Review Article - Clinical Practice (2021) Volume 18, Issue 6
Remastered vs. brand new techniques in the treatment of the calcified coronary disease
- Corresponding Author:
- Carlos Salazar
Interventional cardiology unit
Hospital Universitario Mayor Mederi, Colombia
E-mail: chsalazart@gmail.com
Abstract
Calcified coronary artery disease is one of the subtypes of the complex scenario that occurs daily in coronary intervention, therefore it is necessary to know new techniques to achieve an optimal result. A review of new debulking techniques and the importance of the use of intracoronary imaging.
Keywords
calcific coronary disease, rotational atherectomy, orbital atherectomy, intravascular lithotripsy, laser coronary atherectomy
Introduction
The prevalence of Calcific Coronary Disease (CCD) in the population that is done PCI is 32%, corresponding to 5.9% severe CCD [1]. Fibrocalcific deposits increase the stenosis rigidity, making the stent implantation more complex. Risk factors such as chronic inflammatory conditions that lead to calcium deposits in the coronary arteries are the following: smoking, sedentary lifestyle, obesity, old age, family history, dyslipidemia, hypertension, diabetes, metabolic syndrome, and chronic kidney disease. These conditions cause endothelial injury and subsequent cellular dysfunction, escalating in an inflammatory response from leukocytes and vascular smooth muscle cells which generates calcium deposits in the intima and media of the coronary vascular wall and predisposing to future atherothrombotic events [1-3].
Definition
CCD is an increase in mineral content, which progressively becomes radio-opaque, such as bone, and appears when performing fluoroscopy, performing an angiographic qualitative evaluation. The angiographic criteria are moderate calcification is defined as an evident density during the cardiac cycle before contrast injection; nevertheless, severe calcification is the evidence of radio-opacity independent of the cardiac cycle, before contrast injection and involving both sides of the arterial wall [3,4]. Moderate or severe CCD is defined as a type B lesion by AHA/ACC, historically a success probability between 60% to 85% and a moderate risk of complications of the procedure are expected; has been independently associated with increased Major Adverse Cardiovascular Events (MACE) and long-term rates of In- Stent Restenosis (ISR), stent thrombosis, Target Lesion Revascularization (TLR), Myocardial Infarction (MI), and death. Currently, if the operator establishes an adequate approach to treat the calcific stenosis, it would have a high probability of success, exceeding 99% in the DES era [3].
The technical failure in the inability to advance the stent occurs more frequently in patients with CCD than those who are not (8.2% vs. 1.8%, respectively) [5]. The possible consequences of the CCD are the following:
• PCI failure due to non-dilatable stenosis
• balloon dysfunction or burst
• iatrogenic coronary dissection
• coronary perforation
• stent under expansion
• high incidence of MACE, instant restenosis, periprocedural MI, stent thrombosis and TLR [1,2]
At present, some of the drug-eluting stents have a polymer coating that facilitates delivery of the neointimal inhibition medication, but this can sometimes present structural damage when attempting to implant the device in severe calcified stenosis. When presenting polymer damage this can facilitate the alteration in the coating of the struts of the stent and increasing the risk of stent thrombosis. In this situation, it is important to do a plaque preparation technique to avoid unfavourable cardiovascular outcomes.
Intravascular imaging in CCD
Fluoroscopy is quite specific, but not sensitive for the detection of CCD, it only detects 38% of the CCD; in the case of performing intracoronary imaging, the detection capacity for example with IVUS is increased up to 73% [6]. Detection of calcified stenosis with imaging is detected in 83% of lesions by IVUS (40MHz), 77% by OCT, and 40% by angiography. The calcium with IVUS appears as shading in the deep structures of the artery with an acoustic signature that is brighter than the reference adventitia. In the cross-section, it allows a description of the degrees of commitment defined as the calcified arch, its distance, and distribution, including discrimination of deep versus superficial calcium. The severity defined by IVUS corresponds to a large arc of calcified surface involving three quadrants. On the other hand, the calcium with OCT is defined as a well-delineated signal with a poor signal region and smoothly defined edges. It allows the measurement of the size and depth of calcium, although it can be attenuated by an underlying lipid plaque that can attenuate light and compromise the accuracy of its measurement [3,7].
Imaging-based calcium score has been developed and validated; OCT score 2 points for maximum calcium angle >180, 1 point for maximum calcium thickness >0.5 mm, and 1 point for calcium length >5 mm, for a total calcium score of 0-4 points. Lesions with score 0 the stent expansion was 99% (IQR 93-108), score 1 was 85% (IQR 78-93), score 2 was 86% (IQR 77- 100), score 3 was 80% (IQR 73-85), and score 4 was 78% (IQR 70-86); p<0.01. IVUS score, with 1 point for circumferential calcium=360, 1 point for calcium arc >270º that is >5 mm in length, 1 point for vessel size (media-to-media) ≤ 3.5 mm adjacent to the maximum calcium, and 1 point for protruding calcific nodule [8]. Consequently, OCT-based calcium score and IVUS-based calcium score of ≥ 3 may indicate the need for calcium modification technique to bring a successful result of the PCI with adequate stent expansion TABLE 1 [8-10].
| Imaging-based calcium score | |||
|---|---|---|---|
| OCT Score | IVUS Score | ||
| Calcium thickness | ≤ 0.5 mm=0 point | Circumferential Calcium | <360º=0 point |
| >0.5 mm=1 point | 360º=1 point | ||
| Calcium Arc | ≤ 90º=0 point | Length of Calcium >270º | ≤ 5 mm=0 point |
| 90º -180º=1 point | >5 mm=1 point | ||
| >180º=2 points | Diameter | >3.5 mm=0 point | |
| Calcium Length | ≤ 5 mm=0 point | ≤ 3.5 mm=1 point | |
| >5 mm=1 point | Calcified Nodule | Absent=0 point | |
| Present=1 point | |||
TABLE 1. Imaging-based calcium score to choose the need for a debulking technique to treat calcific stenosis [8-10].
Advance plaque modification techniques in CCD
Given the systemic nature of atherosclerosis and the late development of calcification with a severe condition, the natural history of calcified disease will correspond to a complex multivessel coronary disease. Accordingly, patients with severe calcified disease should always be discussed in a heart team to evaluate the risk as to the benefit of the different intervention options (PCI vs. CABG), and according to the selected method should discuss the best therapeutic strategy in an individualized way [3]. To be successful in treating calcified coronary lesions, an adequate therapeutic strategy must be carried out, to be able to adequately prepare the lesion and also achieve the proper stent implantation; At the moment several strategies and novel devices have been arranged, such as the use of specialized balloons and the different kinds of atherectomy (rotational, laser and orbital) [1].
Currently, clinical recommendation guidelines place rotational atherectomy as a therapeutic possibility for calcific or fibrotic non-dilatable lesions with conventional PCI techniques (IIaC) [1].
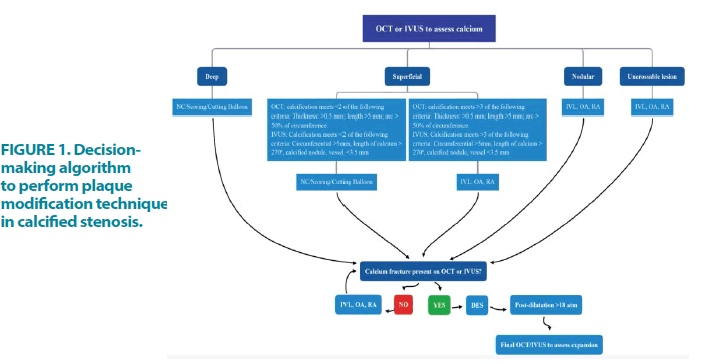
An image-guided intervention algorithm has been used in PCI of calcified coronary disease, recommended by several authors to guide intervention in this complex scenario and reduce unfavourable outcomes FIGURE 1 [11,12].
Rotational atherectomy
The standard debulking technique to perform plaque preparation is rotational atherectomy that produces an enlargement of the lumen by physical removal of the plaque and reduction of its rigidity, facilitating its stent expansion. Commercially available as a Rotablator© and recently introduced RotaPro© (Boston Scientific, Natick, Massachusetts), invented by Auth and described by Ritchie and colleagues, it ablates the plaque using an elliptical head with embedded diamonds, which rotates at a high speed (140,000 rpm to 180,000 rpm) for a helical transmission, progresses gradually through the lesion on a rota wire. The recommended burr size/artery ratio is 0.5-0.7 and preferentially ablates hard and inelastic material, as is calcified plaque. Its high rotational speed facilitates the longitudinal movement of the head through the lesions calcified by an orthogonal displacement of friction. The guidewire helps maintain its abrasive tip coaxially with the lumen of the vessel, although this may not happen in very tortuous or angulated segments predisposing to dissection or perforation [4,5]. Reaches a smooth luminal surface with cylindrical geometry and minimum tissue damage. The increase in vessel lumen is usually greater than that of the burr used [4]. The minimal access required ranges from 6 Fr for up to 1.75 mm burrs, 7 Fr for 2.0 mm burrs, and 8 Fr for 2.15 mm burrs and above.
The rotational atherectomy prepares the plaque facilitating the delivery of interventional devices, favouring the proper stent implantation; this technique is not used frequently because not all operators are familiar with the procedure, its high cost, the high degree of complexity for its implementation, and the lack of evidence about its net clinical benefit [2].
The prospective, randomized ROTAXUS (Rotational Atherectomy before Taxus Stent Treatment for Complex Native Coronary Artery Disease) trial compared a strategy of using RA for lesion preparation with balloon pre dilatation alone before paclitaxel-eluting stent implantation in angiographically moderate-to severely calcified lesions. Among 240 patients, procedural success was higher with RA than with balloon pre-dilatation (92.5% versus 83.3%, p=0.03). Despite greater acute lumen gain with RA, there was higher late lumen loss with RA at 9 months. Rates of restenosis, TLR, definite stent thrombosis, and MACE were not significantly different between the groups at 1-year TABLE 2 and TABLE 3.
| Debulking techniques | ||||
|---|---|---|---|---|
| Rotational atherectomy | Orbital atherectomy | Intravascular lithotripsy | Excimer laser coronary atherectomy | |
| Mechanism of action | High speed concentric rotation of diamond-tipped burr | Elliptical rotation of diamond-coated eccentric crown. | Energy flux density to the calcified stenosis | photochemical, photothermal and photomechanical |
| Device size | 1.25 mm to 2.5 mm | 1.25 mm | 2.5 to 4.0 × 12 mm | 0.9 mm to 2.0 mm |
| Guide catheter | 5 Fr to 8 Fr (Depends on burr size) | 6 Fr | 6 Fr | 6 Fr to 8 Fr (It depends on the size) |
| Device-vessel ratio | 0.4-0.6 | Single size | 1 | 0.5-0.6 |
| Guidewire | Rotawire floppy and extra Support | ViperWire advance and Advance with Flex Tip | Standard coronary guidewire | Standard coronary guidewire |
| Particle size | 5 µm-10 µm | 2 µm | - | <10 µm |
TABLE 2. Comparison between different techniques for stenosis with moderate to severe calcification [11,12].
| Trials of the different plaque preparation techniques for a moderated to severe calcified stenosis | |||||||
|---|---|---|---|---|---|---|---|
| Technique | Clinical study | Year | Number or patients | Procedural success (%) | Acute gain (mm) | MACE (>30 days, (%)) | TLR (%) |
| Rotational atherectomy | Rathore et al | 2010 | 391 | 96.9 | 14.4 (6-9 months) | ||
| ROTAXUS | 2013 | 120 (each arm) | 92.5 | 1.56 ± 0.43 | 24.2 (9 months) | 11.7 (9 months) | |
| Kawamoto, et al. | 2016 | 985 | 99.1 | 2.29 ± 0.64 | 24.9 (2 years) | 16.6 (2 years) | |
| PREPARE.CALC | 2018 | 100 (each arm) | 98 | 1.70 ± 0.42 | 7.0 (9 months) | ||
| Okamoto, et al. | 2018 | 965 | 21.7 (1 year) | 5.8 (1 year) | |||
| Bouisset, et al. | 2020 | 966 | 13.2 (1 year) | 2.4 (1 year) | |||
| Orbital atherectomy | ORBIT I | 2013 | 50 | 94 | 21.2 (5 years) | 3.0 (5 years) | |
| ORBIT II | 2014 | 443 | 88.9 | 23.5 (3 years) | 7.8 (3 years) | ||
| Lee, et al. | 2016 | 458 | |||||
| Okamoto, et al. | 2018 | 184 | 16.3 (1 year) | 1.63 (1 year) | |||
| COAST | 2020 | 100 | 85 | 22.2 (1 year) | 6.3 (1 year) | ||
| ELCA | ERBAC | 1997 | 232 | 77.2 | 1.27 ± 0.45 | 50.3 (1 year) | |
| Bilodeau, et al. | 2004 | 95 | 93 | 47.2 (1 year) | |||
| Badr, et al. | 2013 | 25 | 80 | ||||
| LEONARDO | 2015 | 80 | 91.7 | ||||
| IVL | Disrupt CAD I | 2019 | 60 | 98.3 | 1.7 | 8.3 (6 months) | |
| Disrupt CAD II | 2019 | 120 | 1.63 ± 0.49 | ||||
| Disrupt CAD III | 2020 | 431 | 92.4 | 1.41 ± 0.48 | |||
TABLE 3. Comparison of the different trials with distinct plaque preparation techniques for moderate to severe calcific stenosis [11,12].
Treating non-dilatable stenosis due to CCD with rotational atherectomy can lead to intra-procedural complications. To perform this technique, first, it is necessary to know how to avoid them and be prepared for their corresponding treatment. They are the following: the atheromatous detritus embolism, its therapeutic strategy is to use a small burr, ablate tissue intermittently, and avoid significant decelerations. Another mechanism is platelet activation, aggregation, and its corresponding lysis, treating with optimal antiplatelet therapy, including the initiation of glycoprotein IIb/IIIa inhibitors. Microcirculatory vasospasm may occur, and the use of intracoronary vasodilators is recommended. Bradycardia can occur by neuro-humoral reflex, which is necessary to administer intravenous atropine and even require the use of a transient pacemaker, especially if the stenosis is in the dominant right coronary artery. Another event is intra-procedural hypotension, which requires the initiation of vasopressors, particularly phenylephrine, or the use of mechanical support [4]. In the COAPPCI study comparing rotational atherectomy versus orbital atherectomy, the highest rate of periprocedural MI was found in the RA group, being 13.8% vs. 6.7% p<0.01 [13] TABLE 2 and TABLE 3.
Orbital atherectomy
The Diamondback 360 Coronary OA System (Cardiovascular Systems, Inc., St. Paul, Minnesota) is a newer atherectomy device that uses centrifugal force to modify calcified lesions. The OA system uses a 1.25 mm eccentrically mounted diamond-coated crown connected to a drive shaft and to a controller powered by a pneumatic console that allows for bidirectional modification of calcium at 80,000 rpm or 120,000 rpm. By increasing its elliptical orbit as rotational speed increases, OA allows ablation of calcium using the same device in vessels up to 3.5 mm diameter. Diamond coating of the entire crown makes OA burr entrapment less likely compared with RA. The dedicated 0.014 in Viper Wire for OA is more steerable. In the single-arm ORBIT II study (Evaluate the Safety and Efficacy of OAS in Treating Severely Calcified Coronary Lesions), treatment of de novo severely calcified lesions with this “Classic Crown” system resulted in a low rate of procedural and 1-year target vessel revascularization (5.9%), cardiac death (3.0%), and peri-procedural MI (2%) [14,15]. A new “Micro Crown” OA system has recently been designed in which the diamond-coated crown is similarly 1.25 mm in size, but a diamond-coated distal tip of the shaft allows the Micro Crown to traverse 0.5 mm diameter channels more easily, a 60% reduction in the minimum lesion size the device can treat. The device is also able to produce an orbit like that of the Classic Crown but at lower speeds (low/high speeds=50/80 vs. 80/120,000 rpm) to reduce thermal injury. In the COAST (Coronary Orbital Atherectomy System Study) study, this Micro Crown had a similar procedural success rate compared with the Classic Crown (85.0% vs. 88.9% in ORBIT II, p=0.30) and freedom from MACE at 30 days (85.0% versus 89.6%, p=0.21) [14,15] TABLE 2 and TABLE 3.
Intravascular lithotripsy
The use of the Intravascular Lithotripsy (IVL) balloon in the coronary artery has recently been described for the modification of severely calcified plaques. IVL is a technique based on lithotripsy, a therapeutic strategy used by urology for the treatment of kidney stones. The IVL has integrated lithotripters in the balloon, these produce a shock generation and focusing on the interrogation site. This is defined as energy-flux density that meaning a propagating acoustic wave carries energy. But this kind of acoustic wave needs a transmission medium to generate an effect on the calcified stenoses. For this reason, the balloon preparation with saline solution and contrast (1:1 relation) is essential. Once the balloon is prepared to generate an energy flux density to the calcified stenosis, the mechanisms that affect the area to treat are:
• spall fracture
• shear stress
• super focusing
• squeezing
• cavitation and
• fatigue [16]
For this explanation, the calcified vessel wall requires complete vessel occlusion during a relatively long period due to the necessity of energy transmission. The balloon catheter is connected using a cable to a generator, which is pre-programmed to deliver a specific dose of pulses per treatment [2]. The procedure can be performed either femoral or radial, according to the operator’s preference; the dose of anticoagulation, double antiplatelet therapy, and any other pharmacological strategy is administered according to the local protocol of each institution. The size of the IVL balloon should always be chosen 1:1 concerning the reference of the artery, when placing the balloon in the segment to be treated, it is inflated initially to 4 ATM, that’s when the 10 pulses start to be delivered, each cycle has 10 pulses, and it can be performed for each balloon up to 8 cycles. After the cycle of 10 pulses, the balloon is inflated to 6 ATM inflating it to the vessel size reference. The procedure can be repeated, to perform a minimum of 20 pulses in the target lesion, always maintaining a deflation interval that allows distal perfusion. If the lesion exceeds 12 mm in length corresponding to the balloon, it can be repositioned and the IVL repeated. The repositioning of the balloon occurs in most calcified stenosis [2] TABLE 2 and TABLE 3.
The Disrupt CAD I study was a single-arm, non-randomized, and multicenter study that showed IVL was feasible in all patients (n=60) and facilitated the delivery of stents to all target lesions. The average stenosis was reduced to 12% with an acute diameter gain of 1.7 mm, thus achieving 95% clinical success (residual diameter stenosis <50% without in-hospital MACE). The procedure was safe, with no unresolved dissections, slow flow/no-reflow, embolization, or perforations [17]. In the Disrupt CAD II study, the IVL catheter was successfully delivered to all target lesions and IVL was performed in all patients (n=120). The primary safety endpoint occurred in 5.8% of patients, consisting of 7 non-Q-wave MIs.
There were no incidences of abrupt closure, slow flow/no-reflow, or perforation [18]. In the single-arm Disrupt CAD III study (n=431 patients) the primary safety endpoint of 30-day freedom from MACE was 92.2%; the lower bound of the 95% confidence interval was 89.5%, which exceeded the performance goal of 84.4% (p<0.0001) [19].
Excimer Laser Coronary Atherectomy
Excimer lasers are pulsed gas lasers that use a mixture of a rare gas and halogen as an active medium to generate pulses of short wavelength, high-energy Ultraviolet (UV) light. Excimer Laser Coronary Atherectomy (ELCA) is mediated through 3 distinct mechanisms: photochemical (breakdown of carbon-carbon bonds), photothermal (elevation of the temperature of intracellular water and generation of vapor bubbles at the catheter tip), and photomechanical (expansion and implosion of vapor bubbles disrupting the target lesion). Emits beams in a forward direction and can transmit to deep tissues. Therefore, often this technology is used with stent under-expansion to penetrate plaque behind the stent. Coronary catheters are available in 4 diameters, 0.9 mm (6F-compatible), 1.4 mm and 1.7 mm (7F-compatible), and 2.0 mm (8F-compatible). The recommended catheter size is based on a catheter/vessel diameter ratio of 0.5-0.6 [20-22].
ELCA has several advantages. First, it modifies plaque regardless of stent struts, thereby facilitating balloon expansion, making ELCA particularly beneficial in instant restenosis with stent under expansion. Second, it can be delivered over a standard 0.014-in. coronary interventional guidewire. Third, the laser beam exits forward through the tip of the catheter, supporting the use of ELCA in uncrossable lesions including chronic total occlusions, as it softens plaque and enables balloon advancement. Fourth, ELCA can vaporize thrombi, expanding its use in acute coronary syndromes [20-22].
Early studies reported procedural success rates of 77%-90% with ELCA, complication rates of 4%-7%, restenosis rates in 46%, perforations in 1%-2%, and in-hospital mortality of 0.5%- 1.5%, with no clear benefit over conventional angioplasty TABLE 2 and TABLE 3.
Conclusions
Moderate to severe calcific coronary disease is a complex scenario when the interventionist faces this condition. It occurs in various scenarios such as acute coronary syndrome, in-stent restenosis, bifurcations, left main disease, chronic total occlusions, among others. Given this, the cath lab toolbox should have at least one atherectomy technique in each room. Additionally, it is not only to have an atherectomy technique but to rely on the use of intracoronary imaging both IVUS and OCT to guide the intervention and reduce the unfavourable outcomes that occur daily in this type of disease. At the time of the selection of each described technique, more should be based on the experience of the operator to reduce complications associated with these.
References
- Chambers JW, Behrens AN, Martinsen BJ. Atherectomy devices for the treatment of calcified coronary lesions. Interv Cardiol Clin. 5, 143-151 (2016).
- Ali ZA, Brinton TJ, Hill JM, et al. Optical coherence tomography characterization of coronary lithoplasty for treatment of calcified lesions: First description. JACC Cardiovasc Imaging. 10, 897-906 (2017).
- Tomey MI, Sharma SK. Interventional options for coronary artery calcification. Curr Cardiol Rep. 18, 12 (2016).
- Tomey MI, Kini AS, Sharma SK. Current status of rotational atherectomy. JACC Cardiovasc Interv. 7, 345-353 (2014).
- Baber U, Kini AS, Sharma SK. Stenting of complex lesions: an overview. Nat Rev Cardiol. 7, 485-496 (2010).
- Lee MS, Gordin JS, Stone GW, et al. Orbital and rotational atherectomy during percutaneous coronary intervention for coronary artery calcification. Catheter Cardiovasc Interv. 92, 61-67 (2018).
- Fiorilli PN, Anwaruddin S. How do we treat complex calcified coronary artery disease? Curr Treat Options Cardiovasc Med. 18, 72 (2016).
- Zhang M, Matsumura M, Usui E, et al. TCT-51 IVUS Predictors of stent expansion in severely calcified lesions. J American Colle Cardio. 74, B51 (2019).
- Ali ZA, Galougahi KK. Shining light on calcified lesions, plaque stabilisation and physiologic significance: new insights from intracoronary OCT. EuroIntervention. 13, 2105-2108 (2018).
- Fujino A, Mintz G, Matsumura M, et al. TCT-28 A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. J American Colle Cardio. 70, B12-B13 (2017).
- Galougahi KK, Shlofmitz E, Jeremias A, et al. Therapeutic approach to calcified coronary lesions: disruptive technologies. Curr Cardiol Rep. 23, 33 (2021).
- Rozenbaum Z, Takahashi T, Kobayashi Y, et al. Contemporary technologies to modify calcified plaque in coronary artery disease. Progress Cardio Dis. (2021).
- Meraj PM, Shlofmitz E, Kaplan B, et al. Clinical outcomes of atherectomy prior to percutaneous coronary intervention: A comparison of outcomes following rotational versus orbital atherectomy (COAP-PCI study). J Interv Cardiol. 31, 478-485 (2018).
- Redfors B, Sharma SK, Saito S, et al. Novel micro crown orbital atherectomy for severe lesion calcification: Coronary Orbital Atherectomy System Study (COAST). Circ Cardiovasc Interv. 13, e008993 (2020).
- Genereux P, Lee AC, Kim CY, et al. Orbital atherectomy for treating de novo severely calcified coronary narrowing (1-Year Results from the Pivotal ORBIT II Trial). Am J Cardiol. 115, 1685-1690 (2015).
- Cleveland RO, McAteer JA. Physics of shock-wave lithotripsy. Smith’s Textbook of Endourology. 1, 527-558 (2012).
- Brinton TJ, Ali ZA, Hill JM, et al. Feasibility of shockwave coronary intravascular lithotripsy for the treatment of calcified coronary stenoses. Circulation. 139, 834-836 (2019).
- Ali ZA, Nef H, Escaned J, et al. Safety and effectiveness of coronary intravascular lithotripsy for treatment of severely calcified coronary stenoses. Circ Cardiovasc Interv. 12, 1-10 (2019).
- Hill JM, Kereiakes DJ, Shlofmitz RA, et al. Intravascular lithotripsy for treatment of severely calcified coronary artery disease. J Am Coll Cardiol. 76, 2635-2646 (2020).
- Koster R, Kohler J, Brockhoff C, et al. Laser Coronary Angioplasty: history, present and future. Am J Cardiovasc Drugs. 2, 197-207 (2002).
- Litvack F, Eigler N, Margolis J, et al. Percutaneous excimer laser coronary angioplasty: results in the first consecutive 3.000 patients. The ELCA investigators. J Am Coll Cardiol. 23, 323-329 (1994).
- Topaz O, Bernardo NL, Shah R, et al. Effectiveness of excimer laser coronary angioplasty in acute myocardial infarction or in unstable angina pectoris. Am J Cardiol. 87, 849-855 (2001).