Review Article - Interventional Cardiology (2013) Volume 5, Issue 5
Retrograde approach: a practical guide for maximizing procedural success
- Corresponding Author:
- Colm Gerard Hanratty
Department of Cardiology, Belfast Health and Social Care Trust, Belfast, BT9 5EH, Northern Ireland
Tel: +44 781 688 3487
Fax: +44 289 033 29241
E-mail: colm.hanratty@belfasttrust.hscni.net
Abstract
The first description of a retrograde percutaneous coronary intervention (PCI) technique to open a chronic total occlusion (CTO) was described in 1990 when Kahn and Hartzler described an intervention down a saphenous vein graft (SVG) to access a left anterior descending artery.
Keywords
chronic total occlusion, hybrid algorithm, retrograde percutaneous coronary intervention, reverse controlled antegrade and retrograde subintimal tracking
The first description of a retrograde percutaneous coronary intervention (PCI) technique to open a chronic total occlusion (CTO) was described in 1990 when Kahn and Hartzler described an intervention down a saphenous vein graft (SVG) to access a left anterior descending artery [1]. More recently, accessing the distal target vessel via septal and epicardial collaterals to assist CTO PCI have been reported [2,3]. The Japanese experiences have, in particular, helped refine the procedure to make it routine to the extent that it is now a vital part of a CTO PCI program [4,5]. There has also been significant uptake and rollout in the USA and Europe [5,6].
may be required to complete CTO PCI are: proximal cap ambiguity, ostial occlusions, distal cap at a major bifurcation, small diseased distal vessel, bail out after antegrade techniques fail and the presence of a collateral channel (CC) that can be crossed (interventional collateral). In each scenario, obtaining retrograde access of the distal vessel is more likely to result in a successful PCI. Proximal cap ambiguity refers to a cap that is not easily identified due to the presence of multiple branches or collaterals. Therefore, penetrating through the cap and finding the true vessel may be difficult and potentially hazardous with penetrative wires that are easy to exit. In addition, with ostial lesions getting started can be difficult. Approaching retrogradely in these two situations will allow the operator to clearly identify the proximal cap and antegrade penetration is therefore much safer. With regard to distal diseased vessels or bifurcation – retrograde access will preserve all branches more predictably than antegrade strategies.
In the setting of these anatomical variations and in the presence of an interventional CC, a retrograde attempt may be the initial strategy rather than after a failed antegrade attempt or bail-out.
This review will focus on the relevant practical features to maximize procedural success. It is assumed that there is a clinical indication for the CTO PCI and that the area subtended by the occluded vessel is viable.
Initial setup
▪ Guide catheter selection
As with any coronary procedure there are a range of options available with regards to guide catheter shape and size to facilitate the procedure. Between even high-volume CTO operators, there will be a wide range of shape and size of guiding catheters used. In this review, the CTO vessel will be referred to as the ‘target vessel’ and the coronary artery supplying the CC will be referred to as the ‘donor vessel’.
Contralateral coronary injections are mandatory for contemporary CTO intervention when the vessel distal to the occlusion fills from a CC from a donor artery. Occasionally, the CC are ipsilateral and a single guiding catheter is sufficient for antegrade wire strategies. If then there is a switch to a retrograde strategy, it is possible to complete with a single guide – but this can be difficult owing to all the equipment and a lot of operators will introduce a second guide into the same coronary artery and ‘ping-pong’ the guides accordingly. It is worth putting a guiding catheter in from the start for contralateral injections even if the initial strategy is an antegrade wire escalation one. This means that if the initial strategy fails or stalls, the transition to a retrograde approach will be seamless. Obviously if the initial strategy is a retrograde approach a guiding catheter in the donor artery is mandated.
Having bilateral 8-F guiding catheters offers the full range of interventional options in both the target and donor vessels, and is the setup choice of many operators, especially in the USA. The main reason for requiring an 8-F guiding system is to be in a position to use an anchor balloon to help deliver antegrade dissection reentry equipment (CrossBoss® catheter and Stingray ™ balloon; Boston Scientific, MA, USA) or intravascular ultrasound guide puncture of the proximal cap with a microcatheter in situ. Most other techniques do not mandate 8 F. 8-F guiding catheters are more likely to cause damping and ischemia especially in smaller patients and side-hole catheters may be required, especially with right coronary artery (RCA) occlusions.
Recently there has been a drive to perform standard PCI radially in an effort to reduce vascular complication rates and facilitate early ambulation. With regards to CTO PCI, bilateral radial procedures have been reported; however, most operators do not choose this method owing to many reasons from limited guide catheter size to less comfort associated, especially with left radial intervention. Maximizing comfort for the patient (and the operator) is important with longer, complex procedures as this will probably affect success rates.
A compromise is to use an 8 F target guide to facilitate all CTO PCI options and use a radial 6- or 7-F donor-vessel guide. It is actually possible to insert a standard 8 F guide into some radial arteries with a sheathless configuration [Renfart S, Faurie B, Pers. Comm.].
The 150-cm Corsair® catheter (Asahi Intecc, Nagoya, Japan) has made the need for shortdonor vessel guides almost obsolete. It is possible to run out of catheter length if the donor vessel is filling via a SVG or left internal mammary artery graft. In this case, a short (90 cm) guiding catheter may be useful, but for routine cases standard length guides are adequate.
▪ Anticoagulation
CTO PCIs are often prolonged complex procedures and there may be long periods when the contents of the catheter (especially donor vessel) are static. Standard heparin is cheap, effective, easy to administer, can be monitored easily and the effects can be reversed in the event of a complication or perforation. In the author’s laboratory, a dose of 100 IU/kg is administered at the start of the procedure and activated clotting time is checked every 30 min and top-up doses are given to maintain an ACT above 300 s. Bivalirudin or glycoprotein IIb/IIIa inhibitors are not recommended in these procedures because of concerns in the event of a perforation.
▪ Angiographic assessment
In most cases the collateral filling is from the contralateral coronary artery. Occasionally, the filling is predominately ipsilateral. Even in these cases, a check angiogram of the other coronary artery is useful as the filling pattern may change between the diagnostic study and the intervention date. Even during a case the pattern of filling often changes. As one CC is compromised another will become dominant so having a contralateral catheter ready is sensible.
The coronary angiogram is the key to determining which initial strategy to employ and the anatomical features to look for are described in more depth in another manuscript. Briefly there must be clear visualization of the proximal cap, the distal target vessel and the collateral vessels. A good dual injection is performed to allow careful assessment of the CC and there should be no panning so the direction of filling is appreciated. The pattern of filling is useful in determining the dominance of the collateral. If, for example, there is an occluded RCA, and from a left coronary injection the contrast fills the posterior descending artery first then travels towards the crux and then the posterolateral RCA fills, we can assume that the septal CC are dominant. If, however, the contrast fills the posterolateral RCA, then travels towards the crux and lastly into the posterior descending artery, the dominant collateral filling will be epicardially from the left circumflex. However, often there is bidirectional filling indicating the presence of both septal and epicardial CC.
▪ Collateral channel assessment
A classification system for assessing CC has been proposed based on a modified Rentrop system: CC grade 0: there is no continuous connection, CC1: continuous thread-like connection, CC2: continuous side branch-like (small) connection is present [7]. This scoring system is useful in terms of describing the collaterals, but does not correlate well with the usefulness in terms of retrograde PCI. The presence of CC grade 0 or 1 does not mean that a crossable collateral is absent. Nor does the presence of a CC2 ensure the collateral is interventional, often very dominant collaterals can lead to ischemia when attempting to cross them. Therefore, the practical applicability of this classification is limited in terms of approaching retrograde CTO PCI.
Obtaining retrograde access
There are three possible conduits to obtain retrograde access: septal CC, epicardial CC and surgical conduits – most commonly SVG but occasionally left internal mammary artery or radial bypass grafts.
▪ Choosing an interventional collateral
The term interventional collateral refers to a CC that can be crossed with wire and microcatheter in your laboratory with your skill set. A less experienced CTO operator may only be comfortable with septal collateral crossing, whereas a more experienced CTO operator may choose to cross an epicardial collateral in an ungrafted patient.
The insertion of the CC into the target vessel is also of importance. If the collateral enters the target vessel too close to the distal cap, especially with an unfavorable angle then trying to get started retrogradely can be difficult. Whereas, if a distal collateral were selected then a more favorable angle can be achieved to puncture through the distal cap. Therefore, it may be worth choosing a more distal CC to facilitate a more favorable approach to the distal cap.
▪ Retrograde access via a SVG
If a patient presents with a clinical indication for PCI and has disease of the SVG to an occluded native vessel it may actually be preferable to treat the native occlusion. The long-term outcomes of SVG interventions are poor, whereas outcomes of native vessel PCI are more predictable. With a diseased SVG, an easily accessed patent conduit to the distal target vessel is present and the success rates of retrograde CTO PCI are above 90% when retrograde access has been achieved.
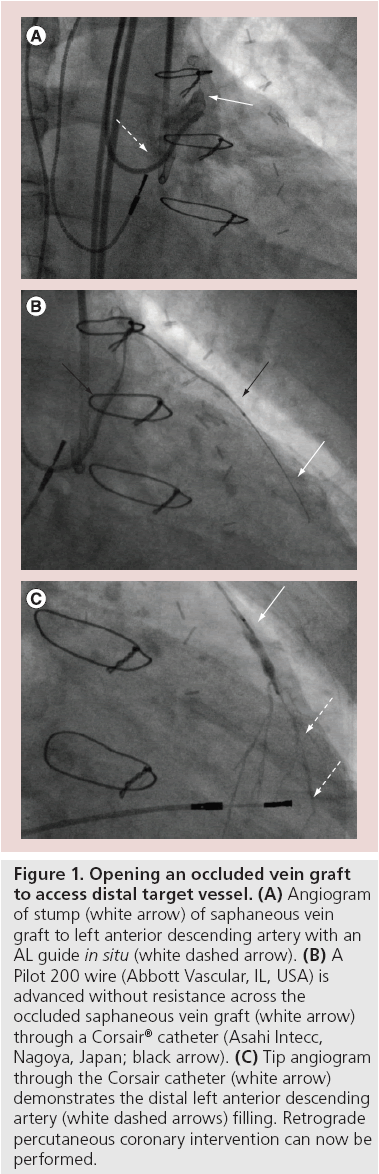
A significant proportion of patients presenting for CTO PCI are due to graft failure when the SVG in particular fails. Accessing the distal target vessel via an occluded SVG is actually often straightforward, especially when the graft is occluded less than 12 months [8]. This is very often a more predictable way to access the distal target vessel compared with septal or epicardial CCs. The safest way to achieve this is to have visualization of the distal target vessel, which usually is filling from a donor vessel CC. Then the SVG can be accessed with a guide (Figure 1), a microcatheter (e.g., Corsair; FineCross™, Terumo Corporation, Tokyo, Japan) and a Pilot 200 wire (Abbott Vascular, IL, USA). This 4.7-g hydrophilic wire will usually track through the occluded SVG easily to the distal target vessel. Confirmation that the wire is in the distal target vessel can be achieved angiographically, then the microcatheter is advanced towards the distal target vessel and a more steerable wire, for example, the Sion (Asahi Intecc) is used to access the proximal portion of the target vessel. Often the angle of the anastamosis can make this wiring challenging. The microcatheter is advanced into the distal target vessel, directed towards the distal cap and then the operator is set up to perform retrograde wire escalation or retrograde dissection re-entry (reverse controlled antegrade and retrograde subintimal tracking [CART]).
Figure 1: Opening an occluded vein graft to access distal target vessel. (A) Angiogram of stump (white arrow) of saphaneous vein graft to left anterior descending artery with an AL guide in situ (white dashed arrow). (B) A Pilot 200 wire (Abbott Vascular, IL, USA) is advanced without resistance across the occluded saphaneous vein graft (white arrow) through a Corsair® catheter (Asahi Intecc, Nagoya, Japan; black arrow). (C) Tip angiogram through the Corsair catheter (white arrow) demonstrates the distal left anterior descending artery (white dashed arrows) filling. Retrograde percutaneous coronary intervention can now be performed.
▪ Retrograde access via septal collaterals
Obtaining retrograde access in a nongrafted (i.e., intact pericardium) is more safely achieved via septal collaterals. There is however more unpredictability about obtaining retrograde access via septal CCs. The septal connections can be arterial–arterial, arterial–venous or arterial–cavity and wiring through these various connections can be frustrating and challenging.
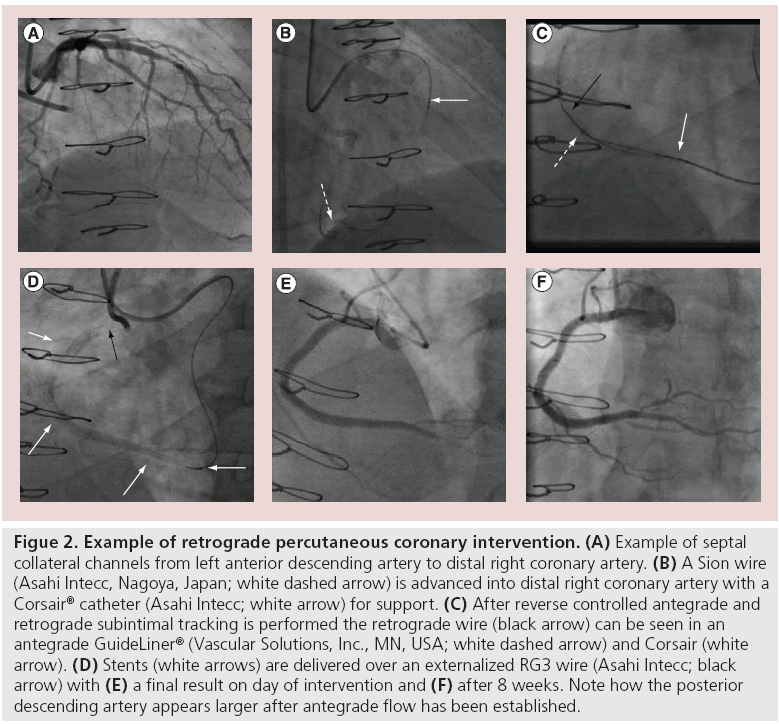
The origin into the septal branch can often be extremely angulated or even retroflex. A workhorse wire with the required tip (often with a significant secondary bend) is used to access the septal branch then a Corsair catheter (150 cm) is advanced into the septal branch. There are two main methods of obtaining retrograde access via septal CCs, the first is ‘septal surfing’ (Figure 2). A Sion wire or Fielder FC (Abbott Vascular, IL, USA) with a short (1 mm) tip with a 40% angle is used to ‘surf ’ the septals CCs. The wire is advanced gently, relying on feel and without contrast. If the wire bunches up it is meeting resistance and is not in the correct channel, and if it starts moving vigorously then it is probably within the ventricular cavity. If either of these scenarios occur then the wire is withdrawn slightly and another channel selected.
Figure 2: Example of retrograde percutaneous coronary intervention. (A) Example of septal collateral channels from left anterior descending artery to distal right coronary artery. (B) A Sion wire (Asahi Intecc, Nagoya, Japan; white dashed arrow) is advanced into distal right coronary artery with a Corsair® catheter (Asahi Intecc; white arrow) for support. (C) After reverse controlled antegrade and retrograde subintimal tracking is performed the retrograde wire (black arrow) can be seen in an antegrade GuideLiner® (Vascular Solutions, Inc., MN, USA; white dashed arrow) and Corsair (white arrow). (D) Stents (white arrows) are delivered over an externalized RG3 wire (Asahi Intecc; black arrow) with (E) a final result on day of intervention and (F) after 8 weeks. Note how the posterior descending artery appears larger after antegrade flow has been established.
If the wire passes without resistance in the anticipated direction then it is allowed to travel. The Corsair catheter can be advanced or withdrawn to allow greater control of the wire.
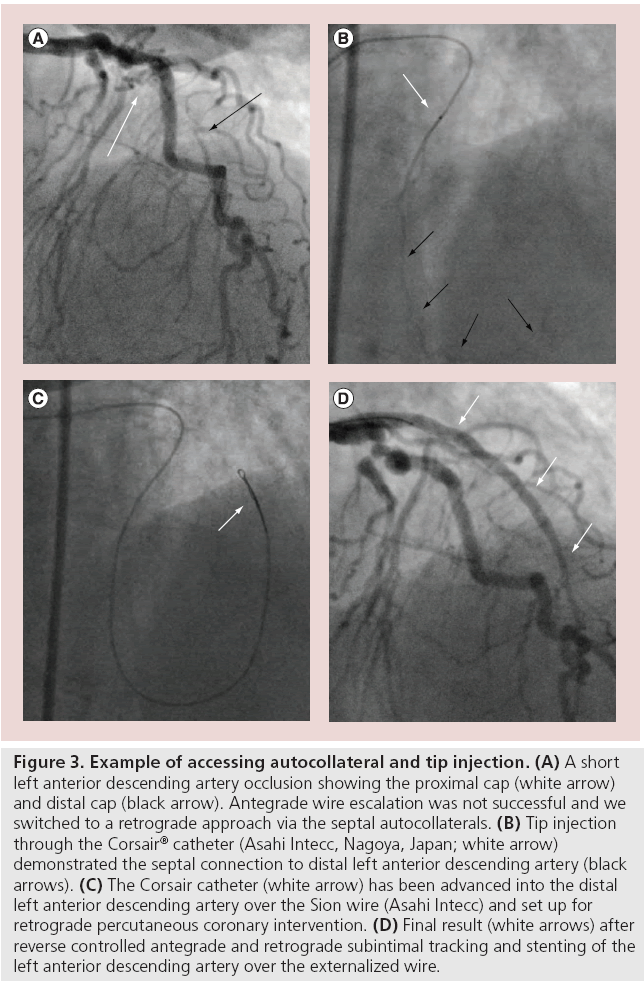
The second method is to perform ‘tip injection’ (Figure 3), using a 3-ml lure-lock syringe and after ensuring there is no air in the catheter neat contrast is injected. If it is not possible to aspirate blood from the Corsair the tip is wedged against the wall of a channel and the operator should not perform injection as this will only cause hydraulic dissection or perforation of the CC. The injected contrast will opacify the CC and a connection will be visible if present. The wire is advanced, followed by the Corsair into the identified channel until resistance is met and then the procedure is performed. If the catheter is in the correct channel, the operator should persist with attempts to wire the distal target vessel. If there is no filling of the distal target vessel then the catheter is not in the correct channel, it is withdrawn and repeat injection performed until the correct channel is identified. This method allows visualization of the CC, but each injection potentially damages the CC plus the contrast can affect wire manipulation, so there is a trade off.
Figure 3: Example of accessing autocollateral and tip injection. (A) A short left anterior descending artery occlusion showing the proximal cap (white arrow) and distal cap (black arrow). Antegrade wire escalation was not successful and we switched to a retrograde approach via the septal autocollaterals. (B) Tip injection through the Corsair® catheter (Asahi Intecc, Nagoya, Japan; white arrow) demonstrated the septal connection to distal left anterior descending artery (black arrows). (C) The Corsair catheter (white arrow) has been advanced into the distal left anterior descending artery over the Sion wire (Asahi Intecc) and set up for retrograde percutaneous coronary intervention. (D) Final result (white arrows) after reverse controlled antegrade and retrograde subintimal tracking and stenting of the left anterior descending artery over the externalized wire.
When the wire reaches the distal target vessel it is manipulated to the distal cap and the Corsair is advanced after. If the Corsair does not pass due to small vessel size of extreme angulation within the septum there are several options. Switching to a fresh microcatheter may be successful. In particular the Corsair catheter can become fatigued or the tip damaged and often a fresh catheter will pass. An anchor balloon in another vessel will help provide more back up to advance the microcatheter, but this is difficult in a 6-F guiding catheter. The septal CC can be dilated with a small balloon. A long, small (1.25 or 1.5 × 20 mm rapid exchange) balloon is recommended and gentle, long inflations (6 or 8 atm for 20–30 s) performed along the CC. After the inflations the microcatheter is advanced.
▪ Retrograde access via epicardial collaterals
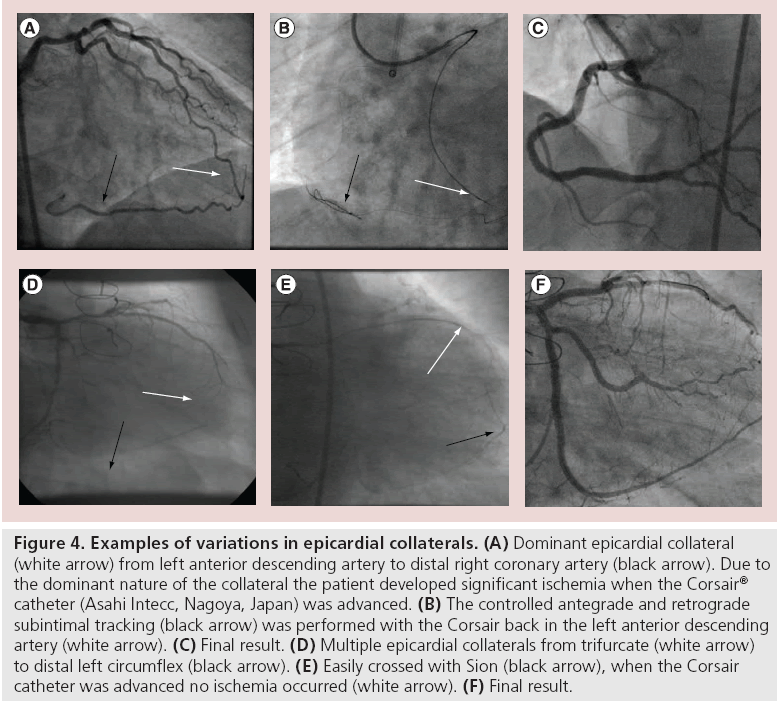
Epicardial CCs range from straightforward short collaterals, for example, from circumflex to postero-lateral RCA, to extremely challenging ‘telephone cord’-like tortuous, high-frequency collaterals, for example, left anterior descending artery to right ventricular branch collaterals. While epicardial collaterals are potentially more dangerous – a perforation will more likely cause pericardial tamponade – they are more predictable. It will become apparent very rapidly whether the collateral is interventional or not and little time is spent finding this out (Figure 4). By contrast, septal CCs are much more unpredictable and frustrating, since some CCs are clearly visualized, but the wire can not go through. In many of those occasions, either minute bending or branching will be the reason of difficulty. Often an operator will spend much time attempting and failing to cross a septal CC.
Figure 4: Examples of variations in epicardial collaterals. (A) Dominant epicardial collateral (white arrow) from left anterior descending artery to distal right coronary artery (black arrow). Due to the dominant nature of the collateral the patient developed significant ischemia when the Corsair® catheter (Asahi Intecc, Nagoya, Japan) was advanced. (B) The controlled antegrade and retrograde subintimal tracking (black arrow) was performed with the Corsair back in the left anterior descending artery (white arrow). (C) Final result. (D) Multiple epicardial collaterals from trifurcate (white arrow) to distal left circumflex (black arrow). (E) Easily crossed with Sion (black arrow), when the Corsair catheter was advanced no ischemia occurred (white arrow). (F) Final result.
Again the Sion wire is the wire of choice when attempting to cross epicardial collaterals. Ensure the wire is ahead of the microcatheter at all times as the Corsair is likely to damage the collateral. If the microcatheter will not cross after the wire has accessed the distal target vessel the options are limited. Aggressive manipulation of equipment will most likely lead to CC injury or perforation. An anchor balloon, guide catheter extension for extra support (GuideLiner®, Vascular Solutions, MN, USA; Guidezilla™, Boston Scientific, MA, USA) or fresh microcatheter are the only other options. Obviously dilating the CC with a balloon is not an option.
Retrograde CTO PCI techniques
Having achieved retrograde access to the distal target vessel it is now possible to attempt retrograde CTO PCI. There are two main umbrella terms to describe the techniques employed: retrograde wire escalation and retrograde dissection re-entry [9].
Retrograde wire escalation is selected as the initial strategy when the CTO is <20 mm [9], the principles are the same as for antegrade wire escalation and the only difference is the direction from which the CTO is approached. The plan is to stay within the occlusion and wire from true lumen to proximal true lumen. A Fielder XT (Asahi) can be selected to probe for microchannels, if this does not traverse the CTO then consider a switch to a Pilot 200 wire (4.7-g jacketed wire) or a penetrative wire, for example, Confianza Pro (Asahi). If it is not possible to wire the true lumen – usually because the wire travels subinitmally – then the operator is in a good position to complete the procedure using retrograde dissection re-entry [9].
Retrograde dissection re-entry is performed if the wire escalation strategy fails or if the CTO segment is long (>20 mm) or the vessel course is ambiguous in the CTO segment (poorly understood course). The main method of dissection re-entry is the CART procedure. The subintimal space is accessed antegradely and retrogradely and equipment is overlapped. The author favors the use of subintimal tracking either with wires or specific dissection tools, for example, CrossBoss catheter (antegradely only). The subintimal space is accessed with a penetrative wire (Confianza Pro) and the microcatheter is advanced about 1 mm into the space. A small loop or ‘knuckle’ is formed on a polymerjacketed wire (either Fielder XT or Pilot 200 wire). This knuckled wire is used to advance either antegrade or retrogradely (or both) within the subintimal space. It is possible to advance wires without knuckling; however, this requires more skill, as long (>20 mm) occlusions often take more time to cross [9]. The knuckle wire technique has several advantages over direct wiring – it is safe and the blunt knuckle will not exit the vessel unless it is forced out a small branch. Long area of occlusion are traversed in relatively short periods of time as the knuckle wire cleaves through planes. There is currently plenty of debate regarding which technique is preferable and time and data will guide us to the best treatment.
When the equipment is overlapped a balloon is inserted into the subintimal space and inflated to connect the spaces. The balloon can come either retrogradely (CART) or antegradely (reverse CART). The reverse CART is the most common procedure performed today as it is easier to bring down an antegrade balloon into the subintimal space, plus the development of Corsair catheter has greatly facilitated retrograde manipulation of guide wires. Once the spaces have been connected, a steerable wire is used to manipulate through the dissection planes into the target-vessel guide catheter or into the aorta.
▪ Externalization of a guide wire
With a retrograde wire in the target-vessel guide it is straightforward to complete the procedure. The Corsair catheter is advanced into the target guide, and a balloon in the distal part of the guide will help ‘trap’ the retrograde wire. It is now fixed and this will help facilitate the advancement of the Corsair through the occluded segment into the guide. When the Corsair is within the guide the short wire is swapped for a externalization wire (330 cm RG3 Ashai Intecc; 335 cm Viper- Wire™, Cardiovascular Systems, MN, USA). The wire is passed antegradely through the Corsair and then out the back of the hemostatic valve at the back end of the target-vessel guide. When the wire is externalized with approximately 15–20 cm of wire outside; the Corsair catheter is withdrawn back through the occlusion segment and even back into the collateral. There is now an externalized wire system which will facilitate antegrade balloon and stent delivery.
Occasionally, it can be challenging to wire retrogradely through dissection planes and then into the target-vessel guide or into the aorta – especially if the disruption is over a long length. To assist with this, it is possible to shorten the distance the wire needs to travel by advancing a guide extension catheter into the occlusion from above (GuideLiner and Guidezilla). It is then a relatively easy to wire into the GuideLiner especially if it is filling the dissection plane. Once the wire is within the guide extension catheter, it can be trapped and the exchange performed as above.
If it is not possible to wire either a guide or guide-extension catheter and the wire is in the aorta, snaring is a rapid and efficient way to achieve access to the target-vessel guide. The three leaf EN Snare® (Merit Medical Center, UT, USA) is easier to use than a gooseneck snare. The wire is first advanced through one of the three loops, and then snared and pulled into the guide and out through the hemostatic valve. The tip which was caught by the snare will have to be cut off to facilitate balloon delivery. It is preferable to snare the externalization wire, but this mandates the Corsair being advanced through the occlusion and into the aorta. Sometimes this is not possible – in this instance it is preferable to snare the soft part of the short wire as close to the transition as possible. The Corsair is then pulled into the guide, the wire is disentangled then removed and externalization is performed as above. In the unlikely scenario of not being able to disentangle the wire, the operator can pull it through the Corsair and out the target vessel guide attached to the snare.
▪ Removal of retrograde equipment
Care is required when removing the retrograde system after the target vessel has been treated. An externalized wire can generate a lot of friction and it is important to manipulate the Corsair back up into at least the stented segment, if not the guide, before withdrawing the externalized wire. This will avoid a potential ‘cheese cutting’ effect of the wire. When the Corsair has been advanced, the operator should pull the externalized wire back with steady force. It is also important to watch that both donor-vessel and target-vessel guides are not drawn into the coronary artery with potential of vessel injury as the wire and Corsair are withdrawn. The operator should always check the donor vessel has not been injured before removing the guide.
▪ Complications specific to retrograde approach
Complications specif ic to the retrograde approach can be categorized into donor vessel or CC complications.
Working in the donor vessel to access CC can lead to vessel injury or dissection especially if the guiding catheter is moving in and out as the Corsair is being manipulated. Vessel spasm can occur if equipment is in situ for long periods – especially if the donor vessel is heavily diseased. Thrombi can form in the CC and can be withdrawn into the donor vessel when Corsair is withdrawn. Care is required when manipulating equipment and it is important to visualize the donor guide and prevent deep intubation. Close checking of ACT will reduce the likelihood of thrombus formation. It is important to check the donor vessel before equipment is withdrawn while there is still an option to fix any complication.
The main risk to the CCs is perforation. In the septum this most commonly causes hematoma which often resolves spontaneously. Even more extensive injury can settle spontaneously with time [10]. It would be very unusual to have to intervene on a septal vessel injury; however, there are reports of septal hematoma causing ‘dry’ tamponade where the septum swells and causes cavity obstruction or outflow problems. This is an extremely rare occurrence. It is for this reason that the septals are the preferred choice for CC crossing for most operators. Epicardial CCs are very easy to injure and perforate. In the setting of previous coronary artery bypass graft and adhesions within the pericardium this is less of a concern but can be a major issue in the ungrafted patient. In addition, the flow into the perforation is often bidirectional and coiling or embolization of the CC has to be performed from both sides.
Conclusion
In conclusion, retrograde CTO PCI is an integral part of any significant CTO program and at least a third of all cases are suitable for the retrograde approach. There is a learning curve involved in gaining the skill set required, but this is simply a matter of teaching and training. However, the rewards make this investment worthwhile as it is associated with very high success rates when the distal target vessel has been accessed. There are ongoing studies to look at the longer-term outcomes of these procedures both in the form of registries and clinical trials.
Future perspective
The patient population attending for coronary intervention continues to age with increasing comorbidity and frailty. Technology continues to advance steadily. As a result, coronary artery disease, which was deemed either suitable for only surgical revascularization or medical therapy, is now being treated successfully percutaneously. A major reason for referral for coronary artery bypass graft is the presence of an occluded artery. With the improved technology and techniques, including teaching and training, more patients with CTOs will be treated with PCI. This area will evolve to the extent that every major hospital will have a meaningful CTO program and patients will be offered revascularization based on merit and not whether there is someone capable of performing the technique or not.
Financial and competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
▪ Contemporary chronic total occlusion percutaneous coronary intervention (PCI) mandates being able to perform retrograde PCI safely and effectively.
▪ Set up and preprocedural planning are critical to success.
▪ Set up can involve different access site and guide catheter selection. Being aware of the pros and cons of each is important.
▪ Being able to identify and select an interventional collateral channel is key to success.
▪ Once retrograde access has been achieved completing the PCI is achieved in 95% of cases.
▪ There are various steps required to complete the PCI and these are listed in the review.
▪ Complications can occur and being mindful of the various issues that cause these complications is important.
References
- Kahn JK, Hartzler GO. Retrograde coronary angioplasty of isolated arterial segments through saphenous vein bypass grafts. Cathet. Cardiovasc. Diagn. 20, 88–93 (1990).
- Surmely JF, Tsuchikane E, Katoh O et al. New concept for CTO recanalization using controlled antegrade and retrograde subintimal tracking: The CART technique. J. Invasive Cardiol. 18, 334–338 (2006).
- Lane RE, Ilsley CD, Wallis W, Dalby MC. Percutaneous coronary intervention of a circumflex chronic total occlusion using an epicardial collateral retrograde approach. Catheter. Cardiovasc. Interv. 69, 842–844 (2006).
- Tsuchikane E, Yamane M, Mutoh M et al.; Retrograde Summit Investigators. Japanese multicenter registry evaluating the retrograde approach for chronic coronary total occlusion. Catheter. Cardiovasc. Interv. doi:10.1002/ ccd.24823 (2013) (Epub ahead of print).
- Thompson CA, Jayne JE, Robb JF et al. Retrograde techniques and the impact of operator volume on percutaneous intervention for coronary chronic total occlusions an early US experience. JACC Cardiovasc. Interv. 2, 834–842 (2009).
- Sianos G, Barlis P, Di Mario C et al.; EuroCTO club. European experience with the retrograde approach for the recanalisation of coronary artery chronic total occlusions. A report on behalf of the EuroCTO club. EuroIntervention 4, 84–92 (2008).
- Werner GS, Ferrari M, Heinke S et al. Angiographic assessment of collateral connections in comparison with invasively determined collateral function in chronic coronary occlusions. Circulation 107, 1972–1977 (2003).
- Brilakis ES, Banerjee S, Lombardi WL. Retrograde recanalization of native coronary artery chronic occlusions via acutely occluded vein grafts. Catheter. Cardiovasc. Interv. 75, 109–113 (2010).
- Brilakis ES, Grantham JA, Rinfret S et al. A percutaneous treatment algorithm for crossing coronary chronic total occlusions. JACC Cardiovasc. Interv. 5(4), 367–379 (2012).
- Fairley SL, Donnelly PM, Hanratty CG, Walsh SJ. Images in cardiovascular medicine. Interventricular septal hematoma and ventricular septal defect after retrograde intervention for a chronic total occlusion of a left anterior descending coronary artery. Circulation 122, e518–e521 (2010).