Drug Evaluation - Imaging in Medicine (2010) Volume 2, Issue 4
Regadenoson myocardial perfusion imaging
Gilbert Zoghbi†1,2 and Ami E Iskandrian11Division of Cardiovascular Disease, University of Alabama at Birmingham, AL, USA
2Birmingham VA Medical Center, AL, USA
- *Corresponding Author:
- Gilbert Zoghbi
920 FOT, 1960 6th Avenue South
Birmingham, AL 35294, USA
Tel.: +1 205 975 0825
Fax: +1 205 934 0973
E-mail: gzoghbi@uab.edu
Abstract
Vasodilator stress myocardial perfusion imaging accounts for approximately half of the stress studies in the USA. Regadenoson, a selective A2A receptor agonist, was recently approved for clinical use by the US FDA. By contrast, compared with adenosine, regadenoson is administered as a single- and fixed-dose bolus, has comparable hyperemic effect and a better tolerability profile. This article will address and highlight the various regadenoson attributes, properties and the preclinical and clinical studies that have been conducted to date
Keywords
adenosine; myocardial perfusion imaging; regadenoson; SPECT
Stress myocardial perfusion imaging (MPI) is commonly used for the evaluation of coronary artery disease. Exercise is the preferred stress modality in patients who can achieve acceptable exercise end points [1]. Vasodilator stress MPI is reserved for approximately 50% of patients who cannot perform exercise owing to exercise limitations, presence of left bundle branch block or electronically paced rhythms [1]. The vasodilator stress agents include the direct nonselective adenosine agonists, such as adenosine and adenosine triphosphate and the newly approved selective A2A receptor agonist, regadenoson (Lexiscan®, Gilead Sciences Inc., CA, USA).
Adenosine receptor
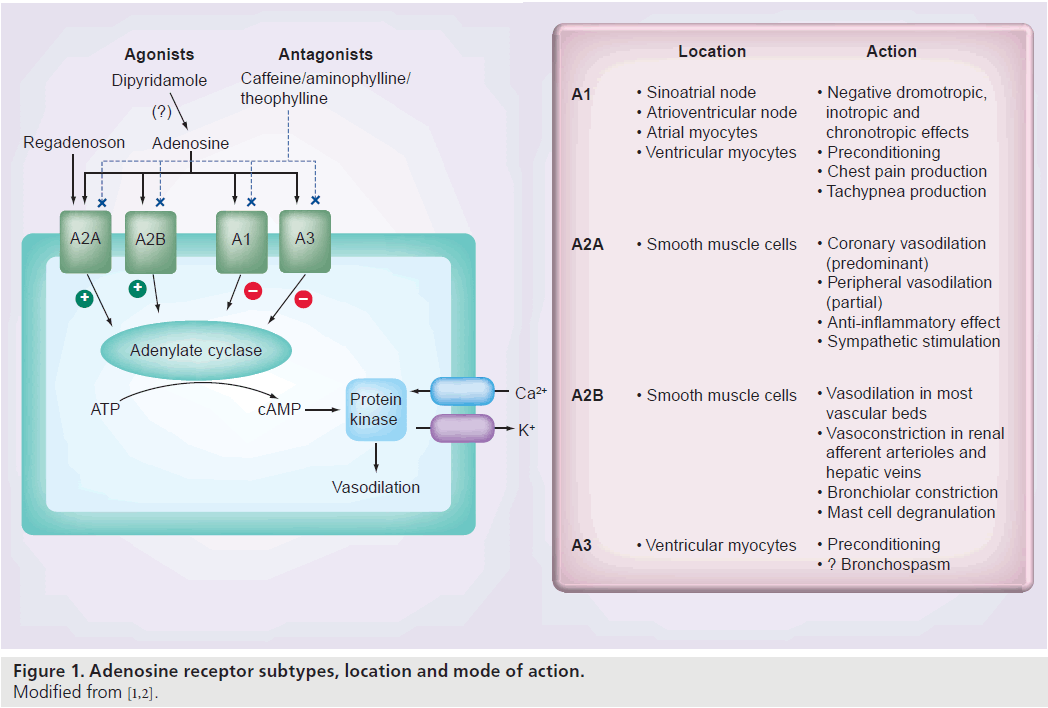
The distribution and action of the four adenosine receptor subtypes (A1, A2A, A2B and A3) in the human heart are shown in Figure 1 [1,2]. The A1 and A3 receptors are linked to a Gi protein whose activation reduces adenylyl cyclase activity, which decreases intracellular cAMP. The A2A and A2B receptors are linked to a Gs protein whose activation increases adnenylyl cyclase activity, which increases intracellular cAMP [3,4]. Increased intracellular cAMP stimulates and opens the potassium channels that hyperpolarize vascular smooth muscle cells and inhibit voltage-gated calcium channels and intracellular calcium release, resulting in smooth muscle relaxation and arteriolar vasodilatation [4,5]. MPI relies on the disparity of myocardial blood flow (MBF) between the different coronary beds. Stimulation of the A2A receptor is the desired effect for vasodilator MPI, whereas stimulation of the other adenosine receptors causes undesirable side effects. The selective A2A agonists have some of the attributes of the ideal vasodilator agent, which are listed in Box 1. Several selective A2A agonists have been studied including apadenoson (BMS068645 or ATL146e), regadenoson (CVT-3146) and binodenoson (MRE-0470 and WRC-04). Regadenoson is the only agent that has been so far approved by the US FDA for stress MPI [6].
Pharmacology, pharmacokinetics & pharmacodynamics
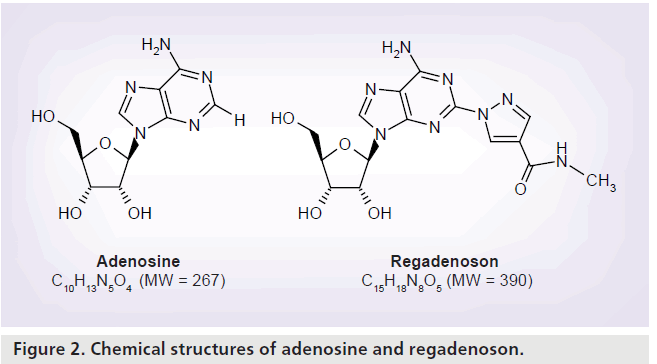
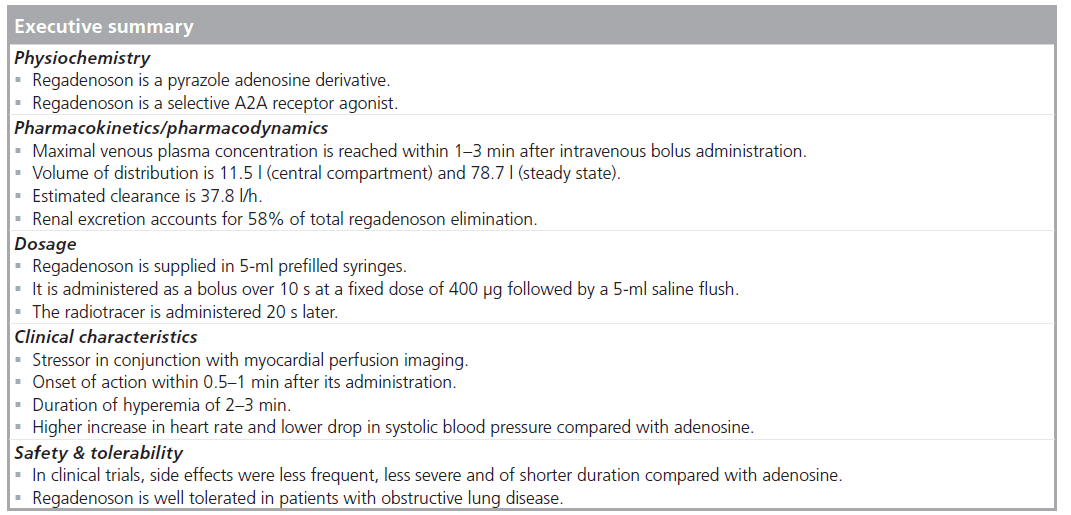
Regadenoson is a 2-[N-1-(4-N-methylcarboxamidopyrazolyl)] pyrazole adenosine derivative (Figure 2) [7]. Unlike adenosine, regadenoson is not rapidly metabolized in plasma by the adenosine deaminase or the cell membrane nucleoside transporter allowing its administration as an intravenous bolus rather than a continuous intravenous infusion [8]. In a population pharmacokinetic and pharmacodynamic analysis of regadenoson in healthy male volunteers, adverse events (related to vasodilatation and increase in heart rate [HR]) were more prevalent at regadenoson doses greater than 3 μg/kg, with a dose-related effect [9]. The maximum tolerated doses of regadenoson were 10 μg/kg in the standing position and 20 μg/kg in the supine position [9]. Regadenoson was rapidly distributed throughout the body, followed by a terminal elimination half-life of approximately 2 h after intravenous bolus dose administration [9]. The regadenoson maximal venous plasma concentration was reached within 1–3 min after intravenous bolus administration [8]. Regadenoson has volumes of distribution of 11.5 l (central compartment) and 78.7 l (steady state) and an estimated clearance of 37.8 l/h [9].
Renal excretion accounts for 58% of the total regadenoson elimination [9]. The clearance, volume of distribution and terminal half-life of regadenoson were not dose dependent [8]. The maximum HR increase (Emax) and the plasma regadenoson concentration causing a 50% increase in Emax (EC50) were 76 beats/min and 12.3 ng/ml, respectively [9]. In subjects with varying degrees of renal function (creatinine clearance of 15–132 ml/min) elimination halflife of regadenoson (single intravenous bolus dose of 400 μg) was prolonged with decreasing renal function [10]. Regadenoson maximum plasma concentrations, number and severity of adverse events did not differ significantly by renal function [10]. Regadenoson dose adjustments are not needed in subjects with impaired renal function [10].
Administration
Regadenoson is supplied in 5‑ml prefilled syringes and is administered as a bolus over 10 s at a fixed dose of 400 μg followed by a 5‑ml saline flush [2]. The radiotracer is administered 20 s later [2].
Adenosine receptor effects
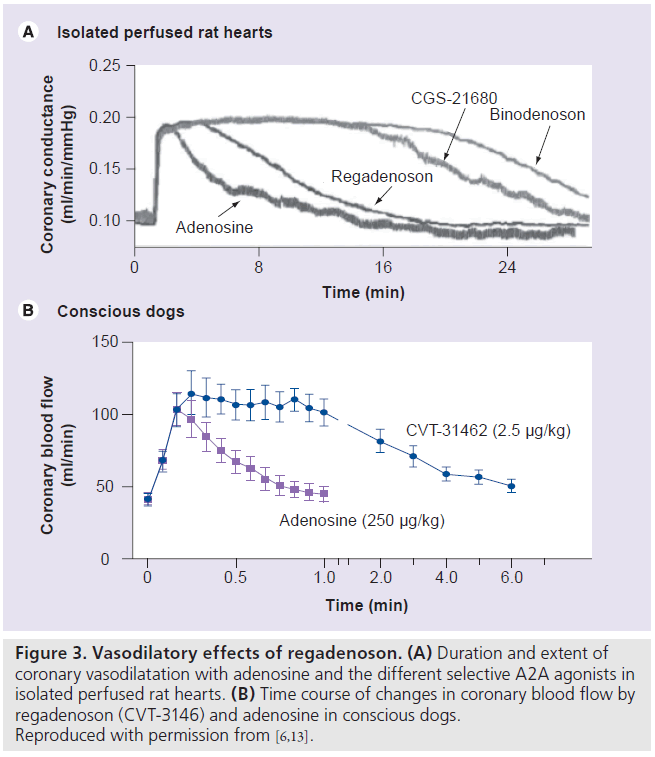
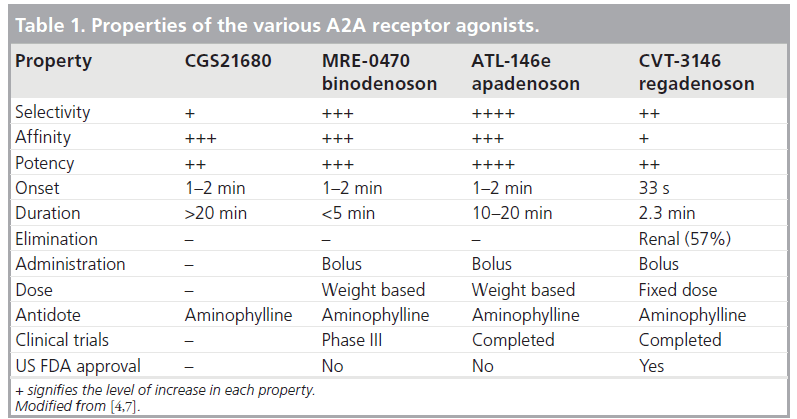
The properties of the various selective A2A agonists are listed in Table 1. Regadenoson is 100‑times more potent at the A2A receptor than is adenosine [4,7]. Adenosine receptor radioligand binding and receptor functional studies in animals demonstrated greater affinity and more selectivity of regadenoson for the A2A receptor compared with the other A1, A2B and A3 receptors [11]. In a study of isolated rat hearts, regadenoson increased coronary conductance by its action on the A2A receptors with no effect on atrioventricular (AV) conduction times, which are mediated by the A1 receptor [11]. Regadenoson had a greater than 13-fold affinity to the A2A receptor compared with the A1A receptor [11]. Receptor affinity (Ki) is the ratio of the rate of drug dissociation to the rate of drug association to the particular receptor [11]. Low-affinity agonists have a higher Ki and a shorter duration of action compared with high-affinity agonists [11]. Regadenoson (Ki = 1200 nmol/l) has a higher affinity at the A2A receptor compared with adenosine (Ki = 2700–5600 nmol/l) but a lower affinity compared with CGS-2168 (Ki = 157 nmol/l) and binodenoson (Ki = 21 nmol/l) [6,11]. Despite its lower affinity to the A2A receptor compared with the other selective A2A agonists, regadenoson produced a maximal coronary vasodilator response of equivalent magnitude and of shorter duration than the higher affinity A2A agonists (Figure 3A) [6,11]. The coronary arteries have a high density of A2A receptors resulting in a large capacity for spare receptor, which allows a lower affinity drug to elicit a potent and rapid vasodilatory response [6,11]. Thus, regadenoson offers a better choice of stress agent compared with the other selective and higher affinity agonists by virtue of a potent vasodilator response with a rapid onset and a shorter duration of action [6,11].
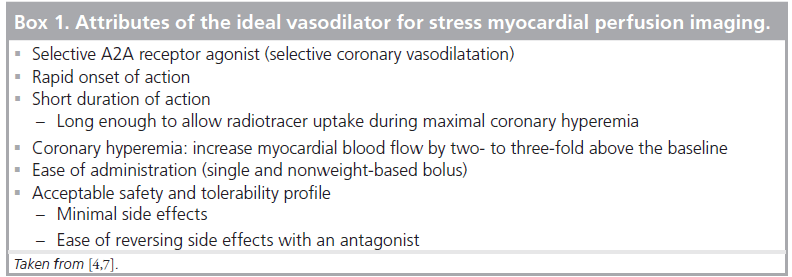
Figure 3.Vasodilatory effects of regadenoson. (A) Duration and extent of coronary vasodilatation with adenosine and the different selective A2A agonists in isolated perfused rat hearts. (B) Time course of changes in coronary blood flow by regadenoson (CVT-3146) and adenosine in conscious dogs. Reproduced with permission from [6,13].
Preclinical studies
Coronary vasodilatation
Intravenous regadenoson (0.1–5.0 μg/kg) and adenosine (13–267 μg/kg) were studied in conscious dogs [12]. Regadenoson caused a dose-dependent increase of coronary blood f low (CBF) with a maximal increase of 221 ± 18% above baseline compared with an equi-effective maximal increase of 227 ± 11% with adenosine [12]. However, regadenoson was more potent than adenosine (median effective dose [ED50] = 0.34 ± 0.08 μg/kg vs ED50 = 51 ± 15 μg/kg, respectively; p < 0.05) [12]. The duration of CBF greater than or equal to twofold baseline was 97 ± 14 s for a 10‑s injection of 2.5 μg/kg of regadenoson, 221 ± 20 s for a 30‑s injection of 2.5 μg/kg of regadenoson, 247 ± 39 s for a 5 μg/kg regadenoson injection and only 24 ± 2 s after injection of 267 μg/kg of adenosine [12]. In another study of conscious dogs, intravenous regadenoson (0.1–2.5 μg/kg) or adenosine (10–250 μg/kg) caused a similar dose-dependent increase in CBF and dosedependent decrease in the late diastolic coronary resistance [13]. Regadenoson at 2.5 μg/kg caused a longer-lasting increase in CBF compared with adenosine at 250 μg/kg, although the maximal increase of CBF was similar for both stressors (Figure 3B) [13]. However, regadenoson (ED50 = 47 ± 7.77 μg/kg) was 100‑times more potent than was adenosine (ED50 = 0.45 ± 0.07 μg/kg). The 2.5 μg/kg of regadenoson caused significantly longer coronary vasodilatation than the 250 μg/kg adenosine dose [13].
The effects of regadenoson (2.5 μg/kg intravenous bolus) and adenosine (4.5-min infusion at 280 μg/kg) on hemodynamics and thallium-201 (201-Tl) and technetium-99m (99m‑Tc) sestamibi biodistribution and kinetics were studied in anesthetized dogs [14]. Regadenoson and adenosine had comparable hyperemic responses and similar biodistribution and clearance of the 201-Tl and tracers [14]. The arterial blood clearance half-time was significantly faster for 99m‑Tc sestamibi than for 201-Tl for both vasodilator stressors [14]. 201-Tl offered significant ex vivo regadenoson perfusion images over 99m-Tc sestamibi in the imaged excised dog hearts [14]. The relative microsphere flow deficit during regadenoson stress was significantly greater with 201-Tl compared with 99m‑Tc sestamibi and the ex vivo SPECT perfusion defect score was larger with 201-Tl (22 ± 2.8%) than with 99m‑Tc sestamibi (17 ± 1.7%) [14].
Hemodynamic effects
In a study of conscious dogs, intravenous regadenoson (0.1–2.5 μg/kg) compared with intravenous adenosine (10–250 μg/kg) caused a smaller decrease in total peripheral resistance and a smaller increase in the lower body flow [13]. Regadenoson (2.5 μg/kg) did not affect renal vascular resistance and caused minimal decrease in renal blood flow (-11 ± 4%) [13]. Adenosine caused a dose-dependent renal vasoconstriction with a 683 ± 197% increase in renal vascular resistance and an 85 ± 4% decrease in renal blood flow with the 250 μg/kg adenosine dose [13]. Regadenosn and adenosine caused similar dose-dependent vasodilatation in the mesentery [13]. The regadenoson 2.5 μg/kg and the adenosine 250 μg/kg doses caused similar increased in cardiac outputs [13]. Intravenous administration of regadenoson 1 μg/kg to anesthetized closed-chest dogs caused a 3.3‑fold increase in the average peak CBF velocity compared with only 1.1‑fold increase in the peak peripheral flow velocity [6]. In the study of Trochu et al., adenosine doses of 134 and 267 μg/kg increased left ventricular (LV) systolic pressure by 12 and 18%, respectively, with no changes with regadenoson [12]. Adenosine and regadenoson increased LV dp/dt by 29 and 39%, respectively [12]. Regadenoson at a dose of 2.5 μg/kg caused a 13 mmHg decrease in mean blood pressure (BP) compared with an 18 mmHg decrease with adenosine [12]. The effect on the systemic vascular resistance was less with regadenoson (-20 vs -45%) while both adenosine and regadenoson caused a similar approximately 45% decrease in mesenteric vascular resistance [12]. These results show the relative selectivity of regadenoson to the coronary circulation compared with the peripheral circulation by virtue of its selective action on the coronary A2A receptors.
Regadenoson caused a higher increase in HR for a longer duration compared with adenosine [12]. Dhalla et al. studied the mechanism of regadenoson-induced tachycardia in awake rats [15]. Regadenoson (0.3–50 μg/kg) caused a dose-dependent increase in HR and a decrease in mean BP at the higher doses [15]. Pretreatment with an A2A receptor antagonist attenuated the BP decrease and the HR increase caused by regadenoson. Pretreatment with metoprolol (1 mg/kg) attenuated the HR increase but had no effect on the hypotension caused by regadenoson. A ganglionic blocker prevented the tachycardia, even though mean BP was further reduced. Regadenoson (10 μg/kg) significantly increased plasma norepinephrine levels by twofold [15]. Regadenoson (5 and 10 μg/kg) did not cause a significant change in HR after administration of propranolol in dogs [16]. The dissociation of HR and mean BP suggests that regadenoson- mediated tachycardia may not entirely be baroreflex-mediated but more so due to a direct stimulation of the sympathetic nervous system via activation of A2A adenosine receptors [15].
Effects of other drugs
In the study by Trochu et al. discussed earlier, aminophylline 20 mg/kg abolished the peak CBF and hemodynamic effects caused by regadenoson 1 μg/kg and attenuated the effects of the 2.5 μg/kg dose [12]. The effects of caffeine on regadenoson (5 μg/kg) coronary vasodilatation and hemodynamics were studied in 16 conscious dogs. Caffeine at 1, 2, 4 and 10 mg/kg did not significantly affect the hyperemic MBF but decreased the duration of hyperemia by 17 ± 4%, 48 ± 8%, 62 ± 5% and 82 ± 5%, respectively [17]. Caffeine at 4 and 10 mg/kg significantly attenuated the effects of regadenoson on mean arterial BP and HR [17].
Clinical studies
The preclinical studies showed that regadenoson is a potent and selective A2A receptor agonist with lower A2A receptor affinity compared with other selective agonists that can achieve equivalent magnitude of coronary hyperemia to adenosine for a period that is optimal for radionuclide imaging and with fewer effects on the peripheral circulation and on AV conduction [6].
Coronary vasodilatation
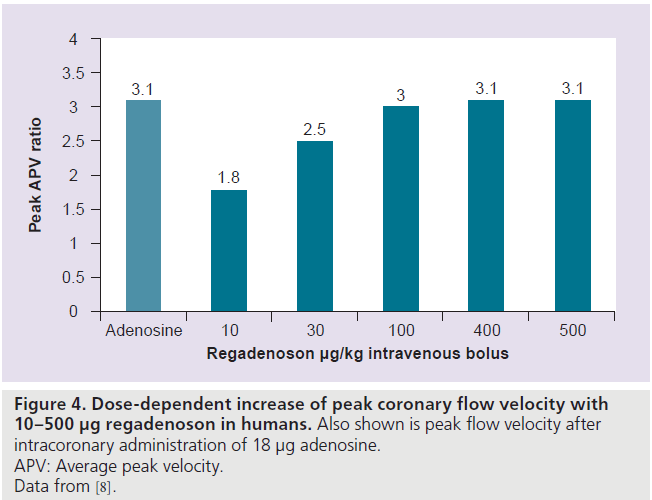
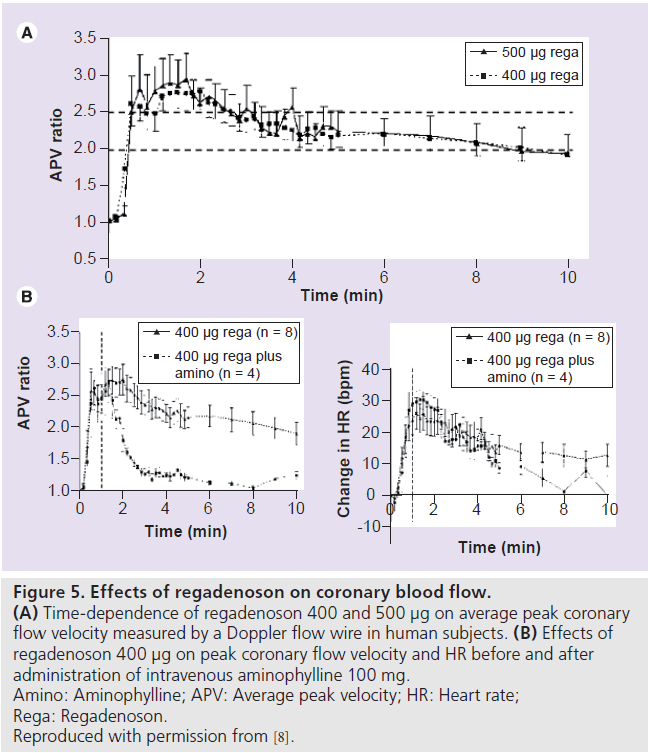
In an open-label, dose-escalation study of rapid intravenous bolus of regadenoson (10–500 μg) in 34 subjects, regadenoson increased peak CBF velocity (measured by a Doppler flow wire) by up to 3.4‑fold in a dose-dependent manner (Figure 4). The mean time to 85% of maximum peak CBF velocity or greater was 33 s (range: 20–40 s) and was independent of regadenoson dose [8]. The peak CBF velocity was reached within 0.5–2.3 min, with the 400 μg dose [8]. The mean duration of greater than 2.5‑fold increase of peak CBF velocity was 2.3 and 2.4 min for the 400 and 500 μg regadenoson doses, respectively (Figure 5A) [8].
Figure 4.Dose-dependent increase of peak coronary flow velocity with 10–500 µg regadenoson in humans.Also shown is peak flow velocity after intracoronary administration of 18 µg adenosine. APV: Average peak velocity. Data from [8].
Figure 5.Effects of regadenoson on coronary blood flow. (A) Time-dependence of regadenoson 400 and 500 µg on average peak coronary flow velocity measured by a Doppler flow wire in human subjects. (B) Effects of regadenoson 400 µg on peak coronary flow velocity and HR before and after administration of intravenous aminophylline 100 mg. Amino: Aminophylline; APV: Average peak velocity; HR: Heart rate; Rega: Regadenoson. Reproduced with permission from [8].
Hemodynamics
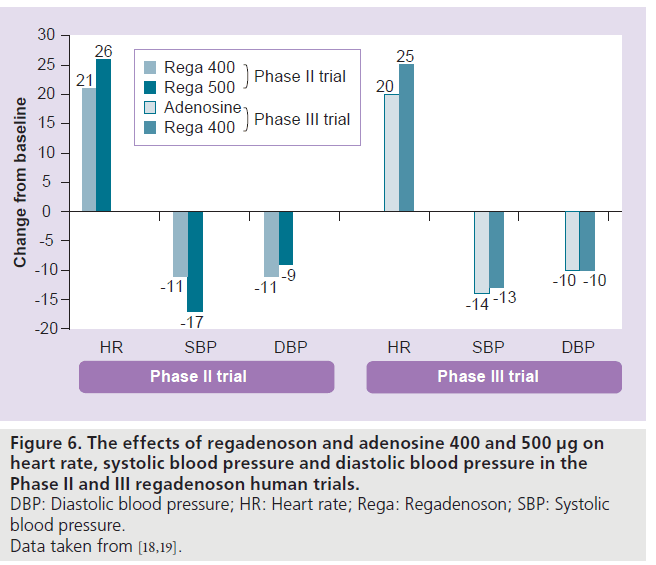
In the study by Lieu et al., regadenoson (10–500 μg) caused a dose-dependent increase in HR and no dose-dependent drop in BP. The HR increased by 21 ± 21 beats/min, systolic BP decreased by 24 ± 15 mmHg and the diastolic BP decreased by 12 ± 10 mmHg after the regadenoson 400‑μg dose [8]. The maximal changes in BP occurred within 5 min and returned to near baseline within 15 min [8]. In the Phase II and III trials, regadenoson caused a decrease in systolic and diastolic BP and an increase in HR (Figure 6) [18,19]. The peak increase in HR was greater with regadenoson than adenosine, but the BP nadirs were similar [19]. The maximum increase in HR occurred within 2 min and returned to near baseline within 15 min of regadenoson administration [18]. For the 400‑μg regadenoson group, a decrease of systolic BP greater than 25 mmHg occurred in one out of 18 subjects with no drop in systolic BP less than 90 mmHg [18]. The greatest drop in systolic BP occurred at 3 min after regadenoson administration [18]. The adenosine- or regadenoson- mediated increase in HR was blunted in patients with diabetes mellitus (DM) and was further blunted in patients who were on insulin therapy [20]. This blunting of HR response is likely mediated by sympathetic denervation that more commonly occurs in diabetic patients.
Figure 6.The effects of regadenoson and adenosine 400 and 500 µg on heart rate, systolic blood pressure and diastolic blood pressure in the Phase II and III regadenoson human trials. DBP: Diastolic blood pressure; HR: Heart rate; Rega: Regadenoson; SBP: Systolic blood pressure. Data taken from [18,19].
In summary, regadenoson caused a faster and greater increase in HR and comparable decreases in systolic and diastolic BP to adenosine [19]. The recovery of HR and BP was faster with adenosine compared with regadenoson [19].
Effect of other drugs
In the study by Lieu et al., 100 mg intravenous aminophylline was given to four subjects 1 min after the 400 μg regadenoson bolus [8]. Aminophylline administration reduced the duration of greater than twofold increase in peak CBF velocity from 6.9 to 0.6 min but did not affect the increase in HR (Figure 5B) [8]. In a double-blind, randomized, placebo-controlled crossover study, 21 subjects received placebo/caffeine (200‑mg capsule) and 20 subjects received caffeine/placebo during 15OH 2O PET. MBF was measured 2 h after receiving placebo or caffeine capsules at rest and after administration of a regadenoson 400‑μg bolus. The regadenoson-induced coronary flow reserve was not significantly different after placebo (2.97 ± 0.16) or caffeine (2.75 ± 0.16). Caffeine ingestion blunted the regadenoson-mediated HR increase with no effect on the BP [21].
Regadenoson MPI
In the Phase II, multicenter, open-label crossover trial, 36 patients who initially had ischemia on adenosine (140 μg/kg/min intravenous infusion over 6 min) 99m-Tc sestamibi SPECT underwent a repeat regadenoson (400 or 500 μg intravenous bolus) 99m-Tc sestamibi SPECT within 2–46 days [18]. The SPECT images were interpreted in a random order by three blinded observers [18]. The agreement rates of the regadenoson 400‑ and 500‑μg doses for the visual presence of ischemia were 89 and 76%, respectively, compared with adenosine SPECT [18]. The overall agreement rates between regadenoson and adenosine for visual and quantitative analysis were 57 and 69%, respectively [18]. On the basis of the results of this study, the regadenoson 400‑μg dose was adopted as the dose for use with MPI and was the dose used in the Phase III trial.
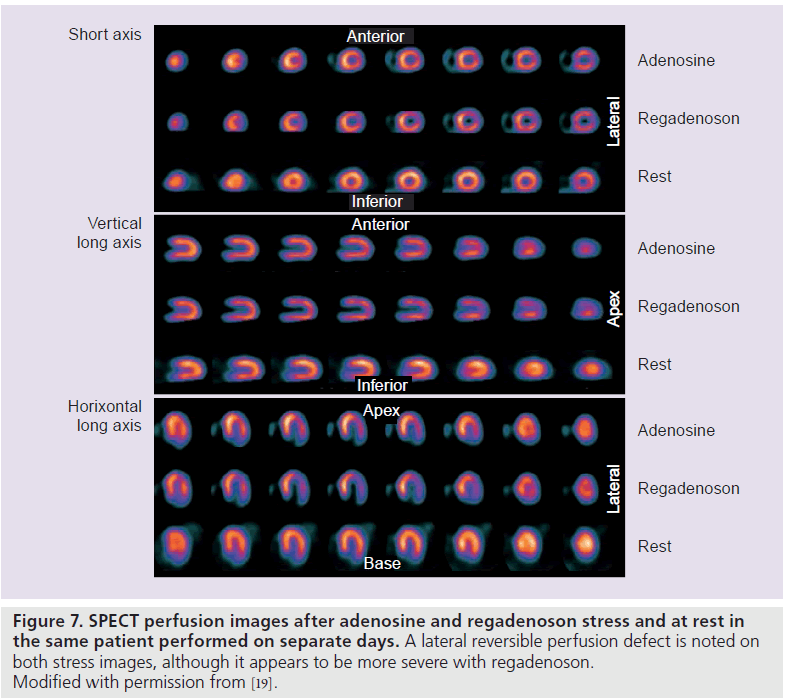
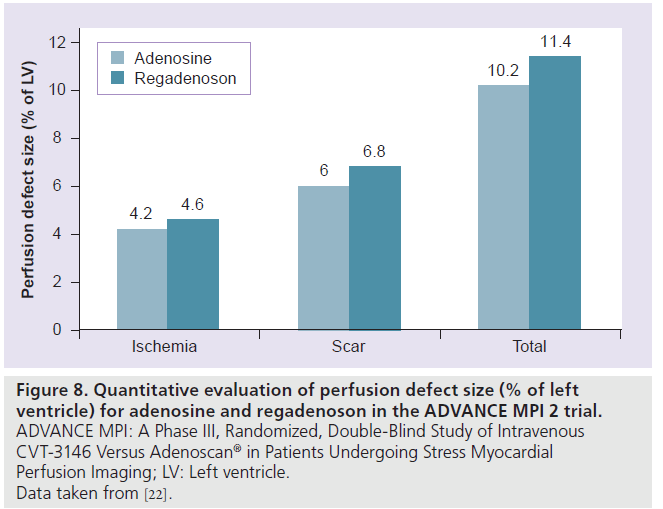
The Phase III, multicenter, international, double-blinded trial, A Phase III, Randomized, Double-Blind Study of Intravenous CVT-3146 Versus Adenoscan® in Patients Undergoing Stress Myocardial Perfusion Imaging (ADVANCE MPI 2), involved 784 patients who underwent two sets of SPECT studies: an initial qualifying adenosine SPECT study followed by a 2:1 randomization to regadenoson (400‑μg rapid intravenous bolus) or adenosine (140‑μg/kg/min intravenous infusion over 6 min) SPECT [19]. The SPECT images were interpreted by three blinded readers. The average agreement rate was 0.63 ± 0.03 for regadenoson–adenosine and 0.64 ± 0.04 for adenosine–adenosine SPECT images (above the prespecified noninferiority margin) [19]. Quantitative analysis showed greater than 90% agreement rates for adenosine– regadenoson and adenosine–adenosine groups (Figure 7) [19]. The image quality was considered good or excellent in 90% of adenosine studies and in 88% of regadenoson studies [19]. In a subsequent quantitative analysis from the ADVANCE MPI 2 Trial, there were no differences between total and ischemic perfusion defect extent and severity observed between regadenoson and adenosine SPECT images (Figure 8) [22]. Linear regression showed excellent correlation between adenosine and regadenoson for total (r = 0.97; p < 0.001) and ischemic (r = 0.95; p < 0.001) perfusion defects [22].
Figure 8.Quantitative evaluation of perfusion defect size (% of left ventricle) for adenosine and regadenoson in the ADVANCE MPI 2 trial. ADVANCE MPI: A Phase III, Randomized, Double-Blind Study of Intravenous CVT-3146 Versus Adenoscan® in Patients Undergoing Stress Myocardial Perfusion Imaging; LV: Left ventricle. Data taken from [22].
The Integrated ADVANCE MPI Trial assessed the effects of age, gender, obesity and DM on the results of regadenoson and adenosine MPI [23]. The agreement rates of adenosine and regadenoson images were similar within age, gender, BMI and DM subgroups [23]. For both stress agents, image quality was similar within the subgroups except for the gender subgroup where image quality was better in men compared with women [23].
Safety & adverse effects
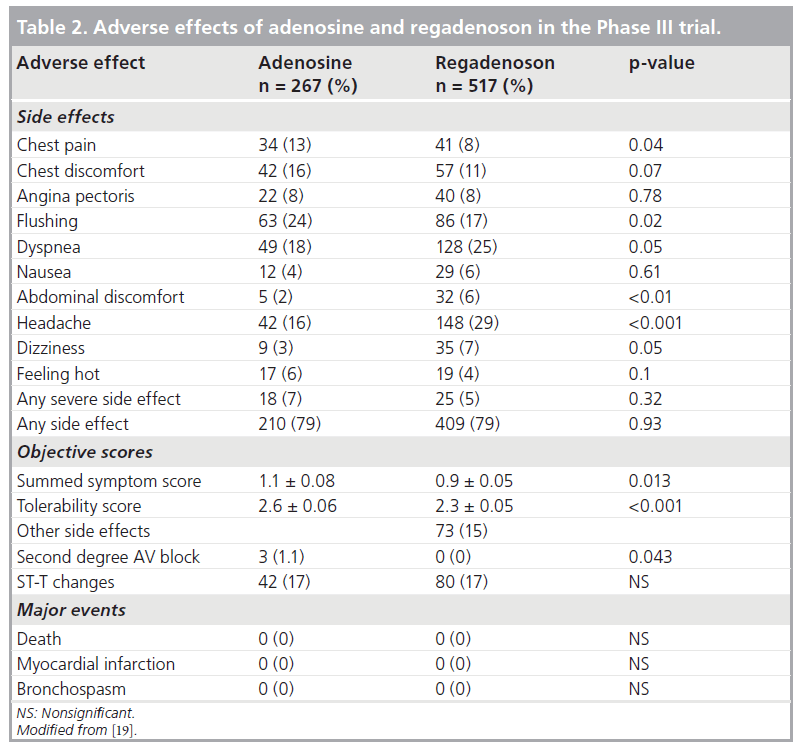
In the Phase II trial, overall, regadenoson was well tolerated and had mild and self-limiting side effects that occurred in 72% of subjects and resolved spontaneously within 10 min [18]. The 500‑μg group had a higher incidence of side effects (83 vs 61% in the 400‑μg group) and more pronounced hemodynamic changes (Figure 6) [18]. There were no occurrences of bronchospasm, second- or third-degree AV blocks, myocardial infarction or death [18]. In the Phase III trial, a summed symptom score of flushing, chest pain and dyspnea was significantly less with regadenoson than adenosine and regadenoson was better tolerated than adenosine [19]. Table 2 summarizes the various side effects observed in the Phase III trial. Despite the high A2A regadenoson selectivity, subjective side effects still occurred albeit briefer and with lesser severity. The doses of regadenoson used in the clinical trials might be causing regadenoson to be less selective for the A2A receptor [1]. Furthermore, dyspnea and chest pain might be induced by sympathetic stimulation via the A2A receptor rather than solely through the A1 receptor [1]. However, the more serious side effects such as hypotension, AV blocks and bronchospasm have not been observed so far. The combination of low-level exercise with regadenoson was shown to reduce the incidence of side effects in one study [24]. In the Integrated ADVANCE MPI Trial that assessed the effects of age, gender, obesity and DM on the safety of regadenoson and adenosine MPI, regadenoson caused less chest pain, flushing, and throat, neck, or jaw pain, but more headache and gastrointestinal discomfort and a lower combined symptom score in nearly all subgroups [23]. Based on the tolerability score, women felt less comfortable than men with both stress agents [23].
Regadenoson MPI in special patient populations & situations
Patients with lung disorders
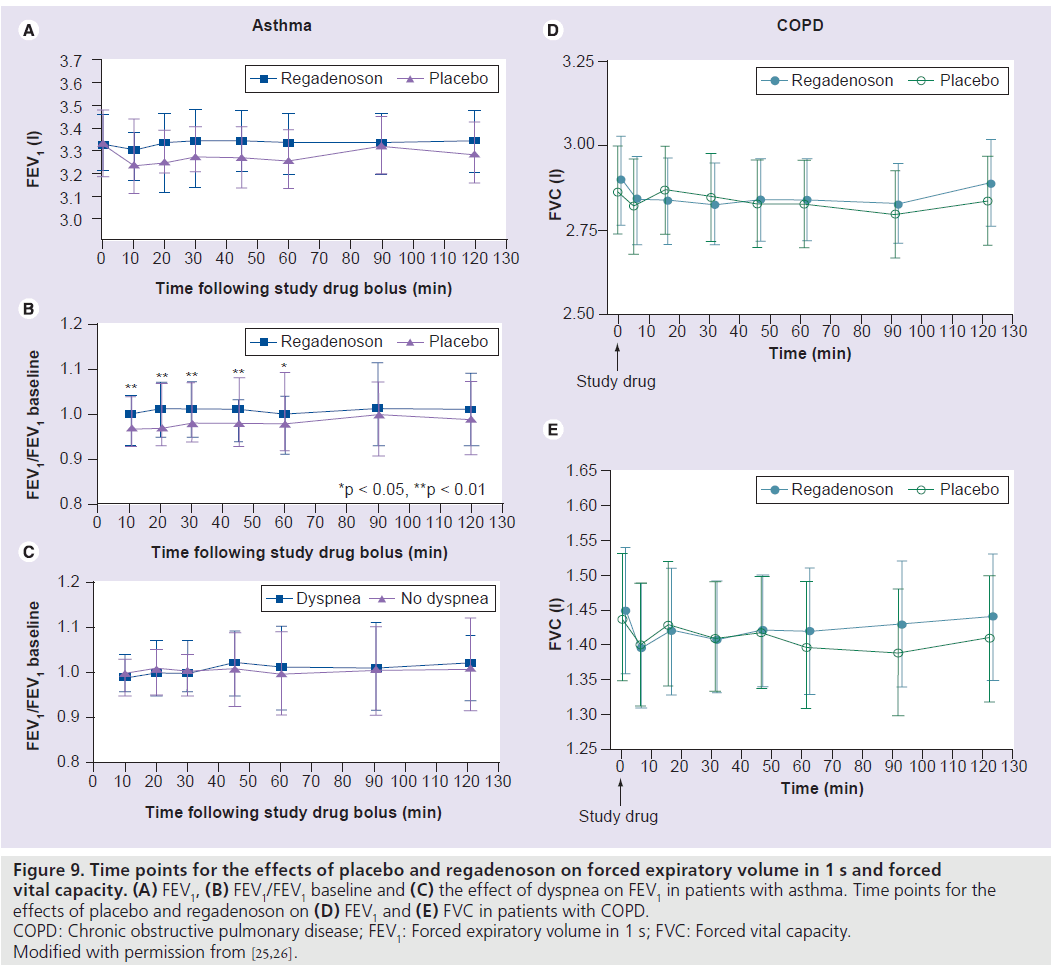
Patients with asthma or chronic obstructive pulmonary disease are at an increased risk of adenosine-induced bronchospasm via activation of the A2B and A3 adenosine receptors [25]. The safety of regadenoson was studied in a randomized, double-blind, placebo-controlled crossover trial that enrolled 48 patients with mild-to-moderate asthma and a positive adenosine monophosphate challenge test [25]. Patients were randomized to regadenoson/placebo or placebo/regadenoson in a 1:1 ratio where each patient received regadenoson 400 μg and a matching placebo on two separate treatment days separated by a 1–14‑day washout period [25]. All patients abstained from the use of bronchodilators for at least 6 h prior to the study. There was no significant difference in the mean forced expiratory volume in 1 s (FEV1) at any time-point between regadenoson and placebo (Figure 9A) [25]. The mean ratio of the FEV1 at each tested time-point relative to the baseline FEV1 was significantly higher after treatment with regadenoson compared with placebo from 10 to 60 min after treatment (Figure 9B) [25]. Bronchoconstrictive reactions (>15% reduction of FEV1 within 2 h compared with baseline) occurred in the moderate asthma group and were similar in the regadenoson and placebo groups (4.3 vs 4.2%; p = nonsignificant), were clinically silent and did not require any therapy [25]. Dyspnea occurred in 34% of patients after regadenoson and in none of the patients after placebo. The occurrence of dyspnea was not associated with a decrease in FEV1 (Figure 9C) and none of the patients in either group had less than 92% oxygen desaturation [25].
In another randomized, double-blind, placebo-controlled crossover trial, the safety of regadenoson was evaluated in 49 patients with moderate-to-severe chronic obstructive pulmonary disease [26]. Short-acting bronchodilators were withheld for at least 8 h before treatment. There were no differences between regadenoson and placebo on repeated (measured at prespecified time intervals up to 2 h post regadenoson or placebo administration) follow-up FEV1, forced vital capacity, respiratory rate, pulmonary examinations or oxygen saturation (Figure 9E & F) [26]. The mean maximum decline in FEV1 was 0.11 ± 0.02 l and 0.12 ± 0.02 l (p = 0.55) in the regadenoson and placebo groups, respectively [26]. New-onset wheezing was noted in 6 and 12% of the regadenoson and placebo groups, respectively (p = 0.33) [26]. Bronchoconstrictive reactions were clinically silent and occurred in 12.2 and 6.1% of the regadenoson and placebo groups, respectively (p = 0.31). Dyspnea was reported in 61% of patients in the regadenoson group and in 0% of patients in the placebo group and was not related to decline in FEV1 or other objective findings. No patient required treatment with bronchodilators or oxygen [26].
Figure 9.Time points for the effects of placebo and regadenoson on forced expiratory volume in 1 s and forced vital capacity. (A) FEV1, (B) FEV1/FEV1 baseline and (C) the effect of dyspnea on FEV1 in patients with asthma. Time points for the effects of placebo and regadenoson on (D) FEV1 and (E) FVC in patients with COPD. COPD: Chronic obstructive pulmonary disease; FEV1: Forced expiratory volume in 1 s; FVC: Forced vital capacity. Modified with permission from [25,26].
Patients with renal disease
The safety of regadenoson, which is renally excreted, was studied in 277 consecutive patients with end-stage renal disease (ESRD) who underwent regadenoson SPECT for clinical indications and were compared with a cohort of 134 patients with normal renal function [27]. The patients with ESRD were younger than the controls. The ESRD patients had similar rates of abnormal MPI (19 vs 18% compared with controls; p = nonsignificant) and lower LV ejection fractions (57 ± 12% vs 64 ± 12% compared with controls; p < 0.001). The changes in HR and systolic BP from peak stress to baseline were 20 ± 12 beats/min versus 22 ± 13 beats/min and -11 ± 24 mmHg versus -12 ± 23 mmHg in the ESRD and control groups, respectively (p = nonsignificant for both). Side effects were reported in very few patients in either group during the stress test and no patient had serious adverse events in either group [27]. Thus, regadenoson was well tolerated in patients with ESRD.
Regadenoson in combination with exercise MPI
Regadenoson with low-level exercise in patients undergoing MPI was evaluated in a randomized, double-blind, placebo- and active-controlled study [24]. Patients who underwent standard adenosine MPI for clinical indications were randomized in a 2:1 manner to low-level exercise with bolus intravenous injection of regadenoson (RegEx; n = 39) or placebo (PlcEx; n = 21) [24]. Adverse events occurred in 95, 77 and 33% of patients receiving adenosine, RegEx and PlcEx, respectively. Peak HR was greater following RegEx compared with that following PlcEx and adenosine [24]. BP changes from baseline were not different between RegEx and PlcEx. There was no second-degree or higher-degree AV block following RegEx or PlcEx whereas no patient developed second-degree AV block following adenosine. The mean heart-to-liver and heart-to-gut ratios were improved on RegEx versus Adenosine [24]. Compared with adenosine, 70% of patients felt RegEx was better based on a tolerability questionnaire [24]. One drawback of this study is that there was no regadenoson-only arm for comparison.
Conclusion & future perspective
Regadenoson, a selective A2A receptor agonist, has many of the features of an ideal vasodilator stressor. Regadenoson was FDA approved in April 2008. Its use as a stressor instead of adenosine or dipyridamole is gaining more acceptance and adoption. The other A2A receptor agonists are still in various stages of development though their longer duration of action compared with regadenoson could be a drawback. The FDA’s Cardiovascular and Renal Drugs advisory committee met in July 2009 and voted 11 to five against binodenoson (CorVue®, King Pharmaceuticals, TN, USA) since the data presented to them did not prove the drug was as effective as adenosine. The fate of binodenoson is not known at this time.
Regadenoson’s ease of administration as a single-weight unadjusted dose, fast onset of action and short duration of action sufficient to cause a hyperemic response, and better sideeffect profile compared with adenosine will likely lead to its use as a stressor with other imaging modalities. Regadenoson will also have a promising role as a stressor for invasive hemodynamic evaluation of coronary stenoes with pressure or Doppler wires in the cardiac catheterization laboratory. The current cost of regadenoson is comparable to adenosine, although this could change in the future when adenosine becomes generic. However, one must keep in mind that the throughput in a stress laboratory is more efficient with regadenoson.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Zoghbi GJ, Iskandrian AE: Pharmacological stress testing. In: Nuclear Cardiac Imaging: Principles and Applications. Garcia EV, Iskandrian AE (Eds). Oxford University Press, NY, USA, 293–315 (2008).
- Gemignani AS, Abbott BG: The emerging role of the selective A(2A) agonist in pharmacologic stress testing. J. Nucl. Cardiol. 17(3), 494–497 (2010).
- Olah ME, Stiles GL: Adenosine receptor subtypes: characterization and therapeutic regulation. Annu. Rev. Pharmacol. Toxicol. 35, 581–606 (1995).
- Hendel RC, Jamil T, Glover DK: Pharmacologic stress testing: new methods and new agents. J. Nucl. Cardiol. 10(2), 197–204 (2003).
- Sato A, Terata K, Miura H et al.: Mechanism of vasodilation to adenosine in coronary arterioles from patients with heart disease. Am. J. Physiol. Heart Circ. Physiol. 288(4), H1633–H1640 (2005).
- Cerqueira MD: The future of pharmacologic stress: selective A2A adenosine receptor agonists. Am. J. Cardiol. 94(2A), 33D–40D; discussion 40D–42D (2004).
- Al Jaroudi W, Iskandrian AE: Regadenoson: a new myocardial stress agent. J. Am. Coll. Cardiol. 54(13), 1123–1130 (2009).
- Lieu HD, Shryock JC, von Mering GO et al.: Regadenoson, a selective A2A adenosine receptor agonist, causes dose-dependent increases in coronary blood flow velocity in humans. J. Nucl. Cardiol. 14(4), 514–520 (2007).
- Gordi T, Frohna P, Sun HL et al.: A population pharmacokinetic/ pharmacodynamic analysis of regadenoson, an adenosine A2A-receptor agonist, in healthy male volunteers. Clin. Pharmacokinet. 45(12), 1201–1212 (2006).
- Gordi T, Blackburn B, Lieu H: Regadenoson pharmacokinetics and tolerability in subjects with impaired renal function. J. Clin. Pharmacol. 47(7), 825–833 (2007).
- Gao Z, Li Z, Baker SP et al.: Novel shortacting A2A adenosine receptor agonists for coronary vasodilation: inverse relationship between affinity and duration of action of A2A agonists. J. Pharmacol. Exp. Ther. 298(1), 209–218 (2001).
- Trochu JN, Zhao G, Post H et al.: Selective A2A adenosine receptor agonist as a coronary vasodilator in conscious dogs: potential for use in myocardial perfusion imaging. J. Cardiovasc. Pharmacol. 41(1), 132–139 (2003).
- Zhao G, Linke A, Xu X et al.: Comparative profile of vasodilation by CVT-3146, a novel A2A receptor agonist, and adenosine in conscious dogs. J. Pharmacol. Exp. Ther. 307(1), 182–189 (2003).
- Mekkaoui C, Jadbabaie F, Dione DP et al.: Effects of adenosine and a selective A2A adenosine receptor agonist on hemodynamic and thallium-201 and technetium-99msestaMIBI biodistribution and kinetics. JACC Cardiovasc. Imaging 2(10), 1198–1208 (2009).
- Dhalla AK, Wong MY, Wang WQ et al.: Tachycardia caused by A2A adenosine receptor agonists is mediated by direct sympathoexcitation in awake rats. J. Pharmacol. Exp. Ther. 316(2), 695–702 (2006).
- Zhao G, Serpllion S, Shryock J et al.: Regadenoson, a novel pharmacologic stress agent for use in myocardial perfusion imaging, does not have a direct effect on the QT interval in conscious dogs. J. Cardiovasc. Pharmacol. 52(5), 467–473 (2008).
- Zhao G, Messina E, Xu X et al.: Caffeine attenuates the duration of coronary vasodilation and changes in hemodynamics induced by regadenoson (CVT-3146), a novel adenosine A2A receptor agonist. J. Cardiovasc. Pharmacol. 49(6), 369–375 (2007).
- Hendel RC, Bateman TM, Cerqueira MD et al.: Initial clinical experience with regadenoson, a novel selective A2A agonist for pharmacologic stress singlephoton emission computed tomography myocardial perfusion imaging. J. Am. Coll. Cardiol. 46(11), 2069–2075 (2005).
- Iskandrian AE, Bateman TM, Belardinelli L et al.: Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: results of the ADVANCE Phase 3 multicenter international trial. J. Nucl. Cardiol. 14(5), 645–658 (2007).
- Hage FG, Heo J, Franks B et al.: Differences in heart rate response to adenosine and regadenoson in patients with and without diabetes mellitus. Am. Heart J. 157(4), 771–776 (2009).
- Gaemperli O, Schepis T, Koepfli P et al.: Interaction of caffeine with regadenosoninduced hyperemic myocardial blood flow as measured by positron emission tomography: a randomized, double-blind, placebocontrolled crossover trial. J. Am. Coll. Cardiol. 51(3), 328–329 (2008).
- Mahmarian JJ, Cerqueira MD, Iskandrian AE et al.: Regadenoson induces comparable left ventricular perfusion defects as adenosine: a quantitative analysis from the ADVANCE MPI 2 trial. JACC Cardiovasc. Imaging 2(8), 959–968 (2009).
- Cerqueira MD, Nguyen P, Staehr P et al.: Effects of age, gender, obesity, and diabetes on the efficacy and safety of the selective A2A agonist regadenoson versus adenosine in myocardial perfusion imaging integrated ADVANCE-MPI trial results. JACC Cardiovasc. Imaging 1(3), 307–316 (2008).
- Thomas GS, Thompson RC, Miyamoto MI et al.: The RegEx trial: a randomized, double-blind, placebo- and active-controlled pilot study combining regadenoson, a selective A(2A) adenosine agonist, with low-level exercise, in patients undergoing myocardial perfusion imaging. J. Nucl. Cardiol. 16(1), 63–72 (2009).
- Leaker BR, O’Connor B, Hansel TT et al.: Safety of regadenoson, an adenosine A2A receptor agonist for myocardial perfusion imaging, in mild asthma and moderate asthma patients: a randomized, double-blind, placebo-controlled trial. J. Nucl. Cardiol. 15(3), 329–336 (2008).
- Thomas GS, Tammelin BR, Schiffman GL et al.: Safety of regadenoson, a selective adenosine A2A agonist, in patients with chronic obstructive pulmonary disease: a randomized, double-blind, placebocontrolled trial (RegCOPD trial). J. Nucl. Cardiol. 15(3), 319–328 (2008).
- Aljaroudi W, Hermann D, Hage F et al.: Safety of regadenoson in patients with end-stage renal disease. Am. J. Cardiol. 105(1), 133–135 (2009).
- Botvinick EH: Current methods of pharmacologic stress testing and the potential advantages of new agents. J. Nucl. Med. Technol. 37(1), 14–25 (2009)