coating irregularities, but the incidence and size of irregularities differed among the DES types examined. Some irregularities were located at characteristic spots of the stent that were exposed to increased mechanical stress during the process of stent expansion [26]. Cracks in the coating were found in phosphorylcholine-based Endeavor stents (referred to as Endeavor stents) and Endeavor Resolute, while Taxus Liberté was the only DES type that showed webbing with large bare-metal exposure [20]. Areas with bare-metal aspects were largest on Endeavor Sprint, while on Xience V the incidence of bare-metal areas was particularly low and their dimensions were small. The findings of this study suggested that there was on average an improvement in coating morphology from the early to the newer generation DES.
Each DES type showed some characteristic irregularities that were to some extent specific to that DES type. The geometry of the stent platform, details of the process of coating the stent, and both composition and physical characteristics of the coating (e.g., elasticity) may contribute to the reproducible shape and location of certain irregularities [19].
▪ Coating irregularities of unexpanded DES
Characteristic coating irregularities and their specific distribution patterns on different DES types, as reported above, raised the question of whether stent manufacturing and handling (e.g., the configuration of the stent in its folded state) might be involved in the formation of coating irregularities. For instance, adhesion of polymer coating to adjacent stent struts could promote certain irregularities and defects. We therefore examined both unexpanded and expanded samples of five contemporary DES with SEM to obtain insights into the potential mechanisms of formation of coating irregularities [14]. A total of 1200 SEM images of 15 expanded and 15 unexpanded stent samples of Cypher Select® Plus (sirolimus-eluting stents with PEVA/ PBMA copolymer-based coating; Cordis, NJ, USA), Taxus Liberté, Endeavor, Xience V and Endeavor Resolute were examined. Coating irregularities were identified on both unexpanded and expanded stent samples. In addition, in unexpanded DES samples, characteristic spots (predilection sites) of coating irregularities were carefully examined, taking the known individual distribution pattern of coating irregularities in the various DES types into account. We considered coating irregularities on unexpanded stents to be precursors to corresponding coating irregularities on expanded stents if they differed morphologically from those observed on the expanded samples, but shared the same characteristic location [14].
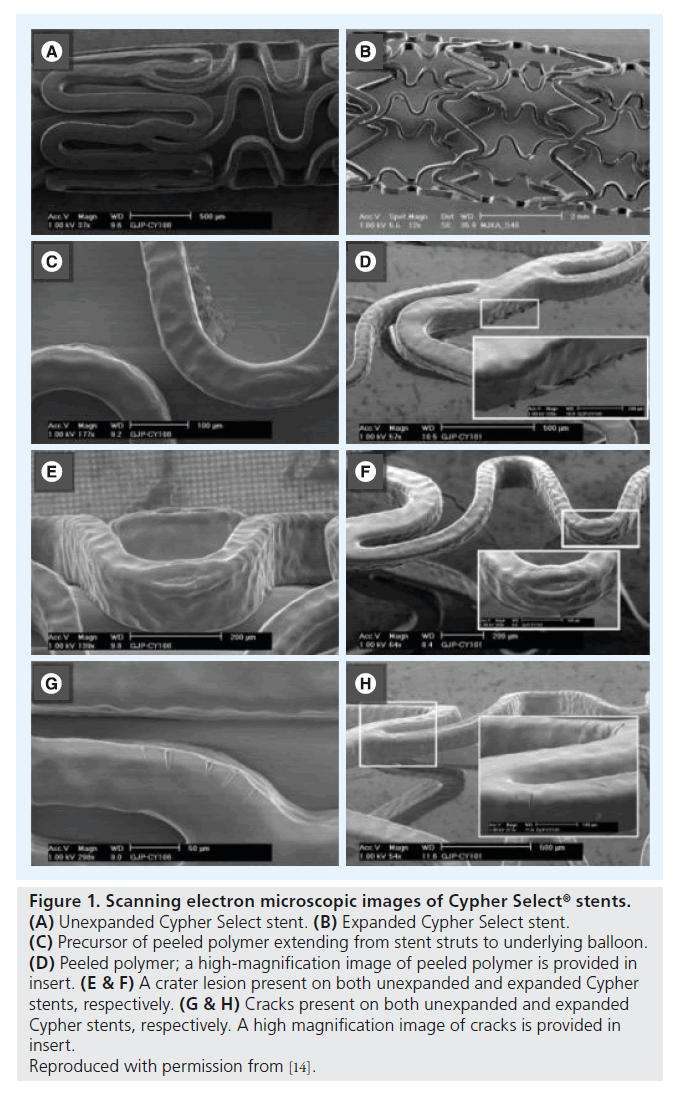
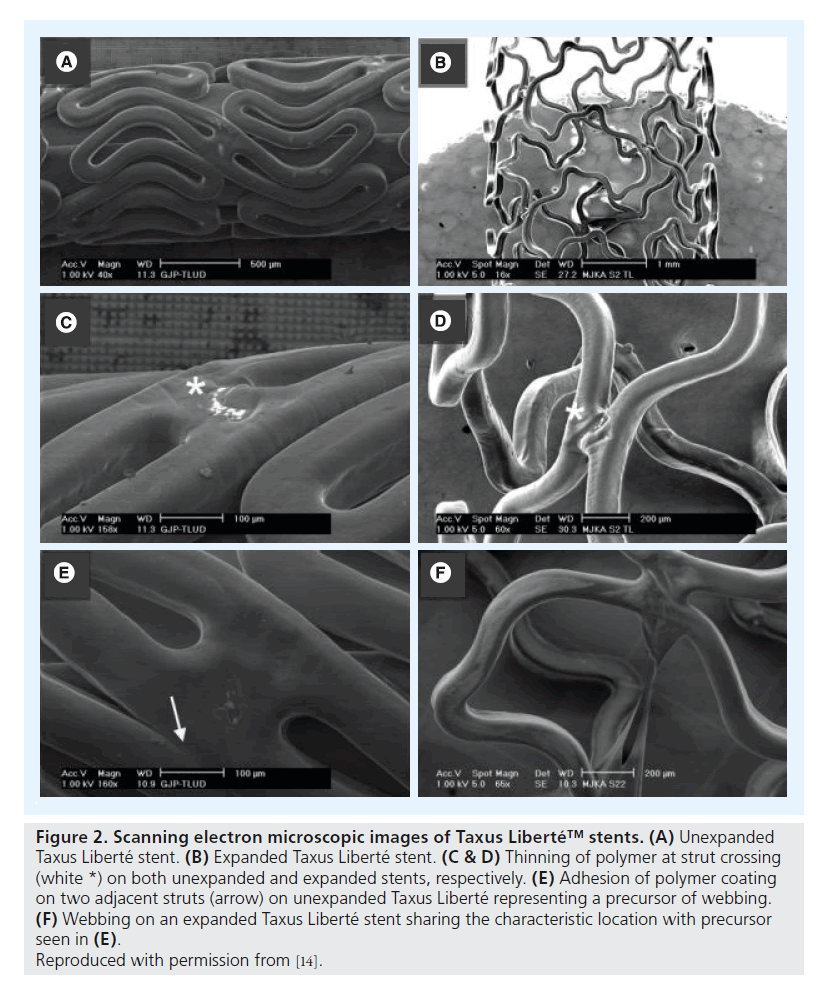
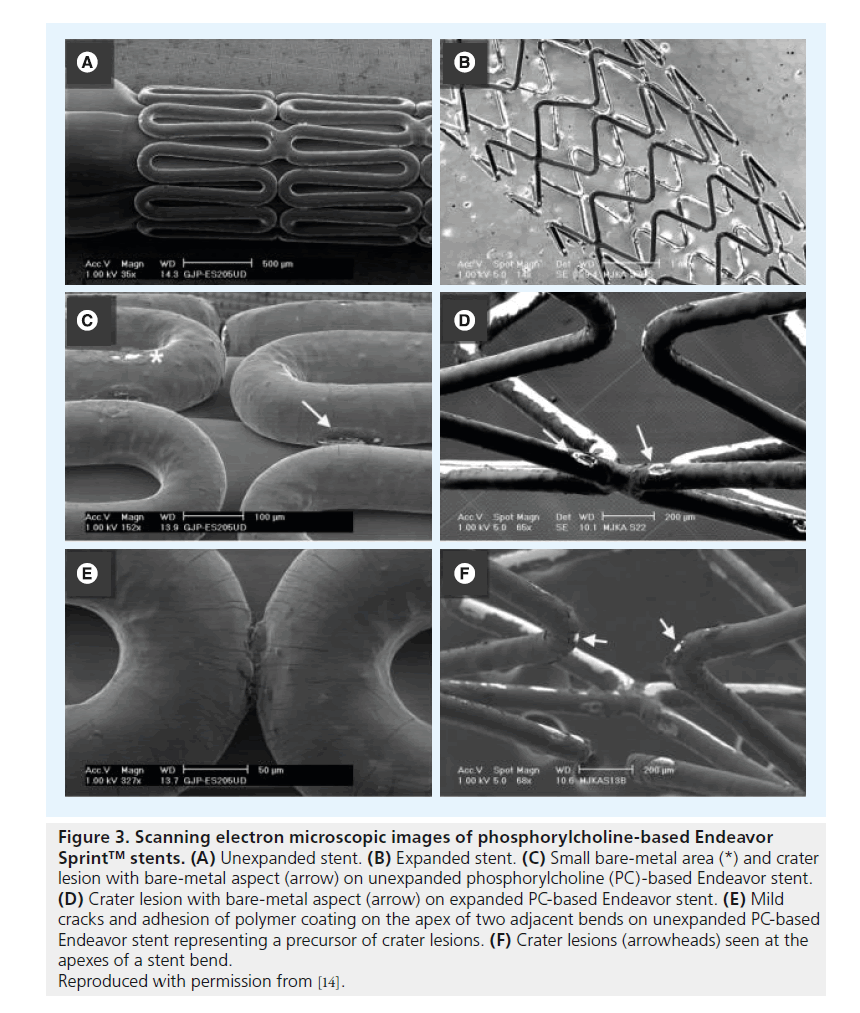
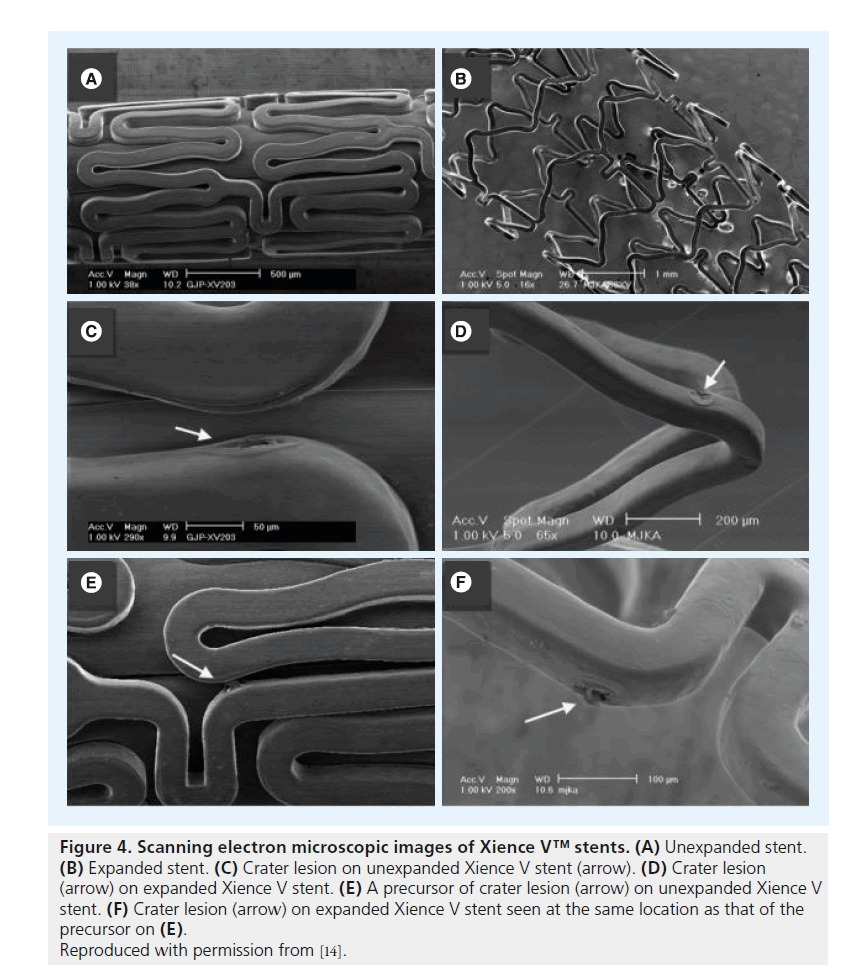
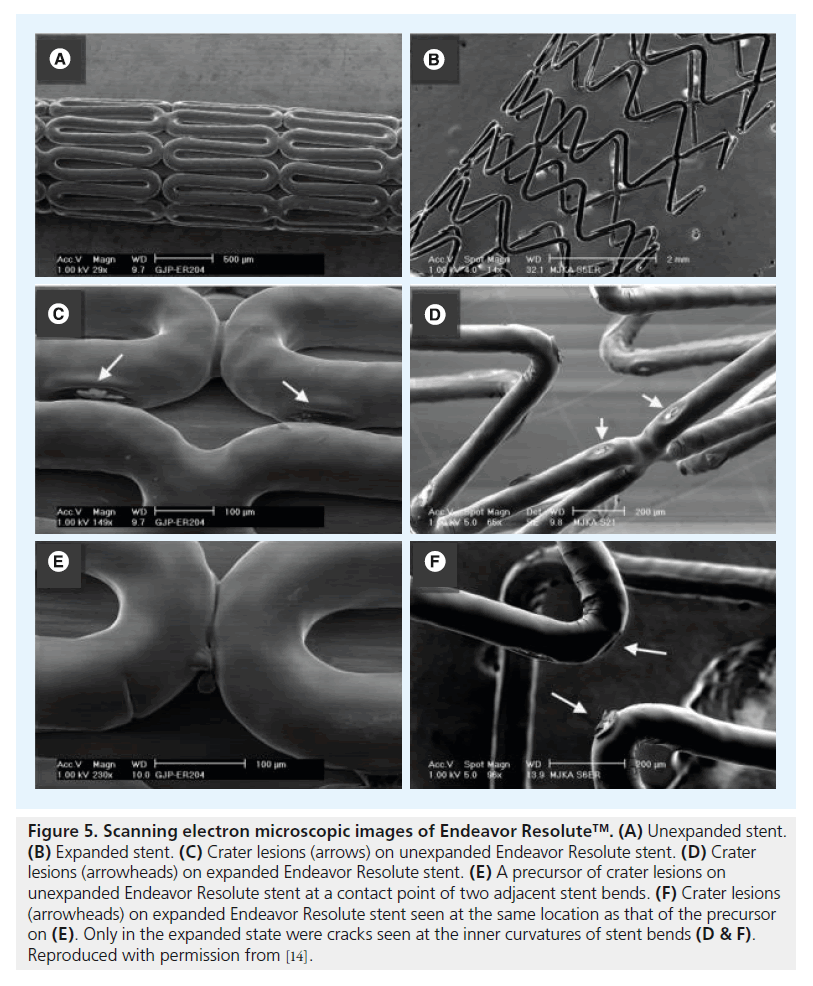
Assessment of unexpanded Cypher stents revealed (small) crater lesions and cracks together with precursors of ‘peeling’ (Figure 1). Unexpanded Taxus Liberté showed thinning of the polymer, small bare-metal areas, wrinkles and one type of precursor (Figure 2). Unexpanded Endeavor stents showed cracks, small bare-metal areas, crater lesions and their precursors (Figure 3). Assessment of unexpanded Xience V and Endeavor Resolute mainly revealed crater lesions and their precursors. Unexpanded and expanded DES did not significantly differ in the frequency of coating irregularities and precursors, with the only exception being more bare-metal areas on samples of expanded Taxus Liberté (Figures 4 & 5).
Figure 1. Scanning electron microscopic images of Cypher Select ® stents. (A) Unexpanded Cypher Select stent. (B) Expanded Cypher Select stent. (C) Precursor of peeled polymer extending from stent struts to underlying balloon. (D) Peeled polymer; a high-magnification image of peeled polymer is provided in insert. (E & F) A crater lesion present on both unexpanded and expanded Cypher stents, respectively. (G & H) Cracks present on both unexpanded and expanded Cypher stents, respectively. A high magnification image of cracks is provided in insert.
Reproduced with permission from [14].
Figure 2. Scanning electron microscopic images of Taxus Liberté™ stents. (A) Unexpanded Taxus Liberté stent. (B) Expanded Taxus Liberté stent. (C & D) Thinning of polymer at strut crossing (white *) on both unexpanded and expanded stents, respectively. (E) Adhesion of polymer coating on two adjacent struts (arrow) on unexpanded Taxus Liberté representing a precursor of webbing. (F) Webbing on an expanded Taxus Liberté stent sharing the characteristic location with precursor seen in (E).
Reproduced with permission from [14].
Figure 3. Scanning electron microscopic images of phosphorylcholine-based Endeavor Sprint™ stents. (A) Unexpanded stent. (B) Expanded stent. (C) Small bare-metal area (*) and crater lesion with bare-metal aspect (arrow) on unexpanded phosphorylcholine (PC)-based Endeavor stent. (D) Crater lesion with bare-metal aspect (arrow) on expanded PC-based Endeavor stent. (E) Mild cracks and adhesion of polymer coating on the apex of two adjacent bends on unexpanded PC-based Endeavor stent representing a precursor of crater lesions. (F) Crater lesions (arrowheads) seen at the apexes of a stent bend.
Reproduced with permission from [14].
Figure 4. Scanning electron microscopic images of Xience V™ stents. (A) Unexpanded stent. (B) Expanded stent. (C) Crater lesion on unexpanded Xience V stent (arrow). (D) Crater lesion (arrow) on expanded Xience V stent. (E) A precursor of crater lesion (arrow) on unexpanded Xience V stent. (F) Crater lesion (arrow) on expanded Xience V stent seen at the same location as that of the precursor on (E).
Reproduced with permission from [14].
Figure 5. Scanning electron microscopic images of Endeavor Resolute™. (A) Unexpanded stent. (B) Expanded stent. (C) Crater lesions (arrows) on unexpanded Endeavor Resolute stent. (D) Crater lesions (arrowheads) on expanded Endeavor Resolute stent. (E) A precursor of crater lesions on unexpanded Endeavor Resolute stent at a contact point of two adjacent stent bends. (F) Crater lesions (arrowheads) on expanded Endeavor Resolute stent seen at the same location as that of the precursor on (E). Only in the expanded state were cracks seen at the inner curvatures of stent bends (D & F).
Reproduced with permission from [14].
Notably, for 72% of the types of coating irregularities on expanded DES specimens, a matching irregularity and/or its precursor was observed on the unexpanded DES specimens [14]. This implies that most coating irregularities, or the potential to develop them, are inherent to the unexpanded DES. The configuration of unexpanded stents, as well as the physical properties of DES coatings, may be important determinants of the formation of coating irregularities. While crushing forces of the balloon during normal stent deployment do not play a major role.
DES coating integrity following stent postdilation maneuvers
As a following step, we then assessed the effect of postdilation on DES coating morphology [25]. In clinical practice, oversized postdilation of DES is performed in certain anatomical situations to avoid stent malapposition. Particularly in long lesions or major bifurcations, extremely oversized partial postdilation of stents may be required, which exposes DES coatings to extreme forces.
▪ Effect of stent postdilation on frequency & extent of DES coating irregularities
Changes in stent geometry as a result of postdilations can be well assessed with micro-computed tomography, a technique that has previously been established for stent examination by Ormiston et al., who used it for the investigation of bifurcation stenting [27]. This technique was also adopted by other research groups for the analysis of stent geometry [28,29]. Our group has used micro-computed tomography to assess the effect of aggressive partial postdilation of DES on stent geometry and to examine the potential relationship between SEM findings and stent deformation [26]. Partial stent postdilation resulted in the formation of three stent regions with different degrees of geometric deformation: the postdilated; nonpostdilated; and transitional stent region. Stent cells with the largest size were found in the transitional area for most DES types [26]. The different geometric deformations expose the DES coating to a variable amount of mechanical stress, which may promote the formation of dissimilar types and frequencies of coating irregularities on different types of DES.
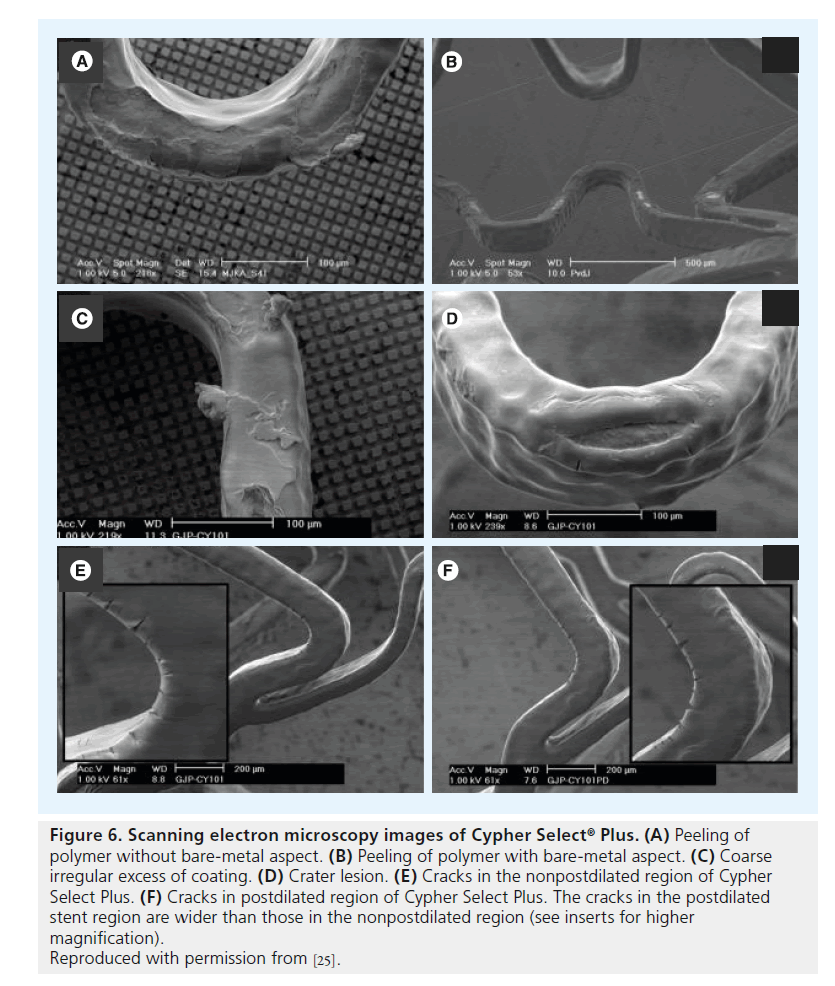
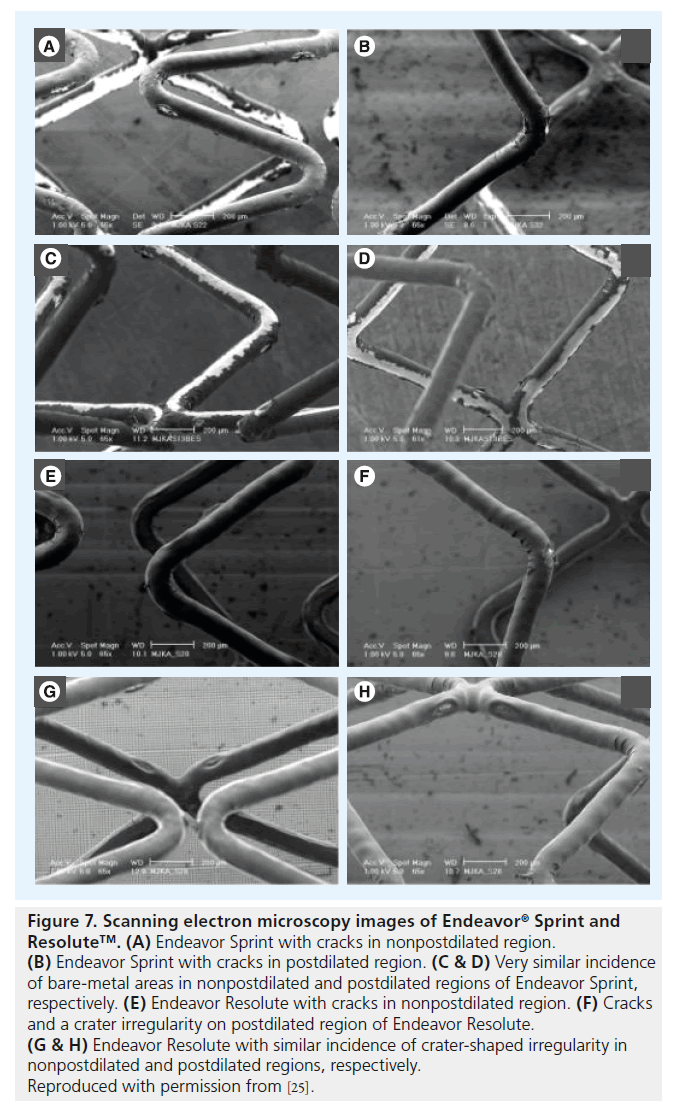
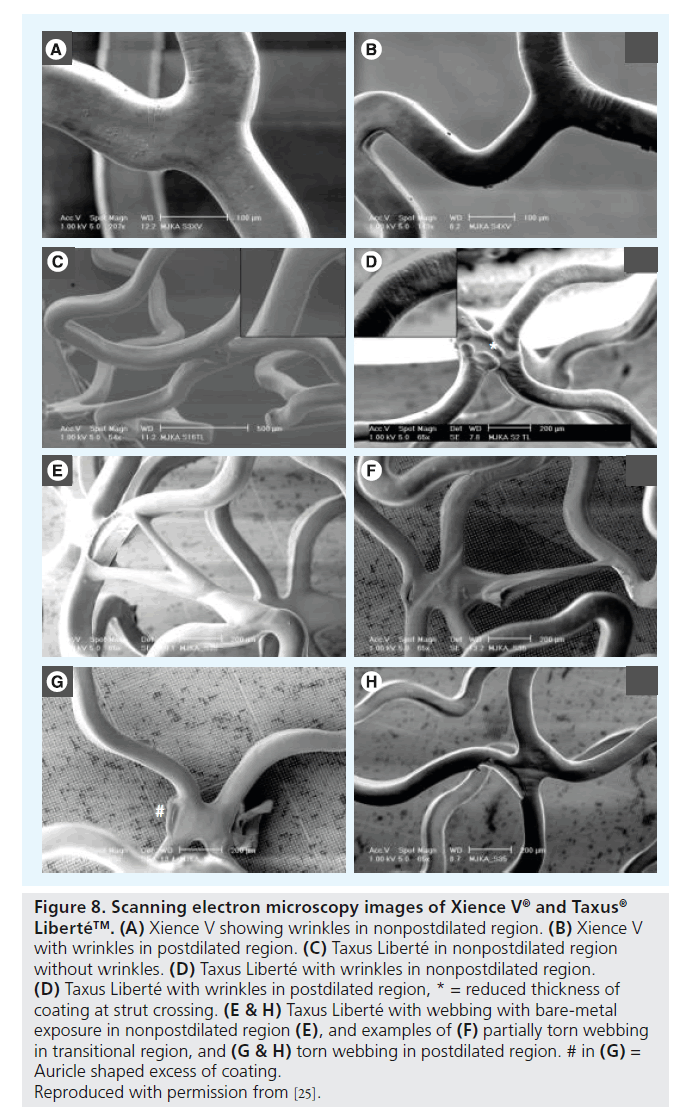
Extremely oversized partial postdilation of 3.5-mm stents (nominal size) with noncompliant 5.0 mm balloons at 18 atm was performed in a total of 15 DES samples (Cypher Select Plus, Taxus Liberté, Endeavor, Endeavor Resolute, and Xience V; three samples each), followed by SEM assessment of shape and incidence of DES coating irregularities [25]. SEM examination of a total of 660 images demonstrated that shape and incidence of coating irregularities in the postdilated and/or transitional regions of the stents differed only mildly from the nonpostdilated regions. Cypher Select Plus showed more peeling without the bare-metal aspect in the postdilated and transitional regions, and cracks were wider in the postdilated and transitional regions (Figure 6). In Taxus Liberté, one additional type of irregularity (torn webbing) and more wrinkles were observed. In Endeavor Resolute (Figure 7), wider cracks were found in the extremely postdilated region only; and Endeavor (Figure 7) and Xience V (Figure 8) showed no differences in the shape or incidence of coating irregularities between oversized and nonoversized stent regions [25].
Figure 6. Scanning electron microscopy images of Cypher Select ® Plus. (A) Peeling of polymer without bare-metal aspect. (B) Peeling of polymer with bare-metal aspect. (C) Coarse irregular excess of coating. (D) Crater lesion. (E) Cracks in the nonpostdilated region of Cypher Select Plus. (F) Cracks in postdilated region of Cypher Select Plus. The cracks in the postdilated stent region are wider than those in the nonpostdilated region (see inserts for higher magnification).
Reproduced with permission from [25].
Figure 7. Scanning electron microscopy images of Endeavor ® Sprint and Resolute™. (A) Endeavor Sprint with cracks in nonpostdilated region. (B) Endeavor Sprint with cracks in postdilated region. (C & D) Very similar incidence of bare-metal areas in nonpostdilated and postdilated regions of Endeavor Sprint, respectively. (E) Endeavor Resolute with cracks in nonpostdilated region. (F) Cracks and a crater irregularity on postdilated region of Endeavor Resolute. (G & H) Endeavor Resolute with similar incidence of crater-shaped irregularity in nonpostdilated and postdilated regions, respectively.
Reproduced with permission from [25].
Figure 8. Scanning electron microscopy images of Xience V ® and Taxus ® Liberté™. (A) Xience V showing wrinkles in nonpostdilated region. (B) Xience V with wrinkles in postdilated region. (C) Taxus Liberté in nonpostdilated region without wrinkles. (D) Taxus Liberté with wrinkles in nonpostdilated region. (D) Taxus Liberté with wrinkles in postdilated region, * = reduced thickness of coating at strut crossing. (E & H) Taxus Liberté with webbing with bare-metal exposure in nonpostdilated region (E), and examples of (F) partially torn webbing in transitional region, and (G & H) torn webbing in postdilated region. # in (G) = Auricle shaped excess of coating.
Reproduced with permission from [25].
Thus, even very aggressive postdilation resulted in no more than mild differences in coating irregularities between postdilated and nonpostdilated regions of durable polymercoated DES [25]. As a consequence, examination of less aggressive postdilation protocols would have been superfluous.
▪ Examination of DES after bifurcation stenting & simultaneous kissing balloon inflations
Restenosis rates after treatment of bifurcation lesions have been reduced since the introduction of DES, but in bifurcation lesions the ostium of the side branch still remains a predilection site for the development of restenosis [30–33]. Damage to the coating of DES arising from maneuvers that are performed during bifurcation stenting could potentially promote restenosis. Various techniques of bifurcation stenting have been suggested and applied. The diversity of all of these techniques prevents comprehensive SEM assessment of all the approaches applied for bifurcation stenting and imposes a more focused assessment of the most frequently applied stenting procedures.
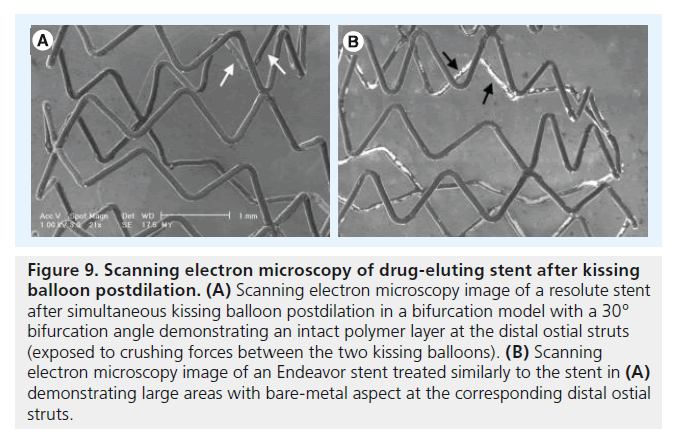
Postdilation of stents in a main branch and a side branch by simultaneous kissing balloon inflation is a frequently applied method that often leads to favorable acute results in both branches [34,35]. Guérin et al. recently reported SEM data on the consequences of simultaneous kissing balloon inf lations in five types of earlier generation DES (stent diameter of 3.0 mm): Cypher, Cypher Select, Endeavor, Taxus Express and Taxus Liberté. In that study, stents were deployed at the nominal pressure in a nonconstrained fashion; then simultaneous kissing balloon inflations were performed with two semicompliant balloons of a nominal size of 3.0 mm. A semiquantitative analysis of coating damage demonstrated significantly greater damage to the coating on ostial struts in all DES evaluated [16]. Our group currently uses SEM to gain insight into the coating damage of several, mostly contemporary DES. Figure 9 shows an example of the damaged stent coating on the ostial struts of an Endeavor stent after simultaneous kissing balloon inflations in a vessel phantom, as compared with the intact coating on the corresponding struts of an Endeavor Resolute stent following the same maneuver (i.e., the novel DES type shows more favorable coating characteristics). Both Endeavor DES and Endeavor Resolute DES share the same Driver cobalt–chromium stent platform (Medtronic, Medtronic Cardiovascular), but each DES type is covered with different polymer coatings, which clearly suggests that the observed difference in coating irregularities on the depicted stents is most likely related to differences in polymer coatings, which we previously emphasized [36].
Figure 9. Scanning electron microscopy of drug-eluting stent after kissing balloon postdilation. (A) Scanning electron microscopy image of a resolute stent after simultaneous kissing balloon postdilation in a bifurcation model with a 30° bifurcation angle demonstrating an intact polymer layer at the distal ostial struts (exposed to crushing forces between the two kissing balloons). (B) Scanning electron microscopy image of an Endeavor stent treated similarly to the stent in (A) demonstrating large areas with bare-metal aspect at the corresponding distal ostial struts.
SEM examination of novel DES
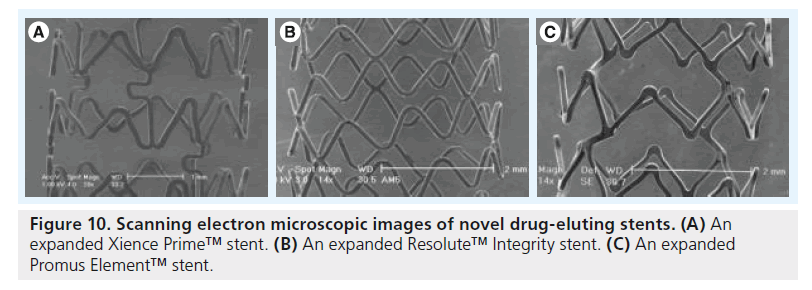
Recently, further stent refinement resulted in the development of so-called third-generation DES, which offer increased stent flexibility. A greater flexibility of the stent platform facilitates not only stent delivery in lesions with challenging anatomy, but also improves DES apposition to the vessel wall [37–39]. This is expected to optimize drug delivery and may reduce the risk of stent thrombosis. However, the changes in stent geometry and flexibility could perhaps also affect DES coating morphology. Systematic SEM examination of these new DES and subsequent comparison with images acquired from predecessor stents may provide insights into the effect of the aforementioned modifications in stent geometry on coating morphology. Figure 10 displays SEM images from ongoing research of our own group in the contemporary durable polymer DES Xience Prime™ (Abbott Vascular), Resolute™ Integrity (Medtronic, Medtronic Cardiovascular) and Promus Element™ (Boston Scientific Corp.).
Limitations of bench-top research with SEM
Bench-side studies do not exactly mimic conditions in vivo. DES with a somewhat less favorable appearance at SEM assessment may be clinically highly efficacious and safe. For instance, a higher hydrophilicity of certain DES coatings may compensate for a somewhat higher incidence of certain irregularities on these coatings [40]. The results of adequately powered comparative clinical studies are most important to form a prudent opinion on a certain type of DES [3–7,41]. However, bench-side studies, such as the assessment of DES with SEM, may represent a valuable addition to clinical research. Bench-top studies with SEM may contribute to the overall picture of a DES, together with stent testing in animal models [42], and the examination of other components of DES [43], such as assessment of the metallic stent platform with micro-computed tomography [13], or through computational methods [44]. Phantoms used in bench-side experiments are often made from silicon or Perspex, and do not perfectly resemble diseased coronary vessels. In clinical practice, potential shear between the abluminal surface of DES and the vessel wall during stent delivery may aggravate coating defects or lead to additional defects, depending on vessel tortuosity and calcification [45,46]. The avoidance of phantoms in unconstrained models eliminates the need for sample harvesting that could otherwise cause artificial defects, while possibly intensifying a few coating irregularities. However, the latter limitation may be more relevant for a DES coated with a biodegradable polymeric coating [47,101]. In a recent study, Yazdani et al. implanted a limited number of biodegradable and biodurable coated DES (n = 3 per group) in healthy porcine coronary arteries with subsequent harvesting of samples through enzymatic digestion of the coronary vessels. They compared DES coating defects after in vivo implantation to defects seen after expansion in vitro. In their study, 90% of the surface area of in vitro implanted samples demonstrated coating with defects versus 60% of the surface of the in vivo implanted stents, suggesting that the in vitro methodology may somewhat overestimate irregularities in biodegradable coating DES compared with biodurable coating DES [47]. Although there is no direct evidence to suggest that coating damage is related to subsequent neointimal proliferation or thrombus formation, there are adequate theoretical concerns that heterogeneous drug distribution on disturbed polymer coating may potentially act as a focus for inflammatory responses [48]. These concerns may be supported by the focal patterns of restenosis, as seen by intravascular ultrasound [49], and presence of thrombi, together with focal restenosis, as seen by angioscopy [50].
Future perspective
Progress in the development of durable coating DES has resulted in coating surfaces that show limited coating irregularities and that are robust in the setting of aggressive postdilation of stents [25]. SEM data suggested that many coating irregularities or their precursors are present on unexpanded DES samples [14]. This finding convinced us that many coating irregularities result from adhesion between coating material on adjacent stent struts, and for that reason, persistent attention to the process of loading coatings on stent platforms and the handling of freshly coated durable coating DES is warranted. Differences in the elastic properties of coatings on different DES may explain the generation of different coating irregularities as a result of shear stress or adhesion of adjacent stent struts. In certain types of DES coating, the presence of cracks at high shear-stress locations suggests that these particular coatings are less elastic than other DES coatings that show wrinkles at corresponding sites. Moreover, highly elastic polymer coatings will develop only strands (webbing) at points of adhesions of coating on adjacent stent struts, while in DES with less elasticity of the coatings, adhesions may result in other forms of coating irregularities such as crater lesions. Nevertheless, without exact knowledge of all technical details of the complex process of DES production, it is very difficult to attribute all irregularities observed to a specific cause.
Early-generation biodegradable coating DES recently demonstrated favorable long-term results as compared with first-generation durable coating DES [51,52]. Similar to the progress in the field of durable polymer DES, innovations in DES with biodegradable coatings have been made [53,54], which may trigger further research with SEM.
We feel that there is still a need to carefully assess novel DES with SEM. Such research can provide valuable information that may complement preclinical and clinical studies. While stent manufacturers have the obligation to continuously monitor the quality of their products and will generally strive to perfect their devices, SEM assessment as part of an external research program has been shown to provide valuable additional data that allow comparison of different DES types [13]. In view of this, interventional cardiologists may use complementary information from bench-side research as one of the pieces of available evidence to tailor therapy.
Financial & competing interests disclosure
C von Birgelen is consultant to Abbott, Medtronic and Boston Scientific; has received travel expenses from Biotronic; and has received a speaker’s honorarium from MSD and institutional grants from Abbott, Medtronic, Boston Scientific and Biotronic. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Surface of drug-eluting stents & rationale for morphologic assessment
▪ Coating irregularities or defects might locally disturb drug elution kinetics and elicit neointimal response.
▪ Drug-eluting stents (DES) efficacy may be locally reduced as a result of locally decreased thickness or absence of the coating.
▪ Displacement of coating could lead to (micro)vascular obstruction.
▪ Increased roughness of the DES surface may be associated with increased thrombogenicity.
Scanning electron microscopic assessment of DES coatings
▪ Scanning electron microscopy (SEM) is highly suitable for examination of DES coating morphology due to its 3D character and high resolution.
▪ Based on the thickness and/or displacement of coating, coating irregularities are classified into four main categories.
▪ Full quantitative analysis of coating irregularities allows accurate comparison between different DES types.
▪ After gentle stent deployment DES coating morphology showed some characteristic irregularities that were, to some extent, specific to DES types. Data suggest improvement in coating morphology between early and newer generation DES.
Coating irregularities of unexpanded DES
▪ For the majority of coating irregularities on expanded DES, a matching irregularity and/or its precursor was observed on the unexpanded DES samples.
▪ Most coating irregularities or the potential, to develop them, are inherent to the unexpanded DES.
▪ The configuration (stent design) of unexpanded stents, as well as the physical properties of DES coatings, may be important for formation of coating irregularities.
▪ Crushing forces of the balloon during normal stent deployment play no major role.
DES coating integrity following stent postdilation maneuvers
▪ Very aggressive postdilation resulted in no more than mild differences in coating irregularities between postdilated and nonpostdilated regions of durable polymer DES.
▪ Semiquantitative analysis of coating damage after simultaneous kissing balloon postdilation demonstrated a significantly greater damage to the coating on ostial struts in all early-generation DES evaluated.
SEM examination of novel DES
▪ Stent refinement resulted in the development of third-generation DES with increased stent flexibility.
▪ The effect of modifications in stent geometry on coating morphology may be examined by systematic SEM examination of novel DES and by comparing the findings with data from predecessor stents.
Future perspective
▪ Continuous attention to the process of loading coatings on stent platforms and handling of freshly coated durable coating DES is warranted.
▪ Introduction of newer generation biodegradable coating-based DES may trigger further SEM research.
References
Papers of special note have been highlighted as:
▪ of interest
- Morice MC, Serruys PW, Sousa JE et al. A randomized comparison of a sirolimuseluting stent with a standard stent for coronary revascularization. N. Engl. J. Med. 346(23), 1773–1780 (2002).
- Moses JW, Leon MB, Popma JJ et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med. 349(14), 1315–1323 (2003).
- Kedhi E, Joesoef KS, McFadden E et al. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet 375(9710), 201–209 (2010).
- Sen H, Tandjung K, Basalus MW et al. Comparison of eligible non-enrolled patients and the randomised TWENTE trial population treated with Resolute and Xience V drug-eluting stents. EuroIntervention 8, 336–344 (2012).
- Serruys PW, Silber S, Garg S et al. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N. Engl. J. Med. 363(2), 136–146 (2010).
- Stone GW, Rizvi A, Sudhir K et al. Randomized comparison of everolimus- and paclitaxel-eluting stents. 2 year follow-up from the SPIRIT (Clinical evaluation of the XIENCE V everolimus eluting coronary stent system) IV trial. J. Am. Coll. Cardiol. 58(1), 19–25 (2011).
- von Birgelen C, Basalus MW, Tandjung K et al. A randomized controlled trial in second-generation zotarolimus-eluting resolute stents versus everolimus-eluting xience V stents in real-world patients: the TWENTE trial. J. Am. Coll. Cardiol. 59(15), 1350–1361 (2012).
- Windecker S, Serruys PW, Wandel S et al. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomised non-inferiority trial. Lancet 372(9644), 1163–1173 (2008).
- Garg S, Serruys PW. Coronary stents: current status. J. Am. Coll. Cardiol. 56(Suppl. 10), S1–S42 (2010).
- Perez de Prado A, Perez-Martinez C, Cuellas C et al. Preclinical evaluation of coronary stents: focus on safety issues. Curr. Vasc. Pharmacol. doi:10.1161/ 01.CIR.0000145164. 85178.2E (2012) (Epub ahead of print).
- Foerst JR, Ball TC, Vorpahl M, Virmani R, Kaplan AV. Post-mortem micro computed tomography evaluation of very late stent thrombosis. J. Am. Coll. Cardiol. 56(2), e3 (2010).
- Wilson GJ, Huibregtse BA, Pennington DE, Dawkins KD. Comparison of the SYNERGY with the PROMUS (XIENCE V) and bare metal and polymer-only element control stents in porcine coronary arteries. EuroIntervention 8(2), 250–257 (2012).
- Basalus MW, von Birgelen C. Benchside testing of drug-eluting stent surface and geometry. Int. Cardiol. 2(2), 159–175 (2010).
- Basalus MW, Tandjung K, van Westen T et al. Scanning electron microscopic assessment of coating irregularities and their precursors in unexpanded durable polymerbased drug-eluting stents. Catheter Cardiovasc. Interv. 79(4), 644–653 (2012).
- Denardo SJ, Carpinone PL, Vock DM, Batich CD, Pepine CJ. Changes to polymer surface of drug-eluting stents during balloon expansion. JAMA 307(20), 2148–2150 (2012).
- Guérin P, Pilet P, Finet G et al. Drug-eluting stents in bifurcations: bench study of strut deformation and coating lesions. Circ. Cardiovasc. Interv. 3(2), 120–126 (2010).
- Ijichi T, Nakazawa G. TCT-224: coating irreguralities of drug-eluting stents as assesed by scanning electron microscopy. J. Am. Coll. Cardiol. 58(Suppl. 20), B60 (2011).
- Finn AV, Nakazawa G, Kolodgie FD, Virmani R. Temporal course of neointimal formation after drug-eluting stent placement: is our understanding of restenosis changing? JACC Cardiovasc. Interv. 2(4), 300–302 (2009).
- Byrne RA, Joner M, Kastrati A. Polymer coatings and delayed arterial healing following drug-eluting stent implantation. Minerva Cardioangiol. 57(5), 567–584 (2009).
- Basalus MW, Ankone MJ, van Houwelingen GK, de Man FH, von Birgelen C. Coating irregularities of durable polymer-based drug-eluting stents as assessed by scanning electron microscopy. EuroIntervention 5(1), 157–165 (2009).
- Otsuka Y, Chronos NA, Apkarian RP, Robinson KA. Scanning electron microscopic analysis of defects in polymer coatings of three commercially available stents: comparison of BiodivYsio, Taxus and Cypher stents. J. Invasive Cardiol. 19(2), 71–76 (2007).
- Basalus MW, van Houwelingen KG, Ankone M, de Man FH, von Birgelen C. Scanning electron microscopic assessment of the biodegradable coating on expanded biolimus-eluting stents. EuroIntervention 5(4), 505–510 (2009).
- Hecker JF, Scandrett LA. Roughness and thrombogenicity of the outer surfaces of intravascular catheters. J. Biomed. Mater. Res. 19(4), 381–395 (1985).
- Enderle JD, Bronzino JD. Introduction to Biomedical Engineering. Academic Press, MA, USA (2011).
- Basalus MW, Tandjung K, van Apeldoorn AA, Ankone MJ, von Birgelen C. Effect of oversized partial postdilatation on coatings of contemporary durable polymer-based drug-eluting stents: a scanning electron microscopy study. J. Interv. Cardiol. 24(2), 149–161 (2011).
- Basalus MW, van Houwelingen KG, Ankone MJ, Feijen J, von Birgelen C. Microcomputed tomographic assessment following extremely oversized partial postdilatation of drug-eluting stents. EuroIntervention 6(1), 141–148 (2010).
- Ormiston JA, Webster MW, Webber B, Stewart JT, Ruygrok PN, Hatrick RI. The “crush” technique for coronary artery bifurcation stenting: insights from microcomputed tomographic imaging of bench deployments. JACC Cardiovasc. Interv. 1(4), 351–357 (2008).
- Hikichi Y, Inoue T, Node K. Benefits and limitations of cypher stent-based bifurcation approaches: in vitro evaluation using micro-focus CT scan. J. Int. Cardiol. 22(2), 128–134 (2009).
- Mortier P, Van Loo D, De Beule M et al. Comparison of drug-eluting stent cell size using micro-CT: important data for bifurcation stent selection. EuroIntervention 4(3), 391–396 (2008).
- Steigen TK, Maeng M, Wiseth R et al. Randomized study on simple versus complex stenting of coronary artery bifurcation lesions. Circulation 114(18), 1955–1961 (2006).
- Lefevre T, Louvard Y, Morice MC et al. Stenting of bifurcation lesions: classification, treatments, and results. Catheter Cardiovasc. Interv. 49(3), 274–283 (2000).
- Colombo A, Moses JW, Morice MC et al. Randomized study to evaluate sirolimuseluting stents implanted at coronary bifurcation lesions. Circulation 109(10), 1244–1249 (2004).
- Pan M, Suárez de Lezo J, Medina A et al. Drug-eluting stents for the treatment of bifurcation lesions: a randomized comparison between paclitaxel and sirolimus stents. Am. Heart J. 153(1), 15 (2007).
- Sharma SK. Simultaneous kissing drugeluting stent technique for percutaneous treatment of bifurcation lesions in large-size vessels. Catheter Cardiovasc. Interv. 65(1), 10–16 (2005).
- Sharma SK, Choudhury A, Lee J et al. Simultaneous kissing stents (SKS) technique for treating bifurcation lesions in medium-tolarge size coronary arteries. Am. J. Cardiol. 94(7), 913–917 (2004).
- von Birgelen C, Basalus MW. On the loss of the phosphorylcholine-based DES coating on the abluminal surface of endeavor stents. Catheter Cardiovasc. Interv. 76, 158–159 (2010).
- Capodanno D, Tamburino C. Unraveling the EXCEL: promises and challenges of the next trial of left main percutaneous coronary intervention. Int. J. Cardiol. 156(1), 1–3 (2012).
- Sarno G, Lagerqvist B, Carlsson J et al. Initial clinical experience with an everolimus eluting platinum chromium stent (Promus Element) in unselected patients from the Swedish coronary angiography and angioplasty registry (Scaar). Int. J. Cardiol. doi:10.1016/j. ijcard.2011.12.057 (2012) (Epub ahead of print).
- Turco MA. The Integrity bare-metal stent made by continuous sinusoid technology. Expert Rev. Med. Devices 8(3), 303–306 (2011).
- Hezi-Yamit A, Sullivan C, Wong J et al. Impact of polymer hydrophilicity on biocompatibility: implication for DES polymer design. J. Biomed. Mater. Res. A. 90(1), 133–141 (2009).
- Tandjung K, Basalus MW, Sen H et al. DUrable polymer-based sTent CHallenge of Promus ElemEnt versus ReSolute integrity (Dutch peers): rationale and study design of a randomized multicenter trial in a Dutch all-comers population. Am. Heart J. 163(4), 557–562 (2012).
- Nakazawa G, Finn AV, Ladich E et al. Drug-eluting stent safety: findings from preclinical studies. Expert Rev. Cardiovasc. Ther. 6(10), 1379–1391 (2008).
- Ako J, Bonneau HN, Honda Y, Fitzgerald PJ. Design criteria for the ideal drug-eluting stent. Am. J. Cardiol. 100(8B), 3M–9M (2007).
- Mortier P, De Beule M, Dubini G, Hikichi Y, Murasato Y, Ormiston JA. Coronary bifurcation stenting: insights from in vitro and virtual bench testing. EuroIntervention 6(Suppl. J), J53–J60 (2010).
- Kitahara H, Kobayashi Y, Yamaguchi M et al. Damage to polymer of undelivered sirolimuseluting stents. J. Invasive Cardiol. 20(3), 130–133 (2008).
- Wiemer M, Butz T, Schmidt W, Schmitz KP, Horstkotte D, Langer C. Scanning electron microscopic analysis of different drug eluting stents after failed implantation: from nearly undamaged to major damaged polymers. Catheter Cardiovasc. Interv. 75(6), 905–911 (2010).
- Yazdani SK, Vorpahl M, Nakano M, Su SH, Kolodgie FD, Virmani R. In vitro and in vivo characterisation of biodegradable polymerbased drug-eluting stent. EuroIntervention 7(7), 835–843 (2011).
- Farooq V, Gogas BD, Serruys PW. Restenosis: delineating the numerous causes of drug-eluting stent restenosis. Circ. Cardiovasc. Interv. 4(2), 195–205 (2011).
- Fujii K, Mintz GS, Kobayashi Y et al. Contribution of stent underexpansion to recurrence after sirolimus-eluting stent implantation for in-stent restenosis. Circulation 109(9), 1085–1088 (2004).
- Oikawa Y, Yajima J, Costa MA et al. Intravascular ultrasound, angioscopic and histopathological characterisation of heterogeneous patterns of restenosis after sirolimus-eluting stent implantation: insights into potential “thromborestenosis” phenomenon. EuroIntervention 6(3), 380–387 (2010).
- Raber L, Kelbaek H, Ostoijc M et al. Comparison of biolimus eluted from an erodible stent coating with bare metal stents in acute ST-elevation myocardial infarction (COMFORTABLE AMI trial): rationale and design. EuroIntervention 7(12), 1435–1443 (2012).
- Stefanini GG, Kalesan B, Serruys PW et al. Long-term clinical outcomes of biodegradable polymer biolimus-eluting stents versus durable polymer sirolimus-eluting stents in patients with coronary artery disease (Leaders): 4 year follow-up of a randomized non-inferiority trial. Lancet 378(9807), 1940–1948 (2003).
- Hamon M, Normandy F. First-in-man experience with the DES Orsiro in the treatment of patients with single de novo coronary artery lesions (Bioflow-I).
- Late breaking first-in-human trials session. Presented at: EuroPCR 2011. Paris, France, 17–20 May 2011. 54 Meredith IT, Verheye S, Dubois CL et al. Primary endpoint results of the EVOLVE trial: a randomized evaluation of a novel bioabsorbable polymer-coated, everolimuseluting coronary stent. J. Am. Coll. Cardiol. 59(15), 1362–1370 (2012).
▪ Comprehensive review on drug-eluting stents.
▪ Review on the use of scanning electron microscopy and micro-computed tomography for examination of drug-eluting stents.
▪ Recent bench-side study examining coating irregularities on the surface of unexpanded drug-eluting stents.
▪ Recent bench-side study examining the release of free particles after drug-eluting stent expansion.
▪ Bench-side study examining the relationship between the extent of stent dilation and the severity of coating irregularities.
▪ Review on preclinical studies on drug- eluting stents.
▪ Review on components of drug-eluting stents.
▪ Interesting review on possible mechanisms involved in restenosis.
▪ Website
101. Ormiston J, Peal WMR. Polymer integrity after cypher and taxus stent implantation: a scanning electron microscope study. www.tctmd.com