Case Report - International Journal of Clinical Rheumatology (2017) Volume 12, Issue 4
Recurrent life-threatening bleeding due to acquired clotting factor XIII inhibitor in a Chinese patient with systemic lupus erythematosus
- *Corresponding Author:
- Jacky Ka Hing Chan
Department of Medicine
Intensive Care Unit
Tseung Kwan O Hospital
Tseung Kwan O, Hong Kong
E-mail: ckhj01@ha.org.hk
Abstract
A 52-year-old Chinese male with systemic lupus erythematosus presented with recurrent retroperitoneal haematoma and finally died of aggressive bleeding complications related to acquired clotting factor XIII inhibitor. We review different aspects of this rare but severe bleeding disorder with normal baseline coagulation studies.
Keywords
recurrent, bleeding, clotting factor XIII inhibitor, systemic lupus erythematosus
Introduction
Inhibitor associated clotting factor XIII deficiency is a potentially life-threatening bleeding disorder with normal baseline coagulation studies, and may be associated with various drugs and autoimmune diseases. Failure to recognize this rare condition may lead to catastrophic outcome. We report a 52-year-old male with systemic lupus erythematosus (SLE) presented with recurrent spontaneous haematoma due to acquired inhibitor to clotting factor XIII, resulting in fatal outcome. In this report, we also discuss thoroughly on the biology of factor XIII, clinical features, investigations and management of patients with acquired factor XIII inhibitor.
Case
A 52 year-old Chinese male presented with thrombocytopenia, haemolytic anaemia, proteinuria and was diagnosed systemic lupus erythematosus (SLE) in 2006. Renal biopsy showed lupus nephritis WHO class IIb. There was also skin involvement and biopsy revealed bullous lupus erythematosus. He had spontaneous spleen rupture with hemoperitoneum in 2007 and spontaneous haematoma in left iliopsoas muscle in 2014. Both episodes were managed with blood product transfusion and recombinant factor VIIa (rFVIIa). He developed acute kidney injury with serum creatinine up to 306 μmol/L. Baseline coagulation studies including prothrombin time (PT), activated partial thromboplastin time (aPTT); platelet, fibrinogen and bleeding time were unremarkable. Mild lupus anti-coagulant was present in the serum. He was then followed up by private rheumatologist and put on mycophenolate mofetil (MMF).
He was admitted again on 4th July 2016 for two days history of right lower quadrant and right hip pain. It was increasing in severity and exacerbated by right hip flexion. There was no history of recent trauma. He had no loin pain, bowel or urinary symptoms. He had no fever, joint pain or oral ulcers. Blood pressure was 115/75 mmHg and pulse rate was 76/min. Examination of abdomen demonstrated old incisional hernia and right lower quadrant tenderness. Range of movement of right hip was limited because of pain. Haemoglobin level dropped from the baseline of 11.4 g/dL to 9.7 g/dL. White cell count was normal and platelet count was 358 x 109. International normalized ratio (INR) was 1.1 and aPTT was 23.4. Fibrinogen was mildly raised to 4.27 g/L. Erythrocyte sedimentation rate was 82 and C-reactive protein was 9.1 mg/L. He had normal renal function but impaired liver function. Anti-double stranded DNA was <30 IU/ml and Complement 3(C3) was static at 0.62 g/L. X-ray right hip showed no fracture or dislocation. Contrast computer tomography (CT) scan of abdomen and pelvis showed a large acute right retroperitoneal and psoas haematoma with tiny actively bleeding sites.
He was assessed by both orthopaedic surgeon and general surgeon. There was no urgent surgical intervention required. His CT result was also reviewed by radiologist and his condition was not amenable with embolization. His haemoglobin level further dropped to 5.7 g/dL three days after admission despite packed cells transfusion. He developed shock with systolic blood pressure about 90 mmHg and tachycardia. Fluid replacement, packed cells transfusion and dopamine infusion were initiated. He was then transferred to intensive care unit for further management on 7th July 2016.
He received repeated packed cells transfusion but there was no improvement in haemoglobin level. There was ongoing abdominal distension and progressive increase in oxygen requirement due to diaphragmatic splinting and fluid overload. Assessment was also performed by haematologist and rheumatologist. Bleeding episodes were either related to clotting factor inhibitor or vasculitis. Inhibitors to various clotting factors were sent for investigation. Initiation of immunosuppressant was planned if the results came back to be positive.
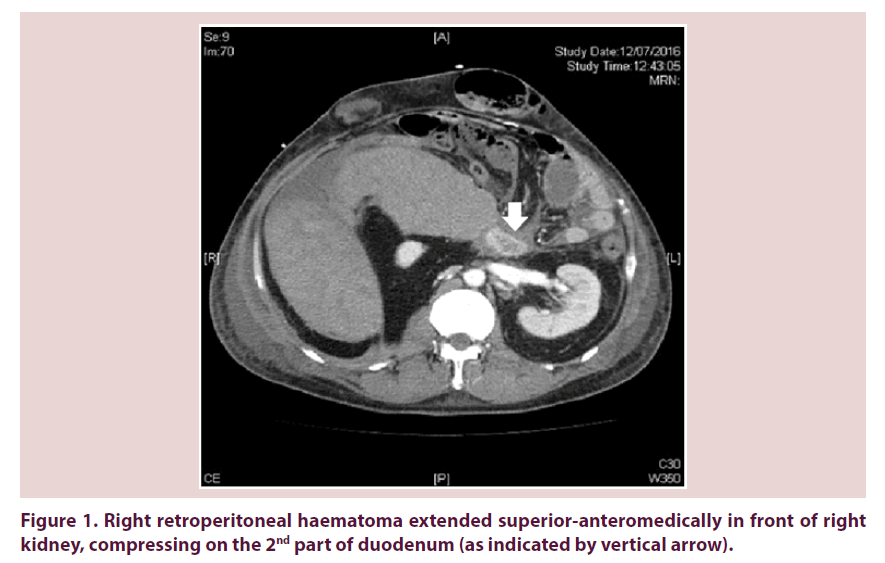
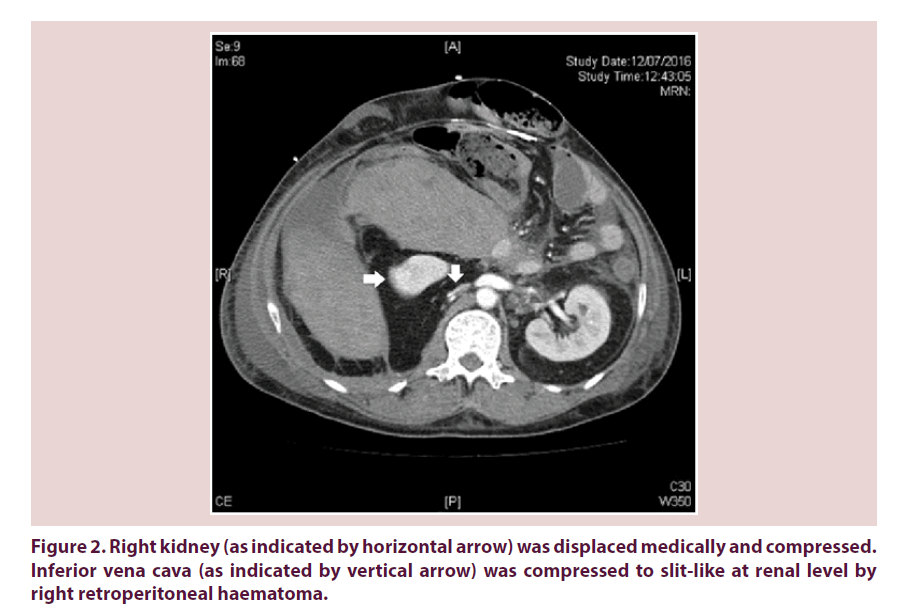
Our patient required escalating dose of inotropes on 12th July 2016 and recombinant factor VIIa was arranged for control of bleeding. Urgent contrast CT abdomen and pelvis was done. Retroperitoneal haematoma showed interval enlargement and measured 15 cm x 13 cm x 16.6 cm. It extended superioranteromedically in front of right kidney, compressing on the 2nd part of duodenum (Figure 1). Right kidney was displaced medically and compressed (Figure 2). Inferior vena cava (IVC) was also compressed to slitlike at renal level (Figure 2). Venous flow was diverted to pelvic veins and hepatic IVC was flatted due to impaired venous return.
Our patient developed pulseless electrical activity (PEA) shortly after transfer back from CT suite. Cardiopulmonary resuscitation (CPR) with fluid and blood product replacement was started immediately. He failed to regain spontaneous circulation after ninety minutes of CPR and finally succumbed. The cause of PEA was postulated to be obstructive shock related to high retroperitoneal haematoma compressing on IVC.
Results of clotting factor inhibitors (Table 1) were available one month after patient’s death. Level of clotting factor XIII (F13) was markedly reduced. Clot lysis was still seen on 1:1 mixing with normal control plasma. Level of factor VII (F7) was moderately reduced whereas level of factor V (F5) was mildly reduced. This was probably related to impairment of liver function. Elevated factor VIII (F8) and Von Willebrand factor represents an acute phase response. Overall findings are suggestive of the presence of specific clotting factor XIII (F13) inhibitor.
| Values | Ref. Range | |
|---|---|---|
| Clotting factors group | ||
| Factor II | 0.75 | 0.5-1.5 IU/ml |
| Factor V | 0.45(L) | 0.5-1.5 IU/ml |
| Factor VII | 0.36(L) | 0.5-1.5 IU/ml |
| Factor VIII | 3.62(H) | 0.5-1.5 IU/ml |
| Factor IX | 1.47 | 0.5-1.5 IU/ml |
| Factor X | 0.95 | 0.5-1.5 IU/ml |
| Factor XIII | 0.01(L) | 0.5-1.5 IU/ml |
| Factor XIII screen | Abnormal | Normal |
| Inhibitor group | ||
| PT inhibitor | Normal | Normal |
| Von Willebrand factor group | ||
| VWF | 3.15(H) | 0.5-2.0 IU/ml |
Table 1. Results of clotting factor inhibitors.
Our patient, who had a history of systemic lupus erythematosus (SLE) on treatment, developed repeated episodes of post-traumatic or spontaneous haematoma related to acquired clotting factor XIII inhibitor and was complicated by lethal consequence.
Discussion
Factor XIII is the final enzyme in the coagulation cascade and is used for catalyzing intermolecular cross-linking of fibrin polymers, thus increasing the mechanical rigidity of fibrin clot. It exists as a tetrameric zymogen of two A subunits and two B subunits. Plasma thrombin, together with fibrinogen and calcium, convent it into an active transglutaminase (factor XIIIa) by enhancing cleavage of the activation peptide from A subunit, followed by dissociation of the A and B subunits [1]. Enzymatically active factor XIIIa catalyzes calcium-dependent cross-linking of fibrin monomers to form insoluble fibrin polymers that stabilize the platelet-fibrin clot at a site of haemorrhage due to vascular injury. Factor XIIIa can further modify the clot structure by covalently cross-linking antifibrinolytic proteins such as alpha2-antiplasmin (a rapid and effective inhibitor of the fibrinolytic enzyme plasmin) and thrombin-activatable fibrinolysis inhibitor (TAFI) into fibrin. Factor XIIIa prevents alpha2-antiplasmin from being expelled from plasma clot during compaction and strongly suppresses fibrinolysis [2]. Anti-fibrinolytic action of factor XIIIa is exclusively mediated by alpha2-antiplasmin and depending on the age of clot. A number of factor XIII gene polymorphism involving missense, nonsense and splicing mutations and nucleotide deletions have been identified; these contributed to factor XIII deficiency.
Congenital factor XIII deficiency is a rare cause of a lifelong bleeding disorder, and homozygotes for this autosomal recessive disorder possess less than 1% of factor XIII activity. Patients present with recurrent soft tissue bleeding and delayed wound healing. Women have increased incidence of fetal loss during pregnancy whereas menorrhagia is not commonly seen. The development of factor XIII inhibitors represents another cause of depressed factor XIII activity. It has been described in patients with congenital factor XIII deficiency with repeated transfusion. However, most inhibitors are IgG antibodies developed in patients without preexisting factor deficiency [3]. Most auto-antibodies are targeting at the subunit A of factor XIII, although antibody against the B subunit was reported to be the novel form of acquired factor XIII deficiency [4]. Clinical hallmark is delayed bleeding after trauma, surgery or invasive procedure, since the initial clot formed is mechanically weak and unstable. Severe subcutaneous or retroperitoneal bleeding usually occurs. It is often difficult to control resulting in high mortality. Our patient presented with repeated episodes of spontaneous retroperitoneal haematoma leading to devastating complications. It was reported that one patient with acquired inhibitor to factor XIII presented with high renal pelvic haematoma compressing on IVC [5], similar to our patient. At the same time, this patient had deep vein thrombosis (DVT) and pulmonary embolism (PE) and was successfully managed by balancing anticoagulation and haemostatic therapy.
Twenty-eight cases of acquired factor XIII inhibitors were identified in a systemic review published in 2013 [6]. Most occurred in elderly patients with median age of 65.5 years old. Slight predominance in women was observed. The bleeding was often severe requiring packed cells transfusion. Spontaneous or post-traumatic haematomas, especially muscular, subcutaneous or retroperitoneal and post-surgical or postdental extraction haemorrhage were the most frequent first presenting bleeding symptoms, being present in 79% and 25% of patients. Drugs were associated in about 40% of cases, such as isoniazid (most common), phenytoin, penicillin, ciprofloxacin, procainamide and practolol.
About one fourth to one third of patients with acquired factor XIII inhibitor was associated with autoimmune diseases such as SLE. Like our patient, a 17 year old female with SLE and class IV lupus nephritis was reported to have spontaneous haematoma and delayed major bleeding after invasive procedure due to acquired factor XIII inhibitor [7]. She was successfully managed with plasma-derived factor XIII concentrate, immunosuppressant and plasma exchange. Associations with rheumatoid arthritis, diabetes mellutis and haematological disorder such as monoclonal gammopathy of undetermined significance were also reported. No underlying cause was identified in about one third of cases.
Acquired factor XIII inhibitors produce a unique pattern in coagulation testing. The PT and aPTT, which measure fibrin generation, are normal. Fibrinogen level, platelet count, platelet function assay and bleeding time are all normal. Clot lysis test is abnormal, since the absence of fibrin cross-linking makes the clot to lyse more rapidly in 5 molar urea, 2 percent acetic acid or 1 percent monochloroacetic acid. This test is only sensitive at very low level of factor XIII (zero or very close to zero) and will be normal if the factor XIII activity level rises up to 1-3% [8]. Our patient had clot lysed despite 1:1 mixing of normal control plasma, suggesting the very low level of factor XIII due to the presence of acquired inhibitor. Mild to moderate deficiency in factor XIII are better diagnosed by the use of quantitative assay, such as amine incorporation and ammonia release assays, which measure the transglutamine activity of factor XIII. As these assays are not universally available, hence the clot solubility test remains the most common assay used. Normal factor XIII activity levels ranges from 50 to 220%. Plasma levels between 5 and 30% have shown to be sufficient in prevention of spontaneous bleeding. Whole blood haemostasis examining the viscoelastic properties such as thromboelastography (TEG), rotational thromboelastography (ROTEM) and thrombin generation test (TGT) can also be employed to analyse continued clot formation in whole blood and plasma, and to monitor different phases of thrombin production. Other recently developed factor XIII assays consist of automated quantitative ammonia release assay as an alternative to clot solubility test, a microtiter plate assay for factor XIII subunit A-chain-fibrin interactions, and a reversed activity staining procedure for detecting an acquired antibody against factor XIII. There was also a rapid immunochromatographic test to detect antifactor XIII A subunit antibodies, which can diagnose 90% of cases [9]. This can be a pointof- care test to facilitate prompt diagnosis and appropriate treatment.
Treatment of factor XIII deficiency caused by acquired inhibitor is difficult due to severity of bleeding manifestations and mostly warrants a whole armamentarium of therapeutic tools. Targets should be control of bleeding and eradication of inhibitor. High dose plasma-derived or recombinant factor XIII concentrates (50-150 U/kg) [10] produces adequate haemostasis in most cases, overcoming the inhibitor activity without inducing an anamnestic response. It was reported to be useful in the initial treatment of bleeding symptoms in a 75-year-old man with acquired factor XIII deficiency and severe bleeding after tooth extraction [11]. Replacements with fresh frozen plasma or cryoprecipitates are rarely effective in improving factor XIII activity. The use of recombinant factor VII (rFVIIa), as in our patient, was reported in two patients. The efficacy of this bypassing agent is yet to be proven.
If offending drug is suspected, the medication should be discontinued. Prompt clinical improvement may occur, but excessive bleeding may persist for weeks to months. Combined immunosuppressive therapy is usually required in those associated with autoimmune diseases, as in our patient. Steroid (1-2 mg/kg/day) for 4-6 weeks and cyclophosphamide are the most common first-line immunosuppressant. They are used in 16 out of 28 patients with factor XIII inhibitors, restoring factor activity in 62% of the treated patients [6]. Plasmapheresis can remove autoantibodies to factor XIII resulting in clinical improvement. Despite these immunosuppressive treatments, it was reported that patient still died of haemorrhage after sustained clinical remission for more than 3 years [12]. Use of intravenous immunoglobulin is reported in two patients with positive response in one of them. Successful use of monoclonal anti-CD20 antibody rituximab was observed in all the three patients treated. More data are warranted to consider rituximab as first-line treatment and it should be used as second-line treatment in patients not responsive to steroid or combination of steroid and cyclophosphamide or in those contraindicated to other immunosuppressive agents.
With respect to the clinical outcome of patients with factor XIII inhibitors, mortality rate is about 30% with a higher incidence in those present with cerebral haemorrhage [6]. More than half of reported cases undergo partial or complete remission. One fourth completely resolved without eradication therapy and most of them are associated with drugs.
Conclusion
Our case report documents that inhibitor associated clotting factor XIII deficiency is a rare but life-threatening disease. It is often overlooked as usual baseline coagulation studies are essentially normal. High vigilance and prompt recognition of this disease is of paramount importance, especially in patients with associated clinical condition such as offending drugs or autoimmune diseases. This definitely facilitates early and appropriate management resulting in improvement of outcome and saving of patients’ lives.
References
- Jerrold HL, Charles G. Biology of Factor XIII and clinical manifestations of Factor XIII deficiency. Transfusion. 53, 1120–1131 (2013).
- Dingeman CR, Shirley UW. Inhibition of Fibrinoloysis by Coagulation Factor XIII. BioMed Research International. 2017(1209676), 1–19 (2017).
- Thomas FG, Barry C. Case report of an acquired factor XIII inhibitor: diagnosis and management. Proc (Bayl Univ Med Cent). 19: 221–223 (2006).
- Eva A, Agota S, Adrienne K et al. Severe bleeding complication caused by an autoantibody against the B subunit of plasma factor XIII: a novel form of acquired factor XIII deficiency. Blood. 113(3), 723–725 (2009).
- Ogawa Y, Yanagisawa K, Souri M et al. Successful Management of a Patient with Autoimmune Haemorrhaphilia due to Anti-Factor XIII/13 Antibodies Complicated by Pulmonary Thromboembolism. Acta Haematol. 137(3), 141–147 (2017).
- Massimo F, Francesco F, Silvia C et al. Acquired FXIII inhibitors: a systemic review. J. Thromb. Thrombolysis. 36: 109–114 (2013).
- Rabik CA, Atkinson MA, Sule S et al. Treatment of an acquired Factor XIII inhibitor in an adolescent with systemic lupus erythematosus and renal failure. Transfusion. 57(9), 2159–2163 (2017).
- Hsieh L, Nugent D. Factor XIII deficiency. Haemophilia. 14, 1190–1200 (2008).
- Osaki T, Sugiyama D, Magari Y et al. Rapid immunochromatographic test for detection of anti-factor XIII A subunit antibodies can diagnose 90% of cases with autoimmune haemorrhaphilia XIII/13. Thromb. Haemost. 113 (6), 1159–1382 (2015).
- Massimo F, Giancarlo C, Antonio C et al. Acquired inhibitors of clotting factors: AICE recommendations for diagnosis and management. Blood Transfusion. 13, 498–513 (2015).
- Hayashi T, Kadohira Y, Morishita E et al. A case of acquired FXIII deficiency with severe bleeding symptoms. Blood Coagul. Fibrinolysis. 24(1), 85–89 (2013).
- Kotake Y, Souri M, Takada K et al. Report of a patient with chronic intractable autoimmune haemorrhaphilia due to anti-factor XIII/13 antibodies who died of haemorrhage after sustained clinical remission for 3 years. Int. J. Haematol. 101(6), 598–602 (2015).