Review Article - Imaging in Medicine (2012) Volume 4, Issue 1
Reducing the side effects of external beam radiotherapy in prostate cancer: role of imaging techniques
Emma J Alexander*‡, Victoria A Harris‡, Aslam Sohaib and David DearnaleyThe Royal Marsden Hospital NHS Foundation Trust & Institute of Cancer Research, Downs Road, Sutton, Surrey, SM2 5PT, UK
- *Corresponding Author:
- Emma J Alexander
The Royal Marsden Hospital NHS Foundation Trust & Institute of Cancer Research
Downs Road Sutton, Surrey, SM2 5PT, UK
Tel.: +44 208 661 3271
Fax: +44 208 643 8809
E-mail: emma.alexander@icr.ac.uk
Abstract
Imaging plays an important role in prostate radiotherapy. New imaging technologies have improved our ability to visualize tumors within the gland and allow accurate guidance of treatment delivery. Imaging is increasingly an integral part of ‘state of the art’ radiation planning and delivery, which gives the promise of increasing cancer control while reducing the side effects of treatment.
Keywords
cone-beam CT; dominant intraprostatic lesion; electronic portal imaging device; image-guided radiotherapy; intensity-modulated radiotherapy; intraprostatic lesion; megavoltage CT; MRI; multiparametric imaging; prostate radiotherapy; tomography; toxicity
Prostate cancer is the most common male cancer with an annual incidence of 36,000 cases in the UK [301]. Management of these patients includes surveillance and radical treatment with either surgery or radiotherapy. Active surveillance of prostate cancer is a possible management strategy for men with low-risk disease, avoiding treatment- related morbidity but requiring a robust follow-up strategy, which may include imaging to assess disease progression. Other radical treatment options include radiotherapy and surgery. Radiotherapy is the most commonly given curative option in the UK, with around 10,000 men treated with external beam radiotherapy annually [1].
Radical radiotherapy is delivered with the intention of delivering a sufficient dose of radiation to provide tumor control while minimizing dose to the neighboring normal tissues (dose-limiting). External beam radiotherapy is an established treatment for localized prostate cancer and has survival and biochemical control rates similar to that seen with surgery and in selected cases, brachytherapy [2]. There is increasing evidence to suggest its benefit in locally advanced disease [3]. A number of randomized controlled trials have demonstrated biochemical disease control (measured by prostate-specific antigen [PSA]) when using escalated doses of radiation [4–8] but late morbidity is increased in the groups of patients receiving higher doses. Prostate cancer can be indolent and have a long natural history; therefore it is essential to keep treatment-related morbidity to a minimum. In the post-PSA era, more men are being treated for prostate cancer and at an increasingly younger age. Consequently, late sequelae and survivorship issues are important.
Recent advances in radiation technology such as intensity-modulated radiotherapy (IMRT) enable complex 3D dose distributions and steep dose gradients to be delivered. This allows further dose escalation to the target along with the potential of sparing dosage to nearby organs at risk (OARs), thereby increasing the therapeutic ratio. Advances in imaging technologies have potential for more precise staging and delineation of disease in prostate cancer. Image-guided radiotherapy (IGRT) is becoming widespread and more sophisticated techniques are now available to improve verification of target position prior to treatment. This raises the possibility of a reduction in planning safety margins around targets with the further potential to decrease dose to the OARs. At present the full potential of these recent advances is limited by:
▪ The current use of CT images in the localization of the prostate in the planning stage of radiotherapy. It is not possible to identify the primary tumor within the prostate gland with CT. Furthermore, clinically important normal structures are difficult to identify;
▪ The day-to-day variability in prostate position during the course of treatment, which may result in a reduced dose being delivered to the prostate cancer and a greater dose to the nearby normal structures. This can be improved with effective verification procedures during treatment.
In this review we discuss how improvements in imaging, localization of the prostate and its verification during treatment could improve treatment accuracy with a consequent reduction in side effects.
The role of imaging in diagnosing prostate cancer
Screening
Screening for prostate cancer using PSA testing is not done routinely in the UK at present, but increasing numbers of men are having this test and being found to have an elevated result [9]. A disadvantage of using PSA for screening is the risk of ‘overdiagnosis’. For example, Schroder et al. state that 1410 men would need to be screened and 48 treated to prevent one prostate cancer death [10]. While PSA-screening would continue to identify large numbers of men with an elevated result, the number of men requiring treatment could be reduced if we were able to correctly identify indolent cancers that do not require immediate treatment. This has implications for radiotherapy resources and also potential avoidance of treatment-related toxicity for the patient. Although no imaging modalities are currently used in screening, in future using a combination of imaging along with other tests could provide risk calculators/nomograms that may identify clinically significant tumors, and thus, a subsequent reduction in unnecessary treatment.
Diagnosis
Diagnosis of prostate cancer is made histologically following biopsy, typically under transrectal ultrasound (TRUS) guidance. Gland volume can also be measured. On TRUS malignant nodules most commonly appear as hypoechoic lesions within the peripheral zone of the gland but may also appear iso- or hyper-echoic. The low sensitivity and specificity of TRUS in the detection of prostate cancer means that it is not suitable for screening or diagnosis. Since TRUS is unreliable in differentiating benign prostatic tissue from tumor tissue, cancerous lesions cannot be specifically targeted at the time of biopsy [11]. TRUS is used to identify the prostate to enable systematic sampling of the prostate gland at the time of biopsy. Initially, sextant biopsies were performed, but this has been superseded by 12-core biopsy, which gives a better diagnostic yield. Alternative techniques are saturation biopsies where large numbers of biopsies (typically >20) are taken, or template-mapping biopsies where biopsies are taken at 5-mm intervals throughout the base and the apical parts of the prostate gland. Using MRI to localize the site of prostate cancer prior to biopsy is usually performed in patients with elevated PSA and previous negative TRUSbiopsy. Prebiopsy MRI is not routinely performed but is currently being considered within a clinical trial setting.
Staging
Multiple imaging techniques may be used in the staging of prostate cancer, including MRI, CT and technetium-99 bone scintigraphy.
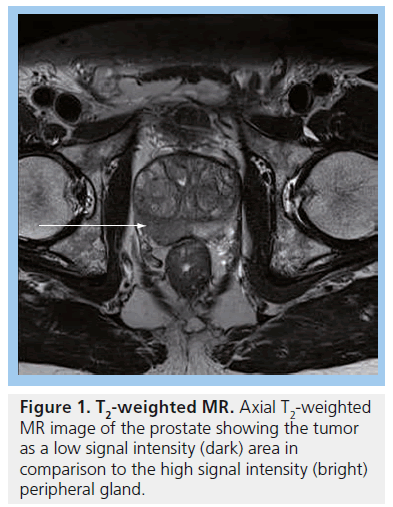
MRI is used for local staging of the pelvis and can identify high-risk features (e.g., with extracapsular spread, seminal vesicle invasion or node-positive). MRI findings have been shown to contribute significantly to the accurate staging of prostate cancer [12] and should be performed at least 6 weeks after biopsy since the biopsy changes signal characteristics and it can be difficult to distinguish between tumor and postbiopsy hemorrhage. On a standard T2-weighted MRI of the prostate, tumor tissue is seen as a focus of low signal intensity relative to the surrounding peripheral zone of the prostate. In the presence of benign prostatic hypertrophy, nodules of tumor tissue can be difficult to identify among the heterogeneous signal intensity seen within the central prostate gland [13]. The accuracy of T2-weighted MRI in the detection of prostate cancer is approximately 67% and increases to 77% when endorectal coil technique is used [14].
MRI methods are now available where the contrast is determined by an aspect of tissue function rather than anatomy (e.g., hypoxia, perfusion, metabolism). These functional MRI methods are being increasingly explored and are showing promise in improving the localization of tumor tissue. These techniques are discussed later in this article.
CT scanning is used in cases where there is suspicion of involved lymph nodes beyond the pelvis, and/or where there is suspicion of visceral metastases. Bone scintigraphy with technetium-99-methyldiphosphonate is used in patients who are symptomatic or have a PSA greater than 10 ng/ml and poorly differentiated cancers as a means of identifying bone metastases.
Accurate tumor staging allows important clinical decisions to be made regarding the most suitable treatment techniques for an individual patient, improving on, for example, the advisability of brachytherapy or prostatectomy, the need for lymph node treatment and the duration of neoadjuvant and adjuvant hormonal therapy.
Past & present techniques for localization of the prostate in radiotherapy planning
Prostate radiotherapy was historically planned using 2D orthogonal f ield radiographs, upon which a standard size field was drawn. Urethrograms could be performed at the time of radiotherapy planning, whereby contrast inserted via the urethra would demonstrate the position of the proximal urethra and bladder neck, allowing better localization. Planning was done manually, and absence of 3D images meant that no information regarding dose to nearby normal tissues was available. Furthermore, it was difficult to assess the position of the prostate and whether it had been displaced by nearby structures such as the rectum. Radiotherapy dose was low by modern standards owing to constraints imposed by nearby normal tissues and lack of available dose–volume information for these structures. This type of treatment planning, termed conventional planning, was superseded by the use of CT planning, which has allowed greater precision with reduction in treatment-related toxicity [15].
CT images are good at distinguishing between structures with different x-ray attenuation properties, such as air, fat, soft tissue and bone. However, it is difficult to distinguish between adjacent structures on CT if they possess similar x-ray attenuation [16], as is seen in the pelvis. Therefore, CT scanning can accurately display prostatic size and shape, but is limited in delineating the internal gland anatomy [17]. Widespread access to CT scanners has resulted in CT-based planning of prostate radiotherapy becoming standard practice. Images are acquired on a standard CT scanner using a flat-top couch and positioning lasers, and are exported to radiotherapy planning software via a Digital Image Communications in Medicine™ (Medical Imaging and Technology Alliance, VA, USA) link [18]. At the time of contouring, all diagnostic images may be available for reference, but are not uploaded onto the planning software for direct use in treatment planning. The dose delivered to the prostate is limited by the radiation tolerance of nearby organs.
As imaging of the prostate develops it is becoming increasingly feasible to identify areas within the prostate that represent active tumor tissue; these are referred to as dominant intraprostatic lesions or intraprostatic lesions (IPL). If these areas can be accurately identified and the dose escalated to these areas alone (while sparing nearby normal tissues) it is anticipated that tumor control will improve and normal tissue toxicity could be decreased.
Dose escalation to the prostate & IPLs
Traditionally the whole prostate is treated as a clinical target volume (CTV) with three or four radiation fields. Over the past decade, 3D conformal radiotherapy (3DCRT) has been developed, a benefit of which is the increased shielding with multileaf collimators or lead blocks, resulting in decreased toxicity [15]. 3DCRT results in better shaping of the high-dose region around the prostate gland, and avoidance of nearby normal tissues. This has allowed for dose escalation to the prostate gland, which has been shown to increase biochemical relapse-free survival, and may reduce distant metastases and increase cause-specific survival [7,19–22], although these benefits have not yet been confirmed in a randomized controlled trial [4,6,7,23,24] and are at the expense of increased toxicity [6,7]. Data suggests that higher doses to the prostate (>80 Gy) would reap further benefits in terms of biochemical relapse-free survival [22,25–28]. A meta-analysis has shown an almost linear correlation between dose and biochemical control between doses of 64 Gy and 79.2 Gy [28] and the benefits in terms of local control are estimated at 1.8–2.2% per additional Gy of dose [17,28]. Unfortunately Phase III trials of 3DCRT have also shown increased late morbidity in the higher dose groups (74–78 Gy) [7,29].
IMRT techniques enable more complex dose distributions with the production of steep dose gradients and ability to conform to concave shapes in the target. The potential of this technology is the ability to purposely create areas of dose inhomogeneity within the prostate, enabling higher doses to be delivered to tumorous tissue identified within the gland, maintaining standard doses to the remainder of the gland and sparing the nearby OARs.
Identification of tumor nodules or IPLs is not possible with CT scans. MRI has superior softtissue contrast with respect to CT and can identify tumor nodules. There have been technological improvements in MRI with the introduction of increased field strengths, endorectal coils and improved multiparametric imaging techniques, which are discussed later in this article.
Studies investigating IMRT to deliver a simultaneous boost to IPLs in prostate radiotherapy have been published. Four feasibility studies (either case studies or small planning studies) have used a boost dose of 90 Gy with a prostate dose of either 70 Gy [30,31] or 75.6 Gy [32,33] and show acceptable acute toxicity or normal tissue complication probability. Fonteyne and colleagues from Ghent University Hospital (Ghent, Belgium) treated 230 patients with IMRT of which 118 patients had an IPL definable and were boosted to a median dose of 81 Gy; no increase in acute toxicity was reported with this regime [34]. Three further studies have recently been published. The first was a clinical study treating 50 patients with 3DCRT to a dose of 64 Gy and delivering an IMRT boost using an endorectal balloon for rectal immobilization [35]. In this Phase I trial the boost was given in two fractions and was dose escalated starting at 5 Gy, up to 6 Gy, 7 Gy and finally 8 Gy (equivalent to total dose of 104 Gy in 2 Gy per fraction). After median follow-up of 72 months for the low-dose group and 60 months for the high-dose group the results showed an excellent 5-year biochemical relapse-free survival of 98%, but high rates of lower gastrointestinal (GI) toxicity, (probability of G2 ≥ late genitourinary and GI toxicity of 17.8 and 27.8%) comparable to some of the higher rates of toxicity seen in the 3DCRT Phase III dose-escalation trials [35]. In the second, a planning study, the dose to the prostate was maintained at 75.6 Gy with maximal boost achieved being 200% (151.2 Gy). This boost level was achieved in 50% of the IPL patients without violating radiotherapy toxicity scoring system dose constraints [36]. Third, in a further planning study, the Ghent group have assessed different IMRT methods and found volumetric arc therapy delivered significant theoretical advantages over three- and five-field static IMRT in terms of rectal sparing especially at the lower dose levels [37]. Dose escalation with three-field IMRT was not possible within their dose constraints above a median of 93 Gy. Recently, a Phase III trial has opened in Europe led by the University Medical Center in Utrecht (The Netherlands). The FLAME trial randomizes patients between the control arm treatment of 77 Gy to the whole prostate and the experimental arm of 95 Gy to delineated IPL(s) with 77 Gy to the remainder of the prostate gland [38]. IPLs will be identified using anatomical and multiparametric techniques with the use of diffusion weighted (DW) MRI and dynamic contrast-enhanced MRI.
Identification of IPL, radiotherapy planning and radiotherapy delivery are all heavily reliant on accuracy; accurate IPL identification is of no clinical benefit if the IPL cannot be identified at radiotherapy planning, and treatment designed appropriately. Furthermore, if patient setup during treatment is not sufficiently accurate there is a risk that the high-dose boost intended for the IPL could miss, treating either the other regions of the prostate, or more worryingly, the nearby OARs, potentially resulting in more treatment-related toxicity.
Developments in prostate cancer localization
MRI
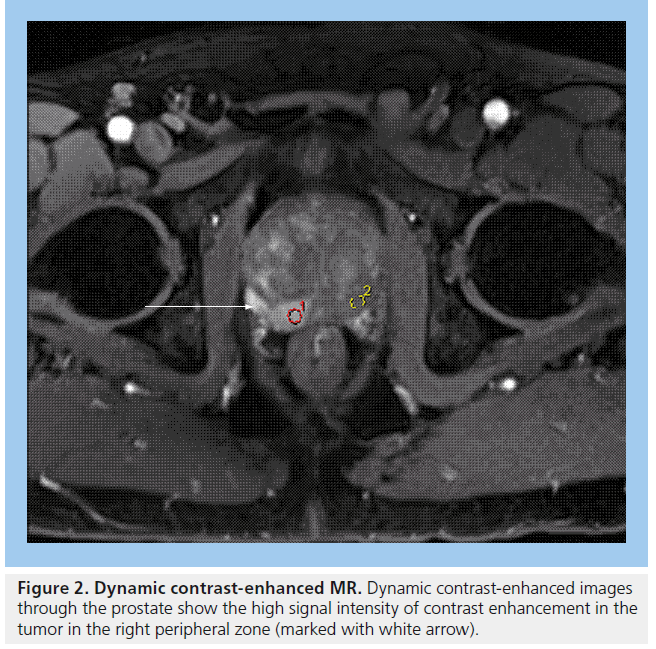
MRI plays an important role in modern radiotherapy planning since conventional morphological MRI techniques offer improved tissue contrast in comparison with CT scanning [39]. Diagnostic MRI results are considered at the time of treatment planning, in making decisions such as whether to include the pelvic lymph nodes within the radiation field. Typically, planning CT scans are performed and the diagnostic MR images are consulted at the time of prostate gland delineation. In particular, capsular breach or seminal vesicle involvement on MRI will impact on the volume localized on the planning CT scan. MRI can aid identification of the prostatic apex and help distinguish between bladder base and anterior rectal wall [39], resulting in better shaping of treatment fields, which can reduce the risk of treatment-related toxicity to nearby normal tissues [40]. Conventional MRI for prostate cancer typically includes T2-weighted images, which show tumors arising from the peripheral zone as dark in comparison with the normally bright peripheral zone (Figure 1). Gadolinium enhancement and washout assists in differentiating between normal prostate and tumor (Figure 2).
Difficulties encountered when using MRI scanning include: inability of patient to tolerate the scan owing to claustrophobia and patients with pacemakers or other implants or devices that are not suitable for MRI owing to the magnetic fields involved.
MRI/CT image coregistration & data fusion
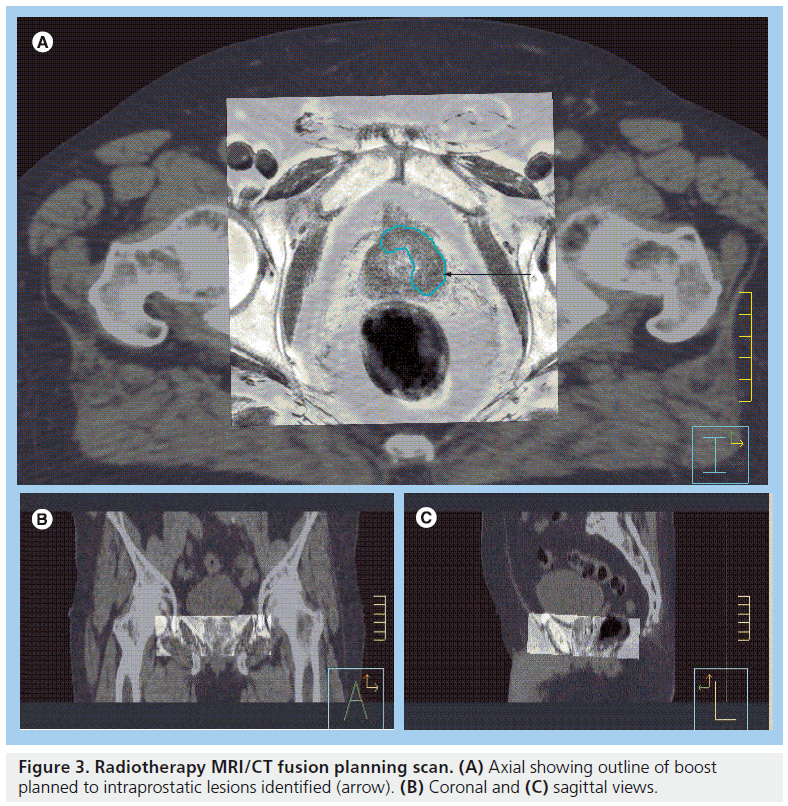
Most radiotherapy treatment planning software now allows image registration and mapping of scan information from one imaging study to another. Radiotherapy planning CT scans can therefore be coregistered with diagnostic MR scans to a common coordinate system. This process is termed ‘data fusion’ [41]. Via a ‘split-screen’, the two sets of images can be instantly reviewed and used to help delineate the target area. This technique of data fusion has been shown to enable improved target definition including delineation of IPLs (Figure 3) [42,43]. There are several published studies that suggest the prostate volume defined on MRI is between 13 and 40% smaller than the volume defined on CT [40,43–47,301]. MR images are subject to geometric distortion, which should ideally be corrected for when coregistering MR data with undistorted CT images [48]. Owing to this geometric distortion and also owing to the lack of electron density information (which is needed when calculating dose distributions in radiotherapy planning) MRI cannot be used easily without CT in radiotherapy planning systems to create treatment plans [16].
The use of an endorectal coil causes anatomical distortion of the prostate gland and should be avoided in MRI scans used for radiotherapy planning. In addition, flat rather than curved MRI couch tops are used when planning radiotherapy to aid coregistration.
Multiparametric MRI
Cancer growth is accompanied by neovascularization in order to meet metabolic demands of the tumor, and these processes are exploited by multiparametric MR techniques. Patients who receive radical radiotherapy treatment are often initially treated with luteinizing-releasing hormone agonist therapy for 3–6 months in order to shrink their prostate cancer. This combined modality approach is particularly appropriate for National Comprehensive Cancer Network intermediateand high-risk cancers. However, after hormonal therapy or radiotherapy treatment MRI is poor at localizing the tumor. Therefore images need to be acquired prior to starting the hormone therapy and coregistered with anatomical information prior to planning radiotherapy treatment.
Multiparametric MRI may help differentiate tumor nodules from benign nodules within the prostate, increasing specificity. MR techniques being studied in IPL definition include DW-MRI, MR spectroscopy (MRS) and dynamic contrastenhanced- MRI [49]. Studies have shown that a combination of techniques may improve sensitivity and specificity. The combination of two parameters is associated with an improvement in prostate cancer detection over the use of any one parameter alone, however, there is contradictory evidence regarding whether the use of a third parameter further improves rates of detection [50,51]. A combination of multiparametric MR techniques may provide additional information, which permits the design of high-dose radiotherapy boost to a dominant intraprostatic lesion [30].
Types of multiparametric MRI DW-MRI
On DW-MRI, image contrast is based on diffusion of water molecules. Diffusion of water within the body is limited by the natural barrier effect caused by cell membranes [52]. Tumors have increased cellular density compared with normal tissue, therefore show less water diffusion with a lower apparent diffusion coefficient (ADC) than less cellular (nontumor) region [16]. A combination of DW-MRI images and ADC maps are used in the evaluation of prostate cancer (Figure 4), with areas of prostate cancer appearing darker on ADC map (representing a low ADC value) than normal prostate tissue [52]. DW-MRI has the advantage of rapid acquisition and appears to be a promising technique for prostate tumor definition increasing specificity to 61–89% [53–55].
Dynamic contrast-enhanced-MRI
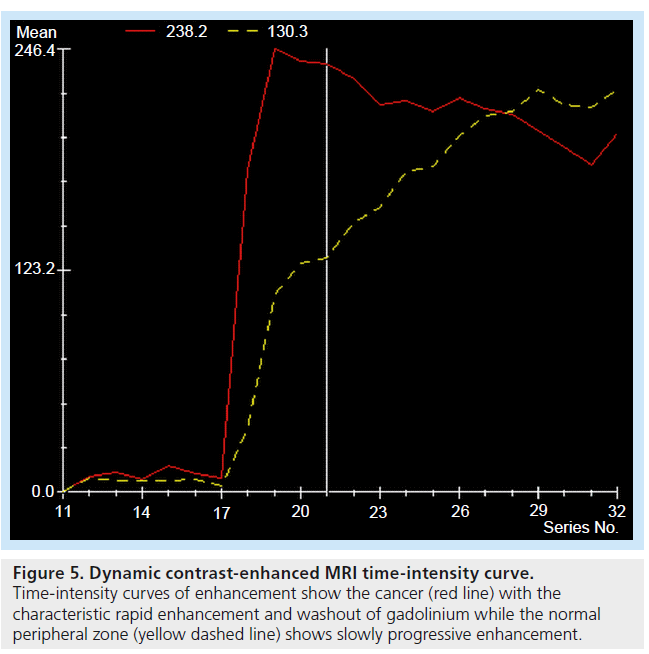
This technique facilitates disease staging when added to standard T2-weighted MRI [56–59]. It exploits the vascular dynamics seen in neoangiogenesis/ abnormal tumor vasculature, and uses fast MR sequences performed following administration of contrast agent [16]. The enhancement seen during uptake and washout of the contrast can be used to evaluate tissue perfusion (Figure 5), which is compared with standard enhancement patterns seen in normal tissue and malignant disease [60]. Sensitivity and specificity of tumor detection are 81 and 79% for peripheral zone cancer and 37 and 97% for central gland cancer, respectively [56].
MRS
This technique enables the detection of molecular markers (e.g., choline, citrate and polyamines) within the prostate, and is performed in conjunction with MRI, which provides the anatomical component of this imaging. Significant reduction in citrate and elevation in choline levels have been documented in prostatic adenocarcinoma compared with normal prostatic tissue [61]. MRS has high specificity for tumors over 1 cm² and may potentially distinguish tumors in the transitional zone, which although less common can account for up to 17–25% of prostate cancer cases [50,62]. However, there is conflicting data as to the utility of MRS, with a study by the American College of Radiology imaging network suggesting MRS is no better at localizing prostate cancer than MRI alone [63].
Figure 2. Dynamic contrast-enhanced MR. Dynamic contrast-enhanced images through the prostate show the high signal intensity of contrast enhancement in the tumor in the right peripheral zone (marked with white arrow).
PET/CT
This technique combines anatomical and functional data sets to provide detailed information about tumor location and metabolic activity. 18F-fluorodeoxyglucose (18FDG) is the most commonly used PET tracer in oncology [64]. However, use of 18FDG is limited in imaging prostate cancer owing to both the low-glucose metabolism of prostate cancer, the physiological urinary excretion of 18FDG, which may interfere with imaging of the pelvis [65].
There are other tracers available for use in PET imaging; of particular interest are Choline radiolabeled isotopes such as 11C- and 18F-Choline. These have negligible urinary excretion, making it useful in visualizing genitourinary cancer. The main role of 11C-choline PET/CT is in restaging biochemically relapsed prostate cancer, and it is not widely used for primary prostate cancer detection owing to limited reported sensitivity (71.6%) and specificity (42.6%) in localizing tumors within the prostate gland [66], although it remains an area of active research.
11C-acetate has been evaluated as an image guide in patients with localized intracapsular prostate cancer with promising results [67].
Localization of lymph nodes
The probability of locoregional control of nodepositive prostate cancer can be increased by delivering an adequate radiotherapy dose to the involved lymph nodes. Both CT and MRI techniques are used to identify involved lymph nodes, however, the accuracy rates for both modalities show wide variation. For nodal involvement in pelvic malignancy, the sensitivity of CT ranges from 24 to 78%, and the specificity from 84 to 97% [68–70]. With MRI, the sensitivity ranges from 24 to 75% and the specificity from 78 to 99% [68,71–74]. Guidelines have been published that define the upper limit of normal for lymph node size in different regions of the pelvis using either CT [75] or MRI [76]. However, using lymph node size criteria does have a limitation, namely that enlarged reactive lymph nodes will be falsely positive and small involved nodes will be falsely negative. Fusion of DW-MRI with T2-weighted MRI can improve the identification of pelvic lymph nodes compared with T2-weighted MRI alone [77].
Ultrasmall superparamagnetic iron oxide particles (USPIOs) have been reported to discriminate between normal and abnormal lymph nodes [16]. This technique has reported high sensitivity and specificity [78]. Unfortunately, USPIOs did not gain approval from the US FDA or EMA. However, previous work while USPIOs were undergoing clinical evaluation has shown that lymph node positions can be mapped [79–81], thus aiding target definition in radiotherapy treatment planning, particularly when giving a boost treatment to involved nodes. These maps have also allowed guidelines for pelvic lymph node delineation to be developed to ensure adequate coverage of at-risk nodes, but avoiding nearby normal tissues [82].
As mentioned earlier, 18FDG PET/CT is of limited use in imaging prostate cancer, but even 11C-choline PET/CT is limited at detecting small neoplastic nodal deposits (specificity 99.8%, sensitivity 41.4%) [83].
Verification of treatment planning
Principles of IGRT
In recent years there has been a rapid expansion in the technology of image guidance, which mirrors the expansion of radiation delivery advances. There have been great changes to the verification process from 10–20 years ago. At that time, weekly poor-contrast megavoltage (MV) portal films were used to distinguish bony anatomy as a surrogate for the neighboring target. Now there is a minimum standard of electronic portal imaging devices (EPIDs). These capture better quality MV images using amorphous silicon detectors and software that automatically demagnifies and matches it to reference (planning) images. Larger centers may also be equipped with more sophisticated methods of verification to be discussed in this section.
IMRT has been shown to reduce the toxicity of radiotherapy with respect to 3D conformal techniques [84–86]. This is due to the creation of steeper dose gradients and a higher degree of conformity around the target with consequent increased shielding of nearby OARs. Increased conformity, however, increases the risk of geographical miss of the target organ. IGRT is therefore needed to verify the position of the target, particularly if reducing treatment margins. Images should ideally be obtained immediately before the radiation beam is switched on (online verification).
Conventionally, offline verification of target position was performed to establish and correct for systematic error. Serial images were often taken in the first week of treatment. Assessment of these images and any corrections were performed following radiation delivery and the patient was imaged weekly to confirm correct positioning. The study of prostate motion has shown that the gland can move 1 cm or more in relation to bony and skin landmarks [59,87–91]. Prostate movement is related to changes in bladder and rectal filling and increased rectal distension has been shown to be inversely correlated with biochemical outcomes in prostate radiotherapy [92,93]. These data have shown that geometric uncertainty associated with a large rectum can negate the benefits gained from dose escalation, further highlighting the need for accurate treatment verification and image guidance. The large interfractional displacements that have been demonstrated in prostate position during a course of radiotherapy have led to an increasing use of daily online verification to improve treatment accuracy [94,95].
More recently, methods of tracking intrafraction prostate motion have become available and data are accumulating regarding the magnitude of this problem. Imaging is therefore beginning to play a very large role in radiation delivery as well as target delineation.
Figure 3. Radiotherapy MRI/CT fusion planning scan. (A) Axial showing outline of boost planned to intraprostatic lesions identified (arrow). (B) Coronal and (C) sagittal views.
Improved accuracy of therapy in itself will hopefully have an effect in reducing toxicity, at least in reducing those thought to be due to focal high-dose areas such as rectal bleeding. The expectation is that IGRT will enable a reduction in the safety margins currently used in radiotherapy. Margins are required to overcome some of the uncertainties that arise during treatment planning and delivery. It is the reduction in planning margins that will have a greater effect in the dose reduction to OARs and therefore toxicity outcomes. Here we will discuss the available technologies for IGRT and their scope for reduction in margins and toxicity in prostate radiotherapy.
Figure 5.Dynamic contrast-enhanced MRI time-intensity curve. Time-intensity curves of enhancement show the cancer (red line) with the characteristic rapid enhancement and washout of gadolinium while the normal peripheral zone (yellow dashed line) shows slowly progressive enhancement
Ultrasound
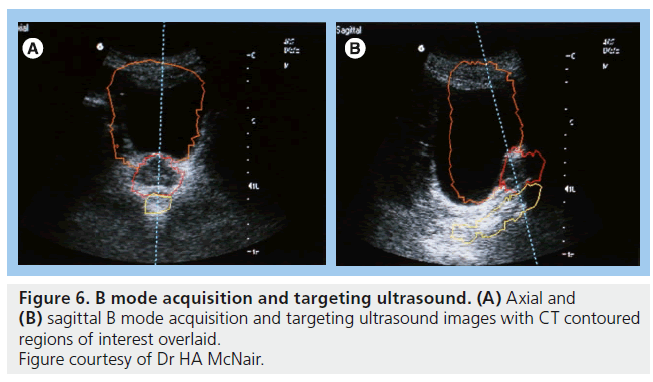
Ultrasound of the prostate is an attractive verification technique as it is noninvasive and does not use ionizing radiation. B mode acquisition and targeting (BAT) is the most extensively studied ultrasound modality (Figure 6). The obvious advantage is the ability to localize the prostate daily without additional radiation. Studies using ultrasound have highlighted the degree of prostate movement with respect to bony or skin landmarks and all studies demonstrate a considerable random error, representing this movement [96–101]. To date two studies have demonstrated a decrease in acute GI toxicity [102,103] and a possible decrease in late GI toxicity [103,104], but both used sequential historical controls for comparison and there is a need for additional, preferably randomized, evidence to confirm a benefit.
There are several disadvantages to the use of BAT for verification purposes. First, interobserver variability can be as high as 7 mm [105,106]. This is not an insignificant level with respect to quality assurance tolerance levels in radiotherapy departments. Second, image quality can be poor. The largest reported study assessed 147 patients with BAT and found unacceptable image quality in 5.1% [107]. Poor image quality can be due to inability to maintain a full bladder, bladder filling, obesity, depth of isocenter and position of prostate relative to pubic symphysis [98,99,108]. Third, there is the possible displacement or deformity of the prostate from the pressure of the ultrasound probe. Studies have shown possible displacements of up to 1 cm with moderate probe pressure or incorrect handling [109,110] with mean displacements between 0 and 3 mm [99,101,108,111]. Finally, there is a concern that use of ultrasound can introduce a systematic error when results are compared to CT and gold seed fiducial images, particularly in the superior–inferior direction [98,105,106,112]. Possible causes of systematic error include the displacement of the prostate and difficulty visualizing the inferior portion of the gland. Similar problems have been encountered using 3D ultrasound for localization [113].
The problems with confirming accuracy of ultrasound for daily IGRT mean that its clinical value remains controversial. It does not appear that margins can be significantly reduced using the system [96,101,114]. It may be useful in departments with no access to more sophisticated techniques or patients with rectal distension and large anterior–posterior movements. One study hypothesizes that the use of daily BAT IGRT can negate the geographical miss and therefore poor outcomes of patients with rectal distension [115].
Fiducial markers & EPIDs
The use of gold seeds as fiducial markers (FMs) using EPIDs in prostate radiotherapy has been studied for over 15 years [88,116]. Although invasive, implantation has been demonstrated to be tolerable [117–119] and the position of FMs is generally stable within the prostate throughout treatment [88,120–123]. The largest study (n = 914) examining these issues to date, suggested a discontinuation of FM IGRT in 0.05% owing to migration of seeds and a 0.05% rate of G3 toxicity (urosepsis) [124]. FMs are visible on kilovoltage (KV) and MV images are not affected by patient factors such as obesity. Intraobserver variability in matching FMs from treatment images to planning images is generally submillimeter [106,125,126]. EPIDs use the MV treatment beam to image leading to other advantages such as the imaging dose being accounted for in treatment and simultaneous verification of the radiation field with respect to the prostate can take place. These factors along with the widespread availability of EPID technology make it a popular verification technique.
There are a wealth of data analyzing margin recipes with online and offline protocols of skin, bone and fiducial setups. The data are in general agreement that CTV to planning target volume (PTV) margins of over 8 mm (1–1.5 cm) are required to ensure adequate coverage of prostate if bone or skin is used for setup [127–129]. With an online EPID and FM IGRT protocol, data suggest that CTV to PTV margins can be reduced to 2.5–7.2 mm [89,91,130–135]. Displacements are most often demonstrated in anterior–posterior and superior–inferior directions consistent with anticipated directions from bladder and rectal changes. With increasing use of daily online verification to reduce interfraction setup errors, intrafraction random errors due to prostate movement become the limiting factor in further reduction of planning safety margins [91,131,136]. Several studies have also assessed prostate intrafraction motion by repeating EPIDs after radiation delivery. Margins to account specifically for intrafraction motion of the prostate vary from 1.0 to 3.4 mm [91,137,138].
FM have been shown to be superior for prostate position verification compared with techniques for soft-tissue localization such as BAT ultrasound and cone beam CT (CBCT) [105,139,140]. This is due to the ease of matching FMs, which can be done either manually or automatically with high reproducibility. Examination of the delivered doses of treatment plans (dosimetry) using an EPID/FM IGRT protocol has demonstrated an improvement in measures of normal tissue doses such as dose-volume histograms and normal tissue complication probability [141]. Phase II studies reporting toxicity data from high-dose prostate radiotherapy using EPID/FM IGRT compare favorably to non-IGRT dose escalated data but no randomized evidence is available [6,7,19,142].
KV CT
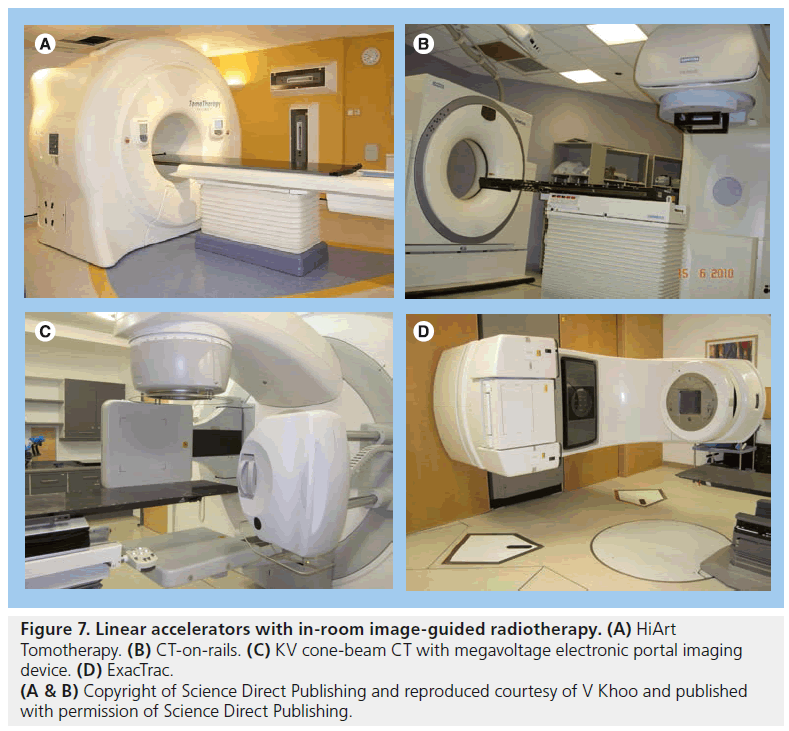
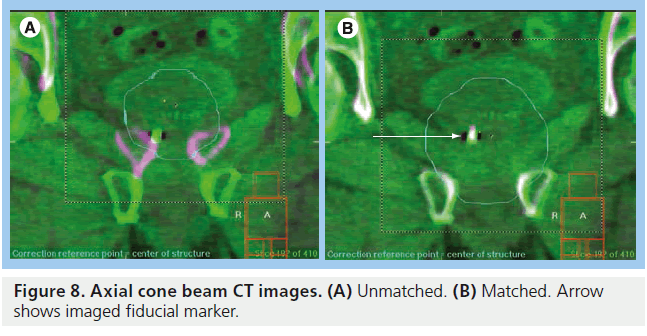
CT images give volumetric data that can be used to assess target and OAR motion as well as further information regarding tumor progression or response to treatment. Modern in-room CT-guided images for radiotherapy can be performed with various techniques. ‘CT on rails’ uses a conventional type of CT scanner (i.e., fan-beam type). The treatment couch moves in the room on a set of rails between the CT scanner and the linear accelerator so that images can be acquired just before the patient is in the final set-up position (Figure 7B) [143,144]. CT on rails produces highquality images but the patient must be realigned before radiation delivery commences and so is less able to assess intrafraction motion. An alternative method is with an ‘on-board CBCT’ (Figure 7C). Here a large flat panel detector is built into the linear accelerator perpendicular to the treatment head and serial digital radiographs taken over a single rotation of the gantry are reconstructed into 3D CT images. This system can also be used for KV fluoroscopy and 4D CT [145–147]. CBCT can be performed once the patient has been set up to the treatment isocenter and reconstructed images have submillimeter spatial resolution [148]. Images must be taken in between radiation beam delivery rather than simultaneously. CBCT images are of poorer quality than ‘CT on rails’ but the data give a better estimation of intrafraction motion (Figure 8).
Figure 7.Linear accelerators with in-room image-guided radiotherapy. (A) HiArt Tomotherapy. (B) CT-on-rails. (C) KV cone-beam CT with megavoltage electronic portal imaging device. (D) ExacTrac. (A & B) Copyright of Science Direct Publishing and reproduced courtesy of V Khoo and published with permission of Science Direct Publishing.
In-room CT can be used to measure prostate displacement using either contour based- or automated gray scale-based soft-tissue matching [149]. Data have shown soft-tissue alignment is superior to bone alignment alone [150] but FM alignment has been found to be equivalent or superior to CT soft tissue as it has lower rates of inter- and intraobserver availability, is less time-consuming and requires less physician input [151,152]. A study comparing FM IGRT using CT on rails, CBCT and EPIDs found that all techniques have some minor errors in alignment using the fiducial markers. It concludes that CBCT reduces systematic error compared with CT on rails due to the advantage of having the same isocenter for imaging and treatment [153].
Two of the most promising uses for in-room CT imaging are the potential for using volumetric information to create adaptive radiotherapy plans and to assess dosimetry. In adaptive radiotherapy the patient is replanned using information gained from their initial treatment imaging, for example the first five fractions. CBCT adaptive plans have been shown to significantly reduce PTV margins and consequently rectal dose with acceptable coverage of CTV throughout treatment course [154]. Dosimetric studies using CT data with and without FMs for alignment have also demonstrated the negative impact on dosimetry from interfraction changes in rectal volumes and noted the danger of reduced margins in the presence of moderate and large uncorrected prostate displacements [128,155,156]. The use of online EPID and CBCT/FM IGRT may allow margins as small as 4 mm (3 mm posteriorly) [128] with adequate CTV coverage, although the uncertainty caused by inter- and intra-observer organ delineation between each CBCT needs to be considered. Calculating dosimetric data from CBCT is a laborious process and is not compatible with routine treatment at present.
KV planar fluoroscopy
KV fluoroscopy uses the same gantry-mounted equipment as KV CBCT. It takes 2D monoscopic images in a fixed position perpendicular to the treatment beam gantry angle and the approximate fluoroscopic time is 30–40 s per treatment beam. Adamson and Wu have shown that this technique can be used with FMs to successfully monitor ‘beam on’ intrafraction prostate motion [147]. There are several limitations to this technique at present that may be overcome in the future. First, it can only be assessed offline, although it potentially could be used in real time. Second, owing to the 2D nature of acquisition, translation into 3D localization information has some technical limitations, which reduce the accuracy of monitoring certain types of motion, in particular high-frequency oscillations (however, drifting motions, which are more dosimetrically important, can be accurately measured in 3D). Third, there is some degradation to image quality when the MV beam is on and this leads to decreased accuracy in certain gantry angles. Finally, rotational errors cannot be accounted for with this technique as yet. This strategy has been used to assess intrafraction prostate motion and shown to be able to reduce planning margins using both population margin recipes and patient-specific adaptive radiotherapy [157].
Stereoscopic KV imaging
Stereoscopic x-ray imaging uses two x-ray sources and detectors, which take orthogonal planar images simultaneously. The advantages of stereoscopic x-ray imaging are that 3D and 4D information can be obtained and doses are low in comparison with other techniques (Table 1). These systems can track movement of external and internal markers in near-real time, which allows beam on management, such as treatment gating. Several systems now have an automated couch with six degrees of freedom, which accounts for rotational movements: pitch, yaw and roll. The couch can adjust to these complex displacements as well as translational errors. Disadvantages are the lack of volumetric and soft-tissue data; surrogates such as bone or markers must be used and depending on which part of the body is imaged there may be compromises in image quality due to overlapped structures [158]. Examples of stereoscopic x-ray systems are VERO™ (BrainLAB AG, Germany and Mitsubishi Heavy Industries, Japan), EXACTRAC® (BrainLAB AG, Germany) and Cyberknife® (Stanford, CA, USA).
VERO uses x-ray sources and detectors are fixed within an O-ring structure through which the radiotherapy couch moves, with similar appearances to a CT ring. EXACTRAC 6D x-ray system is an example of a peripheral (mounted around treatment room) stereoscopic system integrated with an infrared optical positioning system and a couch with six degrees of freedom. The KV x-ray sources are mounted in the floor with the flat panel detectors mounted on the ceiling and the sources are oblique with respect to the patient (Figure 7D). There are few data describing the use of these systems specifically in prostate cancer at present.
Cyberknife is a 6 MV linear accelerator mounted onto a robotic arm capable of delivering radiation from multiple noncoplanar directions without movement of the patient. It is currently being used for stereotactic radiosurgery. These regimes use extreme hypofractionation; therefore the accuracy of treatment is imperative. Cyberknife has a peripheral stereoscopic planar KV imaging system. Stanford University (CA, USA) has published several studies assessing the use of prostate stereotactic body radiotherapy. Images are taken and fed back every 40 s then matched to the planning images. Adjustments are then made in near-real time. Prostate movements of less than 5 mm can be adjusted by alterations in the robotic beam positioning. If the prostate moves more than 5 mm the radiation must be interrupted to allow the couch to be repositioned. Intrafraction tracking data using Cyberknife has shown prostate movement to be erratic and unpredictable with excursions of 3–5 mm common and maximal motions of 9 mm seen [159]. The unpredictability of movement is not only interpatient but also intrapatient and intrafraction. This suggests a need for intrafraction monitoring of prostate movement if small margins are to be safely used with these types of regimes, which deliver high doses of hypofractionated radiation over a protracted fraction time (50–70 min). Using a protocol that images every 30 s keeps prostate displacements under 2 mm in 95% of measurements, if this is extended to a 120 s protocol the figure drops to 86% [159]. Even with a 30 s protocol short-lived high-frequency displacements may be missed. Five-year data on biochemical progression- free survival (92.7%) and toxicity have recently been reported for a cohort of 41 patients with favorable risk-localized prostate cancer [160]. The results are promising with very low rates of late toxicity. Using a standard radiotherapy toxicity scoring system, grade 2 GI toxicity or above occurs in 2.5% of patients with urinary toxicity being 9.5%.
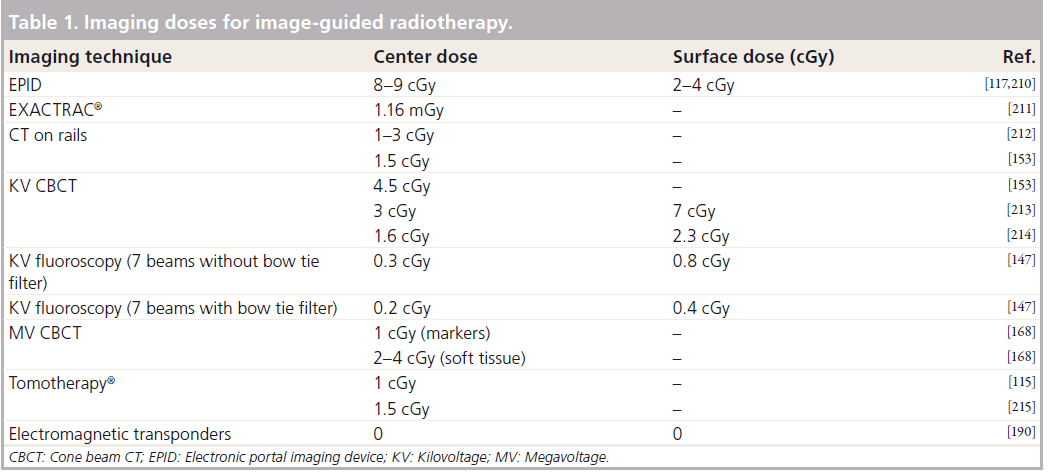
MV CT
MV CT can be used for treatment delivery and image guidance with a single isocentric technique. Advantages are volumetric and dosimetric information and the use of images acquired with the treatment beam. Other advantages are improvement in the artifacts caused by prostheses and dental implants with respect to KV CT [161]. There are various ways of acquiring MV CT data: through the linear accelerator and large flat detector (MV cone-beam) [162,163], using linear accelerator treatment head and an attached arc of linear detectors (produces a single slice CT [164]) or using a MV beam source within a fan-beam CT for treatment delivery and array of linear detectors for image acquisition (Tomotherapy®, Accuray, Sunnyvale, CA, USA), which can be serial or helical [165]. CBCT equipment can be used for MV fluoroscopy and tomosynthesis (using multiple radiographs but limited gantry rotations) [166]. Acceptable MV CBCT images can be acquired at low-doses of 2–15 cGy with acquisition and processing time comparable to KV CBCT (3 min) and without artifacts from FMs [167,168]. Tomosynthesis has the advantages of speed of acquisition and can therefore be used for 4D images and respiratory gating. The disadvantage is the loss of 3D volumetric information and therefore for prostate radiotherapy MV CBCT is a more appropriate method. The main disadvantage of MV IGRT is the image quality, as bone and soft-tissue contrast are poorer owing to the highenergy beam and consequent loss of photoelectric effect. Additional dose can be another potential disadvantage although this may be offset if the imaging dose is included when calculating the monitor units and dose to be delivered with the treatment beam [169,170]. MV CBCT and tomosynthesis are still areas of current research while Tomotherapy is in widespread clinical use.
Tomotherapy
Tomotherapy units (Figure 7A) utilize fan-beam CT and helical delivery and can give a more favorable dose distribution to the rectum and bladder than static field IMRT [171,172]. However, intrafraction motion and its dosimetric consequences are different in helical Tomotherapy from that of static IMRT owing to the nature of the beam delivery. A single narrow rotating beam delivery with a moving couch mean that it is particularly vulnerable to errors in the superior-inferior direction. Treatment beams can be used to image or imaging can be performed separately. Studies assessing interobserver variability of Tomotherapy IGRT agree with the data from KV CT: FMs significantly reduce variability compared with soft-tissue matching and therefore improve accuracy [173]. Algorithms for better soft-tissue matching using MVCT are currently being investigated [174]. MVCT localization has been shown to be superior to ultrasound, both in accuracy and ability to reduce margins [175–177]. This translates to improvements in treatment dosimetry using MVCT IGRT with respect to ultrasound [176]. As with KVCT, there is a measure of uncertainty in organ and target delineation that must be taken into consideration. This is aggravated by the poorer image quality [178]. Dosimetric studies of MVCT and FM have shown the high degree of PTV coverage that can be achieved with this form of IGRT [179]. It has also shown the large interfractional dosimetric variations in the bladder and rectum due to daily variations in volumes of these organs. This stresses an important point that is often overlooked in reports of IGRT: even with the increased accuracy in delivered dose to the prostate, the dose to OARs can be unpredictable owing to daily changes in shape and volume [179,180]. Disadvantages of helical Tomotherapy with respect to other MVCT are the higher integral dose (total dose to target plus all tissues outside target) [181], higher skin dose [182] and longer time taken for scan acquisition and processing [183]. There is also the issue of the short x-ray target lifespan, which typically needs replacing every 10–12 months; this can have a significant effect on dosimetry at the end of lifespan [184,185]. Software packages are now or will soon be available for adaptive radiotherapy treatment plans [180] and to account for intrafraction motion [186,187].
Given the issues of additional dose, studies assessing the use of MV IGRT have looked at the frequency necessary for image guidance. Some studies have suggested a protocol of imaging in the first four fractions to reduce systematic error and assess patient-specific margins [188,189]. Although the population error can be reduced with imaging in the first 3–5 fractions, data show that there remains considerable interpatient variation, and random errors continue to be approximately 3–4 mm throughout treatment even with alternate day imaging. Therefore daily imaging is necessary if margins are to be reduced to levels such as 5 mm [91,115].
Other tracking systems
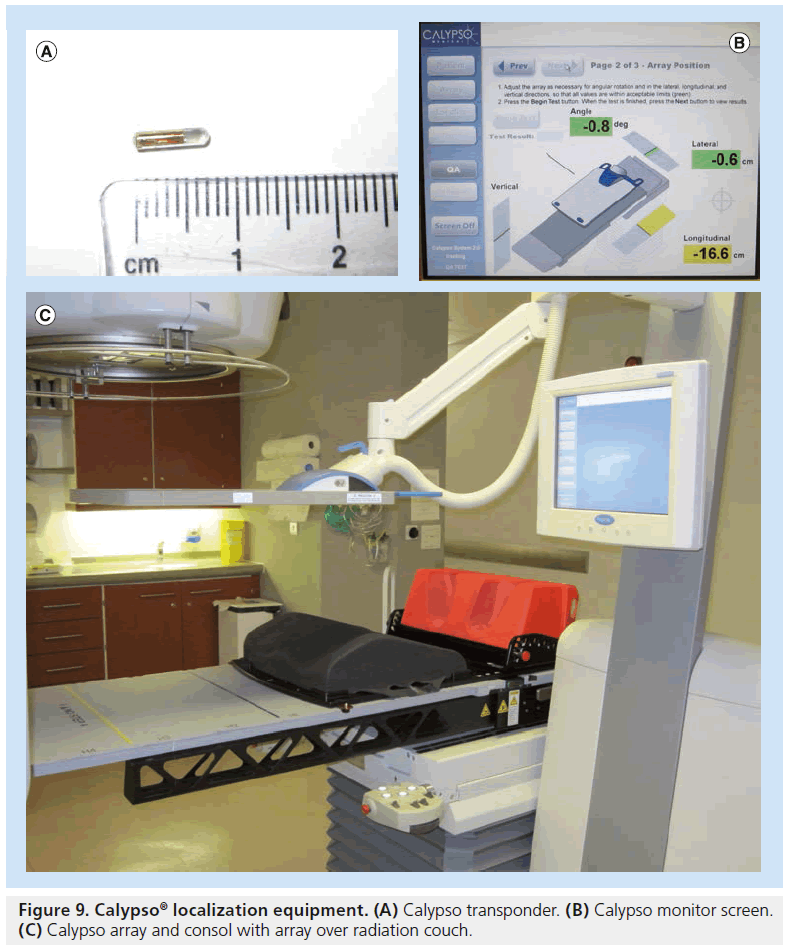
Calypso® Medical (Seattle, Washington, USA) has introduced a wireless in-room electromagnetic (EM) localization system that allows implantation of permanent EM transponders in the same manner as gold seed fiducials. The prostate is localized via the EM transponders using a sensor array that excites the transponders and feeds back positioning relative to the isocenter (Figure 9) [190]. The prostate movement can then be tracked during treatment delivery. Various mechanisms can be put into place to take advantage of this tracking such as gating or realignment of patient if a threshold displacement is reached. It does not involve ionizing radiation and does not require acquisition or reconstruction time and so can shorten the amount of time to setup patients. Disadvantages are first, that the transponders are larger than most commonly used gold seeds, and therefore require larger (14 Gauge) needle guides. Second, the transponder causes considerable artifacts with MRI making target delineation during planning stages or later assessment of disease progression difficult [191]. Calypso® gives researchers a wealth of data regarding intrafraction prostatic motion, which are in agreement with the data produced using Cyberknife® tracking. Intrafraction prostate movement increases with time, is most pronounced in anterior–posterior and superior–inferior directions, can be significant (i.e., >1 cm for individual patients) and it is difficult to predict patterns of movement [90,192]. Movements above 3–5 mm are common [192]. Data have shown considerable improvements in terms of rectal dosimetry when EM transponders are used to localize the patient when compared with skin mark set-up [193]. Dosimetry performed on EM localized prostate patients has shown that margins can be reduced to 2 mm with no significant detriment to CTV coverage and with sparing of bladder and rectum [194]; further improvements could be made with an intrafraction intervene protocol during tracking. Continuous intrafraction tracking has shown to be a significant improvement over pre-, post- and intermittent-intrafraction imaging as such protocols have a low sensitivity unless frequency of intermittent imaging as high as every 15 s [195]. For EM tracking to be used to its full potential it needs to be integrated with couch controls and synchronized with linear accelerator multileaf collimator motion and beam delivery. This is an area of current research [196].
Future perspective
Diagnostic imaging
New technologies may help us to differentiate benign and malignant prostate tissue, thus helping pinpoint areas of disease for biopsy. Examples include power Doppler, which is able to detect isoechoic tumor areas and may increase diagnostic performance and tissue harmonic imaging, which results in increased spatial and contrast resolution with better detection of small lesions. MRI scanning, including multiparametric imaging, prior to biopsy is likely to become more common, allowing targeted biopsies to be performed on suspicious areas within the prostate. MRI-guided prostate biopsy has already demonstrated superior diagnostic yield in comparison with TRUS biopsy [197] and can reduce the number of biopsies that need to be taken [51].
Radiotherapy planning/tumor localization
Open low-field strength MR scanners can act as radiotherapy simulators providing a radiotherapy environment similar to CT simulation for radiotherapy planning [16]. An advantage of this over CT–MRI fusion is that patient immobilization devices that were previously restricted by size with conventional MR scanners could be employed. A previous study of 243 patients revealed that open Low-field MR simulation provided adequate images for radiotherapy planning in up to 95% of cases [47]. MR simulation can better delineate erectile soft tissues to permit dose sparing of these structures by IMRT [198].
The emergence of 3 T and higher field strength scanners will further improve spatial resolution of MR scans. These higher field strengths will improve image quality in DW-MRI and MRS resolution, and provide better temporal resolution in dynamic contrast-enhanced-MRI scanning [199]. Improved imaging will enable easier detection of IPL and radiotherapy boost treatment to these lesions is expected to become commonplace.
At present USPIO agents are not licensed for clinical use. If alternative lymphotrophic MR contrast agents are licensed then it may be possible that they will be regularly used to detect involved lymph nodes. This will allow us to determine which lymph nodes we wish to include in radiotherapy fields and allow shaping of these fields around these nodes to deliver boost treatment.
Verification
Current areas of research and future directions include other forms of tracking prostate intrafraction motion, in-room MR IGRT and dose-guided radiotherapy. Very recently, two further tracking systems have been described in early stages of development. Real Eye uses a single radioactive iridium seed implant (100 mCi) with a detector integrated on the gantry [200]. The seed can be inserted using standard gauge biopsy kits and has minimal artifact on CT and MRI. Owing to neutron interference it can only be used to track movement with lower energy MV beams (4–6 MV). This may limit its use with standard linear accelerator-based treatments, although not with other modalities such as Tomotherapy or Cyberknife. PeTrack is another novel tracking system that uses positron emitting 22Na fiducials. It detects the coincident g‑rays emitted from positron annihilation events and can use this data to track the path along the line of the detectors to the origin of the annihilation event [201]. As 22Na has a long half-life, other isotopes would need to be used when this technology is translated into the clinic. Both systems have a high degree of accuracy in phantoms; clinical data are yet to be published [200,201]. There is potential for automatic detection and tracking of FMs using the MV beam of a linear accelerator. Using the treatment beam for the tracking of FMs without additional dose to the patient would be an advantage. If this could be combined with improvements in digital tomosynthesis and MV CBCT it would allow tracking and volumetric information to be captured using no additional ionizing radiation.
Second, MRI and linear accelerator fusion is currently being investigated. Designs include fusion with a cobalt machine (Viewray®, OH, USA) or linear accelerator. In Utrecht (The Netherlands), research is underway into fusion of a linear accelerator with a 1.5 T MRI [202]. This is a powerful magnet when compared with the other proposed units, such as Viewray (0.3 T) and the Edmonton MRI/linear accelerator (0.8 T) [203]. There are many issues yet to be addressed, such as the impact of the B0 field on dose distribution and the dose deposition from secondary electrons affected by the magnetic field, especially at tissue air interfaces [202].
Third, dose-guided radiotherapy is being investigated. The use of volumetric information from onboard CT imaging integrated with intrafraction target position information allows reconstruction of doses delivered to the target and OARs [204]. This information can be used for adaptive radiotherapy but in the future it is hoped that the possibility of dose-guided radiotherapy may be realized. This would allow images and delivered doses during previous fractions to be amalgamated and the current treatment plan to be reoptimized to compensate for dosimetric errors [205]. This requires automated methods of assessing and constructing delivered dosimetry and improvements in the current levels of accuracy of dosimetric estimations. Dose-guided radiotherapy would be the ultimate in IGRT as it would result in the verification of both positioning of the patient and dose delivered to the target and organs at risk.
Conclusion
Improvements in diagnostic imaging, particularly multiparametric MRI, enable us to better localize the areas of cancer within the prostate that require an additional radiotherapy dose. By accurately defining these areas we can shape radiotherapy fields in a way that avoids the adjacent normal tissues and therefore reduces the possibility of side effects caused by irradiation of these tissues.
Intrafraction motion is now one of the most limiting factors to the increased precision and therefore reduction in toxicity in prostate radiotherapy. Real-time monitoring with the ability to intervene is important, especially if PTV margins are to be reduced to less than 5 mm. Patient specific and adaptive solutions should be examined as well as gating radiation beams to prostate movement. Accuracy is of particular importance with dose escalation to tumor nodules and nodal areas in prostate radiotherapy as the main OARs: rectum, bladder, small bowel and urethra are all in close proximity. Volumetric information to assess the dose and volumes of theses OARs is vital.
There are caveats to the likely reduction in toxicity with increasing radiotherapy targeting. The first is the need for vigilance in quality assurance when using newer technologies. Radiation errors have been documented on numerous occasions with inadequate quality assurance programs when introducing new hardware and software as well as deficiencies in appropriate regulation and supervision [206–209]. Furthermore, inadequate checks or excessive reductions in safety margins may lead to an increased risk of geographical miss. The second caveat relates to toxicities such as a possible increased risk of second malignancies, which may be modeled as a stochastic effect. With IMRT and arc radiotherapy, the amount of tissue irradiated to a low dose is increased. This is sometimes called the low-dose “bath effect”, which could result in an increased risk of a second cancer. Such issues need to be considered when integrating these new developments.
With the rapid development of new technologies bringing the prospect of increased accuracy of localization and treatment delivery, these are exciting times and there is no doubt that the face of prostate cancer planning and verification will change substantially over the next decade.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: * of considerable interest
- Mayles WP. Survey of the availability and use of advanced radiotherapy technology in the UK. Clin. Oncol. (R. Coll. Radiol.) 22(8), 636–642 (2010).
- Kupelian PA, Potters L, Khuntia D et al. Radical prostatectomy, external beam radiotherapy <72 Gy, external beam radiotherapy > or =72 Gy, permanent seed implantation, or combined seeds/external beam radiotherapy for stage T1–T2 prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 58(1), 25–33 (2004).
- Widmark A, Klepp O, Solberg A et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised Phase 3 trial. Lancet 373(9660), 301–308 (2009).
- Dearnaley DP, Hall E, Lawrence D et al. Phase III pilot study of dose escalation using conformal radiotherapy in prostate cancer: PSA control and side effects. Br. J. Cancer 92(3), 488–498 (2005).
- Pollack A, Zagars GK, Starkschall G et al. Prostate cancer radiation dose response: results of the M. D. Anderson Phase III randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 53(5), 1097–1105 (2002).
- Peeters ST, Heemsbergen WD, Koper PC et al. Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized Phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J. Clin. Oncol. 24(13), 1990–1996 (2006).
- Dearnaley DP, Heemsbergen WD, Koper PC et al. Escalated-dose versus standarddose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol. 8(6), 475–487 (2007). & This is the largest reported study confirming increase in disease control in prostate cancer following dose escalation.
- Zietman AL, DeSilvio ML, Slater JD et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA 294(10), 1233–1239 (2005).
- Schroder FH, Hugosson J, Roobol MJ et al. Screening and prostate-cancer mortality in a randomized European study. N. Engl. J. Med. 360(13), 1320–1328 (2009).
- Kelloff GJP, Choyke DS. Coffey DS. Challenges in clinical prostate cancer: role of imaging. Am. J. Roentgenol. 192(6), 1455–1470 (2009).
- Wang L, Hricak H, Kattan MW et al. Prediction of organ-confined prostate cancer: incremental value of MR imaging and MR spectroscopic imaging to staging nomograms. Radiology 238(2), 597–603 (2006). 12 Kwek JW, Thng CH, Tan PH et al. Phasedarray magnetic resonance imaging of the prostate with correlation to radical prostatectomy specimens: local experience. Asian J. Surg. 27(3), 219–226, discussion 225–226 (2004).
- Hricak H, White S, Vigneron D et al. Carcinoma of the prostate gland: MR imaging with pelvic phased-array coils versus integrated endorectal–pelvic phased-array coils. Radiology 193(3), 703–709 (1994).
- Dearnaley DP, Khoo VS, Norman AR et al. Comparison of radiation side-effects of conformal and conventional radiotherapy in prostate cancer: a randomised trial. Lancet 353(9149), 267–272 (1999).
- Khoo VS, Joon DL. New developments in MRI for target volume delineation in radiotherapy. Br. J. Radiol. 79(Spec No. 1), S2–S15 (2006).
- Hricak H, Dooms GC, McNeal JE et al. MR imaging of the prostate gland: normal anatomy. Am. J. Roentgenol. 148(1), 51–58 (1987).
- Baker GR. Localization: conventional and CT simulation. Br. J. Radiol. 79(Spec No. 1), S36–S49 (2006).
- Kuban D, Pollack A, Huang E et al. Hazards of dose escalation in prostate cancer radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 57(5), 1260–1268 (2003).
- Jacob R, Hanlon AL, Horwitz EM et al. The relationship of increasing radiotherapy dose to reduced distant metastases and mortality in men with prostate cancer. Cancer 100(3), 538–543 (2004).
- Kuban DA, Thames HD, Levy LB et al. Long-term multi-institutional analysis of stage T1–T2 prostate cancer treated with radiotherapy in the PSA era. Int. J. Radiat. Oncol. Biol. Phys. 57(4), 915–928 (2003).
- Zelefsky MJ, Reuter VE, Fuks Z, Scardino P, Shippy A. Influence of local tumor control on distant metastases and cancer related mortality after external beam radiotherapy for prostate cancer. J. Urol. 179(4), 1368–1373, discussion 1373 (2008).
- Zietman AL, Bae K, Slater JD et al. Randomized trial comparing conventionaldose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton radiation oncology group/american college of radiology 95–109. J. Clin. Oncol. 28(7), 1106–1111 (2010).
- Kuban DA, Tucker SL, Dong L et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 70(1), 67–74 (2008).
- Zelefsky MJ, Yamada Y, Fuks Z et al. Long-term results of conformal radiotherapy for prostate cancer: impact of dose escalation on biochemical tumor control and distant metastases-free survival outcomes. Int. J. Radiat. Oncol. Biol .Phys. 71(4), 1028–1033 (2008).
- Cahlon O, Zelefsky MJ, Shippy A et al. Ultra-high-dose (86.4 Gy) IMRT for localized prostate cancer: toxicity and biochemical outcomes. Int. J. Radiat. Oncol. Biol. Phys. 71(2), 330–337 (2008).
- Eade TN, Hanlon AL, Horwitz EM et al. What dose of external-beam radiation is high enough for prostate cancer? Int. J. Radiat. Oncol. Biol. Phys. 68(3), 682–689 (2007).
- Viani GA, Stefano EJ, Afonso SL. Higherthan- conventional radiation doses in localized prostate cancer treatment: a meta-analysis of randomized, controlled trials. Int. J. Radiat. Oncol. Biol. Phys. 74(5), 1405–1418 (2009).
- Peeters ST, Lebesque JV, Heemsbergen WD et al. Localized volume effects for late rectal and anal toxicity after radiotherapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 64(4), 1151–1161 (2006).
- van Lin EN, Fütterer JJ, Heijmink SW et al. IMRT boost dose planning on dominant intraprostatic lesions: gold marker-based three-dimensional fusion of CT with dynamic contrast-enhanced and 1H-spectroscopic MRI. Int. J. Radiat. Oncol. Biol. Phys. 65(1), 291–303 (2006). & This is a clinical trial showing proof of principle in treating dominant intraprostatic lesions with intensity-modulated radiotherapy.
- Nutting CM, Corbishley CM, Sanchez-Nieto B et al. Potential improvements in the therapeutic ratio of prostate cancer irradiation: dose escalation of pathologically identified tumour nodules using intensity modulated radiotherapy. Br. J. Radiol. 75(890), 151–161 (2002).
- Xia P, Corbishley CM, Sanchez-Nieto B et al. Forward or inversely planned segmental multileaf collimator IMRT and sequential Tomotherapy to treat multiple dominant intraprostatic lesions of prostate cancer to 90 Gy. Int. J. Radiat. Oncol. Biol. Phys. 51(1), 244–254 (2001).
- Pickett B, Vigneault E, Kurhanewicz J, Verhey L, Roach M. Static field intensity modulation to treat a dominant intraprostatic lesion to 90 Gy compared to seven field 3-dimensional radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 44(4), 921–929 (1999).
- Fonteyne V, Villeirs G, Speleers B et al. Intensity-modulated radiotherapy as primary therapy for prostate cancer: report on acute toxicity after dose escalation with simultaneous integrated boost to intraprostatic lesion. Int. J. Radiat. Oncol. Biol. Phys. 72(3), 799–807 (2008). & This is the largest clinical trial treating patients with dose-escalated radiation boosts. 34 Miralbell R, Mollà M, Rouzaud M et al. Hypofractionated boost to the dominant tumor region with intensity modulated stereotactic radiotherapy for prostate cancer: a sequential dose escalation pilot study. Int. J. Radiat. Oncol. Biol. Phys. 78(1), 50–57 (2010).
- Housri N, Ning H, Ondos J et al. Parameters favorable to intraprostatic radiation dose escalation in men with localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 80(2), 614–620 (2011).
- Ost P, Speleers B, De Meerleer G et al. Volumetric arc therapy and intensitymodulated radiotherapy for primary prostate radiotherapy with simultaneous integrated boost to intraprostatic lesion with 6 and 18 MV: a planning comparison study. Int. J. Radiat. Oncol. Biol. Phys. 79(3), 920–926 (2011).
- van der Heide UA, Korporaal JG, Groenendaal G, Franken SP, van Vulpen M. Functional MRI for tumor delineation in prostate radiation therapy. Imaging Med. 3(2), 219–231 (2011).
- Khoo VS, Padhani AR, Tanner SF, Finnigan DJ, Leach MO, Dearnaley DP. Comparison of MRI with CT for the radiotherapy planning of prostate cancer: a feasibility study. Br. J. Radiol. 72(858), 590–597 (1999).
- Steenbakkers RJ, Padhani AR, Tanner SF et al. Reduction of dose delivered to the rectum and bulb of the penis using MRI delineation for radiotherapy of the prostate. Int. J. Radiat. Oncol. Biol. Phys. 57(5), 1269–1279 (2003).
- Kessler ML. Image registration and data fusion in radiation therapy. Br. J. Radiol. 79(Spec No. 1), S99–S108 (2006).
- Rasch C, Padhani AR, Tanner SF et al. Definition of the prostate in CT and MRI: a multi-observer study. Int. J. Radiat. Oncol. Biol. Phys. 43(1), 57–66 (1999). nn Seminal paper describing the use of MRI and CT scanning in the planning of radiotherapy treatments.
- Roach M 3rd, Faillace-Akazawa P, Malfatti C, Holland J, Hricak H. Prostate volumes defined by magnetic resonance imaging and computerized tomographic scans for three-dimensional conformal radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 35(5), 1011–1018 (1996).
- Jackson AS, Reinsberg SA, Sohaib SA et al. Distortion-corrected T2 weighted MRI: a novel approach to prostate radiotherapy planning. Br. J. Radiol. 80(959), 926–933 (2007).
- Sannazzari GL, Ragona R, Ruo Redda MG et al. CT–MRI image fusion for delineation of volumes in three-dimensional conformal radiation therapy in the treatment of localized prostate cancer. Br. J. Radiol. 75(895), 603–607 (2002).
- Kagawa K, Lee WR, Schultheiss TE et al. Initial clinical assessment of CT–MRI image fusion software in localization of the prostate for 3D conformal radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 38(2), 319–325 (1997).
- Debois M, Oyen R, Maes F et al. The contribution of magnetic resonance imaging to the three-dimensional treatment planning of localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 45(4), 857–865 (1999).
- Krempien RC, Schubert K, Zierhut D et al. Open low-field magnetic resonance imaging in radiation therapy treatment planning. Int. J. Radiat. Oncol. Biol. Phys. 53(5), 1350–1360 (2002).
- Reinsberg SA, Doran SJ, Charles-Edwards EM, Leach MO. A complete distortion correction for MR images: II. Rectification of static-field inhomogeneities by similarity-based profile mapping. Phys. Med. Biol. 50(11), 2651–2661 (2005).
- Ahmed HU, Calleary J, Arya M et al. Re: Dynamic contrast enhanced, pelvic phased array magnetic resonance imaging of localized prostate cancer for predicting tumor volume: correlation with radical prostatectomy findings. A. Villers, P. Puech, D. Mouton, X. Leroy, C. Ballereau and L. Lemaitre, J Urol 2006; 176: 2432-2437. J. Urol. 177(6), 2395, author reply 2395–2396 (2007).
- Riches SF, Payne GS, Morgan VA et al. MRI in the detection of prostate cancer: combined apparent diffusion coefficient, metabolite ratio, and vascular parameters. Am. J. Roentgenol. 193(6), 1583–1591 (2009).
- Franiel T, Stephan C, Erbersdobler A et al. Areas suspicious for prostate cancer: MR-guided biopsy in patients with at least one transrectal US-guided biopsy with a negative finding–multiparametric MR imaging for detection and biopsy planning. Radiology 259(1), 162–172 (2011).
- Afaq A, Koh DM, Padhani A, van As N, Sohaib SA. Clinical utility of diffusionweighted magnetic resonance imaging in prostate cancer. BJU Int. 108(11), 1716–1722 (2011).
- desouza NM, Reinsberg SA, Scurr ED, Brewster JM, Payne GS. Magnetic resonance imaging in prostate cancer: the value of apparent diffusion coefficients for identifying malignant nodules. Br. J. Radiol. 80(950), 90–95 (2007).
- Morgan VA, Kyriazi S, Ashley SE, DeSouza NM. Evaluation of the potential of diffusion-weighted imaging in prostate cancer detection. Acta Radiol. 48(6), 695–703 (2007).
- Kozlowski P, Chang SD, Jones EC, Berean KW, Chen H, Goldenberg SL. Combined diffusion-weighted and dynamic contrastenhanced MRI for prostate cancer diagnosis – correlation with biopsy and histopathology. J. Magn Reson. Imaging 24(1), 108–113 (2006).
- Ogura K, Maekawa S, Okubo K et al. Dynamic endorectal magnetic resonance imaging for local staging and detection of neurovascular bundle involvement of prostate cancer: correlation with histopathologic results. Urology 57(4), 721–726 (2001).
- Futterer JJ, Engelbrecht MR, Huisman HJ et al. Staging prostate cancer with dynamic contrast-enhanced endorectal MR imaging prior to radical prostatectomy: experienced versus less experienced readers. Radiology 237(2), 541–549 (2005).
- Engelbrecht MR, Huisman HJ, Laheij RJ et al. Discrimination of prostate cancer from normal peripheral zone and central gland tissue by using dynamic contrast-enhanced MR imaging. Radiology 229(1), 248–254 (2003).
- Padhani AR, Gapinski CJ, Macvicar DA et al. Dynamic contrast enhanced MRI of prostate cancer: correlation with morphology and tumour stage, histological grade and PSA. Clin. Radiol. 55(2), 99–109 (2000).
- Kayhan A, Fan X, Oto A. Dynamic contrast-enhanced magnetic resonance imaging in prostate cancer. Top. Magn. Reson. Imaging 20(2), 105–112 (2009).
- Casciani E, Polettini E, Bertini L et al. Prostate cancer: evaluation with endorectal MR imaging and three-dimensional proton MR spectroscopic imaging. Radiol. Med. 108(5–6), 530–541 (2004).
- Augustin H, Hammerer PG, Blonski J et al. Zonal location of prostate cancer: significance for disease-free survival after radical prostatectomy? Urology 62(1), 79–85 (2003).
- Weinreb JC, Blume JD, Coakley FV et al. Prostate cancer: sextant localization at MR imaging and MR spectroscopic imaging before prostatectomy – results of ACRIN prospective multi-institutional clinicopathologic study. Radiology 251(1), 122–133 (2009).
- Senft A, de Bree R, Hoekstra OS et al. Screening for distant metastases in head and neck cancer patients by chest CT or whole body FDG–PET: a prospective multicenter trial. Radiother. Oncol. 87(2), 221–229 (2008).
- Shreve PD, Grossman HB, Gross MD, Wahl RL. Metastatic prostate cancer: initial findings of PET with 2-deoxy-2-[F-18] fluoro-d-glucose. Radiology 199(3), 751–756 (1996).
- Giovacchini G, Picchio M, Coradeschi E et al. 11C choline uptake with PET/CT for the initial diagnosis of prostate cancer: relation to PSA levels, tumour stage and anti-androgenic therapy. Eur. J. Nucl. Med. Mol. Imaging 35(6), 1065–1073 (2008).
- Seppala J, Seppänen M, Arponen E, Lindholm P, Minn H. Carbon-11 acetate PET/CT based dose escalated IMRT in prostate cancer. Radiother. Oncol. 93(2), 234–240 (2009).
- Kim SH, Choi BI, Han JK et al. Preoperative staging of uterine cervical carcinoma: comparison of CT and MRI in 99 patients. J. Comput. Assist. Tomogr. 17(4), 633–640 (1993).
- Oyen RH, Van Poppel HP, Ameye FE et al. Lymph node staging of localized prostatic carcinoma with CT and CT-guided fine-needle aspiration biopsy: prospective study of 285 patients. Radiology 190(2), 315–322 (1994).
- Fukuda H, Nakagawa T, Shibuya H. Metastases to pelvic lymph nodes from carcinoma in the pelvic cavity: diagnosis using thin-section CT. Clin. Radiol. 54(4), 237–242 (1999).
- Hawnaur JM, Johnson RJ, Buckley CH, Tindall V, Isherwood I. Staging, volume estimation and assessment of nodal status in carcinoma of the cervix: comparison of magnetic resonance imaging with surgical findings. Clin. Radiol. 49(7), 443–452 (1994).
- Kim SH, Kim SC, Choi BI, Han MC. Uterine cervical carcinoma: evaluation of pelvic lymph node metastasis with MR imaging. Radiology 190(3), 807–811 (1994).
- Jager GJ, Barentsz JO, Oosterhof GO et al. Pelvic adenopathy in prostatic and urinary bladder carcinoma: MR imaging with a three-dimensional TI-weighted magnetization-prepared-rapid gradient-echo sequence. Am. J. Roentgenol. 167(6), 1503–1507 (1996).
- Roy C, Le Bras Y, Mangold L et al. Small pelvic lymph node metastases: evaluation with MR imaging. Clin. Radiol. 52(6), 437–440 (1997).
- Vinnicombe SJ, Norman AR, Nicolson V, Husband JE et al. Normal pelvic lymph nodes: evaluation with CT after bipedal lymphangiography. Radiology 194(2), 349–355 (1995).
- Grubnic S, Vinnicombe SJ, Norman AR, Husband JE. MR evaluation of normal retroperitoneal and pelvic lymph nodes. Clin. Radiol. 57(3), 193–200, discussion 201–204 (2002).
- Mir N, Sohaib SA, Collins D, Koh DM. Fusion of high b-value diffusion-weighted and T2-weighted MR images improves identification of lymph nodes in the pelvis. J. Med. Imaging Radiat. Oncol. 54(4), 358–364 (2010).
- Harisinghani MG, Saini S, Weissleder R et al. MR lymphangiography using ultrasmall superparamagnetic iron oxide in patients with primary abdominal and pelvic malignancies: radiographic-pathologic correlation. Am. J. Roentgenol. 172(5), 1347–1351 (1999). & Paper confirming the value of MRI in combination with ultrasmall superparamagnetic iron oxide particles in the identification of small lymph node metastases.
- Taylor A, Rockall AG, Reznek RH, Powell ME. Mapping pelvic lymph nodes: guidelines for delineation in intensity-modulated radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 63(5), 1604–1612 (2005).
- Dinniwell R, Chan P, Czarnota G et al. Pelvic lymph node topography for radiotherapy treatment planning from ferumoxtran-10 contrast-enhanced magnetic resonance imaging. Int. J. Radiat. Oncol. Biol. Phys. 74(3), 844–851 (2009).
- Shih HA, Harisinghani M, Zietman AL et al. Mapping of nodal disease in locally advanced prostate cancer: rethinking the clinical target volume for pelvic nodal irradiation based on vascular rather than bony anatomy. Int. J. Radiat. Oncol. Biol. Phys. 63(4), 1262–1269 (2005).
- Lawton CA, Michalski J, El-Naqa I et al. RTOG GU Radiation oncology specialists reach consensus on pelvic lymph node volumes for high-risk prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 74(2), 383–387 (2009).
- Schiavina R, Scattoni V, Castellucci P et al. 11C-choline positron emission tomography/computerized tomography for preoperative lymph-node staging in intermediate-risk and high-risk prostate cancer: comparison with clinical staging nomograms. Eur. Urol. 54(2), 392–401 (2008).
- Zelefsky MJ, Levin EJ, Hunt M et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 70(4), 1124–1129 (2008).
- Nutting CM, Rowbottom CG, Cosgrove VP et al. Optimisation of radiotherapy for carcinoma of the parotid gland: a comparison of conventional, three-dimensional conformal, and intensity-modulated techniques. Radiother. Oncol. 60(2), 163–172 (2001).
- Nutting CM, Morden JP, Harrington KJ et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a Phase 3 multicentre randomised controlled trial. Lancet Oncol. 12(2), 127–136 (2011).
- Vigneault E, Pouliot J, Laverdière J, Roy J, Dorion M. Electronic portal imaging device detection of radioopaque markers for the evaluation of prostate position during megavoltage irradiation: a clinical study. Int. J. Radiat. Oncol. Biol. Phys. 37(1), 205–212 (1997).
- Crook JM, Raymond Y, Salhani D, Yang H, Esche B. Prostate motion during standard radiotherapy as assessed by fiducial markers. Radiother. Oncol. 37(1), 35–42 (1995).
- Schallenkamp JM, Raymond Y, Salhani D, Yang H, Esche B. Prostate position relative to pelvic bony anatomy based on intraprostatic gold markers and electronic portal imaging. Int. J. Radiat. Oncol. Biol. Phys. 63(3), 800–811 (2005).
- Kupelian P, Willoughby T, Mahadevan A et al. Multi-institutional clinical experience with the calypso system in localization and continuous, real-time monitoring of the prostate gland during external radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 67(4), 1088–1098 (2007).
- McNair HA, Hansen VN, Parker CC et al. A comparison of the use of bony anatomy and internal markers for offline verification and an evaluation of the potential benefit of online and offline verification protocols for prostate radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 71(1), 41–50 (2008).
- de Crevoisier R, Tucker SL, Dong L et al. Increased risk of biochemical and local failure in patients with distended rectum on the planning CT for prostate cancer radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 62(4), 965–973 (2005).
- Heemsbergen WD, Hoogeman MS, Witte MG et al. Increased risk of biochemical and clinical failure for prostate patients with a large rectum at radiotherapy planning: results from the Dutch trial of 68 Gy versus 78 Gy. Int. J. Radiat. Oncol. Biol. Phys. 67(5), 1418–1424 (2007).
- Greer PB, Dahl K, Ebert MA et al. Assessment of a daily online implanted fiducial marker-guided prostate radiotherapy process. J. Med. Imaging Radiat. Oncol. 52(5), 517–524 (2008).
- Kupelian PA, Lee C, Langen KM et al. Evaluation of image-guidance strategies in the treatment of localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 70(4), 1151–1157 (2008).
- Little DJ, Lee C, Langen KM et al. Use of portal images and BAT ultrasonography to measure setup error and organ motion for prostate IMRT: implications for treatment margins. Int. J. Radiat. Oncol. Biol. Phys. 56(5), 1218–1224 (2003).
- Huang E, Dong L, Chandra A et al. Intrafraction prostate motion during IMRT for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 53(2), 261–268 (2002).
- Morr J, DiPetrillo T, Tsai JS, Engler M, Wazer DE. Implementation and utility of a daily ultrasound-based localization system with intensity-modulated radiotherapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 53(5), 1124–1129 (2002).
- Serago CF, Chungbin SJ, Buskirk SJ et al. Initial experience with ultrasound localization for positioning prostate cancer patients for external beam radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 53(5), 1130–1138 (2002).
- Lattanzi J, McNeeley S, Pinover W et al. A comparison of daily CT localization to a daily ultrasound-based system in prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 43(4), 719–725 (1999).
- Trichter F, Ennis RD. Prostate localization using transabdominal ultrasound imaging. Int. J. Radiat. Oncol. Biol. Phys. 56(5), 1225–1233 (2003).
- Jani AB, Gratzle J, Muresan E et al. Analysis of acute toxicity with use of transabdominal ultrasonography for prostate positioning during intensity-modulated radiotherapy. Urology 65(3), 504–508 (2005).
- Bohrer M, Schröder P, Welzel G et al. Reduced rectal toxicity with ultrasound-based image guided radiotherapy using BAT (B-mode acquisition and targeting system) for prostate cancer. Strahlenther. Onkol. 184(12), 674–678 (2008).
- Jani AB, Gratzle J, Muresan E, Martel MK. Impact on late toxicity of using transabdominal ultrasound for prostate cancer patients treated with intensity modulated radiotherapy. Technol. Cancer Res. Treat. 4(1), 115–120 (2005).
- Langen KM, Pouliot J, Anezinos C et al. Evaluation of ultrasound-based prostate localization for image-guided radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 57(3), 635–644 (2003).
- McNair HA, Mangar SA, Coffey J et al. A comparison of CT- and ultrasound-based imaging to localize the prostate for external beam radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 65(3), 678–687 (2006).
- Chandra A, Dong L, Huang E et al. Experience of ultrasound-based daily prostate localization. Int. J. Radiat. Oncol. Biol. Phys. 56(2), 436–447 (2003).
- Pinkawa M, Pursch-Lee M, Asadpour B et al. Image-guided radiotherapy for prostate cancer. Implementation of ultrasound-based prostate localization for the analysis of inter- and intrafraction organ motion. Strahlenther. Onkol. 184(12), 679–685 (2008).
- McGahan JP, Ryu J, Fogata M. Ultrasound probe pressure as a source of error in prostate localization for external beam radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 60(3), 788–793 (2004).
- Dobler B, Mai S, Ross C et al. Evaluation of possible prostate displacement induced by pressure applied during transabdominal ultrasound image acquisition. Strahlenther. Onkol. 182(4), 240–246 (2006).
- Artignan X, Smitsmans MH, Lebesque JV et al. Online ultrasound image guidance for radiotherapy of prostate cancer: impact of image acquisition on prostate displacement. Int. J. Radiat. Oncol. Biol. Phys. 59(2), 595–601 (2004).
- Van den Heuvel F, Powell T, Seppi E et al. Independent verification of ultrasound based image-guided radiation treatment, using electronic portal imaging and implanted gold markers. Med. Phys. 30(11), 2878–2887 (2003).
- Johnston H, Hilts M, Beckham W, Berthelet E. 3D ultrasound for prostate localization in radiation therapy: a comparison with implanted fiducial markers. Med. Phys. 35(6), 2403–2413 (2008).
- Falco T, Shenouda G, Kaufmann C et al. Ultrasound imaging for external-beam prostate treatment setup and dosimetric verification. Med. Dosim. 27(4), 271–273 (2002).
- 5 Kupelian PA, Willoughby TR, Reddy CA et al. Impact of image guidance on outcomes after external beam radiotherapy for localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 70(4), 1146–1150 (2008).
- Balter JM, Sandler HM, Lam K et al. Measurement of prostate movement over the course of routine radiotherapy using implanted markers. Int. J. Radiat. Oncol. Biol. Phys. 31(1), 113–118 (1995).
- Dehnad H, Nederveen AJ, van der Heide UA et al. Clinical feasibility study for the use of implanted gold seeds in the prostate as reliable positioning markers during megavoltage irradiation. Radiother. Oncol. 67(3), 295–302 (2003).
- Henry AM, Wilkinson C, Wylie JP et al. Trans-perineal implantation of radio-opaque treatment verification markers into the prostate: an assessment of procedure related morbidity, patient acceptability and accuracy. Radiother. Oncol. 73(1), 57–59 (2004).
- Igdem S, Akpinar H, Alço G et al. Implantation of fiducial markers for image guidance in prostate radiotherapy: patient-reported toxicity. Br. J. Radiol. 82(983), 941–945 (2009).
- Kupelian PA, Willoughby TR, Meeks SL et al. Intraprostatic fiducials for localization of the prostate gland: monitoring intermarker distances during radiation therapy to test for marker stability. Int. J. Radiat. Oncol. Biol. Phys. 62(5), 1291–1296 (2005).
- Litzenberg D, Dawson LA, Sandler H et al. Daily prostate targeting using implanted radiopaque markers. Int. J. Radiat. Oncol. Biol. Phys. 52(3), 699–703 (2002).
- Poggi MM, Gant DA, Sewchand W, Warlick WB. Marker seed migration in prostate localization. Int. J. Radiat. Oncol. Biol. Phys. 56(5), 1248–1251 (2003).
- Pouliot J, Aubin M, Langen KM et al. (Non)-migration of radiopaque markers used for on-line localization of the prostate with an electronic portal imaging device. Int. J. Radiat. Oncol. Biol. Phys. 56(3), 862–866 (2003).
- Moman MR, van der Heide UA, Kotte AN et al. Long-term experience with transrectal and transperineal implantations of fiducial gold markers in the prostate for position verification in external beam radiotherapy; feasibility, toxicity and quality of life. Radiother. Oncol. 96(1), 38–42 (2010).
- Ullman KL, Ning H, Susil RC et al. Intra- and inter-radiation therapist reproducibility of daily isocenter verification using prostatic fiducial markers. Radiat. Oncol. 1, 2 (2006).
- 126 Kong V, Lockwood G, Yan J et al. The effect of registration surrogate and patient factors on the interobserver variability of electronic portal image guidance during prostate radiotherapy. Med. Dosim. 36(4), 337–343 (2010).
- van Haaren PM, Bel A, Hofman P et al. Influence of daily setup measurements and corrections on the estimated delivered dose during IMRT treatment of prostate cancer patients. Radiother. Oncol. 90(3), 291–298 (2009).
- Pawlowski JM, Yang ES, Malcolm AW et al. Reduction of dose delivered to organs at risk in prostate cancer patients via image-guided radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 76(3), 924–934 (2010).
- Tanyi JA, He T, Summers PA et al. Assessment of planning target volume margins for intensity-modulated radiotherapy of the prostate gland: role of daily inter- and intrafraction motion. Int. J. Radiat. Oncol. Biol. Phys. 78(5), 1579–1585 (2010).
- Graf R, Wust P, Budach V, Boehmer D. Potentials of on-line repositioning based on implanted fiducial markers and electronic portal imaging in prostate cancer radiotherapy. Radiat. Oncol. 4, 13 (2009).
- Kotte AN, Hofman P, Lagendijk JJ et al. Intrafraction motion of the prostate during external-beam radiation therapy: analysis of 427 patients with implanted fiducial markers. Int. J. Radiat. Oncol Biol. Phys. 69(2), 419–425 (2007).
- Greer PB, Dahl K, Ebert MA et al. Comparison of prostate set-up accuracy and margins with off-line bony anatomy corrections and online implanted fiducialbased corrections. J. Med. Imaging Radiat. Oncol. 52(5), 511–516 (2008).
- Wu J, Haycocks T, Alasti H et al. Positioning errors and prostate motion during conformal prostate radiotherapy using on-line isocentre set-up verification and implanted prostate markers. Radiother. Oncol. 61(2), 127–133 (2001).
- Skarsgard D, Cadman P, El-Gayed A et al. Planning target volume margins for prostate radiotherapy using daily electronic portal imaging and implanted fiducial markers. Radiat. Oncol. 5, 52 (2010).
- Enmark M, Korreman S, Nystrom H. IGRT of prostate cancer; is the margin reduction gained from daily IG time-dependent? Acta Oncol. 45(7), 907–914 (2006).
- Kron T, Thomas J, Fox C et al. Intra-fraction prostate displacement in radiotherapy estimated from pre- and post-treatment imaging of patients with implanted fiducial markers. Radiother. Oncol. 95(2), 191–197 (2010).
- Litzenberg DW, Balter JM, Hadley SW et al. Influence of intrafraction motion on margins for prostate radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 65(2), 548–553 (2006).
- Beltran C, Herman MG, Davis BJ. Planning target margin calculations for prostate radiotherapy based on intrafraction and interfraction motion using four localization methods. Int. J. Radiat. Oncol. Biol. Phys. 70(1), 289–295 (2008).
- Scarbrough TJ, Golden NM, Ting JY et al. Comparison of ultrasound and implanted seed marker prostate localization methods: implications for image-guided radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 65(2), 378–387 (2006).
- Shi W, Li JG, Zlotecki RA et al. Evaluation of kV cone-beam CT performance for prostate IGRT: a comparison of automatic grey-value alignment to implanted fiducial-marker alignment. Am. J. Clin. Oncol. 34(1), 16–21 (2010).
- Gauthier I, Carrier JF, Béliveau-Nadeau D et al. Dosimetric impact and theoretical clinical benefits of fiducial markers for dose escalated prostate cancer radiation treatment. Int. J. Radiat. Oncol. Biol. Phys. 74(4), 1128–1133 (2009).
- Martin JM, Bayley A, Bristow R et al. Image guided dose escalated prostate radiotherapy: still room to improve. Radiat. Oncol. 4, 50 (2009).
- Uematsu M, Fukui T, Shioda A et al. A dual computed tomography linear accelerator unit for stereotactic radiation therapy: a new approach without cranially fixated stereotactic frames. Int. J. Radiat. Oncol. Biol. Phys. 35(3), 587–592 (1996).
- Kuriyama K, Onishi H, Sano N et al. A new irradiation unit constructed of self-moving gantry-CT and linac. Int. J. Radiat. Oncol. Biol. Phys. 55(2), 428–435 (2003).
- Sonke JJ, Zijp L, Remeijer P, van Herk M. Respiratory correlated cone beam CT. Med. Phys. 32(4), 1176–1186 (2005).
- Dietrich L, Jetter S, Tücking T, Nill S, Oelfke U. Linac-integrated 4D cone beam CT: first experimental results. Phys. Med. Biol. 51(11), 2939–2952 (2006).
- Adamson J, Wu Q. Prostate intrafraction motion evaluation using kV fluoroscopy during treatment delivery: a feasibility and accuracy study. Med. Phys. 35(5), 1793–1806 (2008).
- Jaffray DA, Siewerdsen JH, Wong JW, Martinez AA. Flat-panel cone-beam computed tomography for image-guided radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 53(5), 1337–1349 (2002).
- Smitsmans MH, de Bois J, Sonke JJ et al. Automatic prostate localization on cone-beam CT scans for high precision image-guided radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 63(4), 975–984 (2005).
- Fung AY, Grimm SY, Wong JR, Uematsu M. Computed tomography localization of radiation treatment delivery versus conventional localization with bony landmarks. J. Appl. Clin. Med. Phys. 4(2), 112–119 (2003).
- Moseley DJ, White EA, Wiltshire KL et al. Comparison of localization performance with implanted fiducial markers and cone-beam computed tomography for on-line imageguided radiotherapy of the prostate. Int. J. Radiat. Oncol. Biol. Phys. 67(3), 942–953 (2007).
- Barney BM, Lee RJ, Handrahan D et al. Image-guided radiotherapy (IGRT) for prostate cancer comparing kV imaging of fiducial markers with cone beam computed tomography (CBCT). Int. J. Radiat. Oncol. Biol. Phys. 80(1), 301–305 (2011).
- Owen R, Foroudi F, Kron T et al. A comparison of in-room computerized tomography options for detection of fiducial markers in prostate cancer radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 77(4), 1248–1256 (2010).
- Nijkamp J, Pos FJ, Nuver TT et al. Adaptive radiotherapy for prostate cancer using kilovoltage cone-beam computed tomography: first clinical results. Int. J. Radiat. Oncol. Biol. Phys. 70(1), 75–82 (2008).
- Wong JR, Grimm L, Uematsu M et al. Image-guided radiotherapy for prostate cancer by CT-linear accelerator combination: prostate movements and dosimetric considerations. Int. J. Radiat. Oncol. Biol. Phys. 61(2), 561–569 (2005).
- Chen L, Paskalev K, Xu X et al. Rectal dose variation during the course of image-guided radiation therapy of prostate cancer. Radiother. Oncol. 95(2), 198–202 (2010).
- Adamson J, Wu Q. Prostate intrafraction motion assessed by simultaneous kV fluoroscopy at MV delivery II: adaptive strategies. Int. J. Radiat. Oncol. Biol. Phys. 78(5), 1323–1330 (2010).
- Chang Z, Wang Z, Ma J et al. 6D image guidance for spinal non-invasive stereotactic body radiation therapy: comparison between ExacTrac x-ray 6D with kilo-voltage cone-beam CT. Radiother. Oncol. 95(1), 116–121 (2010).
- Xie Y, Djajaputra D, King CR et al. Intrafractional motion of the prostate during hypofractionated radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 72(1), 236–246 (2008).
- Freeman DE, King CR. Stereotactic body radiotherapy for low-risk prostate cancer: five-year outcomes. Radiat. Oncol. 6, 3 (2011).
- Sterzing F, Kalz J, Sroka-Perez G et al. Megavoltage CT in helical Tomotherapy – clinical advantages and limitations of special physical characteristics. Technol. Cancer Res. Treat. 8(5), 343–352 (2009).
- Mosleh-Shirazi MA, Evans PM, Swindell W, Webb S, Partridge M. A cone-beam megavoltage CT scanner for treatment verification in conformal radiotherapy. Radiother. Oncol. 48(3), 319–328 (1998).
- Groh BA, Siewerdsen JH, Drake DG et al. A performance comparison of flat-panel imager-based MV and kV cone-beam CT. Med. Phys. 29(6), 967–975 (2002).
- Nakagawa K, Aoki Y, Tago M, Terahara A, Ohtomo K. Megavoltage CT-assisted stereotactic radiosurgery for thoracic tumors: original research in the treatment of thoracic neoplasms. Int. J. Radiat. Oncol. Biol. Phys. 48(2), 449–457 (2000).
- Ruchala KJ, Olivera GH, Schloesser EA, Mackie TR. Megavoltage CT on a Tomotherapy system. Phys. Med. Biol. 44(10), 2597–2621 (1999).
- Maurer J, Godfrey D, Wang Z, Yin FF. On-board four-dimensional digital tomosynthesis: first experimental results. Med. Phys. 35(8), 3574–3583 (2008).
- Pouliot J, Bani-Hashemi A, Chen J et al. Low-dose megavoltage cone-beam CT for radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 61(2), 552–560 (2005).
- Faddegon BA, Aubin M, Bani-Hashemi A et al. Comparison of patient megavoltage cone beam CT images acquired with an unflattened beam from a carbon target and a flattened treatment beam. Med. Phys. 37(4), 1737–1741 (2010).
- Miften M, Gayou O, Reitz B et al. IMRT planning and delivery incorporating daily dose from mega-voltage cone-beam computed tomography imaging. Med. Phys. 34(10), 3760–3767 (2007).
- Morin O, Gillis A, Descovich M et al. Patient dose considerations for routine megavoltage cone-beam CT imaging. Med. Phys. 34(5), 1819–1827 (2007).
- Shah AP, Chen SS, Strauss JB et al. A dosimetric analysis comparing treatment of low-risk prostate cancer with Tomotherapy versus static field intensity modulated radiation therapy. Am. J. Clin. Oncol. doi:10.1097/COC.0b013e3181967d89 (2009) (Epub ahead of print).
- Wolff D, Stieler F, Welzel G et al. Volumetric modulated arc therapy (VMAT) vs. serial Tomotherapy, step-and-shoot IMRT and 3D-conformal RT for treatment of prostate cancer. Radiother. Oncol. 93(2), 226–233 (2009).
- Langen KM, Zhang Y, Andrews RD et al. Initial experience with megavoltage (MV) CT guidance for daily prostate alignments. Int. J. Radiat. Oncol. Biol. Phys. 62(5), 1517–1524 (2005).
- Yang D, Chaudhari SR, Goddu SM et al. Deformable registration of abdominal kilovoltage treatment planning CT and Tomotherapy daily megavoltage CT for treatment adaptation. Med. Phys. 36(2), 329–338 (2009).
- Lin SH, Sugar E, Teslow T et al. Comparison of daily couch shifts using MVCT (TomoTherapy) and B-mode ultrasound (BAT System) during prostate radiotherapy. Technol. Cancer Res. Treat. 7(4), 279–285 (2008).
- Peng C, Kainz K, Lawton C, Li XA. A comparison of daily megavoltage CT and ultrasound image guided radiation therapy for prostate cancer. Med. Phys. 35(12), 5619–5628 (2008).
- Gayou O, Miften M. Comparison of mega-voltage cone-beam computed tomography prostate localization with online ultrasound and fiducial markers methods. Med. Phys. 35(2), 531–538 (2008).
- Song WY, Chiu B, Bauman GS et al. Prostate contouring uncertainty in megavoltage computed tomography images acquired with a helical Tomotherapy unit during image-guided radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 65(2), 595–607 (2006).
- Kupelian PA, Langen KM, Zeidan OA et al. Daily variations in delivered doses in patients treated with radiotherapy for localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 66(3), 876–882 (2006).
- Varadhan R, Hui SK, Way S, Nisi K. Assessing prostate, bladder and rectal doses during image guided radiation therapy – need for plan adaptation? J. Appl. Clin. Med. Phys. 10(3), 2883 (2009).
- Iori M, Cattaneo GM, Cagni E et al. Dose-volume and biological-model based comparison between helical tomotherapy and (inverse-planned) IMAT for prostate tumours. Radiother. Oncol. 88(1), 34–45 (2008).
- Roland TF, Stathakis S, Ramer R, Papanikolaou N. Measurement and comparison of skin dose for prostate and headand- neck patients treated on various IMRT delivery systems. Appl. Radiat. Isot. 66(12), 1844–1849 (2008).
- Korreman S, Rasch C, McNair H et al. The European society of therapeutic radiology and oncology-European institute of radiotherapy (ESTRO-EIR) report on 3D CT-based in-room image guidance systems: a practical and technical review and guide. Radiother. Oncol. 94(2), 129–144 (2010). & Excellent review of image guidance systems.
- Staton RJ, Langen KM, Kupelian PA, Meeks SL. Dosimetric effects of rotational output variation and x-ray target degradation on helical tomotherapy plans. Med. Phys. 36(7), 2881–2888 (2009).
- Yadav P, Tolakanahalli R, Rong Y, Paliwal BR. The effect and stability of MVCT images on adaptive tomotherapy. J. Appl. Clin. Med. Phys. 11(4), 3229 (2010).
- Lu W, Chen M, Ruchala KJ et al. Real-time motion-adaptive-optimization (MAO) in Tomotherapy. Phys. Med. Biol. 54(14), 4373–4398 (2009).
- Ngwa W, Meeks SL, Kupelian PA et al. Validation of a computational method for assessing the impact of intra-fraction motion on helical tomotherapy plans. Phys. Med. Biol. 54(21), 6611–6621 (2009).
- Broggi S, Cozzarini C, Fiorino C et al. Modeling set-up error by daily MVCT for prostate adjuvant treatment delivered in 20 fractions: implications for the assessment of the optimal correction strategies. Radiother. Oncol. 93(2), 246–252 (2009).
- Beldjoudi G, Yartsev S, Bauman G et al. Schedule for CT image guidance in treating prostate cancer with helical Tomotherapy. Br. J. Radiol. 83(987), 241–251 (2010).
- Balter JM, Wright JN, Newell LJ et al. Accuracy of a wireless localization system for radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 61(3), 933–937 (2005).
- Zhu X, Bourland JD, Yuan Y et al. Tradeoffs of integrating real-time tracking into IGRT for prostate cancer treatment. Phys. Med. Biol. 54(17), N393–N401 (2009).
- Langen KM, Willoughby TR, Meeks SL et al. Observations on real-time prostate gland motion using electromagnetic tracking. Int. J. Radiat. Oncol. Biol. Phys. 71(4), 1084–1090 (2008).
- Rajendran RR, Plastaras JP, Mick R et al. Daily isocenter correction with electromagnetic-based localization improves target coverage and rectal sparing during prostate radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 76(4), 1092–1099 (2010).
- Li HS, Chetty IJ, Enke CA et al. Dosimetric consequences of intrafraction prostate motion. Int. J. Radiat. Oncol. Biol. Phys. 71(3), 801–812 (2008).
- Noel C, Parikh PJ, Roy M et al. Prediction of intrafraction prostate motion: accuracy of pre- and post-treatment imaging and intermittent imaging. Int. J. Radiat. Oncol. Biol. Phys. 73(3), 692–698 (2009).
- Sawant A, Smith RL, Venkat RB et al. Toward submillimeter accuracy in the management of intrafraction motion: the integration of real-time internal position monitoring and multileaf collimator target tracking. Int. J. Radiat. Oncol. Biol. Phys. 74(2), 575–582 (2009).
- Anastasiadis AG, Lichy MP, Nagele U et al. MRI-guided biopsy of the prostate increases diagnostic performance in men with elevated or increasing PSA levels after previous negative TRUS biopsies. Eur. Urol. 50(4), 738–748, discussion 748–749 (2006).
- Buyyounouski MK, Horwitz EM, Price RA et al. Intensity-modulated radiotherapy with MRI simulation to reduce doses received by erectile tissue during prostate cancer treatment. Int. J. Radiat. Oncol. Biol. Phys. 58(3), 743–749 (2004).
- Rouviere O, Hartman RP, Lyonnet D. Prostate MR imaging at high-field strength: evolution or revolution? Eur. Radiol. 16(2), 276–284 (2006).
- Shchory T, Schifter D, Lichtman R et al. Tracking accuracy of a real-time fiducial tracking system for patient positioning and monitoring in radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 78(4), 1227–1234 (2010).
- Chamberland M, Wassenaar R, Spencer B, Xu T. Performance evaluation of real-time motion tracking using positron emission fudicial markers. Med. Phys. 38(2), 810–819 (2011).
- Lagendijk JJ, Raaymakers BW, Raaijmakers AJ et al. MRI/linac integration. Radiother. Oncol. 86(1), 25–29 (2008).
- Raaijmakers AJ, Raaymakers BW, Lagendijk JJ. Magnetic-field-induced dose effects in MR-guided radiotherapy systems: dependence on the magnetic field strength. Phys. Med. Biol. 53(4), 909–923 (2008).
- Chen J, Morin O, Aubin M et al. Dose-guided radiation therapy with megavoltage cone-beam CT. Br. J. Radiol. 79(Spec No. 1), S87–S98 (2006).
- Cheung J, Aubry JF, Yom SS et al. Dose recalculation and the dose-guided radiation therapy (DGRT) process using megavoltage cone-beam CT. Int. J. Radiat. Oncol. Biol. Phys. 74(2), 583–592 (2009).
- Coen JJ, Zietman AL, Rossi CJ et al. Comparison of high-dose proton radiotherapy and brachytherapy in localized prostate cancer: a case-matched analysis. Int. J. Radiat. Oncol. Biol. Phys. 82(1), e25–e31 (2012).
- Trofimov A, Nguyen PL, Efstathiou JA et al. Interfractional variations in the setup of pelvic bony anatomy and soft tissue, and their implications on the delivery of proton therapy for localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 80(3), 928–937 (2011).
- Bogdanich W. As technology surges, radiation safeguards lag. New York Times, 26th January (2010).
- Coen JJ, Zietman AL. Proton radiation for localized prostate cancer. Nat. Rev. Urol. 6(6), 324–330 (2009).
- Murphy MJ, Balter J, Balter S et al. The management of imaging dose during image-guided radiotherapy: report of the AAPM Task Group 75. Med. Phys. 34(10), 4041–4063 (2007).
- Alonso-Arrizabalaga S, Brualla González L, Roselló Ferrando JV et al. Prostate planning treatment volume margin calculation based on the ExacTrac x-ray 6D image-guided system: margins for various clinical implementations. Int. J. Radiat. Oncol. Biol. Phys. 69(3), 936–943 (2007).
- Amies C, Bani-Hashemi A, Celi JC et al. A multi-platform approach to image guided radiation therapy (IGRT). Med. Dosim. 31(1), 12–19 (2006).
- Ding GX, Coffey CW. Radiation dose from kilovoltage cone beam computed tomography in an image-guided radiotherapy procedure. Int. J. Radiat. Oncol. Biol. Phys. 73(2), 610–617 (2009).
- Islam MK, Purdie TG, Norrlinger BD et al. Patient dose from kilovoltage cone beam computed tomography imaging in radiation therapy. Med. Phys. 33(6), 1573–1582 (2006).
- Shah AP, Langen KM, Ruchala KJ et al. Patient dose from megavoltage computed tomography imaging. Int. J. Radiat. Oncol. Biol. Phys. 70(5), 1579–1587 (2008).