Research Article - Neuropsychiatry (2019) Volume 9, Issue 1
Regulation Effects of XPJY Decoction on Antioxidative and Mitochondrial Functions in Intracerebral and Extra Brain Tissue of CUMS Rats
- Corresponding Author:
- Rongjuan Guo
Dongfang Hospital Beijing University of Chinese, Medicine, 100078 Beijing, China
Abstract
Background and objectives
The treatment of somatic symptoms of depression is a medical problem nowadays. More and more evidences show that oxidative stress and mitochondrial dysfunction may be an important pathogenesis of depression and are closely related to somatic symptoms. Currently, studies on oxidative stress and mitochondrial function are mostly concentrated in the brain, with less attention paid to peripheral tissues, and classical SSRIs are not direct antioxidants in treatment. XingPiJieYu (XPJY) Decoction is one of the most widely used clinical formulas of traditional Chinese medicine. Our study aims to exploring whether it has antioxidative and mitochondrial effects on intracerebral and extra brain tissue.
Materials and Methods
The rat model of depression was established by CUMS (chronic unpredictable mild stress, CUMS) for 6 weeks. They were randomly divided into six groups: control group, CUMS group, CUMS+XPJY (3.6g/kg/d, 7.2g/kg/d, 14.4g/kg/d) groups, CUMS+ sertraline (4.5mg/kg/d) group. We used sucrose preference test and forced swimming test to verify the success of the depression model. The contents of CK and SDH in orbital blood were measured weekly as well as the following assay index were measured on 14th day and 42th day, including MDA, ATP and mtDNA in hippocampus, prefrontal cortex, liver, small intestine and gastrocnemius muscle.
Results
At the 14th day, the sucrose preference ratio was decreased. Besides, the immobility time prolonged at the 42nd day. CUMS increased MDA content in hippocampus, prefrontal cortex and gastrocnemius muscle, but decreased mtDNA content in prefrontal cortex at the 14th day; CUMS decreased serum SDH level at the 35th day, but elevated serum CK level at the 42nd day; CUMS increased MDA content in hippocampus, prefrontal cortex, liver and gastrocnemius muscle, while decreased ATP and mtDNA content in hippocampus, prefrontal cortex, liver, small intestine and gastrocnemius muscle at the 42nd day. XPJY decoction at 14.4g/kg, the efficacy trend of which was better than the other drug groups, could prevent the depressive behavior caused by CUMS, reduce the serum CK content and the MDA content of the tissues mentioned above, and increase serum SDH content as well as ATP, mtDNA content in tissues.
Conclusion
CUMS could induce oxidative stress and mitochondrial dysfunction in hippocampus, PFC, liver, small intestine and gastrocnemius muscle in rats. Early application of sertraline can effectively prevent mtDNA damage in hippocampus, PFC and liver, but its regulation on MDA and ATP is not obvious. However, XPJY decoction plays an significant role in antioxidation by means of improving mitochondrial function, eliminating inflammation and increasing 5-HT content. This research provides an important theoretical basis for the clinical application of XPJY.
Keywords
Depression, Chinese herbal formula, XPJY decoction, CK, SDH, MDA, ATP, mtDNA
Introduction
The clinical manifestations of depression include not only mental disorders such as depression, mental retardation and cognitive impairment, but also somatic symptoms such as headache, muscle ache, tension and indigestion [1]. These symptoms often cover up some of the characteristics of depression, so psychiatrists should attach great importance to them. Depression is considered to be a multi system disease. Research [2] has shown that male and female depressive patients with suicidal ideation show more frequent and more serious somatic symptoms than those without suicidal ideation. Somatic symptoms are closely related to the current suicide risk of depressive patients. Therefore, the attention and treatment of depressive patients’ somatic symptoms is a key link in the treatment of depression.
Ann Gardner [3] found that the decrease in ATP production in muscle tissue was found in patients with somatization symptoms, which reveals that the core symptoms of extreme fatigue caused by depression are closely related to energy deficiency and mitochondrial damage. Furthermore, increasing evidence suggests that oxidative stress and abnormal mitochondrial energy metabolism are involved in the pathophysiological process of depression and become the core factors of depression [4-5].For instance, several evidences indicate that oxidative stress in hippocampus plays an important role in the pathogenesis of CUMS-induced rodents [6-12]. It has also been reported that as the levels of free radicals increased, the total antioxidant capacity in patients with clinical depression decreased, while after antidepressant treatment, the free radicals and lipid peroxidation in serum declined, which reveals that the mechanism of depression may be closely related to oxidative-antioxidant stress system [13]. In addition, some research data indicates that the level of ATP in the brain of depressed patients is normally lower as well as the prevalence rates with mitochondrial diseases of depressed patients are usually up to 54%, compared with control subjects [14-15]. From these perspectives, improving oxidative stress and mitochondrial function may provide a new target for the treatment of depression, especially in accompanied somatic symptoms. However, the current research on mitochondrial function of oxidative stress machine in depression are mainly focused on the brain, and are lacking of the analysis on other systems or tissues.
At present, SSRI antidepressants did not seem to improve the oxidative stress and mitochondrial function. Yan Li [16] found that Sertraline uncoupling mitochondria oxidative phosphorylation inhibits the activity of oxidative phosphorylation complexes I and V in rat liver mitochondria. Similar results were found in rat brain research: Fluoxetine reduced the rate of ATP synthesis [17]. Other studies found that sertraline and paroxetine significantly induced ROS production in 24 hours and SSRI notably reduced mtDNA copy number, which compromise the expression of mitochondria [18-19]. However, Nahon demonstrated that fluoxetine could inhibit the opening of the mitochondrial permeability transition (MPT) pore, release of cytochrome c (cytC) and protect against staurosporine-induced apoptotic cell death. Consequently, there are inconsistencies in the results of SSRI in the protection of mitochondria and antioxidant stress, which may be related to the type of cells [20] and needed to be further studied.
XPJY Decoction, a Chinese herbal medicine, which has been confirmed to improve depressive behavior, increase serum 5-HT, decrease serum corticosterone, decrease inflammatory factors in serum and hippocampus, increase the expression of cAMP, PKA, CREB and BDNF in hippocampus of CUMS rats[21-24]. However, whether it can regulate oxidative stress and mitochondrial function remains to be elucidated. Mitochondria are key organelles regulating cellular energy status via oxidative phosphorylation, can produce the energy available for a variety of cells (ATP), which is especially important for the heart, skeletal muscle, brain and other high hypermetabolic tissues. SDH is one of the marker enzymes of mitochondria, existing in the inner membrane of aerobic respiratory mitochondria, the content of which in serum can reflect the energy metabolism of mitochondria. CK is an important kinase, mainly existing in cytoplasm and mitochondria andrelated directly to intracellular energy translocation, muscle contraction and ATP regeneration, the level of which in Serum can reflect the ability of ATP production and skeletal muscle injury. ROS damages mitochondria in two ways, one is the direct oxidation of mitochondrial DNA, the other is the induction of peroxidation of cardiolipin on the mitochondrial inner membrane, which leads to the decrease of ATP synthase activity and ATP production, resulting in a large number of MDA [5]. Therefore, the content of MDA can reflect the level of oxidative stress in tissues.
To sum up, in this study, we used the recognized rat model of CUMS by detecting the contents of SDH, CK, MDA, ATP and mtDNA to explore the following three questions: (1) whether the chronic unpredictable stress of 6 weeks could lead to oxidative stress and mitochondrial dysfunction in hippocampus, prefrontal cortex, liver, small intestine and gastrocnemius muscle of rats. (2) Whether Sertraline’s early application improved the oxidative stress and mitochondrial damage. (3) Whether Chinese herbal medicine XPJY has the function of antioxidant and mitochondrial protection. Besides, this paper would study and reveal the mechanism of it on the premise that its function is established.
Material and Methods
▪ Preparation and compositional analysis of XPJY
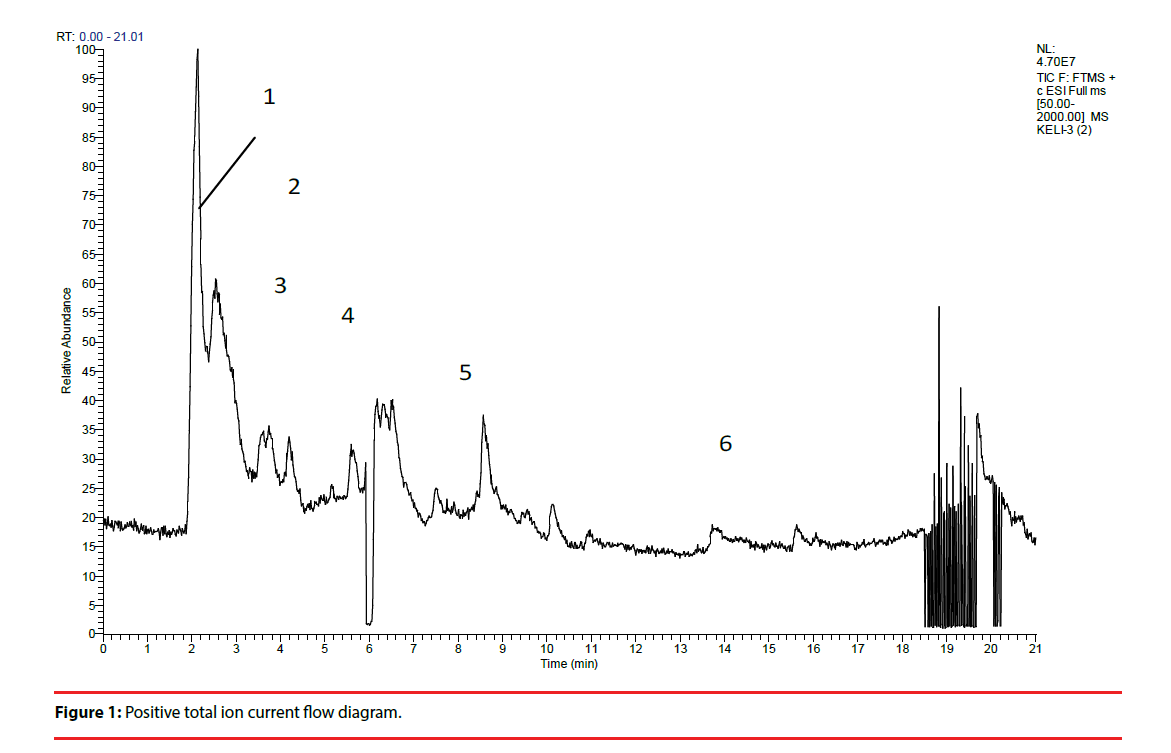
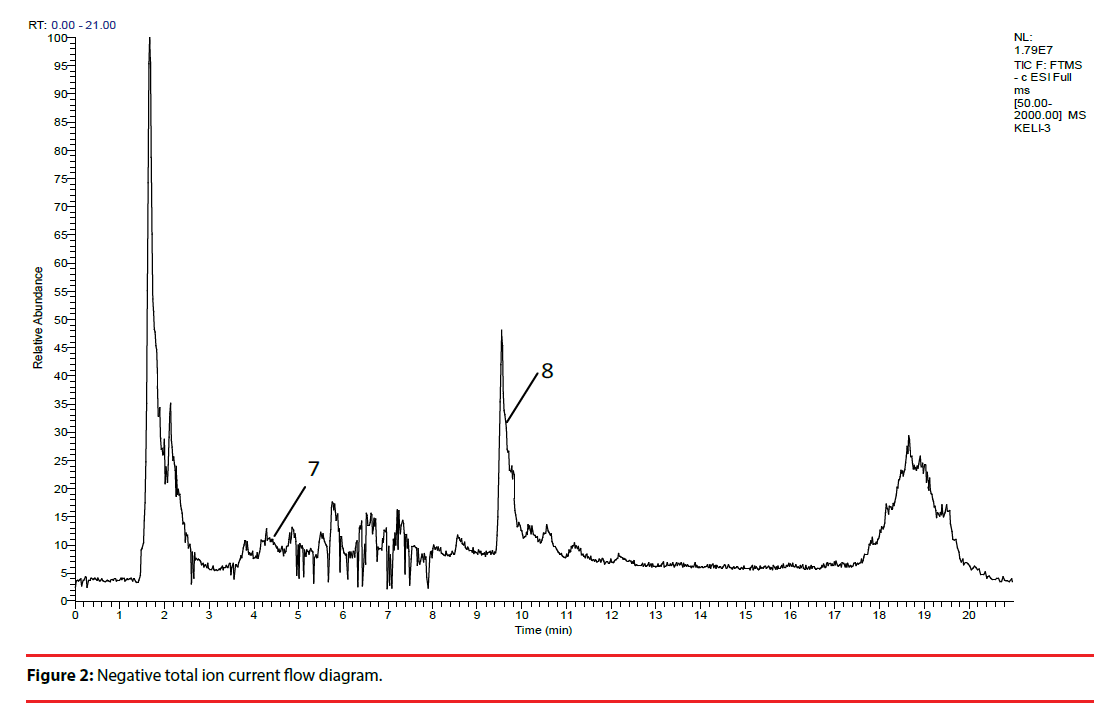
XPJY was composed of the following granules, which were derived from dried raw materials, including American ginseng (Xi-Yang-shen), radix curcumae (Yu-Jin), acori tatarinowii rhizoma (Shi-Chang-pu) and hypericum perforatum (Guan-Ye-lianqiao), and each kind of content 20g. All granules were provided by the medicinal materials company of Beijing KangRenTang Company (Beijing, China), and were analyzed for composition by high resolution mass spectrometry (HRMS). Instrument type: Thermo LTQ-Orbitrap hybrid mass. Chromatographic conditions: column temperature (30°C), flow rate (0.03ml/min), chromatographic column: Thermo scientific 150 × 2.1mm BDSHYPERSIL C18. Mobile phase: A: 0.1% formic acid aqueous solution, B: acetonitrile. Elution procedure: 0-0.3min 5%B, 0.3-0.6min 5%-12%B, 0.6-7min 12%-28%B, 7-15min 28%-32%B, 15-18min 32%-98% B. Mass spectrometry conditions: ion mode: positive ion mode; heater temperature (35°C). capillary temperature (35°C), capillary voltage (35kv), sheath gas velocity (35arb), auxiliary gas velocity (10arb). Quality scanning range: 50-2000, resolution: 30000.Sample treatment method: precise weighed 3.09g particles, placed in a cone flask, precision measurement of 50% methanol solution 60ml added. Ultrasonic 30 minutes. Take the filtrate 1ml, through the 0.45um micro film, to prepare sample solution (Figure 1, Figure 2 and Table 1)
| No. | Retention time (Min) | Compound | Molecular formula | Exact mass | Expected values | Observed values | Error (Ppm) |
|---|---|---|---|---|---|---|---|
| 1 | 2.09 | Aspartate | C4H7NO4 | 133.0375 | 134.0448 | 134.04482 | 0.15 |
| 2 | 2.09 | Valine | C5H11NO2 | 117.079 | 118.0863 | 118.08621 | -0.76 |
| 3 | 2.10 | Glutamate | C5H9NO4 | 147.0532 | 148.0605 | 148.06042 | -0.54 |
| 4 | 2.14 | Proline | C5H9NO2 | 115.0633 | 116.0706 | 116.07047 | -1.2 |
| 5 | 7.5 | Isoscurenol | C15H22O2 | 234.162 | 235.1693 | 235.16953 | 0.98 |
| 6 | 13.79 | Fragrant ginger flavonoids | C15H20O | 216.1514 | 217.1587 | 217.15907 | 1.7 |
| 7 | 4.20 | Malonyl ginsenoside Rb1 | C10H12O2 | 164.0837 | 209.0819 | 209.07996 | -9.27- |
| 8 | 9.51 | Pseudoginsenoside F11 | C42H72O14 | 800.4922 | 799.4849 | 799.47778 | -8.91 |
Table 1: Identification results of ionic compounds.
▪ Animals and grouping
Adult male Wistar rats, weighing 160~180 g, were provided by Institute of Psychology CAS. These animals are raised in single cages in 12 hours of light/12 hours of darkness and are allowed free access to water and food. Before the study, rats were pretreated for 7 days, with 3 minutes of adjustment each day. All animal procedures are in line with the guidelines for the care and use of laboratory animals.
74 rats were randomly divided into 6 groups: control, model, XPJY low-dose, XPJY mediumdose and XPJY high-dose group, 12 rats in each group. Rats in the control group were given normal saline daily for 42 days; rats in the model group were exposed to unpredictable sources of stress and were given normal saline daily for 42 days; rats in the XPJY decoction group were exposed to unpredictable sources of stress and given orally once a day at doses of 3.6 g/kg/d, 7.2 g/kg/d and 14.4 g/kg, respectively, for 42 days; rats in sertraline group were exposed to unpredictable stressors. Sertraline (Pfizer) was intragastrically injected once a day of 4.5 mg/ kg/d, for 42 days. Sertraline and XPJY granules were dissolved in normal saline.
▪ Animal model preparation
The stress scheme was slightly modified from previous study [25]. Briefly, a 6-week chronic unpredictable mild stress (CUMS) model of depressive rats was established. The methods are as follows: inclined rat cage + water deprivation (30 degrees, 12 hours), high frequency flash (12 hours), restraint + tail clamping (5 minutes), noise (12 hours of squeal), cold water swimming (4 degrees, 5 minutes), fasting (12 hours), water deprivation (12 hours), wet mattress (12 hours), warm and hot environment (30-32 degrees, 12 hours), light (24 hours), darkness (24 hours), mixed culture (24 hours), 1. Two kinds of stimulation were arranged by random number table method for 42 days. One or two kinds of stress were given daily, and the same kind of stimulation appeared discontinuously. Except for the blank control group, both the model group and the drug group were subjected to stress.
▪ Sucrose preference test
Two days before the sugar preference test, rats were given two bottles of 0.8% sucrose water; one day before the test, rats were given one bottle of water and one bottle of 0.8% sucrose water, and two bottles were replaced at the midpoint of time; on the test day, rats were given water forbidden for 20 hours, and then one bottle of water and 0.8% sucrose water were given respectively, so that rats could drink water freely for 24 hours, and two bottles were replaced at the midpoint of time. After 24 hours, the drinking condition of rats was counted, and the percentage of sugar water preference was calculated.
▪ Forced swimming test
The forced swimming test was improved on the basis of Porsolt [26]. Forced swimming is a classic behavioral experiment of depression. It is mainly used to investigate animal behavior despair [27]. The size of rat swimming barrel is 25 cm inside diameter and 45 cm in water depth. After swimming in water for 6 minutes, the statistic analysis was made on the immobility time of rats in water for 4 minutes. The immobility criterion was that rats only floated with their heads out of the water and their bodies did not struggle. The longer the immobility time, the more desperate the animals were. During the experiment, rats should be separated from audiovisual interference. Before putting down one rat at a time, the floating feces on the water should be cleaned up. After the rats are taken out of the water, the hair of the rats should be wiped with a towel as far as possible to prevent the rats from catching a cold.
▪ Detection of blood and CK and SDH contents from the orbit
At 2 and 6 weeks, 1.5 mL bloods were taken from the orbit after inhalation of ether. At room temperature, centrifuge was centrifuged at 4°C for 3000 r/ min centrifugation 10 min to separate serum and cryopreserved at -80°C. Serum CK and SDH levels were measured with CK kit and SDH kit instructions. CK units were expressed in U/L and SDH units in U/mL.
▪ Tissue sampling and content determination of ATP and MDA
After 10% chloral hydrate was injected intraperitoneally at the end of the 2nd and 6th weekends, the head was broken after glutaraldehyde perfusion and fixation. The hippocampus, cerebral cortex, liver, skeletal muscle of right lower extremity and small intestine tissue were taken out immediately, washed with normal saline, drained with filter paper and frozen with liquid nitrogen (-196°C) for the determination of MDA content. Specific operation: Take frozen tissue from refrigerator at -80°C, add pre-cooled homogenate medium (including sucrose 250mmol/L, EDTA 0.5mmol/L, Tris-HCL 10mmol/L, adjust PH7.4) at the ratio of 1:9 (W/V), and prepare 10% homogenate. ATP content in tissues was detected by ATP content test kit (chemical method) of Nanjing Jiancheng Institute of Bioengineering, expressed by mmol/g; MDA kit of Nanjing Jiancheng Institute of Bioengineering was used to operate according to the instructions of the kit, and MDA content in tissues was detected by TBA (thiobarbituric acid) method. Mol/g says.
▪ Determination of mtDNA content
Samples were ground with liquid nitrogen, added 1 ml Trizol (invitrogen), absorbed into 1.5 ml EP tube, added 500 UL phenol chloroform, shaking and mixing, stationary for 5 minutes, placed in centrifuge at 4°C for 10 minutes, carefully absorbed supernatant in a new centrifugal tube, added 700 UL isopropanol, mixing, centrifuged at 4°C for 12 000 rpm for 10 minutes. Minutes, carefully go to the supernatant, wash once with 75% ethanol, dry, dissolved in 50 UL DEPC treated water, electrophoretic detection.1 ug RNA was retrieved by superscript III, a reverse transcription kit of invitrogen. After the reaction, it was placed at -20°C. Then, the real-time quantitative PCR reaction was performed on the PRISM 7900 fluorescence quantitative PCR instrument of ABI. Each sample was amplified by primers of the genes to be detected and the internal reference genes. Each reaction was repeated for 3 times, and then the amplification products were fused and analyzed by 1.5% agarose gel electrophoresis. According to the standard curve, the relative copy number of target gene mRNA in the sample to be tested was calculated, and the expression level of target gene mRNA was calculated.
Results
▪ Sucrose preference test
Rats from different groups showed no significant difference in sucrose preference ratio at the 1st day (p>0.05). Significantly decline of sucrose preference ratio was observed at the 14th day, 28th day, and the 42nd day (P<0.05, P<0.05, P<0.01) in the CUMS group. At the 14th day, treatment with sertraline at 4.5mg/kg significantly suppressed the stressinduced decline of sucrose preference ratio (p < 0.05); At the 42nd day, treatment with XPJY decoction at 3.6g/kg, 7.2g/kg, 14.4g/kg and sertraline at 4.5mg/kg significantly suppressed the stress-induced decline of sucrose preference ratio (P<0.01, P<0.01, P<0.01, P<0.01) (Table 2).
| Groups | Sucrose preference ratio (%) | |||
|---|---|---|---|---|
| 1st day | 14th day | 28th day | 42nd day | |
| Control | 84.11 ± 4.19 | 87.33 ± 2.07 | 92.56 ± 0.86 | 92.88 ± 0.12 |
| CUMS | 81.73 ± 4.09 | 86.72 ± 4.01* | 89.89 ± 2.23* | 84.89 ± 1.34** |
| CUMS+XPJY 3.6g/kg | 80.82 ± 2.16 | 85.57 ± 3.21 | 88.12 ± 2.11 | 88.12 ± 2.09△△ |
| CUMS+XPJY 7.2g/kg | 82.01 ± 1.08 | 84.92 ± 3.43 | 91.51 ± 1.09 | 94.77 ± 0.33△△ |
| CUMS+XPJY 14.4g/kg | 81.15 ± 2.20 | 88.19 ± 4.01 | 92.01 ± 2.88 | 93.45 ± 1.19△△ |
| CUMS+ sertraline 4.5mg/kg | 85.04 ± 1.12 | 91.60 ± 2.87△ | 90.90 ± 1.99 | 91.38 ± 1.28△△ |
Table 2: Changes of sucrose preference in different groups.
▪ Forced swimming test
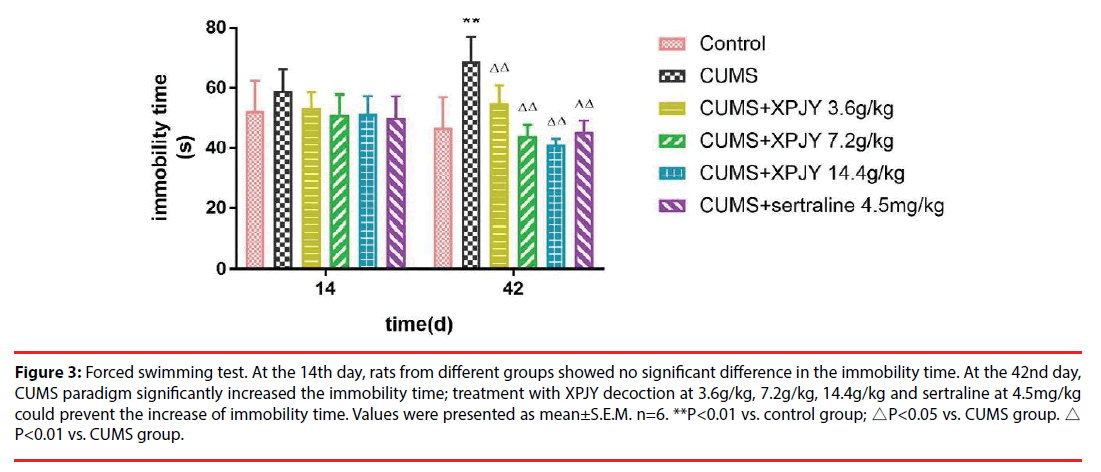
At the 14th day, rats from different groups showed no significant difference in the immobility time (p>0.05). At the 42nd day, in comparison to the control group, the immobility time significantly prolonged in CUMS group (p < 0.01); in comparison to the CUMS group, treatment with XPJY decoction at 3.6g/kg, 7.2g/kg, 14.4g/ kg and sertraline at 4.5mg/kg could shorten the immobility time (p < 0.01, p < 0.01, p < 0.01, p < 0.01) (Figure 3).
Figure 3: Forced swimming test. At the 14th day, rats from different groups showed no significant difference in the immobility time. At the 42nd day, CUMS paradigm significantly increased the immobility time; treatment with XPJY decoction at 3.6g/kg, 7.2g/kg, 14.4g/kg and sertraline at 4.5mg/kg could prevent the increase of immobility time. Values were presented as mean±S.E.M. n=6. **P<0.01 vs. control group; △P<0.05 vs. CUMS group. △ P<0.01 vs. CUMS group.
In this study, after 6 weeks of unpredictable mild stress intervention, the sucrose preference rate of rats in CUMS group decreased significantly and the immobility time prolonged. The rat model of depression was successfully established.
▪ Effects of XPJY on CK content
At the 14th day, 21th day, and 28th day, rats from different groups showed no significant difference in CK content in serum (P>0.05). At the 35th day, compared with the model group, treatment with XPJY decoction at 7.2g/kg could prevent the increased CK content in serum (p<0.05). At the 42nd day, in comparison to the model group, the CK content of serum significantly increased in CUMS group (p<0.05); in comparison to the CUMS group, treatment with XPJY decoction at 7.2g/kg could prevent the increased CK content of serum (p<0.05) (Table 3).
| Day 14 | Day 21 | Day 28 | Day 35 | Day 42 | |
|---|---|---|---|---|---|
| Control | 574.184 ± 118.272 | 543.804 ± 128.982 | 522.538 ± 93.804 | 527.601 ± 102.550 | 549.880 ± 104.201 |
| CUMS | 611.653 ± 154.742 | 697.730 ± 217.605 | 661.274 ± 131.524 | 668.911 ± 149.772 | 724.460 ± 138.986* |
| CUMS+XPJY 3.6g/kg | 596.463 ± 144.457 | 584.311 ± 176.844 | 609.627 ± 131.524 | 569.121 ± 208.448 | 610.640 ± 160.556 |
| CUMS+XPJY 7.2g/kg | 610.640 ± 107.135 | 555.956 ± 146.164 | 550.893 ± 120.460 | 499.331 ± 86.886△ | 539.247 ± 53.828△ |
| CUMS+XPJY 14.4g/kg | 588.788 ± 135.221 | 591.399 ± 261.238 | 585.323 ± 140.637 | 591.399 ± 123.402 | 624.818 ± 164.921 |
| CUMS+ sertraline 4.5mg/kg | 585.323 ± 126.476 | 568.108 ± 165.401 | 573.171 ± 131.525 | 576.209 ± 97.707 | 650.134 ± 112.831 |
Table 3: CK content.
▪ Effects of XPJY on SDH content
At the 14th day, 21th day, and 28th day, rats from different groups showed no significant difference in SDH content in serum (P>0.05). At the 35th day, in comparison to the model group, the SDH content of serum significantly decreased in CUMS group (p<0.05); in comparison to the CUMS group, treatment with XPJY decoction at 7.2g/kg could prevent the decreased SDH content of serum (p<0.05). At the 42nd day, compared with the model group, the SDH content of serum significantly decreased in CUMS group (p<0.05); in comparison to the CUMS group, treatment with XPJY decoction at 7.2g/kg and 14.4g/kg could prevent the decreased SDH content of serum (p<0.05) (Table 4).
| Day 14 | Day 21 | Day 28 | Day 35 | Day 42 | |
|---|---|---|---|---|---|
| Control | 2.025 ± 0.346 | 2.157 ± 0.256 | 2.356 ± 0.630 | 2.265 ± 0.386 | 2.275 ± 0.375 |
| CUMS | 1.914 ± 0.360 | 1.803 ± 0.361 | 1.901 ± 0.409 | 1.732 ± 0.201* | 1.725 ± 0.354* |
| CUMS+XPJY 3.6g/kg | 2.036 ± 0.379 | 2.005 ± 0.412 | 1.914 ± 0.391 | 1.965 ± 0.443 | 2.127 ± 0.466 |
| CUMS+XPJY 7.2g/kg | 1.894 ± 0.329 | 2.167 ± 0.444 | 1.964 ± 0.511 | 2.360 ± 0.422△ | 2.374 ± 0.491△ |
| CUMS+XPJY 14.4g/kg | 2.059 ± 0.392 | 2.299 ± 0.465 | 2.056 ± 0.417 | 2.086 ± 0.376 | 2.461 ± 0.486△ |
| CUMS+ sertraline 4.5mg/kg | 1.853 ± 0.402 | 2.066 ± 0.440 | 1.944 ± 0.444 | 1.987 ± 0.555 | 2.133 ± 0.428 |
Table 4: SDH content.
▪ Effects of XPJY on MDA content
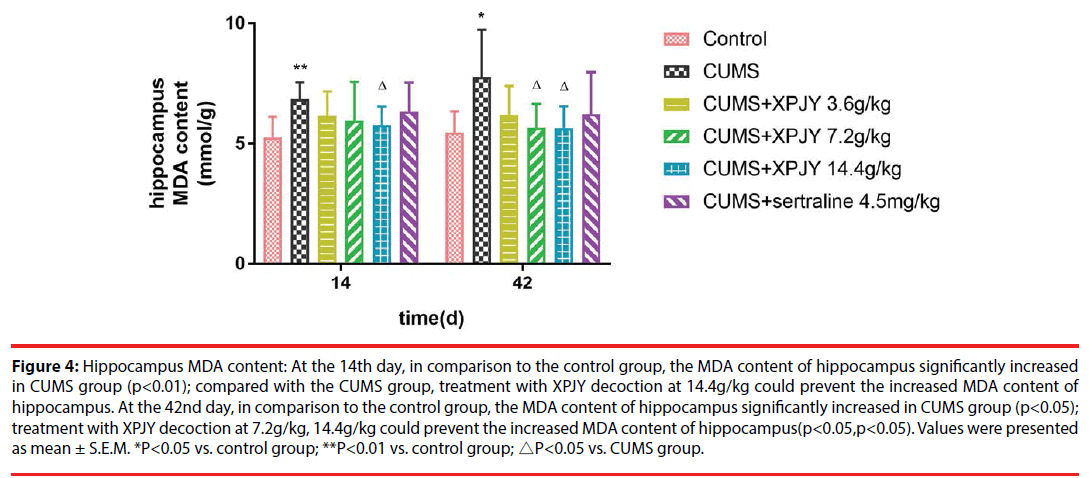
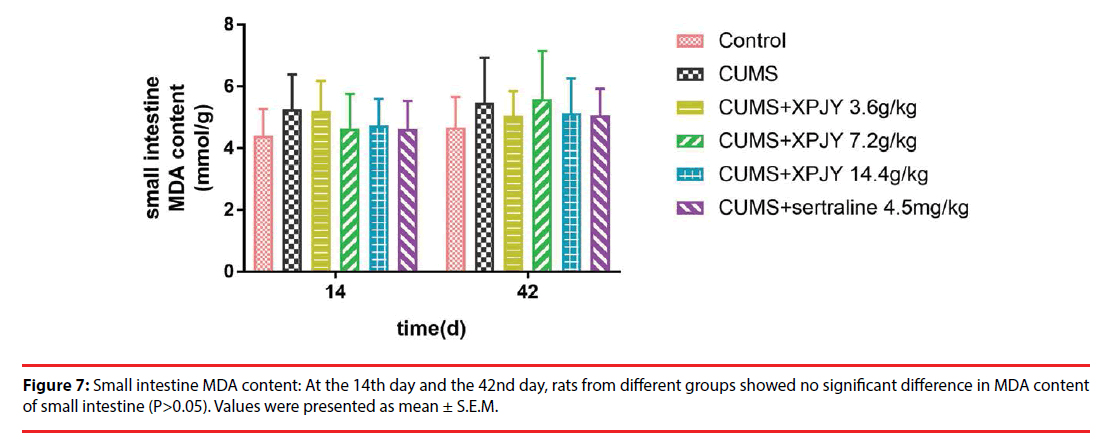
At the 14th day, in comparison to the control group, the MDA content of hippocampus, PFC and gastrocnemius muscle significantly increased in CUMS group (p<0.01, p<0.05, p<0.01); compared with the CUMS group, treatment with XPJY decoction at 14.4g/kg could prevent the increased MDA content of hippocampus and PFC (p<0.05, p<0.05). At the 42nd day, in comparison to the control group, the MDA content of hippocampus, PFC, liver and gastrocnemius muscle significantly increased in CUMS group (p<0.05, p<0.01, p<0.05, p<0.01); compared with the CUMS group, treatment with XPJY decoction at 3.6g/kg could prevent the increased MDA content of liver (p<0.01); treatment with XPJY decoction at 7.2g/ kg could prevent the increased MDA content of hippocampus, PFC, liver and gastrocnemius muscle (p<0.05, p<0.01, p<0.05, p<0.05); treatment with XPJY decoction at 14.4g/kg could prevent the increased MDA content of hippocampus, PFC, liver and gastrocnemius muscle (p<0.05, p<0.01, p<0.01, p<0.05) (Figure 4-8).
Figure 4: Hippocampus MDA content: At the 14th day, in comparison to the control group, the MDA content of hippocampus significantly increased in CUMS group (p<0.01); compared with the CUMS group, treatment with XPJY decoction at 14.4g/kg could prevent the increased MDA content of hippocampus. At the 42nd day, in comparison to the control group, the MDA content of hippocampus significantly increased in CUMS group (p<0.05); treatment with XPJY decoction at 7.2g/kg, 14.4g/kg could prevent the increased MDA content of hippocampus(p<0.05,p<0.05). Values were presented as mean ± S.E.M. *P<0.05 vs. control group; **P<0.01 vs. control group; △P<0.05 vs. CUMS group.
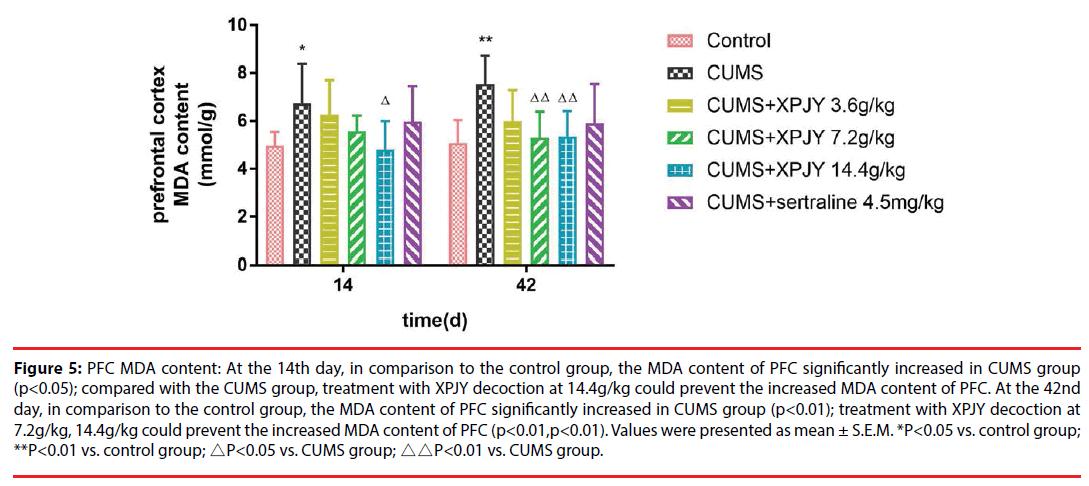
Figure 5: PFC MDA content: At the 14th day, in comparison to the control group, the MDA content of PFC significantly increased in CUMS group (p<0.05); compared with the CUMS group, treatment with XPJY decoction at 14.4g/kg could prevent the increased MDA content of PFC. At the 42nd day, in comparison to the control group, the MDA content of PFC significantly increased in CUMS group (p<0.01); treatment with XPJY decoction at 7.2g/kg, 14.4g/kg could prevent the increased MDA content of PFC (p<0.01,p<0.01). Values were presented as mean ± S.E.M. *P<0.05 vs. control group; **P<0.01 vs. control group; △P<0.05 vs. CUMS group; △△P<0.01 vs. CUMS group.
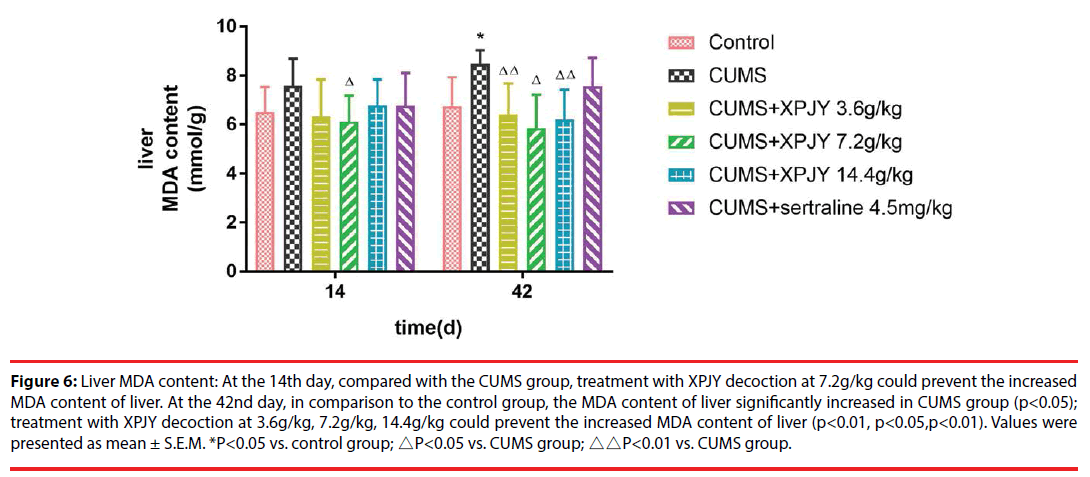
Figure 6: Liver MDA content: At the 14th day, compared with the CUMS group, treatment with XPJY decoction at 7.2g/kg could prevent the increased MDA content of liver. At the 42nd day, in comparison to the control group, the MDA content of liver significantly increased in CUMS group (p<0.05); treatment with XPJY decoction at 3.6g/kg, 7.2g/kg, 14.4g/kg could prevent the increased MDA content of liver (p<0.01, p<0.05,p<0.01). Values were presented as mean ± S.E.M. *P<0.05 vs. control group; △P<0.05 vs. CUMS group; △△P<0.01 vs. CUMS group.
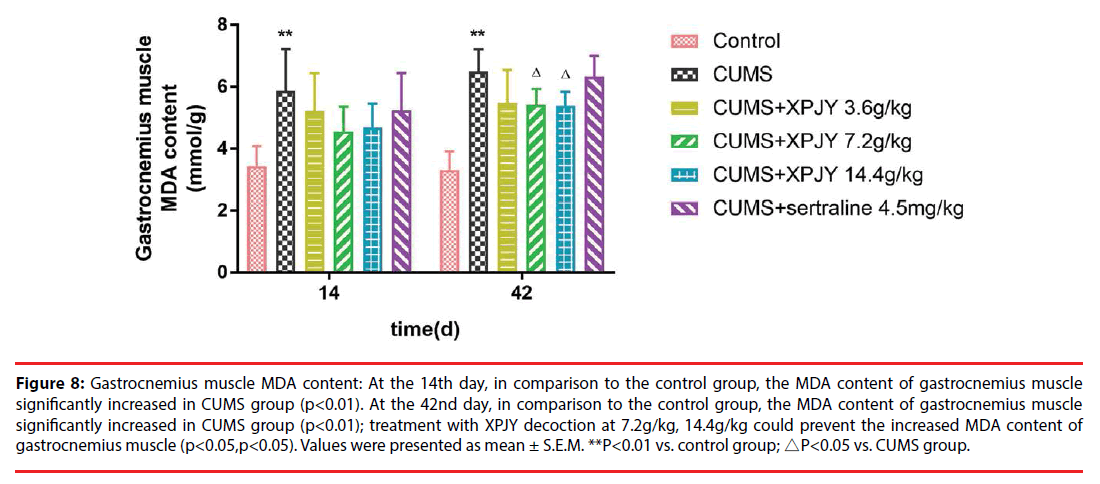
Figure 8: Gastrocnemius muscle MDA content: At the 14th day, in comparison to the control group, the MDA content of gastrocnemius muscle significantly increased in CUMS group (p<0.01). At the 42nd day, in comparison to the control group, the MDA content of gastrocnemius muscle significantly increased in CUMS group (p<0.01); treatment with XPJY decoction at 7.2g/kg, 14.4g/kg could prevent the increased MDA content of gastrocnemius muscle (p<0.05,p<0.05). Values were presented as mean ± S.E.M. **P<0.01 vs. control group; △P<0.05 vs. CUMS group.
▪ Effects of XPJY on ATP content
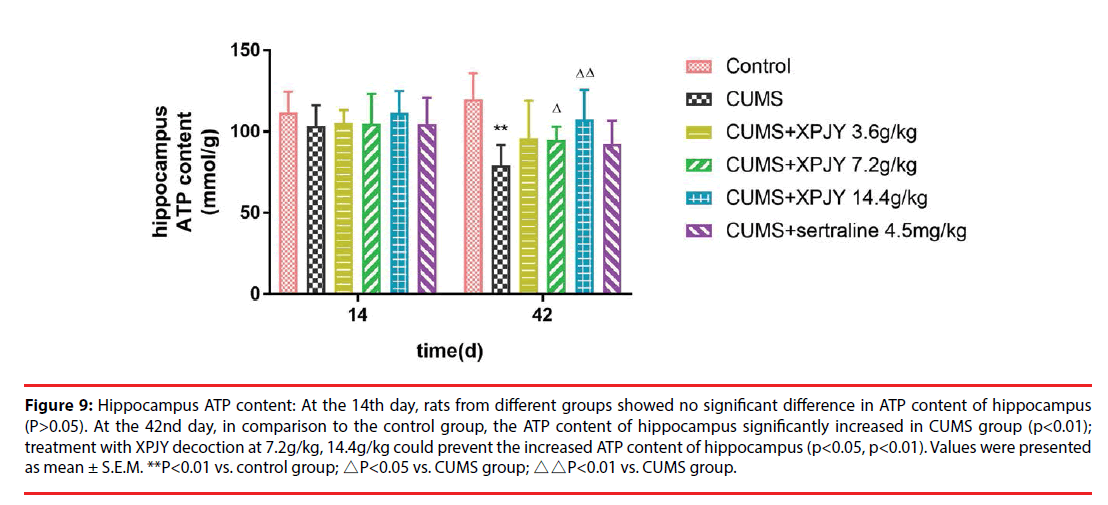
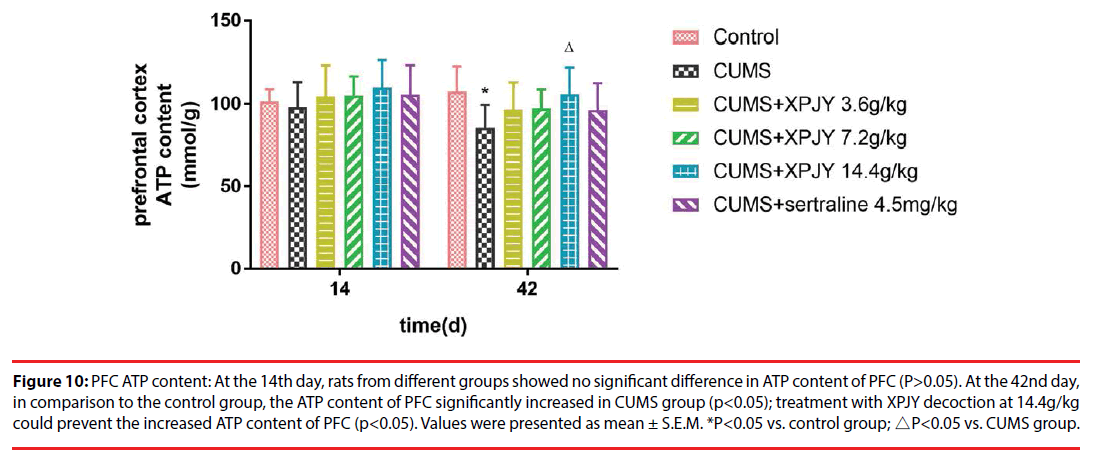
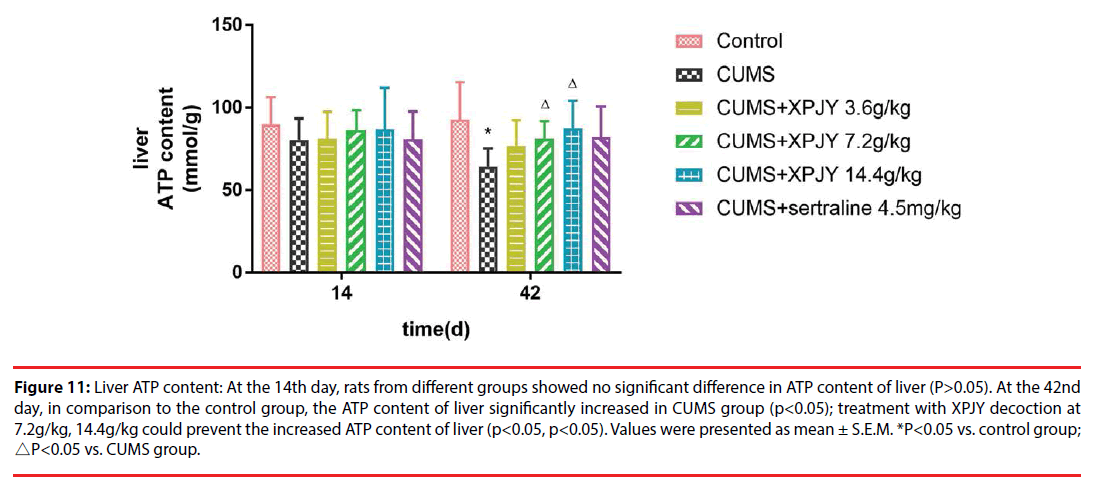
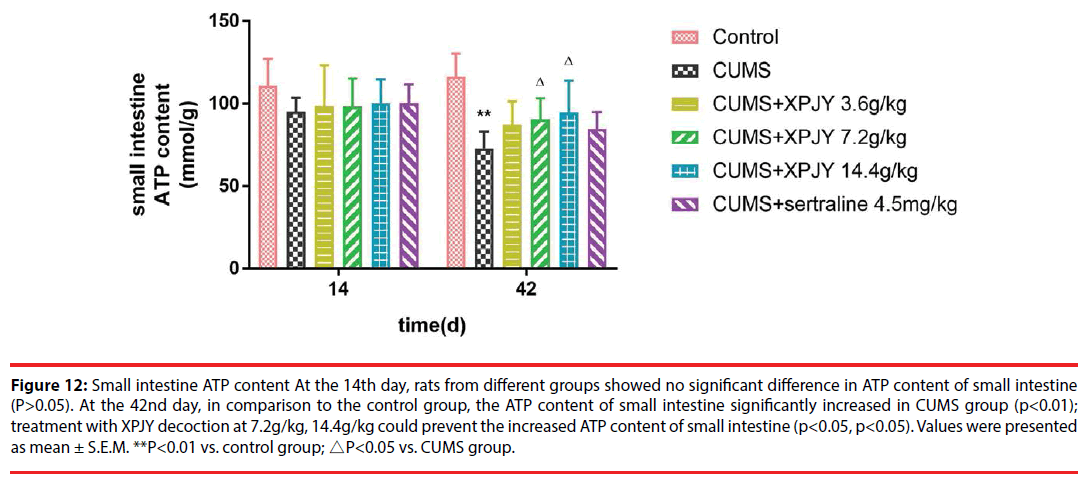
At the 14th day, rats from different groups showed no significant difference in ATP content of hippocampus, PFC, small intestine, and gastrocnemius muscle(P>0.05). At the 42nd day, in comparison to the control group, the ATP content of hippocampus, PFC, liver, small intestine and gastrocnemius muscle significantly decreased in CUMS group (p<0.01, p<0.05, p<0.05, p<0.01, p<0.05); compared with the CUMS group, treatment with XPJY decoction at 7.2g/kg could prevent the decreased ATP content of hippocampus, liver, small intestine and gastrocnemius muscle (p<0.05, p<0.05, p<0.05, p<0.05); treatment with XPJY decoction at 14.4g/kg could prevent the decreased ATP content of hippocampus, PFC, liver, small intestine and gastrocnemius muscle (p<0.01, p<0.05, p<0.05, p<0.05, p<0.05) (Figure 9-13).
Figure 9: Hippocampus ATP content: At the 14th day, rats from different groups showed no significant difference in ATP content of hippocampus (P>0.05). At the 42nd day, in comparison to the control group, the ATP content of hippocampus significantly increased in CUMS group (p<0.01); treatment with XPJY decoction at 7.2g/kg, 14.4g/kg could prevent the increased ATP content of hippocampus (p<0.05, p<0.01). Values were presented as mean ± S.E.M. **P<0.01 vs. control group; △P<0.05 vs. CUMS group; △△P<0.01 vs. CUMS group.
Figure 10: PFC ATP content: At the 14th day, rats from different groups showed no significant difference in ATP content of PFC (P>0.05). At the 42nd day, in comparison to the control group, the ATP content of PFC significantly increased in CUMS group (p<0.05); treatment with XPJY decoction at 14.4g/kg could prevent the increased ATP content of PFC (p<0.05). Values were presented as mean ± S.E.M. *P<0.05 vs. control group; △P<0.05 vs. CUMS group.
Figure 11: Liver ATP content: At the 14th day, rats from different groups showed no significant difference in ATP content of liver (P>0.05). At the 42nd day, in comparison to the control group, the ATP content of liver significantly increased in CUMS group (p<0.05); treatment with XPJY decoction at 7.2g/kg, 14.4g/kg could prevent the increased ATP content of liver (p<0.05, p<0.05). Values were presented as mean ± S.E.M. *P<0.05 vs. control group; △P<0.05 vs. CUMS group.
Figure 12: Small intestine ATP content At the 14th day, rats from different groups showed no significant difference in ATP content of small intestine (P>0.05). At the 42nd day, in comparison to the control group, the ATP content of small intestine significantly increased in CUMS group (p<0.01); treatment with XPJY decoction at 7.2g/kg, 14.4g/kg could prevent the increased ATP content of small intestine (p<0.05, p<0.05). Values were presented as mean ± S.E.M. **P<0.01 vs. control group; △P<0.05 vs. CUMS group.
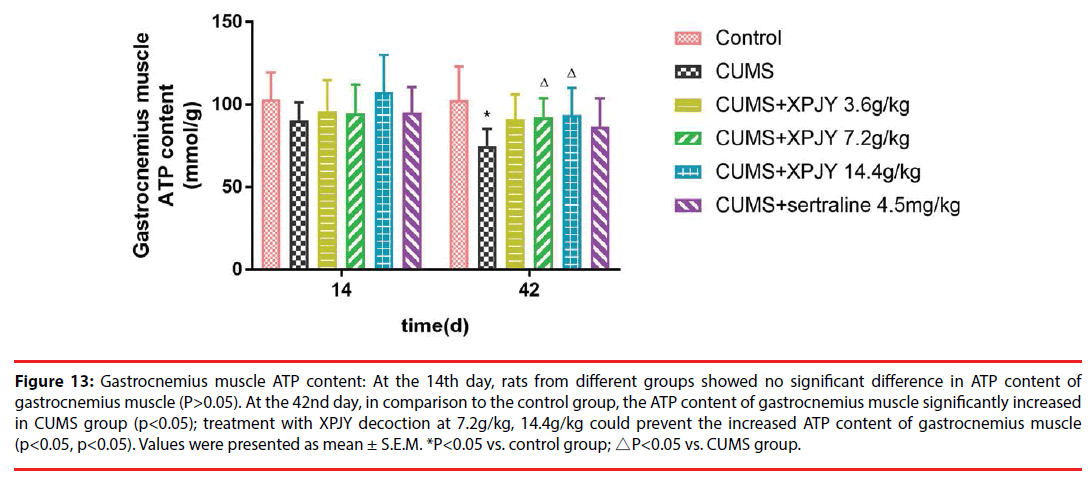
Figure 13: Gastrocnemius muscle ATP content: At the 14th day, rats from different groups showed no significant difference in ATP content of gastrocnemius muscle (P>0.05). At the 42nd day, in comparison to the control group, the ATP content of gastrocnemius muscle significantly increased in CUMS group (p<0.05); treatment with XPJY decoction at 7.2g/kg, 14.4g/kg could prevent the increased ATP content of gastrocnemius muscle (p<0.05, p<0.05). Values were presented as mean ± S.E.M. *P<0.05 vs. control group; △P<0.05 vs. CUMS group.
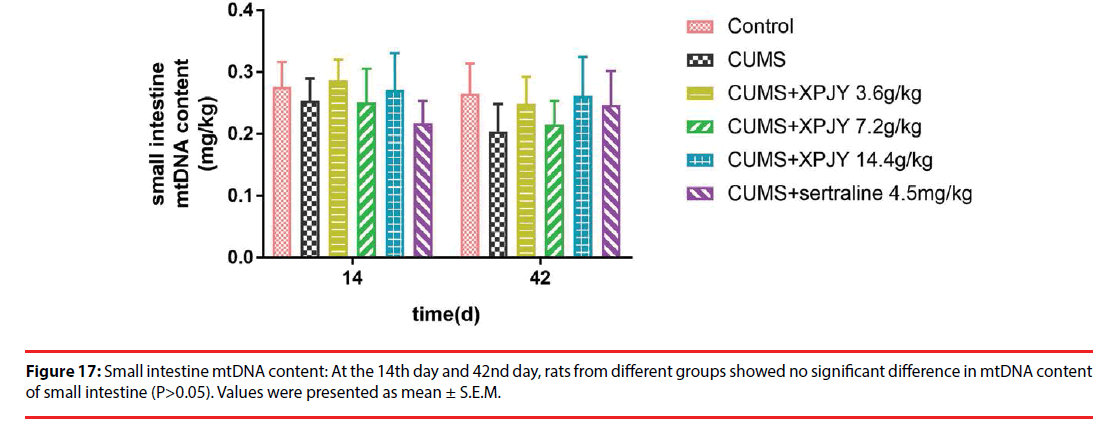
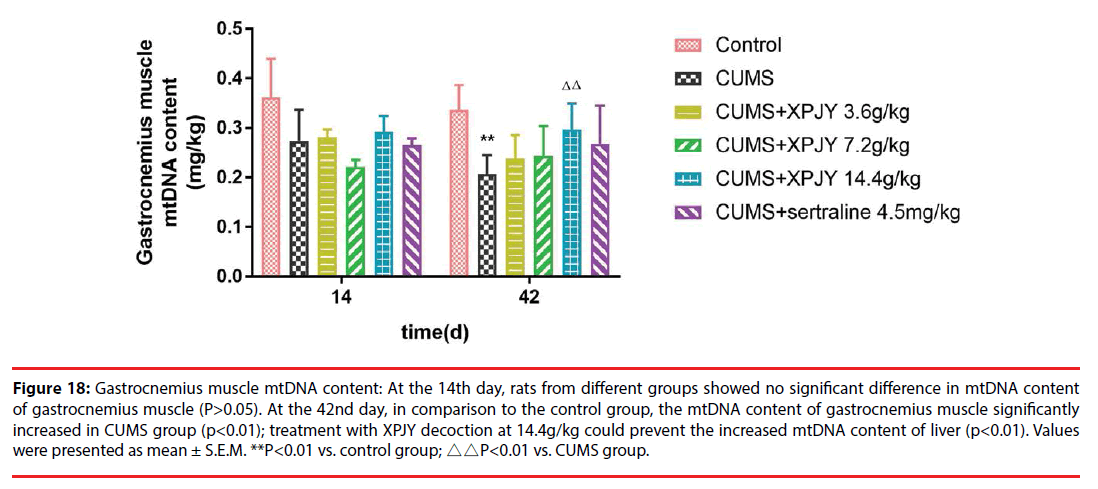
▪ Effects of XPJY on mtDNA content
At the 14th day, in comparison to the control group, the mtDNA content of PFC significantly decreased in CUMS group (p<0.05); compared with the CUMS group, treatment with XPJY decoction at 14.4g/kg could prevent the decreased mtDNA content of PFC (p<0.05). At the 42nd day, in comparison to the control group, the mtDNA content of hippocampus, PFC, liver and gastrocnemius muscle significantly decreased in CUMS group (p<0.01, p<0.05, p<0.01, p<0.01); compared with the CUMS group, treatment with XPJY decoction at 3.6g/kg could prevent the decreased mtDNA content of hippocampus and liver(p<0.05, p<0.05); treatment with XPJY decoction at 7.2g/kg could prevent the decreased mtDNA content of hippocampus (p<0.05); treatment with XPJY decoction at 14.4g/ kg could prevent the decreased mtDNA content of hippocampus, PFC, liver and gastrocnemius muscle (p<0.05, p<0.01, p<0.01, p<0.01); treatment with sertraline at 4.5mg/kg could prevent the decreased mtDNA content of hippocampus, PFC, liver (p<0.05, p<0.05, p<0.01) (Figure 14-18).
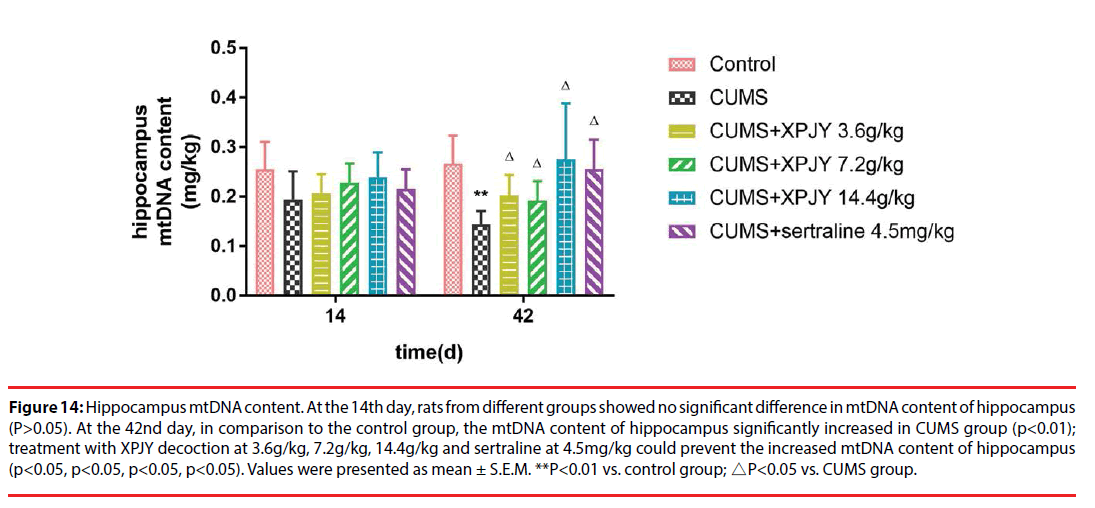
Figure 14: Hippocampus mtDNA content. At the 14th day, rats from different groups showed no significant difference in mtDNA content of hippocampus (P>0.05). At the 42nd day, in comparison to the control group, the mtDNA content of hippocampus significantly increased in CUMS group (p<0.01); treatment with XPJY decoction at 3.6g/kg, 7.2g/kg, 14.4g/kg and sertraline at 4.5mg/kg could prevent the increased mtDNA content of hippocampus (p<0.05, p<0.05, p<0.05, p<0.05). Values were presented as mean ± S.E.M. **P<0.01 vs. control group; △P<0.05 vs. CUMS group.
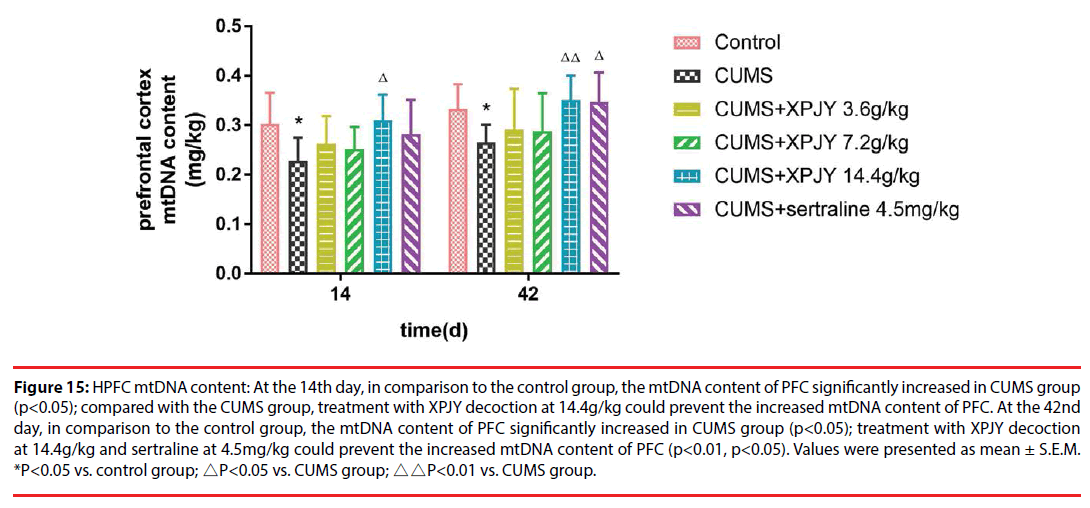
Figure 15: HPFC mtDNA content: At the 14th day, in comparison to the control group, the mtDNA content of PFC significantly increased in CUMS group (p<0.05); compared with the CUMS group, treatment with XPJY decoction at 14.4g/kg could prevent the increased mtDNA content of PFC. At the 42nd day, in comparison to the control group, the mtDNA content of PFC significantly increased in CUMS group (p<0.05); treatment with XPJY decoction at 14.4g/kg and sertraline at 4.5mg/kg could prevent the increased mtDNA content of PFC (p<0.01, p<0.05). Values were presented as mean ± S.E.M. *P<0.05 vs. control group; △P<0.05 vs. CUMS group; △△P<0.01 vs. CUMS group.
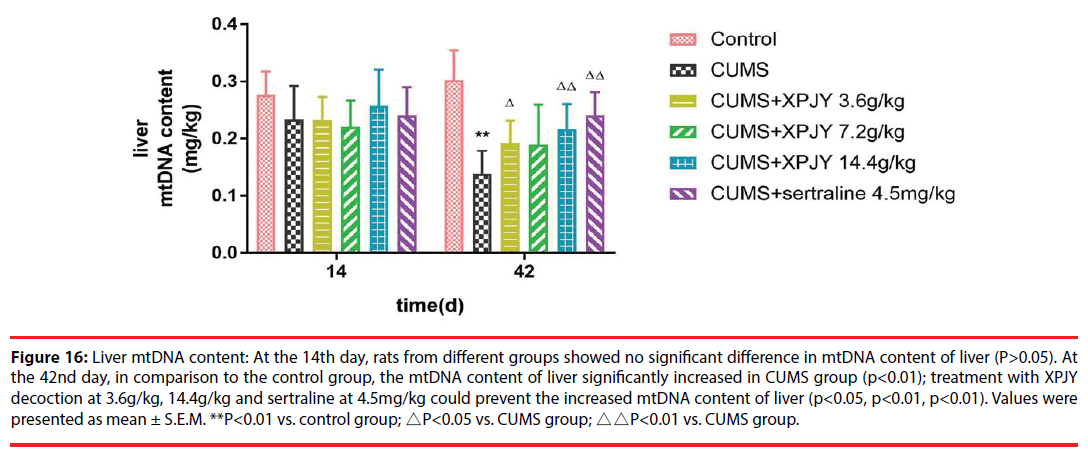
Figure 16: Liver mtDNA content: At the 14th day, rats from different groups showed no significant difference in mtDNA content of liver (P>0.05). At the 42nd day, in comparison to the control group, the mtDNA content of liver significantly increased in CUMS group (p<0.01); treatment with XPJY decoction at 3.6g/kg, 14.4g/kg and sertraline at 4.5mg/kg could prevent the increased mtDNA content of liver (p<0.05, p<0.01, p<0.01). Values were presented as mean ± S.E.M. **P<0.01 vs. control group; △P<0.05 vs. CUMS group; △△P<0.01 vs. CUMS group.
Figure 18: Gastrocnemius muscle mtDNA content: At the 14th day, rats from different groups showed no significant difference in mtDNA content of gastrocnemius muscle (P>0.05). At the 42nd day, in comparison to the control group, the mtDNA content of gastrocnemius muscle significantly increased in CUMS group (p<0.01); treatment with XPJY decoction at 14.4g/kg could prevent the increased mtDNA content of liver (p<0.01). Values were presented as mean ± S.E.M. **P<0.01 vs. control group; △△P<0.01 vs. CUMS group.
Discussion
In present study, rats subjected to two weeks of CUMS exhibited decreased sucrose preference ratio. Moreover, at 2 weeks of stress, MDA content in hippocampus, prefrontal cortex and gastrocnemius muscle increased and mtDNA content in cortex decreased. At 6 weeks of stress, MDA content in hippocampus, prefrontal cortex, liver and gastrocnemius muscle increased and mtDNA content decreased, and ATP content in hippocampus, prefrontal cortex, liver, gastrocnemius muscle and small intestine decreased, and serum CK content increased and SDH content decreased.Rats in group treatment with XPJY decoction at 14.4g/kg, the efficacy trend of which was better than the other drug groups, could decrease MDA content, increase mtDNA and ATP content of the above tissues. Meanwhile, it could also improve the abnormality of serum CK and SDH. However, sertraline had no significant antioxidant effect, but only increased mtDNA content in hippocampus, prefrontal cortex and liver at 6 weeks.
Stressors can lead to increased ROS and oxidative stress through various channels. Firstly, recent studies [28] have reported that stress reduces the expression of BDNF in the prefrontal cortex and hippocampus of rodents. BDNF has antioxidant activity, which can downregulate the oxidative stress and apoptosis of hypothalamic neurons induced by ethanol, the mechanism of its inhibition of oxidative stress may be through regulating the BDNF-TrkB pathway [29-31]. Secondly, the stress source transmits stimulus signals to the hypothalamus through sensory organs, and stimulates the hypothalamus-pituitary-adrenal (HPA) axis to destroy hippocampal neurons, which induces the release of stress hormone corticosterone. Corticosterone can mediate ROS production through the BDNF-TrkB pathway [32]. In addition, unpredictable repeated stimulation can activate the peripheral and central immune system of mice, up-regulate the expression of pro-inflammatory cytokines, and induce aseptic inflammatory response. Inflammatory cells can release a large number of ROS and aggravate oxidative stress [33]. Pressure-induced oxidative stress is mostly studied in hippocampus, cortex, amygdala and other brain tissues. This study found that MDA content in liver and gastrocnemius muscle increased under stress stimulation, indicating that oxidative stress caused by chronic stress involves other tissues outside the brain. This also explains depression from one aspect. The mechanism of gastrointestinal symptoms and muscle soreness symptoms in patients.
Mitochondria are the main source of ROS, which are produced mainly through OXPHOS pathway. Lower levels of ROS play a role in cell differentiation, tissue regeneration, redox biology and promoting adaptation to environmental changes. When premature leakage of electrons to oxygen occurs in the ETC, the production of highly active free radicals such as superoxide and peroxidase increased. Superoxide is the precursor of ROS, and the increase of its content increased the production of ROS and then produces oxidative stress [34]. ROS can cause mitochondrial damage and apoptosis in many ways, which can lead to depression. For example, ROS can induce lipid peroxidation damage in mitochondrial inner membrane and nerve cell membrane, which can lead to depression[35-36]. In addition, reactive oxygen species (ROS) can directly damage mitochondrial DNA[5]. Mitochondria are the first site of oxidative stress damage. Because of the lack of histone protection, antioxidant mechanism and repair mechanism, mtDNA is very vulnerable to oxidative stress damage due to its proximity to reactive oxygen species.It has been found that mtDNA content in anterior cingulate cortex, caudate nucleus, dorsolateral prefrontal cortex, hippocampus, orbitofrontal cortex and thalamus of patients with severe depression is absent in varying degrees. The possible mechanism is that oxidative stress leads to the increase of cellular oxygen free radical content and DNA oxidative damage, among which the frontal cortex is the main one missing area [37]. In addition, some scholars believe that oxidative stress-induced mtDNA damage may also be associated with telomere shortening in oligodendrocytes [38]. ROS can also mediate cell necrosis through endogenous and exogenous pathways, which are related to mitochondrial dysfunction and cell death receptor pathway, respectively. Caspases is a group of aspartate proteolytic enzymes with similar structure in the cytoplasm, which can cleave Asp-X peptide bonds in protein molecules. Its cascade reaction is the junction of multiple apoptotic pathways. ROS can induce caspase cascade reaction, leading to hippocampal neuronal apoptosis, meanwhile, caspase can also increase the level of ROS. Moreover, some scholars have found that the carbonyl content in the brain region of depressive rats is significantly increased. It is believed that oxidative stress leads to the impairment of gamma-aminobutyric acid transport function and then produces depressivelike behavior [39]. In conclusion, oxidative stress can induce cell apoptosis, depression-like behavior and peripheral symptoms by inducing lipid peroxidation damage of biofilm, mitochondrial or cellular DNA damage.
We seem to have got the answer to the questions before. CUMS can lead to oxidative stress and mitochondrial dysfunction in multiple tissues in and out of the brain. The occurrence of oxidative stress is earlier than mitochondrial damage. Although oxidative stress and mitochondrial dysfunction can cause and effect each other and form a vicious cycle, we have reason to believe that oxidative stress in various tissues of CUMS rats plays a more important role in inducing mitochondrial dysfunction. In different tissues, except hippocampus and cortex, gastrocnemius muscle showed earlier increase in MDA content relative to liver and small intestine, which may be due to its higher oxygen consumption. The symptoms of depression include mental disorders and somatic symptoms, such as depression, cognitive and memory impairment, muscle soreness and dyspepsia. The occurrence of these symptoms may be related to cell damage in hippocampus, cortex, liver, intestine, muscle and other tissues, while oxidative stress and mitochondria. Body dysfunction may be an important mechanism of its disease. Therefore, antioxidant therapy is an important link in the treatment of depression, and it is also the key to prevent the further development from chronic stress to mitochondrial damage and cell apoptosis deterioration.
However, there are different opinions about the role of sertraline in the protection of mitochondria and mitochondria. Some scholars found that sertraline treatment significantly induced ROS production in 24 hours, and ATP production in astrocytes treated with sertraline decreased, suggesting that sertraline caused mitochondrial damage [18]. In addition, studies have also shown that sertraline can reduce the content of ATP and mitochondrial respiratory complex in rat liver, induce MPT and uncoupling oxidative phosphorylation, suggesting that sertraline-related hepatotoxicity may be caused by mitochondrial dysfunction [16]. However, this study found that early application of sertraline could reduce liver MDA and increase the content of mtDNA in hippocampus, cortex, liver and ATP content in small intestine when the rats were given for 6 weeks. Sertraline, as SSRIs, could increase serotonin levels in the brain. Some studies have shown that serotonin does have antioxidant effects: 5-HT can increase the level of cAMP through the interaction between 5-HT receptor and G protein-mediated adenylate cyclase, and reduce the expression of cytokines by inhibiting protein kinase A (PKA) pathway to produce anti-inflammatory effect, thereby reducing ROS released by inflammatory cells [40]. In addition, 5-HT can block oxidative reactions in two ways. The hydroxyl radical diffused before free radicals interact with unsaturated lipids in the initial stage of peroxidation, or interfere with the proliferation process of unsaturated lipid chains. Therefore, sertraline may play an antioxidant role by increasing the content of 5-HT. Of course, it may also have other antioxidant mechanisms. The positive effect of sertraline on systemic oxidative stress and mitochondria in depression has been a controversial topic in academic circles at recent years. This study found that although sertraline had the tendency of antioxidant and mitochondrial protection in its early application, its main role was focused on mtDNA, and the regulation of MDA and ATP had a trend of improvement, but had no obvious effect. Apparently, sertraline is not a direct antioxidant.
XPJY decoction, a Chinese herbal formula, have been reported to have an antidepressant effect in rats exposed to chronic stress through improving depressive behavior, increasing serum 5-HT, decreasing serum corticosterone, reducing the inflammatory factors in serum and hippocampus, increasing the expression of cAMP, PKA, CREB and BDNF in hippocampus. This study found that, XPJY also has antioxidant and protective effects on mitochondria. Its regulation on mitochondria is achieved by protecting mtDNA directly. The antioxidant mechanism of XPJY prescription may be achieved through the multi-target effective mechanism of improving mitochondrial function, eliminating inflammation and increasing 5-HT content. Accordingly, in the treatment of depression, XPJY prescription can supplement the scope of SSRIs from the perspective of antioxidation and improving energy metabolism. The combination of Chinese and Western medicine is of great significance to improve the therapeutic effect of central and peripheral injury of depression.
Conclusions
CUMS could induce oxidative stress and mitochondrial dysfunction in hippocampus, PFC, liver, small intestine and gastrocnemius muscle in rats, early application of sertraline can effectively prevent mtDNA damage in hippocampus, PFC and liver, but its regulation on MDA and ATP is not obvious. The effect of XPJY decoction on antioxidant and mitochondrial function is more significant. We have sufficient reason to believe that oxidative stress-induced mitochondrial damage and apoptosis is one of the mechanisms of the occurrence of multiple systemic somatic symptoms in depression and an important link in the treatment of depression. In combination with previous studies [21-24], XPJY prescription has an antidepressant effect in rats exposed to CUMS through increasing serum 5-HT, decreasing serum corticosterone, reducing the inflammatory factors in serum and hippocampus, increasing the expression of cAMP, PKA, CREB and BDNF in hippocampus, regulating oxidative stress and mitochondrial function in multiple tissues. It is highly recommend to be combined with SSRIs in the treatment of patients with depression. Clinical experience has found that the Chinese medicine prescription has remarkable curative effect, and no adverse reactions occur when used alone or in combination. Relevant clinical trials are under way, and the results are expected to appear.
Conflict of Interest
The authors declare that they have no conflicts of interest to this work. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Abbreviations
CUMS: chronic unpredictable stress; XPJY: XingPiJieYu decoction; SPT: sucrose preference test; FST: forced swimming test; CK:creatine kinase; SDH:succinate dehydrogenase; MDA:malondialdehyde; ATP: adenosine triphosphate; mtDNA: mitochondrial DNA; PFC:prefrontal cortex; ETC: electron transport chain.
Acknowledgements
This research was supported by National Natural Science Foundation of China (No 81573843).
Ethics Approval and Consent to Participate
Procedures involving animals and their care were conducted in conformity with NIH guidelines (NIH Pub. No. 85-23, revised 1996) and was approved by Animal Care and Use Committee of Beijing University of Chinese Medicine.
Authors’ Contribution
Conceived and designed the experiments: Rongjuan Guo, Yang Li, Qingjie Yuan, Yao Yu.
Performed the experiments: Yang Li, Qingjie Yuan, Yao Yu, Zhonghui Zhao.
Analyzed the data: Yang Li, Qingjie Yuan, Rongjuan Guo.
Contributed reagents/materials/ analysis tools: Rongjuan Guo, Yang Li, Yao yu, Zhonghui Zhao.
Wrote the paper: Yang Li, Qingjie Yuan, Rongjuan Guo.
General ideas and conception: Rongjuan Guo, Yang Li, Qingjie Yuan.
References
- Simon GE, VonKorff M, Piccinelli M, et al. An international study of the relation between somatic symptoms and depression. N. Engl. J. Med 341(18), 1329-1335 (1999).
- Hong Jin Jeon, Jong-Min Woo, Hyo-Jin Kim, et al. Gender differences in somatic symptoms and current suicidal risk in outpatients with major depressive disorder. Psychiatry. Investig 13(6), 609-615 (2016).
- Ann Gardner, Richard G.Boles. Mitochondrial energy depletion in depression with somatization. Psychother. Psychosom 77(2), 127-129 (2008).
- Roberto Rodrigues, Robert B Petersen, George Perry. Parallels between major depressive disorder and alzheimer’s disease: role of oxidative stress and genetic vulnerability. Cell. Mol. Neurobiol 34(7), 925-949 (2014).
- Josh Allen, Raquel Romay-Tallon, Kyle J Brymer, et al. Kalynchuk. Mitochondria and mood: mitochondrial dysfunction as a key player in the manifestation of depression. Front. Neurosci 12(1), 1-13 (2018).
- Sakr HF, Abbas AM, Elsamanoudy AZ, et al. Effect of fluoxetine and resveratrol on testicular functions and oxidative stress in a rat model of chronic mild stress-induced depression. J. Physiology. Pharmacology 66(4), 515-527 (2015).
- X R Li, Wang T, Qin R. et al. Apigenin ameliorates chronic mild stress-induced depressive behavior by inhibiting interleukin-1β production and NLRP3 inflammasome activation in the rat brain. Behav. Brain. Res 296(1), 318-325 (2016).
- Jindal A, Mahesh R, Bhatt S. Etazolate: a phosphodiesterase 4 inhibitor reverses chronic unpredictable mild stressinduced depression-like behavior and brain oxidative damage. Pharmacol. Biochem. Behav 105(1), 63-70 (2013).
- Filho CB, Jesse CR, Donato F, et al. Chronic unpredictable mild stress decreases BDNF and NGF levels and Na+, K+-ATPase activity in the hippocampus and prefrontal cortex of mice: antidepressant effect of chrysin. Neurosci 289(1), 367-380 (2015).
- Bhatt S, Radhakrishnan M, Jindal A, et al. Neuropharmacological evaluation of a novel 5-HT3 receptor antagonist (6g) on chronic unpredictable mild stressinduced changes in behavioural and brain oxidative stress parameters in mice. Indian. J. Pharmacol 46(2), 191-196 (2014).
- Biala G, Pekala K, Boguszewska-Czubara A, et al. Behavioral and biochemical interaction between nicotine and chronic unpredictable mild stress in mice. Mol. Neurobiol 54(2), 904-921 (2016).
- Umukoro S, Aluko OM, Eduviere AT, et al. Evaluation of adaptogenic-like property of methyl jasmonate in mice exposed to unpredictable chronic mild stress. Brain. Res. Bull 121(1), 105-114 (2016).
- Kotan VO, Sarandol E, Kirhan E, et al. Effects of long-term antidepressant treatment on oxidative status in major depressive disorder: A 24-week follow-up study. Prog. Neuropsychopharmacol. Biol. Psychiatry 35(5), 1284-1290 (2011).
- Moretti A, Gorini A, Villa RF. Affective disorders, antidepressant drugs and brain metabolism. Mol. Psychiatry 8(9), 773-785 (2003).
- Fattal O, Budur K, Vaughan AJ, et al. Review of the literature on major mental disorders in adult patients with mitochondrial diseases. Psychosomatics 47(1), 1-7 (2006).
- Yan L, Letha C, Masahiro H, et al. Mitochondrial dysfunction induced by sertraline, an antidepressant agent. Toxicol. Sci 127(2), 582-591 (2012).
- Curti C, Mingatto FE, Polizello AC, et al. Fluoxetine interacts with the lipid bilayer of the inner membrane in isolated rat brain mitochondria, inhibiting electron transport and F1F0-ATPase activity. Mol. Cell. Biochem 199(1-2), 103-9 (1999).
- Chee-Kin T, Kao-Hui L, Ming-Hsuan L, et al. Antidepressants, sertraline and paroxetine, increase calcium influx and induce mitochondrial damage-mediated apoptosis of astrocytes. Oncotarget 8(70), 115490-115502 (2017).
- Cheng-Chen C, Shaw-Hwa J, Ta-Tsung L, et al. Mitochondria DNA change and oxidative damage in clinically stable patients with major depressive disorder. Plosone 10(5), e0125855 (2015).
- de Oliveira MR. Fluoxetine and the mitochondria: A review of the toxicological aspects. Toxicol. Lett 258(1), 185-191 (2016).
- Wang C, Guo J, Guo R. Effect of XingPiJieYu decoction on spatial learning and memory and cAMP-PKA-CREB-BDNF pathway in rat model of depression through chronic unpredictable stress. BMC. Comple. Altern. Med 17(1), 73 (2017).
- Wang C, Guo R, Zhu X. Effect of Xingpi Jieyu Decoction on learning-memory behavior and inflammatory factors level in depression rats. Beijing J. Trad. Chin. Med 07(1), 503-506 (2014).
- Wang C, Guo R. The Effect of Xingpijieyu decoction on depressive behavior and serum 5-HT as well as corticosterone of depression rats from chronic stress. World Chin. Med 2014(12), 1633-1639 (2014).
- Yuan Q, Guo J, Wang J, et al. Study on syndrome of stagnation of liver qi and spleen deficiency in depression based on corticosterone-inflammation-mitochondrial network and effects of Xingpi Jieyu Decoction. J.Trad. Chin. Med. Pharmacy 05(1), 2241-2245 (2017).
- Jiang P, Zhang WY, Li HD, et al. Stress and vitamin D: altered vitamin D metabolism in both the hippocampus and myocardium of chronic unpredictable mild stress exposed rats. Psychoneuroendocrinology 38(10), 2091-2098 (2013).
- Roger D Porsolt, Le Pichon M, Jalfre M. Depression: A new animal model sensitive to anti-depressant treatment. Nature 266(1), 730-732 (1977).
- Tomoyuki Tashiro, Yuki Murakami, Akihiro Mouri, et al. Kynurenine 3-monooxygenase is implicated in antidepressants-responsive depressive-like behaviors and monoaminergic dysfunctions. Behav. Brain. Res 317(1), 279-285 (2017).
- LT Yi, Li J, Liu BB, et al. BDNFERK-CREB signalling mediates the role of miR-132 in the regulation of the effects of oleanolic acid in male mice. J. Psychiatry. Neurosci 39(5), 348-359 (2014).
- CL Wu, Chen SD, Yin JH, et al. Nuclear Factor-KappaB-Dependent sestrin2 induction mediates the antioxidant effects of BDNF against Mitochondrial Inhibition in Rat cortical neurons. Mol. Neurobiology 53(6), 4126-4142 (2016).
- Boyadjieva NI, Sarkar DK. Cyclic adenosine monophosphate and brain-derived neurotrophic factor decreased oxidative stress and apoptosis in developing hypothalamic neuronal cells: role of microglia. Alcohol. Clin. Exp. Res 37(8), 1370-1379 (2013).
- Min Hu, Wei Z, Chun-Yan W, et al. Hydrogen sulfide protects against chronic unpredictable mild stress-induced oxidative stress in hippocampus by upregulation of BDNF-TrkB pathway. Oxidat. Med. Cellular. Long 225(1), 2153745 (2016).
- Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr. Rev 7(3), 284-301 (1986).
- Fleshner M. Stress-evoked sterile inflammation, danger associated molecular patterns (DAMPs), microbial associated molecular patterns (MAMPs) and the inflammasome. Brain. Behav. Immun 27(1), 1-7 (2013).
- Vakifahmetoglu Norberg H, Ouchida AT, Norberg E. The role of mitochondria in metabolism and cell death. Biochem. Biophys. Res. Commun 482(3), 426-431 (2017).
- Che Y, Zhou Z, Shu Y, et al. Chronic unpredictable stress impairs endogenous antioxidant defense in rat brain. Neurosci. Lett 584(1), 208-213 (2015).
- Islam MT. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res 39(1), 73-82 (2017).
- Mamdani F, Rollins B, Morgan L, et al. The somatic common deletion in mitochondrial DNA is decreased in schizophrenia. Schizophrenia. Res 159(2), 370-375 (2014).
- Szebeni A, Szebeni K, Di Peri T, et al. Shortened telomere length in white matter oligodendrocytes in major depression: potential role of oxidative stress. Int. J. Neuropsychopharmacology 17(10), 1579-1589 (2014).
- Wayhs CAY, Mescka CP, Guerreiro G, et al. Diabetic encephalopathy-related depression: experimental evidence that insulin and clonazepam restore antioxidant status in rat brain. Cell. Biochem. Fun 32(8), 711-719 (2014).
- Paula Kopschina F, Janine D, Hans CK, et al. Anti-inflammatory treatment for major depressive disorder: implications for patients with an elevated immune profile and non-responders to standard antidepressant therapy. J. Psychopharmacol 31(9), 1149-1165 (2017).