Research Article - Neuropsychiatry (2018) Volume 8, Issue 3
Respiratory-Related Evoked Potential Measure of Intrinsic Transient Tracheal Occlusion Elicited Cortical Activations in Rats
- Corresponding Author:
- Pei-Ying S. Chan
Department of Occupational Therapy
Chang Gung University, Taoyuan, Taiwan
Tel: 886-3-2118800 x 5441
Fax: 886-3-2118800 x 5441
Abstract
Respiratory-related evoked potentials (RREP) measured with inspiratory mechanical stimulation have been demonstrated in humans and lambs. Previous studies have shown that the source of the RREP early P1 peak was generated by activation of neurons in the somatosensory (S1) cortex in humans. The RREP in rodents with mechanical loads induced by tracheal occlusions has never been studied before. Therefore, a rat intrinsic transient tracheal occlusion (ITTO) model was developed to test this hypothesis. The electrocorticogram (ECoG) was recorded in 8 chronically instrumented, awake and spontaneously breathing male Sprague-Dawley rats. On the day of recording, the animal was placed into a minimally restrictive restraint apparatus. The ECoG was recorded for 2.5 minutes as control prior to the occlusions. During the occlusion protocol of 10 to 18 minutes (collecting at least 70 occlusions), the occlusions lasted for 3 to 5 breaths and were presented approximately every 30 seconds. Occlusion-elicited evoked potentials were obtained by computer-signal averaging the ECoG activity triggered by the onset of inspiration. Two peaks (peak 1 and peak 2) were observed in the averaged occlusionelicited evoked potentials in all animals. Latencies and amplitudes were identified for each peak in every animal, and each peak was compared among day 1, 3, 5, 7, and 10 using the one-way repeated measures of ANOVA. The short-latency peak 1 showed a trend of increased amplitude at day 3 compared to day 1 (0.52 ± 0.14 V, and 0.23 ± 0.03 V, respectively, p < 0.1), whereas the longer latency peak 2 showed an increase in amplitude at day 3 compared to day 1 (-0.31 ± 0.094 V, and -0.14 ± 0.16 V, respectively; p < 0.05). Besides, peak 2 showed a longer latency on day 10 compared to day 1 in 5 animals. The result demonstrated that the ITTO protocol was feasible to elicit an evoked potential in the S1 cortex in awake, spontaneously breathing rats. The change in peak amplitude and latency may suggest cortical neural changes in respiratory sensory information processing with a chronic load conditioning program. Future studies are in need to further confirm these findings and to investigate the interactions between psychological and physiological factors in respiratory sensation.
Keywords
Respiratory-related evoked potentials, ECoG, RREP
Introduction
Respiratory sensory processing is fundamental to interoceptive awareness of breathing. Cortical awareness of breathing occur when ventilatory status changes. Respiratory afferent inputs regarding ventilatory change are transferred from the periphery (via mechanoreceptors, chemoreceptors, lung afferents and muscle afferents) through the brainstem to subcortical and cortical areas. This sensory activity can be gated out of the higher brain centers [1,2]. It has been suggested that the thalamus is involved in gating-in or -out of respiratory sensory information from the cortical level [3,4]. Phrenic afferents, activated by occlusion and mechanical stimulation, are known to activate neurons in the thalamus [5]. Once the information is gated-in, activations at the cortical level can be measured with different tools, including the respiratoryrelated evoked potential (RREP) method [6].
The RREP method is similar to the eventrelated potential in electroencephalography where a group of specific cortical neurons are activated as a function of repeated stimuli [7]. Respiratory-related sensory inputs stimulated the corresponding afferents that project to a column of cortical neurons which produces a dipole. The polarity, latency, and amplitude of the evoked potential provide inferences to the orientation of the neural cells, the timing and the strength of a dipole activated by respiratory sensory afferents [8]. The RREP elicited by respiratory mechanical loads have been recorded in conscious humans and lambs [6,9-12]. The RREP P1 peak was found to appear at approximately 45 – 70 msec and 35 – 45 msec after the onset of occlusion in adults and children, respectively [10,13-15]. Studies associated with source localization suggested that the early RREP P1 peak was generated by activation of neurons in the S1 cortex in humans [12,16]. The lamb cortical RREP P1 peak was similar to the human RREP where the P1 peak was found approximately 35 – 40 msec after the onset of occlusion, and the recording electrodes were located at the caudal-lateral area of the S1 cortex. In addition to lambs and humans, researchers have found that electrical stimulation and mechanical loads activated somatosensory cortices in cats [17,18].
Cortical electrophysiological recordings in respiratory sensation have not been implemented in rodents because conscious models with electrocorticography were not established. Earlier studies regarding brain activations in response to respiratory afferent stimulation in rodents have been focusing on electrical stimulation or external respiratory loads applied in anesthetized animals [5,19-21]. Recent studies used the intermittent transient tracheal occlusion (ITTO) paradigm to examine brain activations in response to respiratory occlusions in anesthetized rats as well as conscious rats [3,4,22-24]. Tracheal occlusion conditioning was found to modulate gene expression of medial thalamus in conscious rats [4]. This conditioning protocol was also known to elicit stress and anxiety in conscious rats as evidenced by elevated levels of cytochrome oxidase in various locations throughout the brain including thalamus and insular cortex [3]. However, it is unknown if an occlusion paradigm can also elicit a RREP in conscious rodents, and if repeated exposure to ITTO alters the cortical evoke d potential response. Developing a conscious rat model for investigating cortical responses to respiratory mechanosensation in rodents is essential for studying central neural mechanisms with costeffectiveness as compared to using other species.
In the present study, a model was developed to investigate ITTO elicited cortical evoked activity in chronically instrumented, awake, spontaneously breathing male Sprague-Dawley rats. We hypothesized that the RREP in conscious rats would be elicited with ITTO similar to those elicited in lambs and humans. We further hypothesized that repeated ITTO trials over 10 days would modulate the evoked potential responses in conscious rats. Specifically, we analysed and compared the evoked potential peak amplitudes and latencies at day 1, day 3, day 5, day7, and day 10.
Methods
▪ Animals
Eight male Sprague Dawley rats (averaged weight = 240-350g) were housed two per cage in the animal care facility with a 12/12 (light/dark) cycle. This study was carried out in accordance with the recommendations of the Institutional Animal Care and Use Committee (IACUC) in the University of Florida. The experiment protocol was reviewed and approved by the IACUC in the University of Florida.
▪ Experiment preparation
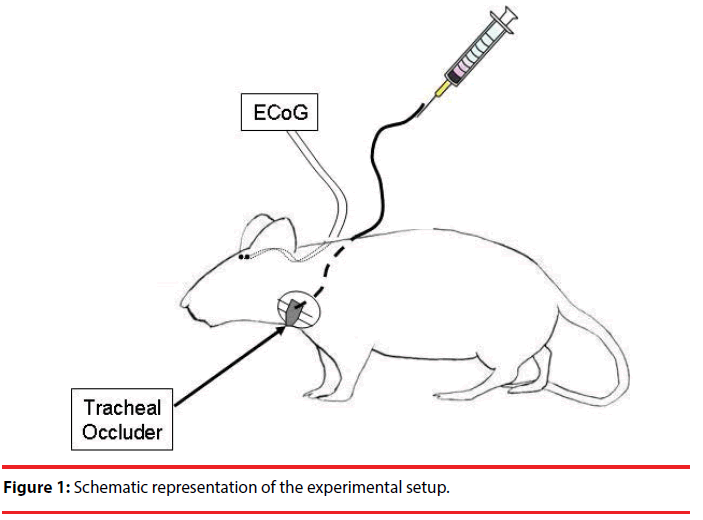
The rats were surgically instrumented under isoflurane anesthetic (2 – 5 % in O2). The depth of the anesthetic was checked by pinching the rear paw of the animal and the anesthetic was supplemented until the withdrawal reflex was absent. The body temperature was measured by a rectal probe and maintained at 38 degrees C with a heating pad. Petroleum ointment was administered to the eyes to prevent from drying. The animals’ incision sites were shaved and sterilized with povidone-iodine topical antiseptic solution. An inflatable balloontype tracheal occluder was implanted around the extrathoracic trachea. Sutures secured the occluder around the trachea, ensuring consistent pressure along the circumference of the trachea during occlusion. The occluder actuating tubing was subcutaneously routed around the neck and exteriorized between the shoulder blades. Please refer to Bernhardt et al.’s (2011) work for a detailed description on the placement of the tracheal occluder.
▪ Protocol
Electrocorticography (ECoG) was recorded by implanting electrodes through two burr holes that were made 2.5 mm lateral to the bregma bilaterally. A wire electrode was inserted through each hole onto the surface of the somatosensory cortex. The animals were allowed to recover for 4-7 days. A schematic representation of the recording is shown in Figure 1. Following recovery, the animals began the ten-day occlusion trial protocol that consisted of one occlusion trial per day for ten days. Animals were placed into a minimally restrictive restraint apparatus and bipolar ECoG was recorded. The occlusion pressure was approximately 800 mmHg to compress the trachea without crashing it. Each occlusion trial consisted of 2.5 minutes of preocclusion baseline recording, 10-18 minutes of uncued occlusion challenges, and 2.5 minutes of post-occlusion recovery. At least 70 occlusions were collected for data analysis. The occlusion conditioning trials consisted of multiple 3-5 breath occlusions followed by approximately 30 seconds of recovery, then another 3-5 breath occlusion. After completion of the occlusion trials, recording continued for a 2.5-minute recovery period. This was repeated for a 10-day period.
▪ Data analysis
Raw ECoG data was sampled at 1 KHz and averaged across trials via the Labchart 7 software (ADInstrument, Bella Vista, Australia). Signal averaging was triggered at the onset of change in balloon pressure and the epochs consisted of 500 msec. A minimum of 40 ITTOs were averaged for observing the evoked potential. ITTOelicited evoked potentials were obtained and identified with peak latencies and amplitudes for every animal after the experimental protocol at day 1, day 3, day 5, day 7, and day 10. Two peaks (peak 1 and peak 2) were identified in the averaged evoked potentials, separately, in the left and right somatosensory cortices for every animal. Peak 1 was identified as the first deflection after the onset of ITTO, and peak 2 as the next deflection after peak 1. The group averaged peak latencies and amplitudes were calculated and compared among and the 5 days with the one-way repeated measures of ANOVA using the SPSS software. The threshold of the significant level was set at p<0.05.
Results
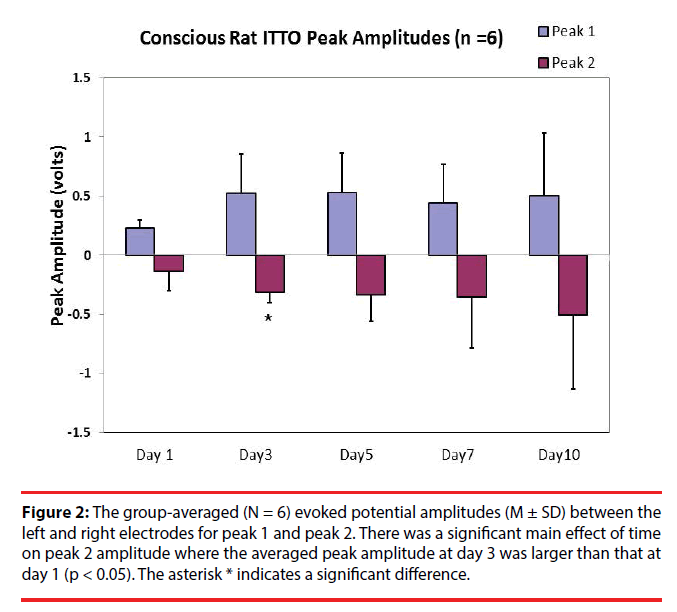
One-way ANOVA with repeated measures were used to compare latencies and amplitudes of the evoked potentials (N = 6) among day 1, day 3, day 5, day7, and day 10. The results revealed that, after multiple comparisons, the averaged peak 1 evoked potential amplitudes were not significantly different (Figure 2) (0.23 ± 0.07 V, 0.52 ± 0.34 V, 0.53 ± 0.34 V, 0.44 ± 0.32 V, and 0.51 ± 0.53 V for day 1, day 3, day 5, day 7, and day 10, respectively; p > 0.05). However there was a trend of increase in amplitudes for peak 1 at day 5 and day 3 compared to that at day 1 (p < 0.1). As for peak 2, there was an effect of time on peak amplitudes. Post-hoc analysis (LSD) revealed that the peak 2 amplitude at day 3 was significantly increased compared to that at day 1 (-0.31 ± 0.094 V, and -0.14 ± 0.16 V, respectively; p < 0.05).
Figure 2: The group-averaged (N = 6) evoked potential amplitudes (M ± SD) between the left and right electrodes for peak 1 and peak 2. There was a significant main effect of time on peak 2 amplitude where the averaged peak amplitude at day 3 was larger than that at day 1 (p < 0.05). The asterisk * indicates a significant difference.
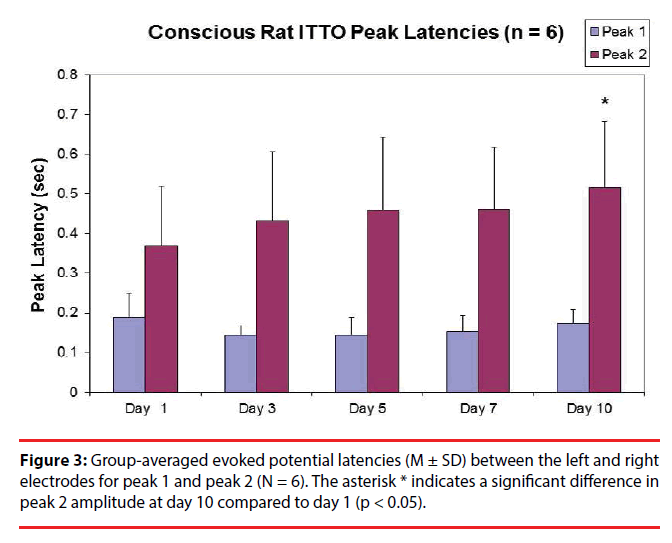
In terms of latencies, after multiple comparisons, one-way ANOVA with repeated measures showed that there were no significant differences among day 10, day7, day 5, day 3 and day 1 (N = 6) for peak 1 (Figure 3) (0.17 ± 0.03 sec, 0.15 ± 0.04 sec, 0.14 ± 0.04 sec, 0.15 ± 0.02 sec, and 0.19 ± 0.05 sec, respectively; p > 0.05). However there was an effect of time on peak 2 latencies among day 1, day 3, day 5, day 7, and day 10 (0.34 ± 0.15 sec, 0.39 ± 0.16 sec, 0.46 ± 0.2 sec, 0.42 ± 0.13 sec and 0.52 ± 0.17 sec, respectively). Posthoc analysis (LSD) revealed that, after multiple comparisons, the group-averaged latency for peak 2 at day 10 was significantly greater than that at day 1 in 5 animals (p < 0.01). In addition, for all animals (n=8) there was a trend of increase in peak 2 latency at day 5 compared to that at day 1 (0.44 ± 0.17 sec and 0.33 ± 0.14 sec, respectively; p < 0.1).
Behaviorally, it was observed that some animals initially appeared to breathe hard to conquer the loads by prolonging inspiratory and/or expiratory time; however, towards the later days of the regime, some animals chose to hold their breaths as long as they could rather than to breathe against the loads immediately.
Discussion
Our results demonstrated that the ITTO protocol was feasible for eliciting evoked potentials in the S1 cortex in awake, spontaneously breathing rats. In contrast to the previous studies where loads were provided for a single breath [25,26], the ITTO in the present study provided sustained loads which lasted for approximately 3 to 5 breaths to the animals, which is similar to some previous studies that caused aversive responses with load compensations in rodents [3,4,24,27]. Based on the previous reports of Pate & Davenport’s (2013) study, conscious animals exposed to ITTO for 10 days were found to have stress and anxiety responses [3]. Bernhardt et al.’s study also demonstrated that the genes associated with anxiety and depression was modulated by chronic tracheal occlusion conditioning [4]. All together, these studies using the ITTO have shown this conditioning paradigm induced a neural state change in the medial thalamus, insular cortex, PAG, brainstem including respiratory nuclei and the intermediate NTS due to load compensation responses. Our current study provides additional information regarding cortical evoked potentials measured by the ECoG in response to the loads generated intrinsically.
The ITTO conditioning protocol for 10 days generated cortical evoked potential responses measured by bilateral electrodes implanted through the burr holes 2.5 mm lateral to the bregma. At least two evoked-potential peaks (peak 1 and peak 2) were observed in the 8 animals. Peak 1 is a short-latency peak with positive deflections observable at approximately 0.1 to 0.2 seconds after the occlusions. Peak 1 increased marginally in amplitudes at day 3 and day 5 and the averaged evoked potential continues to show a robust neural activation throughout the rest of the measuring points. Although within-group variability was present, our finding suggested that the early stage of neural processing of respiratory occlusions could be modulated through the conditioning regimen. The S1 cortex is related to the determination of the spatial, temporal and intensity perception of the respiratory sensory inputs [28,29]. Occlusion is a maximal respiratory resistive load that stimulates the peripheral respiratory receptors then activates the neurons in the S1 cortex. Therefore, in our animals, the trend in the change in peak 1 amplitudes could mean more neurons in the S1 cortex were recruited after undergoing a few days of ITTO protocols, and then the recruitment stabilized. However, it should still be noted that there were significant amount of variations in the evoked potential responses among individual animals, and the experiments need to be replicated essential for further conclusions.
The early peak 1 may be exogenous in nature associated with stimulus intensity and cortical registration of information arrival. The result is similar to some previous findings where shortlatency RREP peak potentials were found unchanged with different external emotional stimulation [30-32]. The 10-day occlusion regimen may have been aversive to the animals, but neural processing of respiratory sensation elicited by the physical properties of the occlusions stabilized within a few days. This is also consistent with another report by von Leupoldt et al. where they examined cortical neural activations in response to sustained loads for 12 consecutive breaths and compared the RREP elicited by early and late stimuli [33]. Their results showed that the RREP N1, P2 and P3 peaks in response to the late stimulus were increased in peak amplitudes compared to that in response to an early stimulus, but not for the RREP early peaks including Nf and P1.
Peak 2 is the longer-latency peak with negative deflections observable after approximately 0.2 seconds after the occlusions. The results showed a trend of increase in P2 latency at day 5 for 8 animals and an increase at day 10 compared to that at day 1 for 5 animals. The fact that peak 2 was present at a much later time-point suggests that this peak may be endogenous in nature. Longer latency for peak 2 after a few days of treatment regime may suggest longer time for neural information processing with this occlusion conditioning regime. This result is comparable to some previous studies in humans [11, 34]. In Chan et al.’s study in the population with generalized anxiety disorder, the patient group was found to demonstrate a longer RREP P3 latency compared to the healthy controls. Von Leupoldt et al. showed decreased RREP P2 and P3 amplitudes in individuals with higher compared to lower anxiety levels, suggesting reallocation of attentional resources. The above evidences suggest that neural processing of respiratory occlusions is a function of emotional status which is affected by the limbic system. Of note is that in the current study, significant variations in terms of latencies and amplitudes exist among animals on top of data loss after 5 days. Further studies are needed to confirm the relationship between the occlusion conditioning and altered respiratory neural information processing time. The ITTO protocol in conscious animals is a valid tool for future studies to examine these relationships.
Emotional responses of animals were usually measured by behavioural measures such as the elevated plus maze measuring the anxiety levels [3]. Although these behaviours were not recorded in the present study with this ECoG paradigm, we observed that these conscious animals voluntarily modified their breathing behaviours in response to respiratory occlusions after being exposed to repetitive stimuli. Based on the observation during the 10-day ITTO regime, it was reasoned that the stress and anxiety due to the repeated ITTO resulted in this “learned helplessness” in our animals. Similar observations were also mentioned in several other studies [3,4,24].
The ITTO paradigm applied in this study used the onset of the balloon pressure, coincident with the closure of the trachea, as the stimulus event for signal averaging. The ITTO may have closed the trachea at any time during the respiratory cycle. There was no specificity for the occlusion occurring during inspiration or expiration. Thus, we do not know if the ITTO elicited RREP’s are from a specific phase of the breathing cycle. In addition, the trachea under the balloonocclusion remained innervated. Activation of the balloon reversibly compressed the trachea and may have stimulated mechanoreceptors in the compression area. Hence, it remains unknown which afferents are mediating the ITTO elicited RREP. The ITTO is selective for the trachea and airway obstruction, suggesting the RREP is tracheal or occlusion related and thus respiratory afferent dependent. The ITTO elicited evoked potential is directly related to activation of the ITTO balloon because no cortical evoked potential was observed in the absence of ITTO tracheal compression.
In summary, the results of the present study demonstrated that respiratory-related evoked potential can be recorded in conscious rats using the ECoG electrodes. The ITTO conditioning protocol generated a short-latency peak 1 and a long-latency peak 2 in the rodents. The conditioning program leads to a marginal increase in peak 1 amplitude and peak 2 latency, suggesting that the 10-day respiratory occlusion regime could induce neural changes in cortical respiratory sensory information processing. Nevertheless, further well-controlled studies are warranted for more definitive conclusions. Future studies are required to present the ITTO during a specific breath phase and to control for direct tracheal afferent stimulation. Further studies are also encouraged to utilize this protocol to examine the effects of external emotional stimulus or environmental contexts on respiratory neural processing in rodents.
Acknowledgement
The authors would like to thank Dr. Mark Hotchkiss for helping with data collection. This study was supported by the Ministry of Science and Technology (MOST-105-2420-H-182- 002-MY3), Chang Gung Memorial Hospital (BMRPB96), and the Chang Gung University research investigator travel grant.
References
- Davenport PW, Vovk A. Cortical and subcortical central neural pathways in respiratory sensations. Respir. Physiol. Neurobiol 167(1), 72-86 (2009).
- Chan PY, Davenport PW. Respiratory related evoked potential measures of cerebral cortical respiratory information processing. Biol. Psychol 84(1), 4-12 (2010).
- Pate KM, Davenport PW. Tracheal occlusion conditioning causes stress, anxiety and neural state changes in conscious rats. Exp. Physiol 98(1), 819-829 (2013).
- Bernhardt V, Hotchkiss MT, Garcia-Reyero N, et al. Tracheal occlusion conditioning in conscious rats modulates gene expression profile of medial thalamus. Front. Physiol 2(1), 24 (2011).
- Zhang W, Davenport PW. Activation of thalamic ventroposteriolateral neurons by phrenic nerve afferents in cats and rats. J. Appl. Physiol 94(1), 220-226 (2003).
- Davenport PW, Friedman WA, Thompson FJ, et al. Respiratory-related cortical potentials evoked by inspiratory occlusion in humans. J. Appl. Physiol 60(1), 1843-1848 (1986).
- Handy TC. Event-related potentials: A methods handbook. MIT press (2005).
- Williamson SJ, Kaufman L. Evolution of neuromagnetic topographic mapping. Brain Topogr 3(1), 113-127 (1990).
- Davenport PW, Hutchison AA. Cerebral cortical respiratory-related evoked potentials elicited by inspiratory occlusion in lambs. J. Appl. Physiol 93(1), 31-36 (2002).
- Webster KE, Colrain IM The respiratoryrelated evoked potential: effects of attention and occlusion duration. Psychophysiology 37(1), 310-318 (2000).
- Chan PY, von Leupoldt A, Liu CY, et al. Respiratory perception measured by cortical neural activations in individuals with generalized anxiety disorder. Respir. Physiol Neurobiol 204(1), 36-40 (2014).
- Logie ST, Colrain IM, Webster KE. Source dipole analysis of the early components of the RREP. Brain. Topogr 11(1), 153-164 (1998).
- Harver A, Squires NK, Bloch-Salisbury E. et al. Event-related potentials to airway occlusion in young and old subjects. Psychophysiology 32(1), 121-129 (1995).
- Chou YL, Davenport PW. The effect of increased background resistance on the resistive load threshold for eliciting the respiratory-related evoked potential. J. Appl. Physiol, 103(1), 2012-2017 (2007).
- Revelette WR, Davenport PW. Effects of timing of inspiratory occlusion on cerebral evoked potentials in humans. J. Appl. Physiol 68(1), 282-288 (1985).
- von Leupoldt A, Keil A, Davenport PW. Respiratory-related evoked potential measurements using high-density electroencephalography. Clin. Neurophysiol 122(1), 815-818 (2011).
- Davenport PW, Reep RL, Thompson FJ. Phrenic nerve afferent activation of neurons in the cat SI cerebral cortex. J. Physiol 588(1), 873-886 (2010).
- Yates JS, Davenport PW, Reep RL. Thalamocortical projections activated by phrenic nerve afferents in the cat. Neurosci. Lett, 180(1), 114-118 (1994).
- Hayward LF, Castellanos M. Activation of the dorsal periaqueductal gray in the rat induces Fos-like immunoreactivity in select non-cholinergic mesopontine neurons. Neurosci. Lett 360(1), 5-8 (2004).
- Malakhova OE, Davenport PW. c-Fos expression in the central nervous system elicited by phrenic nerve stimulation. J. Appl. Physiol 90(1), 1291-1298 (2001).
- Tsai HW, Davenport PW. Tracheal occlusionevoked respiratory load compensation and inhibitory neurotransmitter expression in rats. J. Appl. Physiol 116(1), 1006-1016 (2014).
- Pate KM, Davenport PW. Tracheal occlusions evoke respiratory load compensation and neural activation in anesthetized rats. J. Appl. Physiol 112(1), 435-442 (2012).
- Bernhardt V, Garcia-Reyero N, Vovk A, et al. Tracheal occlusion modulates the gene expression profile of the medial thalamus in anesthetized rats. J. Appl. Physiol 111(1), 117-124 (2011).
- Tsai HW, Condrey J, Adams S, et al. The effect of tracheal occlusion on respiratory load compensation: changes in neurons containing inhibitory neurotransmitter in the nucleus of the solitary tract in conscious rats. Respir. Physiol. Neurobiol 204(1), 138- 146 (2014).
- Forster HV, Lowry TF, Pan LG, et al. Diaphragm and lung afferents contribute to inspiratory load compensation in awake ponies. J. Appl. Physiol 76(1), 330-1339 (1994).
- Watts TL, Wozniak JA, Davenport PW, Hutchison AA: Laryngeal and diaphragmatic activities with a single expiratory load in newborn lambs. Respir. Physiol 107(1), 27-35 (1997).
- Smith BK, Gabrielli A, Davenport PW, et al. Effect of training on inspiratory load compensation in weaned and unweaned mechanically ventilated ICU patients. Respir Care 59(1), 22-31 (2014).
- von Leupoldt A, Dahme B. Cortical substrates for the perception of dyspnea. Chest 128(1), 345-354 (2005).
- von Leupoldt A, Sommer T, Kegat S, et al. The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. Am. J. Respir. Crit. Care. Med 177(1), 1026-1032 (2008).
- Von Leupoldt A, Vovk A, Bradley MM, et al. The impact of emotion on respiratory-related evoked potentials. Psychophysiology 47(1), 579-586 (2010).
- Chenivesse C, Chan PY, Tsai HW, et al. Negative emotional stimulation decreases respiratory sensory gating in healthy humans. Respir. Physiol. Neurobiol 204(1), 50-57 (2014).
- Chan PY, Cheng CH, Jhu YJ, et al. Being Anxious, Thinking Positively: The Effect of Emotional Context on Respiratory Sensory Gating. Front. Physiol 7(1), 19 (2016).
- von Leupoldt A, Bradley MM, Lang PJ, et al. Neural processing of respiratory sensations when breathing becomes more difficult and unpleasant. Front. Physiol 1(1), 144 (2010).
- von Leupoldt A, Chan PY, Bradley MM, et al. The impact of anxiety on the neural processing of respiratory sensations. Neuroimage 55(1), 247-252 (2011).