Review Article - Interventional Cardiology (2014) Volume 6, Issue 1
Renal sympathetic denervation: indications, contemporary devices and future directions
- Corresponding Author:
- Timothy Watson
Green Lane Cardiovascular Service, Auckland City Hospital
Park Road, Auckland, New Zealand
Tel: +60 3 7949 2585
Fax: +60 3 7949 4613
E-mail: timothy.watson@me.com
Abstract
Resistant essential hypertension that does not respond to standard treatment regimens poses an important therapeutic challenge. Catheterbased sympathetic renal denervation (RDN) therapy is an exciting and promising new treatment strategy, with potential to revolutionize the current treatment paradigm in this group of patients. This review will focus on contemporary evidence supporting the utility of RDN, review current and emerging devices, consider potential future treatment indications, and discuss unresolved issues that need to be addressed before RDN can be embraced as mainstream therapy.
Keywords
essential hypertension, renal denervation, resistant hypertension, sympathetic nervous system
Resistant essential hypertension that does not respond to standard treatment regimens poses an important therapeutic challenge. Catheterbased sympathetic renal denervation (RDN) therapy is an exciting and promising new treatment strategy, with potential to revolutionize the current treatment paradigm in this group of patients. This review will focus on contemporary evidence supporting the utility of RDN, review current and emerging devices, consider potential future treatment indications, and discuss unresolved issues that need to be addressed before RDN can be embraced as mainstream therapy.
Burden of hypertension
Hypertension is generally classified as either essential or secondary to a defined etiology (e.g., renal/endocrine disease). In contrast to secondary hypertension, which may respond to treatment of the underlying cause, essential hypertension – which accounts for over 90% of patients with hypertension – is considered a heterogeneous disorder with a multifactorial and poorly understood etiology [1].
Regardless of etiology, hypertension is a major risk factor for cardiovascular disease and is associated with significant morbidity and mortality [2]. Hypertension is a truly global public health epidemic with 47% of all ischemic heart disease worldwide, and 92 million disease-adjusted life years directly attributable to the condition [3]. Historically, hypertension was considered a burden mainly of industrialized nations, largely due to complex genetic–environmental and social interactions [4]. While the disease burden continues to rise among industrialized nations, current trends also show a rapidly rising prevalence in developing nations. Hypertension is projected to affect half of the world’s adult population by 2025 [5,6].
The mainstay of treatment for essential hypertension consists of a combination of lifestyle modification and pharmacological interventions. Although usually effective, there remains an important cohort of patients in whom blood pressure (BP) fails to respond adequately to these measures. In some instances, this may be due to poor compliance or to ‘white-coat’ effect. However, even when these variables are accounted for, there appears to be a group of patients with ‘resistance’ to conventional management strategies. Resistant hypertension is defined as BP that is persistently elevated above 140/90 mmHg despite lifestyle measures (e.g., exercise/salt restriction, among others) and concurrent use of three or more antihypertensive agents [7]. As the prevalence of hypertension has increased, there has also been a corresponding rise in the prevalence of resistant hypertension. The prevalence of resistant hypertension in the USA is estimated at 12% of those diagnosed with hypertension, accounting for approximately 9 million Americans [8]. This is of particular concern as patients with resistant hypertension have a dramatically higher cardiovascular risk with a 50% increase in major events, compared with individuals with controlled BP [9,10].
Given these worrying data and frequent poor response to currently available antihypertensive therapies, recent novel strategies targeting the renal sympathetic nervous system offer the potential for an exciting new option for this previously undertreated group of patients with resistant hypertension.
The renal sympathetic nervous system
The pathophysiology of essential hypertension is complex and multifactorial. It is thought that abnormal renal excretory function plays a central role in the initiation, development and maintenance of the hypertensive process. The kidney has an integral role in sodium and water homeostasis that is central to regulation of arterial pressure. The normal response to an increase in arterial pressure is increased urinary sodium and water excretion, reduced blood volume and thereby reduced arterial pressure. Therefore, any process that affects the excretory function of the kidney can lead to the development of hypertension [11].
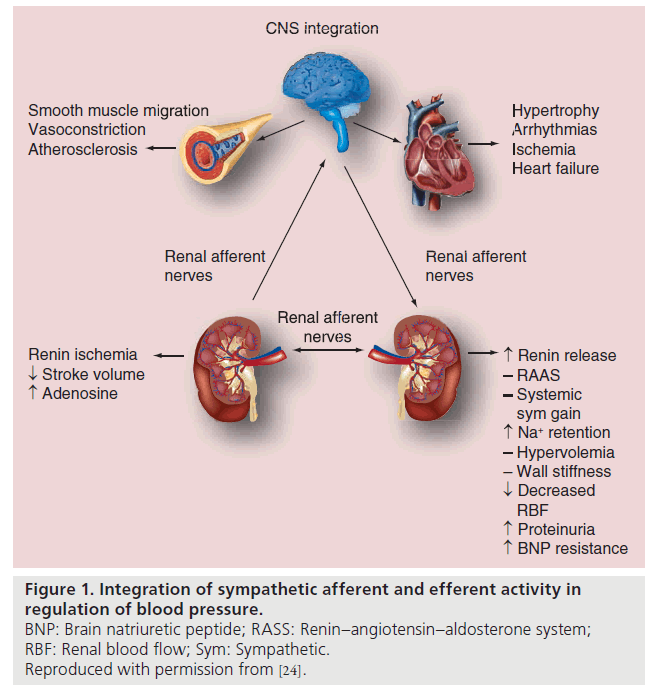
In some patients with resistant hypertension this mechanism is impaired and sympathetic tone appears to be enhanced. The consequent increased renal sympathetic nervous activity results in increased renin secretion, increased renal tubular sodium reabsorption and reduction in both glomerular filtration rate and renal blood flow. Increased sympathetic activity also increases norepinephrine spill over rates from the kidneys in patients with essential hypertension. This effect has been shown to particularly occur in young hypertensive patients [12,13]. The renal sympathetic nervous activity also affects angiotensin II and aldosterone production which both influence diuresis and natriuresis, and are integral to the development of hypertension, heart failure and kidney disease (Figure 1) [14,15]. Therefore RDN to reduce both afferent and efferent sympathetic activity is a logical approach to treat hypertension.
The potential therapeutic benefit of RDN was shown in animal studies designed to determine the role of the renal sympathetic system in the pathophysiology of hypertension, where invasive RDN was achieved using either surgical ligation or surgical stripping with phenol. Following these procedures, hypertension was either prevented or reduced in magnitude [16–18].
The initial clinical experience was with nonselective sympathectomy, first shown to be an effective treatment for hypertension over 50 years ago. This was a radical and invasive surgical approach targeting thoracic, abdominal and pelvic sympathetic nerves. While effective at BP reduction, sympathectomy was associated with a high complication rate and numerous side effects including severe postural hypotension, abdominal pain and impaired regulation of other autonomic functions (e.g., sweating). The advent of effective pharmacological therapy led to sympathectomy being regarded as a procedure of obscure historical and pathophysiologic interest [19–21].
Targeted RDN
In contrast to the initial crude surgical technique, a more targeted approach of sympathetic RDN using catheter-based technology showed promise for treatment of resistant hypertension, with few undesirable side effects [22]. Unpublished preclinical swine studies demonstrated that catheter-based RDN is able to prevent, reverse, or reduce the severity of hypertension, paving the way for human trials. The largest clinical experience to date is with the Symplicity ™ (Medtronic Inc., CA, USA) catheter, designed to deliver radiofrequency (RF) energy through the wall of the renal artery to achieve RDN. It is currently the only commercially available device with randomized controlled trial evidence to support its clinical use [23].
• Symplicity
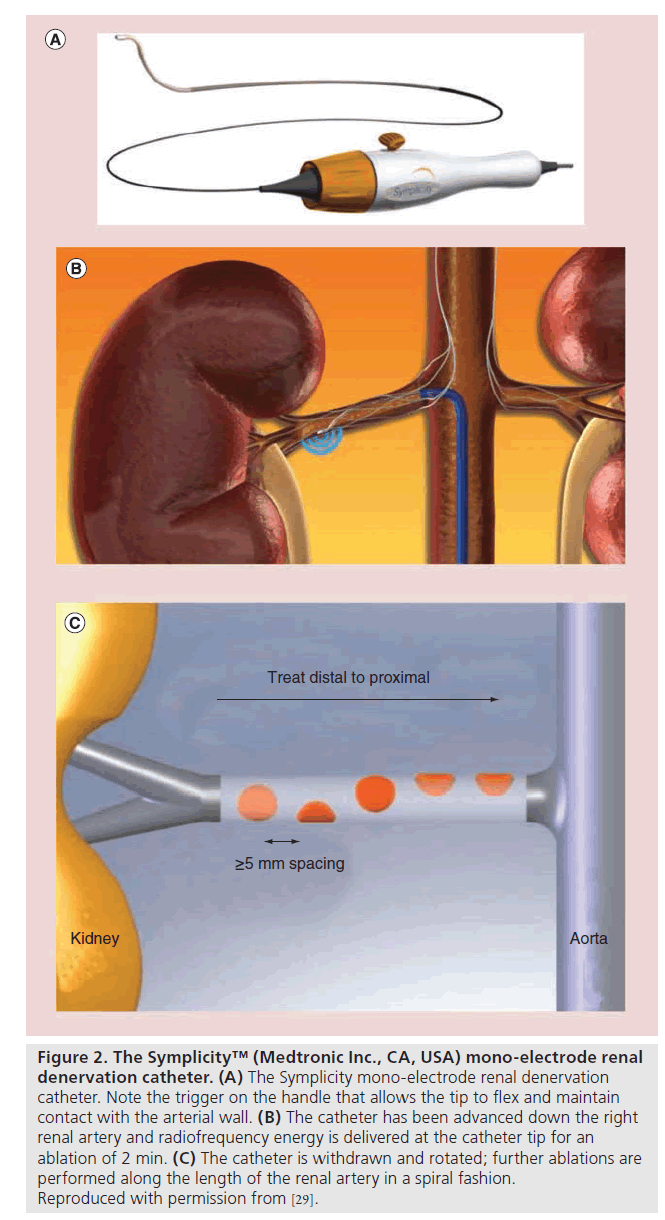
RDN using the Symplicity system requires percutaneous access via the femoral artery. Preceding renal angiography (computed tomography or MRI) is usually undertaken to exclude patients with renal anatomy unsuitable for RDN (e.g., anomalous/accessory vessels), and renal angiography is then performed to confirm renal artery anatomy at the time of the RDN procedure (assessing in particular for anatomical suitability, including vessel caliber, length, angle of origin and the presence of atherosclerotic plaque). Following administration of heparin and appropriate analgesia/sedation, the lumen of the main renal artery is catheterized using a 6 F or larger caliber guide. The Symplicity catheter (unipolar system) is then advanced into the distal portion of the artery, just proximal to the bifurcation (Figure 2). The catheter tip is flexed against the wall to ensure contact and electrical energy alternating at RF is applied for 2 min. The catheter is then withdrawn by 5 mm, rotated and the ablation repeated four- to six-times in a helical manner, prior to repeating the procedure in the contralateral artery ( Figures 2 & 3) [24]. Hence the total treatment time would be 24 min if there were six treatments on each side.
Figure 2: The Symplicity™ (Medtronic Inc., CA, USA) mono-electrode renal denervation catheter. (A) The Symplicity mono-electrode renal denervation catheter. Note the trigger on the handle that allows the tip to flex and maintain contact with the arterial wall. (B) The catheter has been advanced down the right renal artery and radiofrequency energy is delivered at the catheter tip for an ablation of 2 min. (C) The catheter is withdrawn and rotated; further ablations are performed along the length of the renal artery in a spiral fashion. Reproduced with permission from [29].
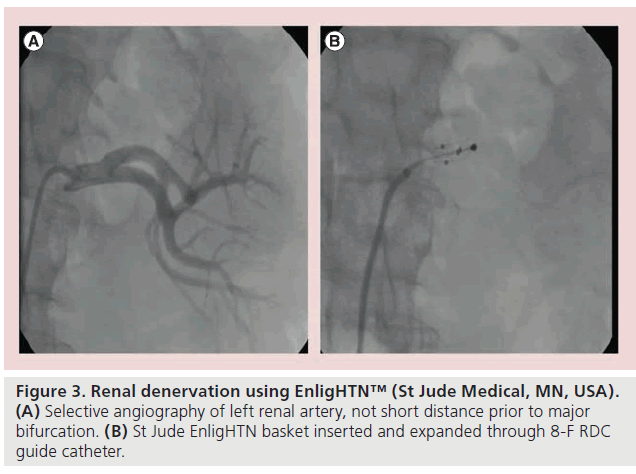
Figure 3: Renal denervation using EnligHTN™ (St Jude Medical, MN, USA). (A) Selective angiography of left renal artery, not short distance prior to major bifurcation. (B) St Jude EnligHTN basket inserted and expanded through 8-F RDC guide catheter.
An initial proof-of-concept and safety study was undertaken using the Symplicity system in 45 patients with resistant hypertension, across sites in Australia and Europe. Participants were required to have an office systolic BP ≥160 mmHg, despite three or more antihypertensive drugs, including a diuretic. This small study reported that RDN with the Symplicity catheter had a good safety profile, and produced a large and sustained office BP reduction of 27/17 mmHg at 12 months [23]. Given these promising results, the initial cohort of 45 nonrandomized patients was expanded to 153 patients, with longer-term follow-up. (Symplicity HTN-1) [25]. The larger cohort had an absolute reduction in office BP of 29/14 mmHg. These patients were considered to be genuinely refractory to treatment as the mean initial office BP was 176/98 despite an average of five antihypertensive agents. The reduction in office BP was sustained out to 24 months after the index procedure, while a small subgroup of the Symplicity HTN-1 cohort followed out to 36 months exhibited ongoing BP response [17,26].
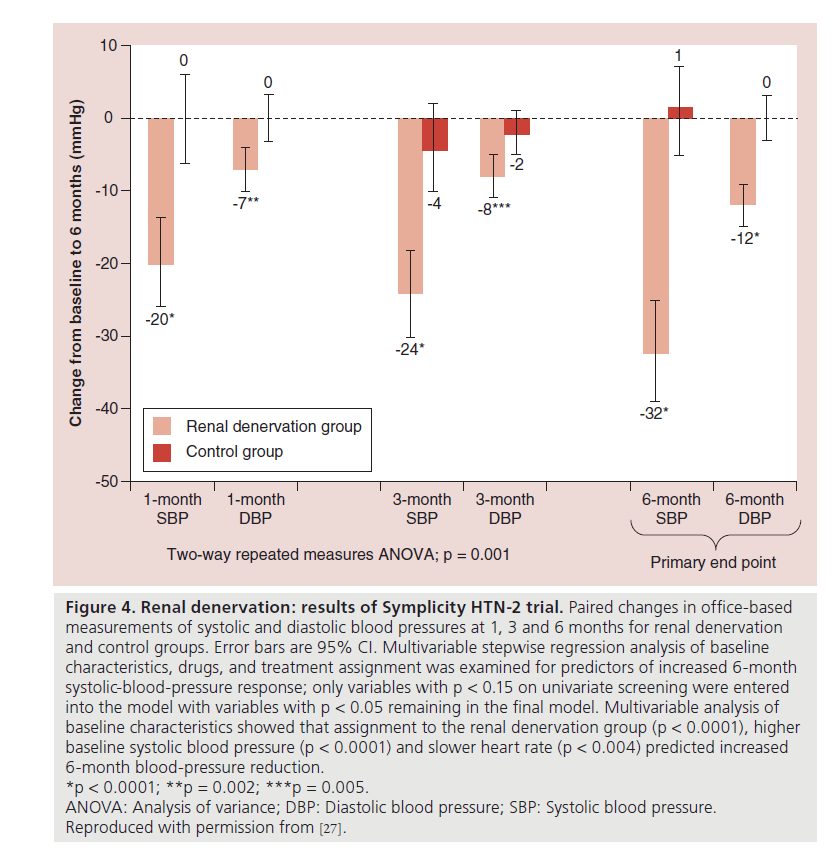
The largest prospective, randomized trial undertaken to date is Symplicity HTN-2. This study included 106 participants randomized to RDN or to a control group, with similar inclusion criteria to Symplicity HTN-1 ( Figure 4). Patients who underwent RDN had a significantly greater reduction in office BP compared with the control group at 6 months (32/12 vs 1/0 mmHg, respectively; p = 0.0001) [27]. This BP reduction was sustained at 1-year follow- up [28].
Figure 4: Renal denervation: results of Symplicity HTN-2 trial. Paired changes in office-based
measurements of systolic and diastolic blood pressures at 1, 3 and 6 months for renal denervation
and control groups. Error bars are 95% CI. Multivariable stepwise regression analysis of baseline
characteristics, drugs, and treatment assignment was examined for predictors of increased 6-month
systolic-blood-pressure response; only variables with p < 0.15 on univariate screening were entered
into the model with variables with p < 0.05 remaining in the final model. Multivariable analysis of
baseline characteristics showed that assignment to the renal denervation group (p < 0.0001), higher
baseline systolic blood pressure (p < 0.0001) and slower heart rate (p < 0.004) predicted increased
6-month blood-pressure reduction.
*p < 0.0001; **p = 0.002; ***p = 0.005.
ANOVA: Analysis of variance; DBP: Diastolic blood pressure; SBP: Systolic blood pressure.
Reproduced with permission from [27].
Both Symplicity HTN-1 and -2 have a number of important shortcomings, including absence of blinding, reliance on office BP measurements for the primary end point, and use of first generation equipment. They were largely studies designed to assess proof-of-concept and evaluate safety of the novel technology. To address some of these concerns, the pivotal US trial – Symplicity HTN-3 – has a prospective, randomized, singleblind study design comparing RDN with a sham procedure. It has completed enrollment and the main results are expected in 2014. The primary end points are change in office systolic BP from baseline to 6 months, and major adverse events to 1 month. The change in 24 h BP from baseline to 6 months is a secondary end point [29]. A recent media release from Medtronic, Inc. stated that while there were no safety concerns, Symplicity HTN-3 did not achieve its primary efficacy end point. The final results and manuscript are yet to be published [101].
• Other devices
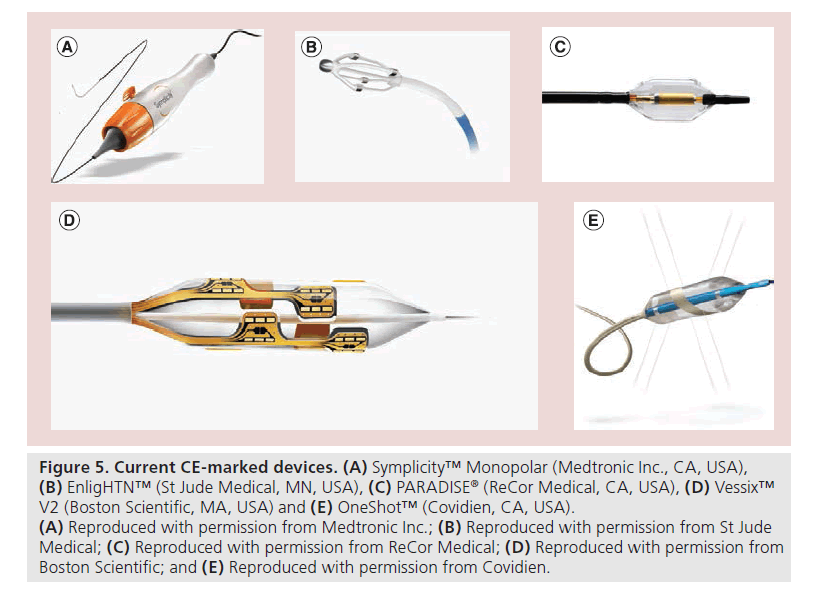
Of the many companies are making devices for RDN, four have currently reached the stage of receiving the EU CE mark ( Figure 5): EnligHTN™ (St Jude Medical, MN, USA), Vessix™ Vascular V2 (Boston Scientific, MA, USA), OneShot™ (Covidien, CA, USA) and PARADISE® (ReCor Medical, CA, USA) [30]. Although none of these systems has randomized trial evidence, early first-in-human evaluations have shown promise. Although the reduction in office BP at 6 months appears similar to that seen in the early Symplicity trials, these studies have in addition captured ambulatory BP data, although formal reporting is awaited.
Figure 5: Current CE-marked devices. (A) Symplicity™ Monopolar (Medtronic Inc., CA, USA), (B) EnligHTN™ (St Jude Medical, MN, USA), (C) PARADISE® (ReCor Medical, CA, USA), (D) Vessix™ V2 (Boston Scientific, MA, USA) and (E) OneShot™ (Covidien, CA, USA). (A) Reproduced with permission from Medtronic Inc.; (B) Reproduced with permission from St Jude Medical; (C) Reproduced with permission from ReCor Medical; (D) Reproduced with permission from Boston Scientific; and (E) Reproduced with permission from Covidien.
The EnligHTN system is 8 F-compatible with four monopolar electrodes mounted on an expandable basket with a deflectable tip, connected to a RF generator. A standard dispersive electrode (grounding pad) is applied to the skin. The EnligHTN I study assessed safety and BP reduction in 46 participants with office systolic BP ≥160 mmHg (or ≥150 mmHg in those with diabetes) on three or more antihypertensive medications. At 6 months follow-up the mean office BP reduction was 26/10 mmHg [31].
The OneShot system is a 9 F-compatible balloon-mounted system with a helical silver monopolar electrode connected to an RF generator. During ablation, the balloon is inflated to nominal size with normal saline at 1 Atm pressure, while saline seeps from the balloon through micropores irrigating, cooling and minimising damage to nontarget tissue. The treatment time is typically 2 min on each side. An initial feasibility study of nine patients showed a reduction in office BP of 31/10 mmHg at 12 months. A randomized, controlled trial is in progress [32,33].
The Vessix V2 is a balloon-delivered system with bipolar RF electrodes mounted in a helical pattern on the balloon. Energy is delivered simultaneously by all electrodes. Typically, only 30 s is require to deliver ablative energy on each side so procedural duration is reduced compared with the Symplicity system. Provisional results of a 120 patient feasibility study, REDUCEHTN, demonstrated a 27/12 mmHg office BP reduction at 6 months [102].
In contrast to the other devices, ReCor system utilizes focused high-energy intravascular ultrasound generated by a transducer located within an 8 F-compatible balloon catheter. During therapy, the balloon is expanded to stabilize the transducer position. The initial cohort of 15 patients exhibited a reduction in office BP of 32/17 mmHg at 6 months [34]. In addition, a number of other devices have early published data but are yet to receive a CE mark (see the ‘Future devices’ section).
• Sustained efficacy
A recent meta-analysis assessed the effect of RDN on BP reduction for patients with resistant hypertension in 12 published studies including two randomized trials (n = 133 patients), and ten observational studies (n = 446 patients). Office BP reduction at 6 months for controlled trials (two studies) and observational studies (ten studies) was 29/11 and 25/10 mmHg, respectively, with no difference in the magnitude of BP reduction between the various catheter systems evaluated in the included studies [35]. A second recent meta-analysis was designed to assess whether RDN resulted in either a ≥10% drop in BP or reduction in the number of antihypertensive agents, and included four studies with 180 patients undergoing RDN and 90 control subjects. Those who underwent RDN had a 50-fold increase in the odds of having at least a 10% BP reduction and a fourfold increase in the odds of being on three or fewer antihypertensive medications (p < 0.0005 and 0.006, respectively) [36]. However the results of both meta-analysis must be interpreted with caution given limitations of the included studies.
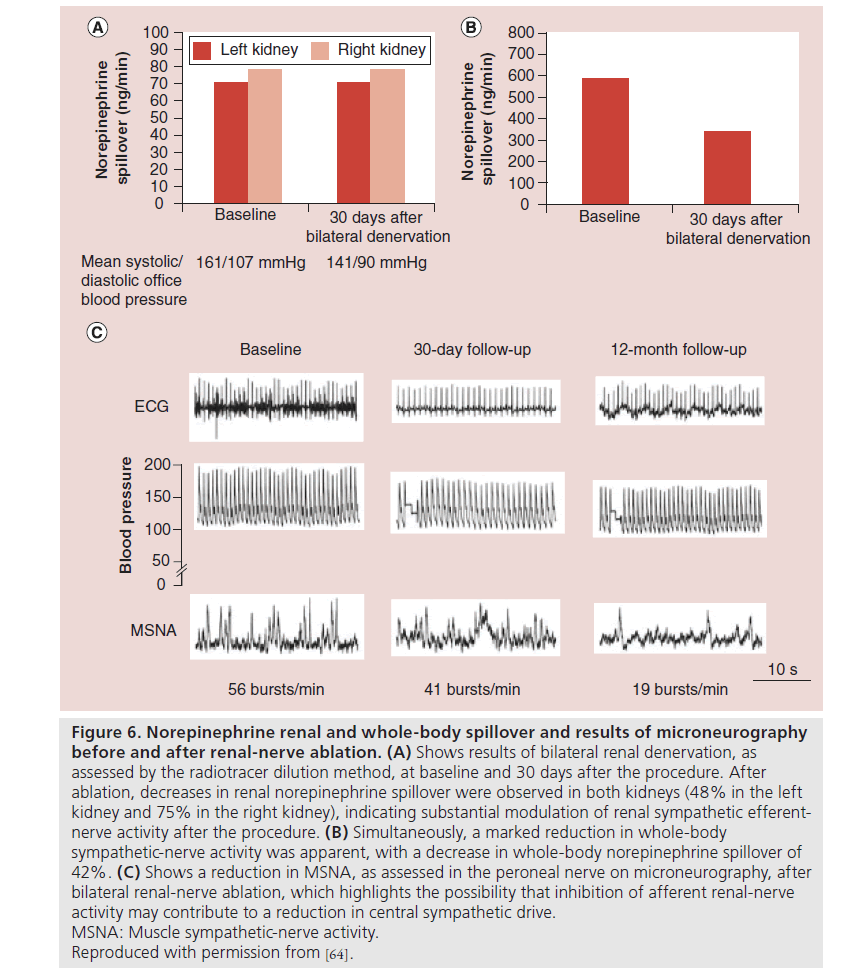
In summary, much of the data supporting use of RDN have been accumulated from proof-of-concept and various first-in-human studies of novel technology, designed largely to assess short-term efficacy and device safety profiles. There is a paucity of randomized trial evidence to support best clinical practice. However pooled meta-analysis data suggests that currently available devices are able to achieve a degree of BP lowering of comparable magnitude to that seen in the early Symplicity trials. In addition, no data yet exist to determine whether the BP reduction observed following RDN translates into improved morbidity and mortality. It is not yet possible to determine whether complete or adequate renal nerve ablation is being achieved during the procedure itself. Interestingly one Symplicity trial substudy assessed postprocedure norepinephrine spillover and demonstrated a significant reduction following RDN. Although this measure requires expertise, this may potentially represent a reliable surrogate marker to indicate effective afferent renal sympathetic ablation [23]. Radiotracer dilution techniques, used in the early proof-of-concept human trial also showed a 47% reduction in norepinephrine spillover assessed 1 month following RDN (Figure 6) [23]. RDN has also been shown to reduce muscle sympathetic nerve activity. A small study of 25 patients who underwent RDN were assessed with both baseline and 3-month follow-up measurements of blood pressure and muscle sympathetic muscle activity. At follow- up in patients who underwent denervation there was a significant reduction in all properties of single unit and multiunit sympathetic muscle activity [37].
Figure 6: Norepinephrine renal and whole-body spillover and results of microneurography before and after renal-nerve ablation. (A) Shows results of bilateral renal denervation, as assessed by the radiotracer dilution method, at baseline and 30 days after the procedure. After ablation, decreases in renal norepinephrine spillover were observed in both kidneys (48% in the left kidney and 75% in the right kidney), indicating substantial modulation of renal sympathetic efferentnerve activity after the procedure. (B) Simultaneously, a marked reduction in whole-body sympathetic-nerve activity was apparent, with a decrease in whole-body norepinephrine spillover of 42%. (C) Shows a reduction in MSNA, as assessed in the peroneal nerve on microneurography, after bilateral renal-nerve ablation, which highlights the possibility that inhibition of afferent renal-nerve activity may contribute to a reduction in central sympathetic drive. MSNA: Muscle sympathetic-nerve activity. Reproduced with permission from [64].
• Safety
Accumulated clinical experience using RDN has confirmed that the procedure has a good safety profile. In the Symplicity HTN-1 and -II, trials the procedure was completed without major complication in 98% of cases [23,24]. Most complications were related to the access site (primarily small hematoma), but one guide catheter-induced renal artery dissection was also documented. In total, 13% of patients also suffered transient bradycardia, sometimes requiring atropine, presumably reflecting a vagal response associated with sheath insertion into the femoral artery or pain during ablation.
Reassuringly no evidence of deterioration in renal function has been reported following RDN. At 1-year follow-up in Symplicity HTN-1, no change in glomerular filtration rate (GFR) was observed. In Symplicity HTN-2 renal function was assessed by serum creatinine, estimated GFR (eGFR), and cystatin C levels, all of which were largely unchanged at 6 months [23,25–27,38]. Patients with an eGFR <45 ml/min/1.73m2 were excluded from the Symplicity trials, so the effect of RDN on long-term renal function in patients with pre-existing severe renal impairment is less certain. A recent small series suggests that RDN in this group of patients is however possible: a series of 15 patients with resistant hypertension and stage 3–4 chronic renal impairment (mean eGFR 31 ml/min/1.73m2) underwent RDN. The postprocedure eGFR remained unchanged suggesting a favorable short-term safety profile in this group of patients [39].
There have been isolated case reports of patients developing a secondary rise in BP following RDN due to the development of renal artery stenosis. Angiographic evidence of dissection was not noted in any of these cases. It is unknown whether the lesion was a direct result of ablation vessel injury or natural progression of pre-existing disease [40,41].
Current guidelines
RDN is not yet commercially available in the USA, the technology being restricted to investigational use in clinical trials. Consequently, no formal consensus guidelines are available. However, the European Society of Hypertension and European Society of Cardiology have both released position statements acknowledging that RDN is an effective therapy when applied to carefully selected patients with proven resistant hypertension [41,42]. Both societies also noted that there were a number of unanswered questions, and recommended the need for expert opinion regarding patient selection, cost–effectiveness and appraisal of benefit/limitations of existing RDN technology.
• Patient selection
Appropriate patient selection is critical, and should be made by a hypertension expert in a specialized center. Optimal medical therapy is an important prerequisite, as there is no evidence that RDN is a substitute for pharmacotherapy. Therefore, those presently considered eligible for RDN should have a documented BP ≥160 mmHg (or ≥150 mmHg in those with diabetes) on at least three antihypertensive medications (of which one should be a diuretic) and appropriate lifestyle measures. Pseudoresistance (white-coat effect) should be excluded with ambulatory BP monitoring, as should secondary causes of hypertension.
Renal arteries must be anatomically suitable for RDN. This requires an adequate length and caliber prior to the first vessel bifurcation – with current technology this is typically a minimum 20-mm length and 4-mm diameter, respectively. The vessels also need to be free of significant stenosis, although there are anecdotal reports of combined denervation and renal artery stenting in patients with renovascular disease [42–44]. Accessory renal arteries can be denervated with some of the devices, although it remains uncertain whether denervating smaller vessels adds clinical benefit. Renal anatomy is best assessed preprocedure with either computed tomography or MRI renal angiography. Ultrasound may suffice as an initial screening tool, for example in those with moderate- to-severe renal impairment, combined with invasive angiography at the time of denervation.
• Cost–effectiveness
Cost–effectiveness analysis is limited by the paucity of clinical trial data (and use of hypertension as a surrogate outcome measure in place of clinical events) and the limited duration of patient follow-up. Despite the initial capital cost of the generator and the associated device and procedure costs, on a per-patient basis, RDN appears to be a highly cost-effective procedure. A recent analysis calculated that the discounted lifetime incremental cost–effectiveness ratio was US$3071 per quality-adjusted life-year. RDN was cost saving for a systolic BP between 160 and 172 mmHg. While RDN has an additional cost at time of therapy, if the BP reduction is sustained long term, it will become increasingly cost effective over time [45].
Future devices
In addition to those devices currently available commercially, numerous novel device technologies are under development, and existing catheter systems continue to be improved. It is likely that many systems will soon be 6 F guide-compatible and able to access the renal arteries via a radial approach, which should reduce vascular access site-related complications. In addition there may be noninvasive denervation procedures, such as externally applied ultrasound.
• Symplicity Spyral
The Symplicity Spyral Multi-Electrode RDN catheter (Medtronic, Inc.) is an evolution of the single electrode Symplicity catheter, using four electrodes to deliver RF energy simultaneously via a highly conformable catheter. The new design was developed to reduce procedure time, and allow improved deliverability and consistency of energy application. An early feasibility study was conducted in 29 patients with similar inclusion and exclusion to Symplicity HTN- 1. At 1-month BP reduction was 16/7 mmHg compared with baseline, while procedural time was reduced by 33 min compared with the single electrode catheter used in Symplicity HTN-2 [103].
Several other ultrasound based systems are also available. TIVUS (Cardiosonic, Tel Aviv, Israel) utilizes high intensity ultrasound delivered endoluminally, and is currently undergoing preclinical evaluation. In contrast to high intensity TIVUS and ReCor systems, the Kona Medical system (Campbell, CA, USA) uses low intensity focused ultrasound directed from an external source. Although early clinical studies used an endoluminal tracer beacon sited in the renal artery, it is hoped that once tissue penetration paths have been fully characterized, the device will become entirely noninvasive.
In addition, adapting standard electrophysiology cryocatheters for RDN procedures has been considered. Theoretic advantages of utilizing this technology relate to concerns that heat generated at the tissue-electrode interface during RF energy delivery may produce superficial injury but limit the depth of the lesion, while creating a nidus for char formation on the catheter. Both ThermoCool (Biosense Webster, CA, USA) and Mariner (Medtronic, Inc.) have tested devices with initial experience being promising.
Another method for achieving RDN is by locally delivered autonomic nerve-blocking drugs. The Bullfrog Microinfusion catheter (Mercator MedSystems, Inc., CA, USA) has a catheter tipped with a balloon-sheathed microneedle. Localized sympathectomy is achieved by delivery of guanethidine through the microneedle after stabilization of the balloon by inflation with saline. Early experimental studies in porcine models demonstrated successful drug delivery and a significant reduction in renal norepinephrine release [46].
Potential benefits other than hypertension
Excessive sympathetic activity is thought to have an important role in many conditions apart from hypertension. Although speculative and as yet untested, RDN may prove to have a therapeutic role for the treatment of diabetes, heart failure, chronic kidney disease and arrhythmias [47].
• Glucose handling
Sympathetic nerve activation has a central role in insulin resistance and the development of diabetes. A recent small series assessed the benefit of RDN on glucose metabolism in patients with resistant hypertension. Patients with treatment- resistant hypertension who underwent bilateral RDN (n = 37) were compared with control patients (n = 13). Those who underwent RDN had significant reductions in fasting glucose levels (118–108 mg/dl; p = 0.039) and basal insulin requirements (20.8 ± 3.0 to 9.3 ± 2.5 μIU/ml; p = 0.006) at 3 months of follow- up [48]. Similarly, improvements in glucose tolerance have been observed in ten patients with resistant hypertension and obstructive sleep apnea following RDN [49].
• Cardiac arrhythmias
Autonomic tone influences chronotropy, dromotropy, sinus node and atrioventricular conduction and therefore RDN may prove useful in the management arrhythmia. In humans, RDN has been shown to reduce both heart rate and increase the PR interval [50]. Animal studies have shown that RDN may improve rate control and reduce the risk of atrial fibrillation [51,52]. In one recent small hypothesis generating study, 27 patients with atrial fibrillation and hypertension undergoing pulmonary vein isolation were randomized to also undergo RDN (n = 13) or control (n = 14) [53]. At 12 months, those who underwent both pulmonary vein isolation and RDN had a significantly lower likelihood of atrial fibrillation recurrence. RDN has also been successfully utilized for ventricular tachyarrhythmia storm in two patients – one with nonobstructive hypertrophic cardiomyopathy and the other with a dilated cardiomyopathy. Following RDN, a marked improvement in ventricular tachyarrhythmia frequency was reported in both subjects [54]. Further experience is needed in both settings prior to drawing firm conclusions.
• Heart failure
In systolic heart failure, pharmacological betablockade has been shown to reduce both cardiovascular morbidity and mortality, presumably at least in part due to modulation of sympathetic nervous activity. Recent data investigating RDN in this context suggests that this intervention can improve symptoms and exercise tolerance at 6 months [55]. Similarly RDN has been shown in refractory hypertension to reduce LV mass and improve diastolic dysfunction [56]. This observation has led to speculation that RDN may improve outcome for those with heart failure with preserved LV ejection fraction. Further studies of the role of RDN in heart failure with normal systolic function are currently underway.
• Unresolved Issues
Uptake of RDN has been rapid and enthusiastic. There are a multitude of different companies developing new percutaneous devices and alternative approaches to achieve sympathetic neuromodulation [46]. However, more information about the downstream effects of RDN is needed before RDN becomes widely accepted into clinical practice.
First, there is accumulating evidence that RDN reduces blood pressure, as yet, no effect on either morbidity or mortality has been demonstrated. There are as yet no studies demonstrating the effect of RDN on myocardial infarction, stroke, heart failure, renal failure and death. Secondly, it is feasible that some of the apparent benefit of RDN may be a placebo effect. The most common cause of ‘refractory’ hypertension is patient noncompliance, and some of the benefit of RDN may be from improved adherence to prescribed drug therapy. Neither of the Symplicity HTN I and II trials used ambulatory BP for the primary end point, instead focussing on office BP as the primary outcome measure [57]. The reduction in ambulatory BP is usually less than that seen with office-based BP measurements, a finding confirmed in a recent series of 109 patients from the European Network Coordinating Research on Renal Denervation. Following RDN, mean ambulatory BP reduction was 5.9 mmHg, compared with a 17.6 mmHg fall in the office-based measurements. In addition, a marked variation in treatment response to RDN was noted, with 23% of patients demonstrating no reduction in ambulatory BP at follow-up [104].
The discordance between office and ambulatory BP responses reported in the trials is interesting, although there are a number of plausible explanations for these findings [57]. First, the baseline office BP measured at the point of recruitment into an RDN trial may be overestimated due to the phenomenon of regression to the mean. That is, a patient is more likely to be deemed suitable for inclusion in a hypertension trial when their BP is above their usual mean. As the variability of office BP is greater than that seen with ambulatory recordings, it is anticipated that the magnitude of response using the latter measure is likely to be smaller. Second, the absence of blinding may introduce potential for observer bias. For example, measurements may be repeated should a particular BP measurement seem incongruous to the anticipated response. Third, the ‘alert response’ and ‘white-coat’ effect may both be more marked at visit one due to the presence of a doctor in the consultation room. By contrast, follow-up measurements may be recorded by other healthcare professionals without the physical presence of a doctor.
Two contemporary large multicenter, randomized trials, Symplicity HTN-3 and EnligHTNment were designed to address some of these issues, while further insights will come from the Symplicity global registry. Symplicity HTN-3 has recently completed recruitment and randomized to either RDN or a sham procedure in a blinded manner. BP reduction is assessed using both office and 24-h BP measurements. The main safety end point is a composite of major adverse events including all cause mortality, vascular complications, and procedure-related complications [29]. However, a recent Medtronic, Inc. media release states that this trial was negative for the primary end point of change in office blood pressure at 6 months [101]. EnligHTNment will be the first trial powered to determine whether RDN for refractory hypertension reduces the risk of myocardial infarction, stroke and death. In total, 4000 patients recruited across 80–150 sites worldwide will be randomized to RDN and optimal medical therapy versus optimal medical therapy alone [105]. The EnligHTN IV trial was designed to assess safety and effectiveness using the EnligHTN Renal Denervation System, with a planned enrolment of 590 patients. The primary effectiveness outcome was reduction in office systolic blood pressure. However, this trial was recently suspended due to poor enrolment [106]. The Symplicity Global Registry, running in parallel to the Symplicity HTN-3 trial, will assess the long-term, real-world effects of RDN in >5000 patients worldwide [58].
There appears to be a nonresponse rate to RDN somewhere in the range of 10–30%. There is currently no way of determining those less likely to respond – the only variable predicting response to date is the magnitude of systolic BP elevation at baseline, with those >170 mmHg more likely to respond [59]. It is hoped that the above large randomized trials and registries will provide further information to detect non-responders. Methods of assessing whether nonresponse might be due to incomplete denervation are also needed.
Future perspective
Given the huge burden of hypertension and its associated cardiovascular risks RDN is an exciting modality that is likely to have a central future role in the treatment of resistant hypertension. Should the long-term efficacy of RDN be proven, and associated with an impact on both morbidity and mortality, the remit of RDN may expand to encompass less severe forms of hypertension, noncompliant patients and those with associated significant renal impairment [59–61]. In addition, studies investigating the role of RDN as part of the management of heart failure and diabetes are underway [59]. The apparent broad clinical benefit of RDN has stimulated interest in developing other neuromodulation devices, some targeting other components of the sympathetic nervous system remote from the kidney. Similarly the prospect for RF ablation to treat other unrelated conditions is also being investigated, including a recent small study from China reporting that pulmonary artery ablation might be an effective treatment for primary pulmonary hypertension [62]. Naturally this requires further exploration.
The present enthusiasm for RDN remains strong but continues to be largely fuelled by emerging data from small proof-of-concept studies. As with all novel technology, it is important to foster the development of a rigorous scientific evidence base and data from large multicenter, double-blinded studies are eagerly awaited. In addition, use of the technology beyond drugresistant hypertension should be in the context of clinical trials closely observing carefully selected patients for both therapeutic response and procedure- related complications [63,64].
Executive summary
Burden of hypertension
• Essential hypertension is a global epidemic that is a major contributor to cardiovascular mortality and morbidity.
• Despite lifestyle measures and medical therapy there is a significant cohort of patients that have hypertension that is resistant to standard treatment strategies.
Catheter-based sympathetic renal denervation
• Catheter-based sympathetic renal denervation (RDN) is a novel iteration of a historical but effective treatment for hypertension.
• A number of different device systems have demonstrated a significant reduction in office blood pressure within the context of several small safety and feasibility studies, although the impact on ambulatory blood pressure appears to be less dramatic.
Conclusion
• Early findings of catheter-based RDN studies have been promising.
• However, (blinded) randomized controlled trial data, including outcome measures is required to further assess the efficacy of RDN and define the place of this procedure in contemporary practice.
Future perspective
• RDN has the potential to become an important adjunct to best medical therapy for the treatment of resistant hypertension. Although the presently available data is inconsistent with a number of methodological flaws, ongoing research including randomized trials incorporating a sham procedure will help to define both the efficacy of RDN compared with best medical therapy, and the place of this procedure in contemporary practice.
• Although currently being investigated primarily as a tool to improve blood pressure, it is feasible that RDN may encompass a broader range of applications where sympathetic nervous system overactivity is detrimental. This includes conditions such as heart failure, where RDN may be useful to augment the effect of current pharmaceutical therapy.
Financial & competing interests disclosure
JA Ormiston serves as an advisory board member and has received minor honoraria from Boston Scientific. He holds a minor share interest in Covidien. RJ Whitbourn has received minor honoraria from Medtronic, Inc. and Kona Medical. He has received institutional grant/research support from Abbott Vascular, Boston Scientific, Kona Medical and Medtronic, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:• of interest
•• of considerable interest
- Carretero OA, Oparil S. Essential hypertension. Part I: definition and etiology. Circulation 101, 329–335 (2000).
- Williams B. The year in hypertension. J. Am.Coll. Cardiol. 55, 65–73 (2009).
- Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet 371, 1513–1518 (2008).
- Bromfield S, Muntner P. High blood pressure: the leading global burden of disease risk factor and the need for worldwide prevention programs. Curr. Hypertens. Rep. 15, 134–136 (2013).
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 365, 217–223 (2005).
- Danaei G, Finucane MM, Lin JK et al.; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Pressure). National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5.4 million participants. Lancet 377, 568–577 (2011).
- Mancia G, Fagard R, Narkiewicz K et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 34, 2159–2219 (2013).
- Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension 57, 1076–1080 (2011).
- Pimenta E, Calhoun DA. Resistant hypertension: incidence, prevalence, and prognosis. Circulation 125, 1594–1596 (2012).
- Daugherty SL, Powers JD, Magid DJ et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation 125, 1635–1642 (2012).
- DiBona GF. The sympathetic nervous system and hypertension: recent developments. Hypertension 43, 147–150 (2004).
- Esler M, Jennings G, Biviano B, Lambert G, Hasking G. Mechanism of elevated plasma norepinephrine in the course of essential hypertension. J. Cardiovasc. Pharmacol. 8(Suppl. 5), S39–S43 (1986).
- Schlaich MP, Lambert E, Kaye DM et al. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and angiotensin neuromodulation. Hypertension 43, 169–175 (2004).
- DiBona GF, Esler M. Translational medicine: the antihypertensive effect of renal denervation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R245–R253 (2010).
- DiBona GF. Sympathetic nervous system and the kidney in hypertension. Curr. Opin. Nephrol. Hypertens. 11, 197–200 (2002).
- Fajardo J, Lopez-Novoa JM. Effect of chemical sympathectomy on renal hydroelectrolytic handling in dogs with chronic caval constriction. Clin. Physiol. Biochem. 4, 252–256 (1986).
- le Noble JL, Janssen BJ, Lappe RW, Brody MJ, Struyker-Boudier HA, Smits JF. Pharmacological evidence for rapid destruction of efferent renal nerves in rats by intrarenal infusion of 6-hydroxydopamine. J. Hypertens. Suppl. 3, S137–S140 (1985).
- le Noble LM, Lappe RW, Brody MJ, Struyker Boudier HA, Smits JF. Selective efferent chemical sympathectomy of rat kidneys. Am. J. Physiol. 249, R496–R501 (1985).
- DiBona GF, Kopp UC. Neural control of renal function. Physiol. Rev. 77, 75–197 (1997).
- Morrissey DM, Brookes VS, Cooke WT. Sympathectomy in the treatment of hypertension; review of 122 cases. Lancet 1, 403–408 (1953).
- Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension; results in 1,266 cases. J. Am. Med. Assoc. 152, 1501–1504 (1953).
- Doumas M, Faselis C, Papademetriou V. Renal sympathetic denervation and systemic hypertension. Am. J. Cardiol. 105, 570–576 (2010).
- Krum H, Schlaich M, Whitbourn R et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 373, 1275–1281 (2009).
- Schlaich MP, Sobotka PA, Krum H, Whitbourn R, Walton A, Esler MD. Renal denervation as a therapeutic approach for hypertension: novel implications for an old concept. Hypertension 54, 1195–1201 (2009).
- Symplicity HTN-1 Investigators. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension 57, 911–917 (2011).
- Krum H, Schlaich M, Sobotka P et al. Long-term follow-up of catheter-based renal denervation for resistant hypertension confirms durable blood pressure reduction. Hypertension 57, 911–917 (2011).
- Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 trial): a randomised controlled trial. Lancet 376, 1903–1909 (2010).
- Esler MD, Krum H, Schlaich M, Schmieder RE, Bohm M, Sobotka PA. Renal sympathetic denervation for treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation 126, 2976–2982 (2012).
- Kandzari DE, Bhatt DL, Sobotka PA et al. Catheter-based renal denervation for resistant hypertension: rationale and design of the Symplicity HTN-3 trial. Clin. Cardiol. 35, 528–535 (2012).
- Bakris GL. Interventional cardiology: indications for renal denervation: a balanced approach? Nat. Rev. Cardiol. 10, 434–436 (2013).
- Worthley SG, Tsioufis CP, Worthley MI et al. Safety and efficacy of a multi-electrode renal sympathetic denervation system in resistant hypertension: the EnligHTN I trial. Eur. Heart J. 34, 2132–2140 (2013).
- Ormiston JA, Watson T, van Pelt N et al. First-in-human use of the OneShot renal denervation system from Covidien. EuroIntervention 8, 1090–1094 (2013).
- Ormiston JA, Watson T, van Pelt N et al. Renal denervation for resistant hypertension using an irrigated radiofrequency balloon: 12-month results from the Renal Hypertension Ablation System (RHAS) trial. EuroIntervention 9, 70–74 (2013).
- Mabin T, Sapoval M, Cabane V, Stemmett J, Iyer M. First experience with endovascular ultrasound renal denervation for the treatment of resistant hypertension. EuroIntervention 8, 57–61 (2012).
- Davis MI, Filion KB, Zhang D et al. Effectiveness of renal denervation therapy for resistant hypertension: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 62, 231–241 (2013).
- Garcia DC, Macedo FYB, Benjo AM et al. Catheter-based renal sympathetic denervation for resistant hypertension: a meta-analysis. Int. J. Cardiovasc. Res. doi:10.4172/2324-8602.1000116 (2013) (Epub ahead of print).
- Hering D, Lambert E, Marusic P. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension 61, 457–464 (2013).
- Krum H, Schlaich MP, Böhm M et al. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet doi:10.1016/S0140-6736(13)62192-3 (2013) (Epub ahead of print).
- Hering D, Mahfoud F, Walton AS et al. Renal denervation in moderate to severe CKD. J. Am. Soc. Nephrol. 23, 1250–1257 (2012).
- Vonend O, Antoch G, Rump LC, Blondin D. Secondary rise in blood pressure after renal denervation. Lancet 380, 778 (2012).
- Kaltenbach B, Id D, Franke JC et al. Renal artery stenosis after renal sympathetic denervation. J. Am. Coll. Cardiol. 60, 2694–2695 (2012).
- Mahfoud F, Luscher TF, Andersson B et al. Expert consensus document from the European Society of Cardiology on catheter- based renal denervation. Eur. Heart J. 34(28), 2149–2157 (2013).
- Schmieder RE, Redon J, Grassi G et al. ESH position paper: renal denervation – an interventional therapy of resistant hypertension. J. Hypertens. 30(5), 837–841 (2012).
- Hering D, Walton A, Krum H, Lambert G, Esler M, Schlaich M. Renal artery ablation reduces blood pressure in a patient with renovascular hypertension resistant to drug and revascularisation therapies. Int. J. Cardiol. 159(2), e35–e36 (2012).
- Geisler BP, Egan BM, Cohen JT et al. Cost–effectiveness and clinical effectiveness of catheter-based renal denervation for resistant hypertension. J. Am. Coll. Cardiol. 60, 1271–1277 (2012).
- Bunte MC, Infante de Oliveira E, Shishehbor MH. Endovascular treatment of resistant and uncontrolled hypertension: therapies on the horizon. JACC Cardiovasc. Interv. 6, 1–9 (2013).
- Mahfoud F, Schlaich M, Kindermann I et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation 123, 1940–1946 (2011).
- Witkowski A, Prejbisz A, Florczak E et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension 58, 559–565 (2011).
- Ukena C, Mahfoud F, Spies A et al. Effects of renal sympathetic denervation on heart rate and atrioventricular conduction in patients with resistant hypertension. Int. J. Cardiol. 167, 2846–2851 (2013).
- Linz D, Mahfoud F, Schotten U et al. Renal sympathetic denervation provides ventricular rate control but does not prevent atrial electrical remodeling during atrial fibrillation. Hypertension 61, 225–231 (2013).
- Zhao Q, Yu S, Huang H et al. Effects of renal sympathetic denervation on the development of atrial fibrillation substrates in dogs with pacing-induced heart failure. Int. J. Cardiol. 168(2), 1672–1673 (2013).
- Pokushalov E, Romanov A, Corbucci G et al. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J. Am. Coll. Cardiol. 60, 1163–1170 (2012).
- Ukena C, Bauer A, Mahfoud F et al. Renal sympathetic denervation for treatment of electrical storm: first-in-man experience. Clin. Res. Cardiol. 101, 63–67 (2012).
- Davies JE, Manisty CH, Petraco R et al. First-in-man safety evaluation of renal denervation for chronic systolic heart failure: primary outcome from REACH-pilot study. Int. J. Cardiol. 162, 189–192 (2013).
- Brandt MC, Mahfoud F, Reda S et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J. Am. Coll. Cardiol. 59, 901–909 (2012).
- Howard JP, Nowbar AN, Francis DP. Size of blood pressure reduction from renal denervation: insights from meta-analysis of antihypertensive drug trials of 4121 patients with focus on trial design: the CONVERGE report. Heart 99(21), 1579–1587 (2013).
- Ukena C, Cremers B, Ewen S, Bohm M, Mahfoud F. Response and non-response to renal denervation: who is the ideal candidate? EuroIntervention 9(Suppl. R), R54–R57 (2013).
- Bhatt DL, Bakris GL. The promise of renal denervation. Cleve. Clin. J. Med. 79, 498–500 (2012).
- Thomas G, Shishehbor MH, Bravo EL, Nally JV. Renal denervation to treat resistant hypertension: guarded optimism. Cleve. Clin. J. Med. 79, 501–510 (2012).
- Doumas M, Douma S. Renal sympathetic denervation: the jury is still out. Lancet 376, 1878–1880 (2010).
- Chen SL, Zhang FF, Xu J et al. Pulmonary artery denervation to treat pulmonary arterial hypertension: a single-center, prospective, first-in-man PADN-1 study, J. Am. Coll. Cardiol. 62, 1092–1100 (2013).
- Jones WS, Vemulapalli S, Patel MR. Interventional treatment of hypertension: a new paradigm. Curr. Cardiol. Rep. 15, 356 (2013).
- Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic- nerve ablation for uncontrolled hypertension. N. Engl. J. Med. 361(9), 932–934 (2009).
• Good summary detailing the role of the sympathetic nervous system in hypertension.
•• Important initial trial highlighting the clinical utility of the Symplicity™ denervation system.
•• This is currently the largest randomized trial for any renal denervation device to date.
• Important document that provides expert consensus on the current role of catheter-based renal denervation in clinical practice.
• Good summary of the current evidence and the European Society of Cardiology’s position on the clinical role of renal denervation.
Websites
101. Renal denervation fails in SYMPLICITY HTN-3. www.medscape.com/viewarticle/818938 (Accessed 10 January 2014)
102. Treatment of Resistant Hypertension Using a Radiofrequency Percutaneous Transluminal Angioplasty Catheter (REDUCE-HTN). http://clinicaltrials.gov/ct2/show/ NCT01541865 (Accessed 16 July 2013)
103. Medtronic’s multi-electrode, simultaneously firing renal denervation catheter shows reduced procedure times while demonstrating significant blood pressure reduction. (Accessed 13 July 2013)
104. O’Riordan M. Disappointing real-world results with renal denervation: BP reductions small with ABPM. www.theheart.org/article/1552223.do (Accessed 13 July 2013)
105. BusinessWire. St Jude Medical initiates landmark study of renal denervation for reduction of heart attack, stroke and death. www.businesswire.com/news/ home/20130215005030/en/St.-Jude-Medical- Initiates-Landmark-Study-Renal (Accessed 13 July 2013)
106. EnligHTN IV renal denervation study called off.www.medscape.com/viewarticle/817482(Accessed 10 January 2014)