Review Article - Interventional Cardiology (2015) Volume 7, Issue 2
Revascularization for stable ischemic heart disease: are there new parallels between percutaneous coronary intervention and coronary artery bypass grafting?
- Corresponding Author:
- T Bruce Ferguson Jr
East Carolina Heart Institute
Department of CV Sciences
Brody School of Medicine at ECU
Greenville, NC, USA
E-mail: fergusont@ecu.edu
Abstract
In patients with stable ischemic heart disease, revascularization is undertaken when optimal medical therapy fails, using percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) as appropriate and determined by the local Heart Team. For years, both revascularization strategies have been based on angiographically assessed anatomy alone. Now, using anatomy and physiology to guide intervention has been definitively shown to be superior to an anatomy-only approach in multicenter trials in PCI, documenting the importance of the regional myocardial perfusion status on outcomes. Since cath-lab physiologic evaluation is a pre-intervention assessment that impacts PCI outcomes, is regional myocardial perfusion important to CABG outcomes as well? Novel intraoperative imaging data are presented to document that in stable ischemic heart disease, competitive flow, graft patency and late mortality in CABG are influenced by the regional myocardial perfusion status of the grafted vessels. This new information should improve the outcomes of CABG as has occurred with PCI.
Keywords
CABG, coronary artery bypass grafting, FFR, fractional flow reserve, fluorescence angiography, iFR, instantaneous diastolic pressure ratio, ischemia, ischemic heart disease, myocardial resistance, PCI, percutaneous cardiovascular intervention, perfusion analysis
Case examples
LAD stenosis: case #1: significant increase in perfusion
This 65-year-old male presented with a non-ST-elevation myocardial infarction (NSTEMI) and severe ischemic cardiomyopathy (EF 10%), and Class III–IV congestive heart failure (CHF). At catheterization he was found to have three-vessel coronary artery disease (CAD). Optimal medical therapy was instituted, and a preoperative MRI showed significant anterolateral ischemia. Based on this, he was recommended for surgical revascularization. The proximal left anterior descending coronary artery (LAD) lesion was interpreted anatomically as 70–75% stenosis, and suitable for bypass grafting.
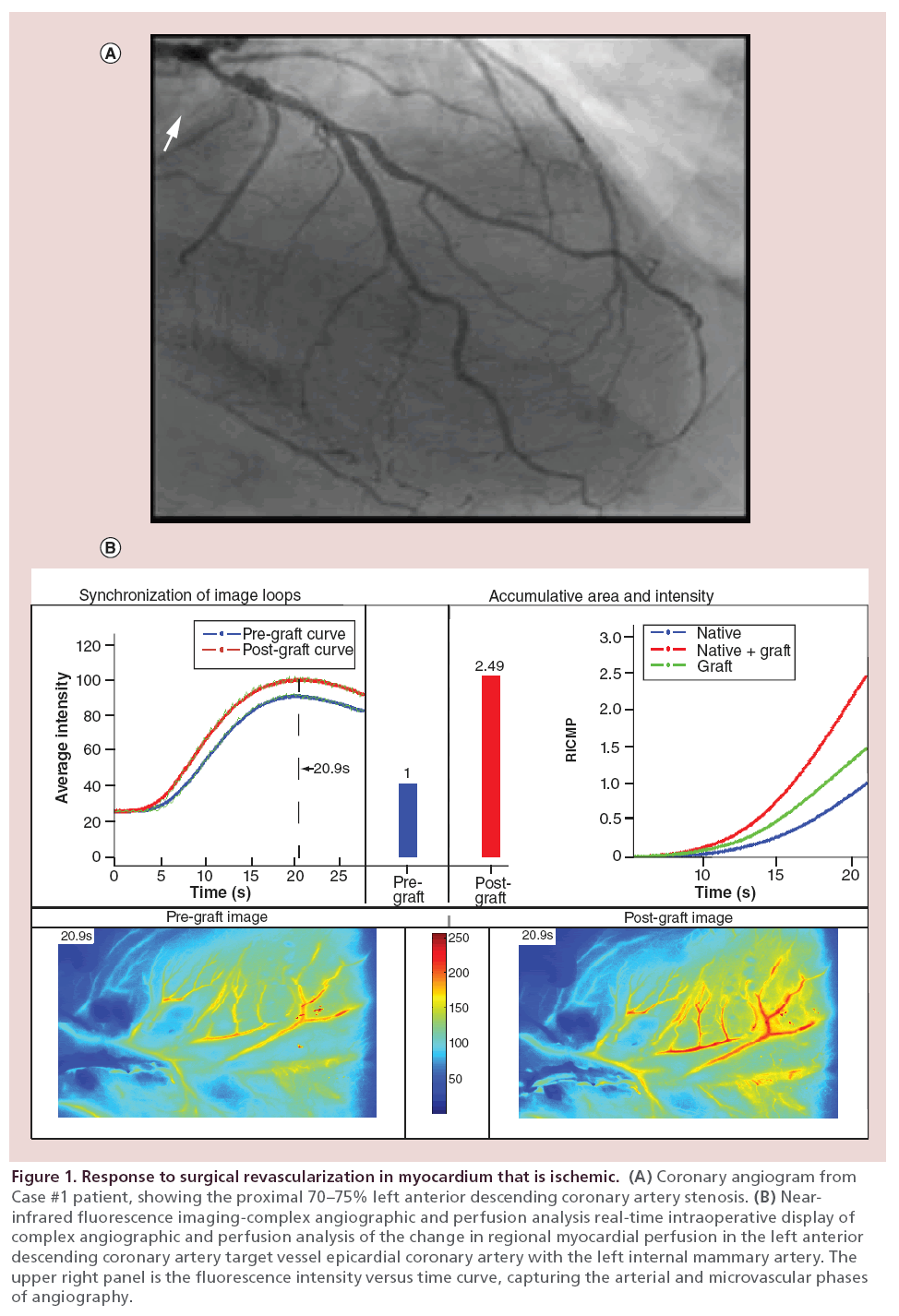
Coronary angiography shows the 70–75% LAD stenosis in the proximal LAD (Figure 1A, arrow). A skeletonized left internal mammary artery (LIMA) was placed to the LAD in a beating-heart, cardiopulmonary bypass (CPB)-supported coronary artery bypass grafting (CABG). There was torrential flow down the LAD. Despite the proximal stenosis and the torrential flow, there was a 2.5-fold increase in perfusion to the anterior wall with grafting as documented by the near-infrared fluorescence (NIRF) Complex Angiography and Perfusion Analysis (CAPA; green line and red bar; Figure 1B).
Figure 1: Response to surgical revascularization in myocardium that is ischemic. (A) Coronary angiogram from Case #1 patient, showing the proximal 70–75% left anterior descending coronary artery stenosis. (B) Nearinfrared fluorescence imaging-complex angiographic and perfusion analysis real-time intraoperative display of complex angiographic and perfusion analysis of the change in regional myocardial perfusion in the left anterior descending coronary artery target vessel epicardial coronary artery with the left internal mammary artery. The upper right panel is the fluorescence intensity versus time curve, capturing the arterial and microvascular phases of angiography.
LAD stenosis: case #2: no increase in perfusion
This 73-year-old male presented with chronic stable angina, status/post proximal LAD stenting 6 years earlier. Nuclear study was equivocal in the anterior wall, and overall ejection fraction was normal with mild lateral wall hypokinesis.
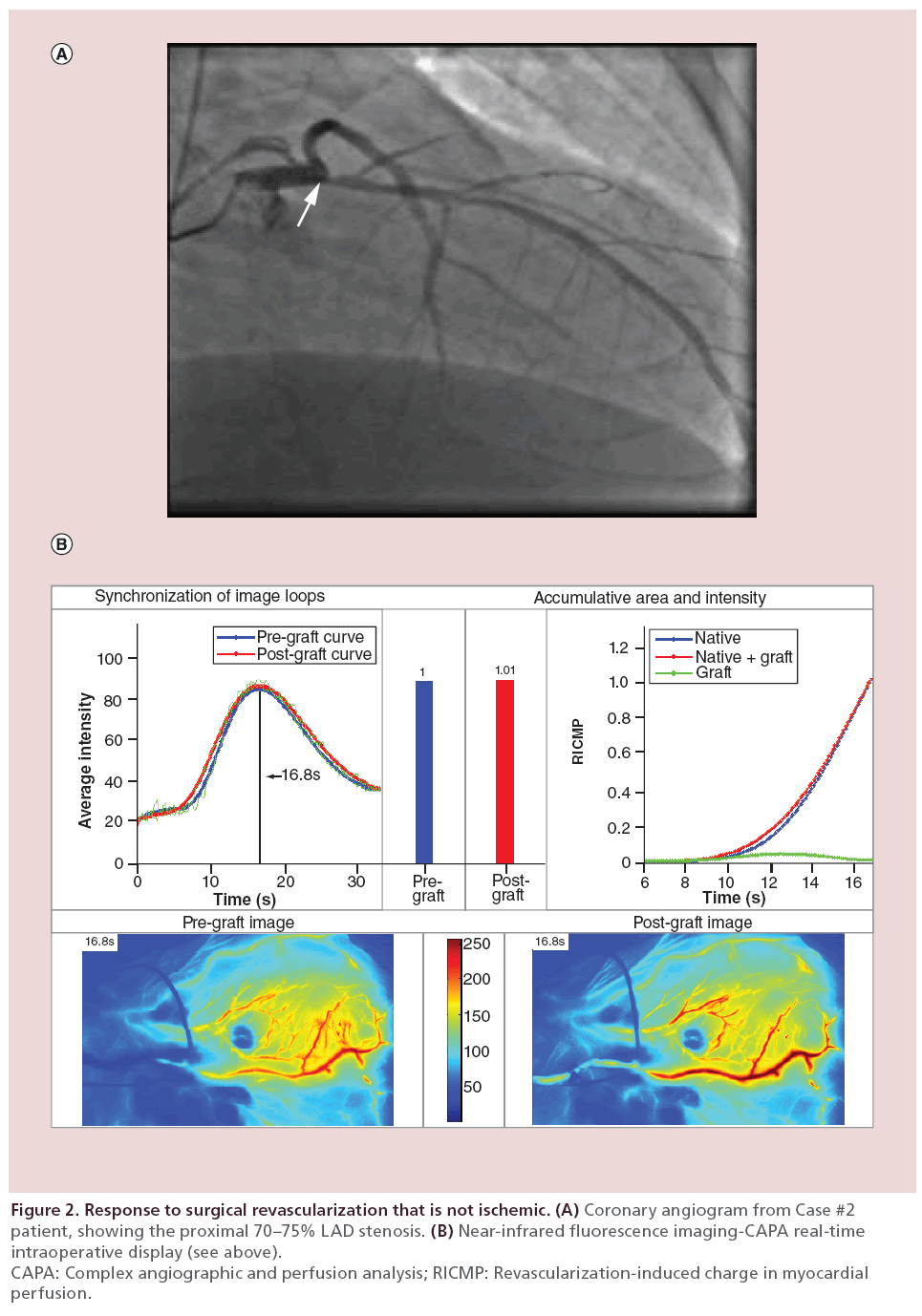
Cardiac catheterization showed a discrete very proximal LAD lesion (Figure 2A, arrow), diagonal and circumflex disease, and an occluded right coronary artery (RCA) with no graftable distal vessels. He underwent an elective off-pump coronary artery bypass grafting (OPCAB) with the right internal mammary artery (RIMA) to the LAD. Despite this proximal stenosis, there was competitive flow between the RIMA graft and the LAD, and no increase in perfusion documented by NIRF and CAPA analysis (Figure 2B, green line and red bar). Other grafts placed were the LIMA to the circumflex marginal, and a saphenous vein graft (SVG) to the DIAG1.
Figure 2: Response to surgical revascularization that is not ischemic. (A) Coronary angiogram from Case #2
patient, showing the proximal 70–75% LAD stenosis. (B) Near-infrared fluorescence imaging-CAPA real-time
intraoperative display (see above).
CAPA: Complex angiographic and perfusion analysis; RICMP: Revascularization-induced charge in myocardial
perfusion.
Please see the remainder of the manuscript for additional details on the NIRF + CAPA analysis platform in CABG.
The anatomic context for revascularization: historical perspective
The development of techniques for routine selective coronary angiography by Mason Sones and colleagues and reported in 1962 provided the anatomy-based framework for revascularization of the heart that has endured for the past 50 years [1]. The initial classification of one-, two- or three-vessel (anatomic disease) persists today in the SYNTAX trial score, for example.
Once stenoses on angiography could be identified, the idea of an important or ‘critical’ anatomic stenosis grew out of important clinical observational and experimental data. William Proudfit at the Cleveland Clinic, a close colleague of Mason Sones, examined the natural history of patients with now angiographically defined coronary artery disease. Proudfit noted that patients with more epicardial coronaries involved (i.e., three- vs two-vessel disease) and with a 50% stenosis defining significant disease (using 1960s cineangiography technology) appeared to have worse survival than patients with fewer coronaries involved [2]. Experimentally, Gould documented in the 1970s that focal stenoses of at least 85% were necessary to significantly reduce resting myocardial blood flow and perfusion to the distal myocardium. Hyperemic flow (mimicking raised demand above resting) could be attenuated by constrictions from on 30% and above depending on lesion length [3].
Once a ‘roadmap’ was available, it did not take long for ‘bypassing of the stenosis’ techniques to be developed by Drs Effler, Favaloro and Loop at the Cleveland Clinic in the mid-1960s. The documentation of an anatomical roadmap, with stenoses, was therefore critical to the development of the techniques for surgical revascularization for patients with clinical ischemia and documented anatomic stenoses. The fundamentals of this anatomical approach and these techniques persist today in CABG as practiced worldwide [4].
Andreas Gruntzig, who developed the technique of percutaneous transluminal coronary angioplasty (PTCA), did in his early work demonstrate a rise in distal pressure after balloon dilatation of a lesion, a precursor to the much later development of clinically useful coronary physiology [5]. Quickly and for many years after the first angioplasty, however, the technical outcome metrics for this second invasive revascularization strategy for ischemic heart disease focused exclusively on the elimination of the visualized angiographic stenosis [6]. Based on Proudfit’s observations and Gould’s experimental data, anatomic lesions defined angiographically as >50% or >70% were felt to be important enough to require intervention (PTCA or grafting).
With the advent of the percutaneous stent era, attention rapidly focused on which of the two technologies was the better approach. As articulated by Taggart, the overwhelming majority of the initial randomized non-inferiority trials of percutaneous coronary intervention (PCI) versus CABG that did suggest equivalence included very low-risk patients, and in particular low-risk surgical patients [7].
In these randomized patients, most had single or twovessel coronary disease with normal ventricular function and minimal if any history of prior myocardial infarction. As we have subsequently learned from more recent trials, intervention of either kind would not be expected to demonstrate much benefit. That is, a target vessel epicardial coronary artery (TVECA) could be stented or grafted, because the myocardial substrate was essentially free of prior disease and the overall global myocardial blood flow and function was normal. Unless these lesions were critically flow limiting, a change in blood flow to the normal myocardium (without evidence of a perfusion deficit or ischemia) would not be expected to be of much physiologic benefit, if it occurred at all.
It must be stated unequivocally that a remarkable amount of observational data from multiple regional and national databases has supported the benefits of this historical anatomy-based surgical revascularization [8–10]. That said, it remains surprising that amid all of the important technologic developments in cardiac surgery at the present time, a serious re-evaluation of an anatomic-only-based approach to surgical revascularization has yet to be entertained by most surgeons. A recent 2014 ‘State of the Art Coronary Artery Bypass Graft’ panel discussed bilateral Internal Mammary Artery (IMA) grafting, stroke prevention and no-touch technique, radial artery and SVG patency issues (including competitive flow), off-pump surgery, minimally invasive CABG/hybrid/robotic procedures and graft assessment [11]. While almost every aspect of the discussion was focused on the anatomic and technical aspects of CABG, there was also a brief discussion about graft indications (anatomy vs physiology), and a potential NHLBI Cardiac Surgery Network FFR-Guided CABG trial. Perhaps unfortunately, most of the panelists discounted the potential importance of preoperative fractional flow reserve (FFR) data to influence decisions at CABG. Those who did not, however, perhaps actually hit the nail on the head, as documented in the two case studies.
This analysis of current evidence and novel intraoperative imaging data support reevaluation of these fundamental tenets of surgical revascularization as timely, not to conflict with this remarkable history of CABG but to indicate how the procedure can continue to be improved in the future.
Disruptive influences on this anatomy-based approach to revascularization
In standard ischemic heart disease (SIHD), the physiology data generated in the catheterization laboratory have documented that the functionality of an anatomic coronary stenosis is determined by the physiology of the vasculature and distal perfused myocardium. This is perhaps the most important new influence that interventional physiology data introduce into the CABG setting. Rather than focusing on just global anatomy and myocardial function/dysfunction, the surgeon is now challenged to consider to concept that the regional myocardium linked to the grafted TVECA influences outcomes.
The ‘hypothesis’ generated from our imaging studies is that in contemporary CABG the regional myocardial perfusion (RMP) status is emerging as a key factor in CABG design, technical strategy and outcomes. In the traditional technical approach with cardiopulmonary bypass and cardioplegic arrest, this has not been a consideration, except in surgical revascularization for acute coronary syndrome with acutely ischemic myocardium. In SIHD, however, this RMP status can be influenced by any combination of a perfusion deficit, stable ischemia, the presence of collateral inflow and outflow from adjacent myocardial regions, or the absence of any of these factors. Importantly, this directs attention to the TVECA ‘unit’, consisting of the TVECA, the graft, the anastomosis and the RMP, as important in determining outcomes on a per graft basis.
RMP status
As mentioned, the fundamental techniques of CABG were developed on patients with proximal coronary lesions, normal distal vasculature and normal ventricular myocardium [4]. Using this surgical strategy based on anatomy, the clinical outcomes for CABG showed steady improvement over the past two decades. However, beginning about 3 years ago, the risk-adjusted isolated coronary bypass mortality has been constant and not declining further across the country, fluctuating around 1.8–2.0%, based on data from STS National Database. This may reflect an asymptote for mortality in this more contemporary patient population, or reflect some other characteristics of the underlying ischemic heart disease process. It also suggests that pushing this mortality benchmark lower might require some re-thinking about the CABG procedure itself.
Today, in excess of 50% of surgical patients have sustained prior myocardial injury. While the overall mean ejection fraction of isolated CABG patients remains nearly normal, the percentage of patients with some impairment of ventricular function has risen steadily over the past decade, to greater than 16%. Almost 20% of CABG patients have CHF. Approximately 35% of patients have undergone prior coronary stenting, and the overall severity (diffuseness of disease, ungraftable coronary arteries, diabetic microvascular disease) of epicardial coronary disease has continued to worsen [12,13]. All of these circumstances have worsened the RMP status of patients coming to CABG. No longer can the impact of placing a bypass graft to a TVECA be evaluated assuming normal regional myocardium distal to the anastomosis. How does the RMP status impact what we already know about CABG graft characteristics of competitive flow, graft failure and completeness of revascularization?
Competitive flow in arterial grafts
Competitive flow (CF) has been the subject of some 200 articles in the coronary surgery literature, including experimental evaluation [14] and demonstration of the dynamic arterial conduit [15]. This literature suggests an inverse relationship between percent angiographic stenosis and CF: if the percent of anatomic stenosis influences CF, and CF influences graft patency, then graft patency should be dependent on the percent stenosis. Based on this assumption, arterial grafts are thought to fail (outside of technical reasons) due to competitive flow between the native TVECA and the graft [11,16,17]. Thus the same anatomic stenoses should have relatively similar degrees of CF.
Interestingly, CF between the TVECA and the graft has never been imaged in real time at surgery until recently, and our real-time imaging data refute this assumption. As illustrated in the ‘Case examples’ section, the Case #2 patient had a 70% stenosis that would be considered significant enough so as not to induce a CF situation, but in fact there was angiographically documented CF between the RIMA and LAD by NIRF angiography. In Case #1, there was a similar 70% stenosis, but torrential flow down the LAD. As noted, despite this there was NO CF with this LIMA graft to the LAD.
So what differentiates these two 70% stenoses? Is it the flow volume down the TVECA? The imaging data in Case #1 demonstrates otherwise. The presence of CF in Case #2 suggests that this patient’s LAD lesion is not hemodynamically significant, despite the numerical assessment. What could account for this numerical similarity but hemodynamic difference? As clearly documented in the CAPA quantifying the change in RMP as a result of bypass grafting in these two cases, it is the functionality of the myocardium, rather than the numerical stenosis, that drives the presence or absence of CF in arterial grafts. Case #1 has a 2.5-fold increase in perfusion with grafting the LAD, while Case #2 has no increase despite a widely patent LIMA graft. As presented last year, approximately 20% of angiographically patent in situ IMA grafts to TVECA with proximal stenoses >70% had no increase in myocardial perfusion documented by NIRF-CAPA analysis; over 90% of these grafts had documented CF with the TVECA [18]. Why does the functional status of the regional myocardium drive the presence or absence of CF in angiographically patent grafts, assessed in real time at surgery?
SVG failure
Critical examination of the angiographic outcomes from CABG in randomized trials where protocolspecified coronary angiography was performed have documented a surprisingly high incidence of SVG failure/occlusion. In the PREVENT IV study, the pergraft occlusion rate was 25% for SVGs, determined angiographically at 12–18 months [19]. In the more recent ROOBY trial, the vein graft occlusion rates in the 64.5% of patients who underwent follow-up angiography, 32% of patients had at least one graft occluded and 20% of SVGs were occluded at 1 year overall [20].
There is some disagreement as to whether SVGs actually exhibit CF with the TVECA. Because it is a ‘pipe’, a SVG might not be influenced by CF because of the volume of flow down the graft versus the TVECA [11]. Indeed, in our real-time imaging of over 1000 grafts at the time of beating-heart CABG, we do not believe we have seen CF in SVGs confirmed to be widely patent by NIRF angiography [21]. This is not true for radial grafts, where approximately 20% of grafts to 70% or greater stenoses in TVECAs show CF.
The incidence of technical issues as a cause of graft failure has been difficult to ascertain, because worldwide only 25% of patients undergo intraoperative evaluation of graft flows or documentation of graft patency [22]. Using NIRF angiography, Taggart, Fremes and others reported a 2–4% intraoperative graft revision rate in small, single-institution studies, a rate much lower than the ultimate graft failure rate [23–25]. Thus if 2–4% of grafts fail from unrecognized acute technical issues, and vein grafts are not subject to CF, then the 25% graft failure rate at 12–18 months must be induced by something other than these two factors. Moreover, graft failure within this intermediate (12–18 months) time frame is not likely associated with progression of the underlying coronary disease.
This high failure rate has also been out of context with otherwise excellent clinical outcomes. In these protocol-specified patients with vein graft occlusions in PREVENT IV, only 7.1% actually presented with clinical symptoms during this 12–18 month interval [26,27]. In fact, the vast majority of these SVG failures were clinically silent; that is, the loss of blood flow down that graft to that myocardium was not associated with a perfusion deficit or ischemia-producing angina or an infarct. In the ROOBY trial, despite a higher SVG failure rate in the OPCAB group, there was no difference in non-fatal myocardial infarction (MI) between groups, and when crossovers were removed from the overall analysis there was no difference in the 1-year primary end point of death from any cause, and non-fatal MI and/or revascularization (30 days to 1 year) [20].
A very recent report from the PREVENT-IV study examined the potential causes of graft failures in that study [28]. The analysis of patients operated upon in 2003–2004 identified angiographic failure with longer operation, endoscopic vein harvesting, poor target artery quality and postoperative clopidogrel/ticlodipine use multivariate risk parameters. Interestingly, both the paper and an accompanying editorial both fail to consider that the physiologic status of the RMP supplied by the grafted coronary artery might also influence graft patency.
Thus in aggregate between 20 and 25% of vein grafts were found to be stenotic or obstructed, most without clinical symptoms, in these studies. Clearly these failures cannot all be attributed to technical issues, competitive flow, vein trauma or postoperative pharmacology. Could the status of the RMP influence graft failure? If these grafts failed without overt clinical sequelae, then were these grafts necessary in the first place?
Multi-arterial grafting
Numerous studies have documented that bilateral IMA (BIMA) grafts convey a late (year 10–20) survival benefit compared with single IMA grafting [29,30]. What is the possible mechanism for this, particularly accounting for an effect that only emerges after 10 years?
In SIHD, the overwhelming determinant of longterm survivability is preserved ventricular function, across an infinite spectrum of anatomic atherosclerotic disease [31–33]. The conveyance of a survival benefit from BIMA grafting must protect and preserve the existing myocardial status, potentially in spite of underlying disease progression. How do two in situ arterial conduits which carry substantially less volume flow that SVGs preserve myocardium? It may be that the biologically active nature of in situ IMA grafts, particularly in producing nitric oxide at the endothelial level distal to the anastomosis, making the distal vasculature more resistant to disease progression [34]. Whatever the exact mechanism(s), the tight coupling between the arterial graft and the distal myocardium is inherently key to the late survival benefit. If this late survival benefit is not based on flow, and not based only on anatomy, then does this dynamic functional revascularization issue directly contribute to this late survival benefit?
The completeness of revascularization
In surgery we have seen the completeness revascularization (CR) definition evolve rather markedly over time [35]. The prevailing context used in the STS Database and elsewhere is the link between anatomy and grafts: ‘anatomic three-vessel disease = three bypass grafts’ [36]. The inadequacy of this definition is illustrated by Dauermann’s description of a more contemporary reasonable incomplete revascularization (IR) [37]. This contemporary departure from a solely anatomy construct for CR/IR is influenced by two factors: the emerging understanding of the importance of anatomic lesion functionality as discussed below and the emerging importance of the RMP status in patients with SIHD.
The SYNTAX trial used the most rigid anatomic basis for defining CR (the SYNTAX score) [38,39]. That said, 56% of PCI patients and 43% of CABG patients had IR by these pre-specified, pre-interventional anatomic criteria. Incomplete revascularization in SYNTAX CABG patients increased with SYNTAX Score tercile, but was not associated with statistically significant major adverse cardiac and cerebrovascular events (MACCE) at 3 or 5 years versus CR. Even with this degree of surgical IR, SYNTAX documented a survival and freedom from late MI benefits versus multi-vessel PCI, in parallel with the increase in IR as defined by terciles [40]. This finding from SYNTAX clearly raises for the surgical community the need to rethink the anatomy-only basis for complete surgical revascularization [41]. How was this mortality benefit achieved, despite this anatomic incomplete revascularization incidence?
The physiology of revascularization: PCI & CABG
Even more compelling data has come from the persistent revolution in PCI involving the limitations of a purely anatomic approach to percutaneous revascularization in SIHD. In aggregate, the results of the DEFER, FAME I and FAME II studies clearly support a reevaluation of the use of a purely anatomic construct for percutaneous revascularization [42–46]. The DEFER 5-year follow-up data clearly showed deferring PCI if the FFR was >0.75 was associated with an annual rate of death or myocardial infarction of 1% [45]. The FAME and FAME II trials of anatomic versus FFRguided multivessel PCI (FFR cut-off of 0.80) unequivocally demonstrates a physiologic-guided approach to PCI is superior to a purely anatomic one, conferring hard clinical benefits along with reduced healthcare costs. These studies have established the principle that the clinical status of the RMP distal to the TVECA is critical in determining in SIHD whether percutaneous intervention should be performed, beyond the anatomic angiographic findings alone.
More recently, studies have documented the potential importance of FFR and functional evaluation in STEMI and NSTEMI patients with acute coronary syndrome [47–51]. While there are limitations to FFR and instantaneous diastolic pressure ratio (iFR) in ACS, and the limitations of FFR and iFR in SIHD are known, the appropriate use of these technologies to derive physiologic functional data and drive decision-making are well documented.
In addressing the discrepancy between anatomicbased intervention and functional-based intervention demonstrated by these studies, the current American Heart Association guidelines now recommend that FFR should be used for assessing lesions of intermediate severity (40–70% stenosis) [52]. These intermediate coronary lesions have long been a target for anatomy-driven PCI, but are now often targets for evaluation with catheterization laboratory coronary physiology (FFR/iFR) prior to PCI [53]. This experience has demonstrated that many visually nonsignificant lesions turning out to be flow limiting by FFR, with the converse also being the case.
FAME documented that more than 50% of lesions with anatomic stenoses between 50 and 70% were not associated with FFR-assessed functionality [43]. Moreover, in the range of 70–90% anatomic stenoses, 20% of lesions were not functionally significant. From a surgical perspective, all surgeons will graft all TVECAs with a stenosis >70% according to the anatomy-based approach to revascularization; it is lesions of this severity range that would be influenced by CF, graft attrition and the status of the RMP. Percutaneous intervention on these intermediate (40–70%) and more severe anatomic (70–90%), nonfunctional lesions was associated with a higher complication rate, less optimal outcomes and increased healthcare costs.
Botman et al. correlated the functionality of the stenosis by FAME criteria in a TVECA bypassed at the time of CABG with angiographic graft patency at 1 year [54]. Overall patency data were 91% of grafts to functional stenoses versus 78.6% to nonfunctional stenoses. Of note, there was no difference in angina class between patients with or without occluded grafts at 1 year, consistent with the PREVENT IV findings. As expected, for nonsignificant stenoses less than 50% the incidence of graft failure was highest by both angiographic and functional criteria. Arterial versus SVG failure rates were 21.9 and 20.0%, respectively, overall for these nonsignificant lesions. In the range of significant stenoses between 70 and 90%, the graft failure rate was 6.7% overall; in this subset, overall failure rates by graft type were 13.7% for arterial grafts and 5.9% for SVGs. In intermediate lesions (50–70% stenosis), patency rates were 91.2 versus 79.8% for functional versus nonfunctional stenoses. Despite this incidence of graft occlusion, only 8/164 patients required clinically driven recatheterization; all eight had failure of a graft beyond a significant (>70%) stenosis. In this only study to correlate functional anatomy with surgical outcome to date, the results are surprisingly consistent with the concept that functionality is as important in surgical revascularization as it is in percutaneous revascularization. The data presented in the two case examples confirm how important these concepts are to the setting of surgical revascularization. If PCI is not appropriate for these lesions, why should vessels with the same nonsignificant, nonfunctional lesions be subject to grafting? Finally, is the 20% nonfunctionality incidence and the 25% graft failure rate incidence in CABG a coincidence?
Emergence of long-term mortality as a consideration following revascularization with CABG & PCI
A recent large-cohort observational study by Froelich et al. demonstrated no difference in late mortality in SIHD and NSTEMI patients with or without functionally guided intervention versus anatomic-guided intervention alone [55]. Similarly, no mortality benefit was demonstrated in the FAME trials with FFR-guided intervention. Malhotra suggested that documentation of the functionality of the intermediate and more severe stenoses generates data to guide a more physiologically complete revascularization, with better clinical and cost outcomes overall [56]. The absence of a mortality benefit is likely because the combination of the anatomy, functionality and myocardial substrate is not severe enough that PCI can affect mortality in these patients.
By contrast, long-term mortality has emerged as an increasingly important consideration in CABG [57]. In parallel with the Froelich study, almost all of the initial PCI versus CABG randomized trials involved patient populations where the mortality risk was so low that differences could not be demonstrated with the small sample sizes; rather, mortality as an outcome was folded into the primary composite outcome for these trials. These trial findings were in contrast to large observational data analyses, where there appeared to be a long-term survival benefit associated with multivessel surgical revascularization compared with multivessel stenting.
The head-to-head trials of CABG versus PCI recently reported have now brought convincing randomized trial data to support these observational database findings. The anatomy-based all-comer SYNTAX trial and the FREEDOM trial in diabetic patients both demonstrated a distinct survival advantage, beginning as early as 2 years following intervention, favoring CABG versus multivessel PCI [39,58].
However, rather than argue the merits of the presence or absence of this benefit, it is interesting to ask the question of why coronary bypass grafting conveys a mortality benefit, whereas multivessel PCI does not, in these supposedly equivalent patient populations? Importantly, SYNTAX and FREEDOM also documented that CABG provides freedom from late myocardial infarction as well; that is, surgical outcomes provide ‘protection’ against these late cardiovascular-related events.
Early in the stenting era, Gersh and Frye hypothesized that because surgical grafts are placed beyond the anatomic stenosis that this somehow protects against the subsequent development of anatomic disease, as compared with PCI [59]. This hypothesis was developed during the anatomic era, and is consistent with an anatomicbased strategy for revascularization. To this hypothesis concept we need to add the emerging science of the different rheological, flow dynamic and microvascular resistance changes that we postulate may occur as a result of anastomosing a graft versus implanting a stent [60–63]. In addition, the FAME data and the concept of functional anatomy now suggest that an alternative explanation, one involving changes to the myocardial substrate, is necessary to fully explain the mortality benefit with CABG.
Importantly, these late outcomes benefits were documented in patients with diabetic microvascular ischemic heart disease and with extensive anatomic disease incompletely revascularized as documented in the higher SYNTAX score terciles. Thus, this CABG population in which there is a mortality and freedom from MI benefit is consistent with patients studied by Seiler and colleagues, with prior myocardial insults and the development of myocardial collateralization [64–66]. Meier and Seiler have documented with sophisticated MRI studies that 85% patients begin collateral development/ augmentation after a single episode of severe angina; the absence of collateral development has been linked with increased mortality, while collateral development has been documented to be protective.
As we have seen, these CF, CR versus IR, graft failure and functionality issues cannot be explained based on the historical anatomic basis for CABG alone, and therefore the importance of RMP abnormalities affecting CABG outcomes can no longer be ignored. In addition to influencing these issues, is the RMP status including collateral flow the physiologic substrate for improved survival with CABG?
Lessons learned from intraoperative imaging & quantification of perfusion change effects of TVECA bypass grafting
In 2005, Novadaq technologies received approval from the US FDA for their NIRF angiography technology called SPY. The technology was initially developed to assess visually coronary bypass grafts at the time of surgical intervention.
We began to use this technology in 2006, and now have prospective experience with over 1000 patients undergoing isolated or combined surgical revascularization. Since the overwhelming majority of these CABG procedures were done either as an OPCAB or a cardiopulmonary bypass-supported beating heart revascularization, we could immediately document the pre-grafting and post-grafting physiology of blood flow and perfusion with this technology [18,21,67,68].
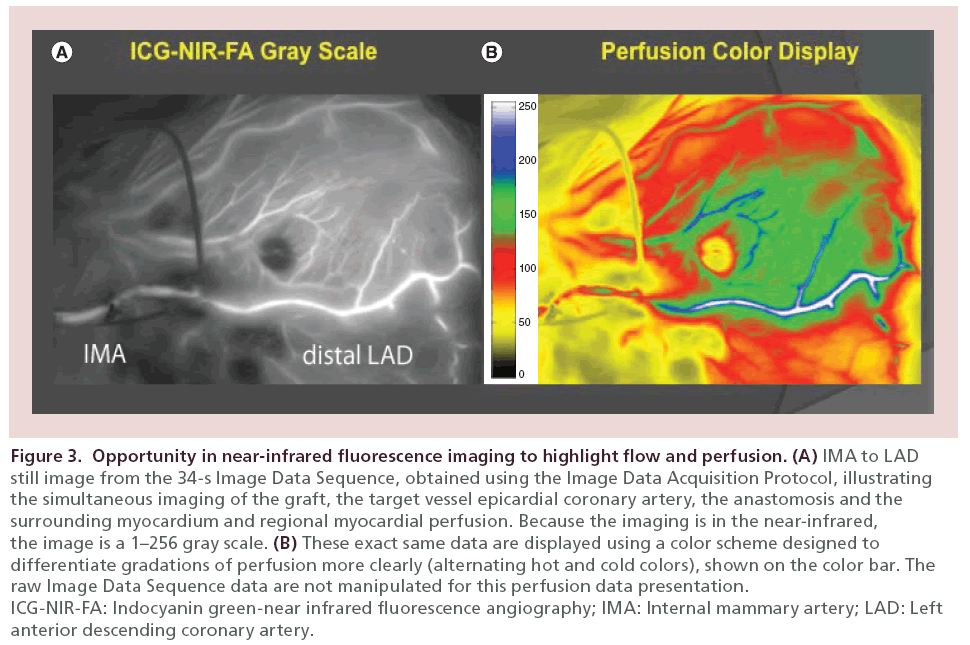
Our first observation was that in the beating heart, the NIRF imaged simultaneous flow down the native epicardial coronary as well as down the bypass graft under physiologic circumstances (Figure 3, left). This illustrated directly the presence or absence of CF. The second observation was that myocardial substrate characteristics could be imaged in real time, including perfusion (Figure 3, right) and the presence of regional collateral flow. The third observation was that the technology could be used to assess RMP to the surrounding tissue supplied by the TVECA, by a relative postgrafting versus pre-grafting relative comparison using a defined Image Data Acquisition Protocol (Figure 4).
Figure 3: Opportunity in near-infrared fluorescence imaging to highlight flow and perfusion. (A) IMA to LAD
still image from the 34-s Image Data Sequence, obtained using the Image Data Acquisition Protocol, illustrating
the simultaneous imaging of the graft, the target vessel epicardial coronary artery, the anastomosis and the
surrounding myocardium and regional myocardial perfusion. Because the imaging is in the near-infrared,
the image is a 1–256 gray scale. (B) These exact same data are displayed using a color scheme designed to
differentiate gradations of perfusion more clearly (alternating hot and cold colors), shown on the color bar. The
raw Image Data Sequence data are not manipulated for this perfusion data presentation.
ICG-NIR-FA: Indocyanin green-near infrared fluorescence angiography; IMA: Internal mammary artery; LAD: Left
anterior descending coronary artery.
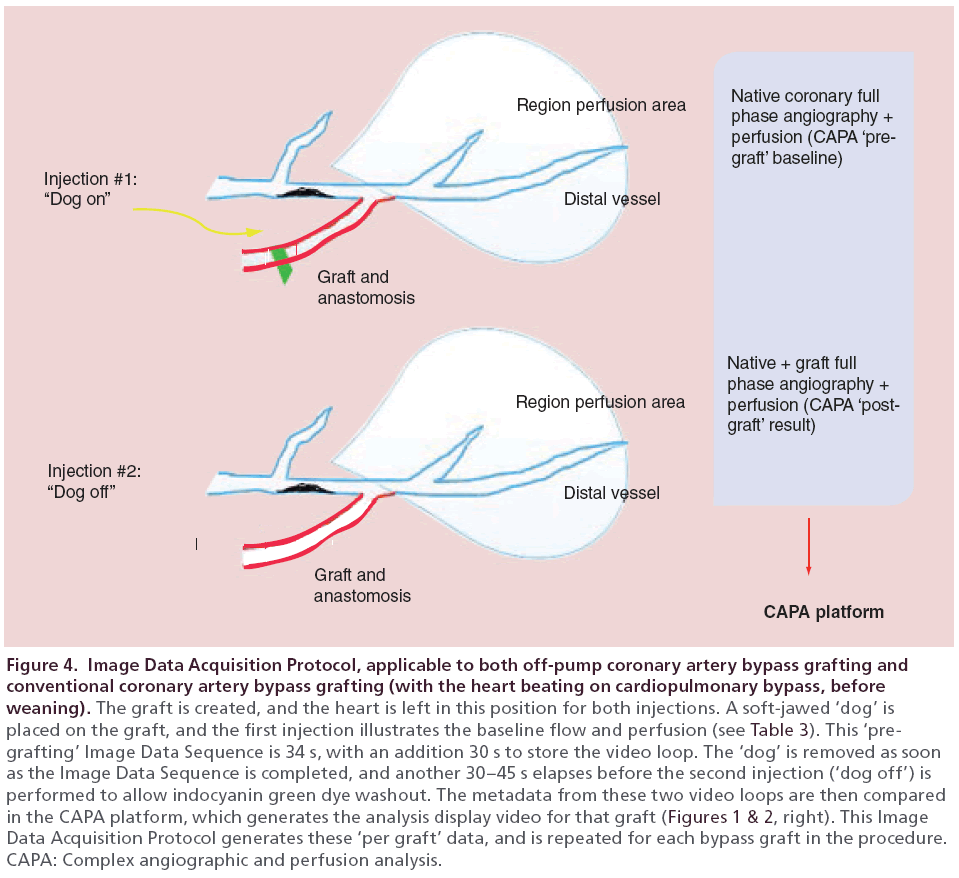
Figure 4: Image Data Acquisition Protocol, applicable to both off-pump coronary artery bypass grafting and conventional coronary artery bypass grafting (with the heart beating on cardiopulmonary bypass, before weaning). The graft is created, and the heart is left in this position for both injections. A soft-jawed ‘dog’ is placed on the graft, and the first injection illustrates the baseline flow and perfusion (see Table 3). This ‘pregrafting’ Image Data Sequence is 34 s, with an addition 30 s to store the video loop. The ‘dog’ is removed as soon as the Image Data Sequence is completed, and another 30–45 s elapses before the second injection (‘dog off’) is performed to allow indocyanin green dye washout. The metadata from these two video loops are then compared in the CAPA platform, which generates the analysis display video for that graft (Figures 1 & 2, right). This Image Data Acquisition Protocol generates these ‘per graft’ data, and is repeated for each bypass graft in the procedure. CAPA: Complex angiographic and perfusion analysis.
Using this large clinical experience, we developed, validated and have tested the CAPA analytical platform that allows for the quantification of the change in RMP immediately before and after coronary bypass grafting to a TVECA. We also developed software to take these regional results and integrate them into a global assessment of the change in RMP obtained with multi-vessel surgical revascularization.
Our studies thus far have demonstrated the following:
• Despite a widely angiographically patent in situ IMA, radial artery or SVG to a TVECA with a minimum 70% angiographic stenosis, an actual increase in RMP only occurs in approximately 77% of grafted TVECAs (see Figures 1 & 2). Table 1 illustrates this for a series of twoand three-vessel consecutive beating heart cases (n = 359 grafts). Since the volume of flow and the nature of the conduit are different, the fact that ∼23% of grafts do not produce a perfusion increase correlates with the FAME study and the Botman study, and supports our hypothesis that RMP status influences the physiologic outcomes from each bypass graft;
| Graft type | Arterial (internal mammary artery,radial) | Saphenous vein graft | |||
|---|---|---|---|---|---|
| 192 | 53% | 167 | 47% | ||
| Increase in regional myocardial perfusion | No Increase in regional myocardial perfusion | ||||
| Total grafts n = 359 | 275 | 77% | 84 | 23% | |
Table 1: Overall regional myocardial perfusion response to bypass grafting.
• Within in situ IMA grafts to TVECAs with 70% or greater stenosis, approximately 22% of widely angiographically patent grafts will demonstrate evidence of CF by imaging (Table 2). In 165 in situ grafts, the range of anatomic percent stenosis ranged from 70 to 100%. In future studies, this CF documented at surgery will need to be correlated with long-term patency. This finding based on standardized intraoperative imaging documents the influence of RMP on the presence or absence of CF in arterial grafts;
| Type | Arterial, n (%) | Saphenous vein graft, n (%) | ||
|---|---|---|---|---|
| In situ internal mammary artery | Radial artery | |||
| Increase in regional myocardial perfusion | 165 (46) 125 (76) |
27(8) 20(74) |
167 (47) 130 (78) |
|
| No increase in regional myocardial perfusion | 40 (24) | 7(26) | 37 (22) | |
| Imaged competitive flow | 37 (93) | 1(0) | 0 (0) | |
Table 2: Regional myocardial perfusion response to bypass grafting by graft type and presence of competitive flow.
• The CAPA image data acquisition sequence illustrates for each graft to a TVECA and for the CABG procedure overall the data in Table 3. Compared with conventional coronary angiography and other intraoperative or post-operative evaluation techniques, this careful, detailed evaluation of the graft, and the physiologic consequences of the graft immediately at the time of surgery, provides considerably more technical and quality data about that graft. In parallel with the physiology of PCI revascularization, this real-time analysis is critically important to document at surgery, and for a better understanding of the physiology of CABG as a revascularization strategy;
| Condition imaged | Physiology | Quantification? |
|---|---|---|
| Temporarily-occluded: ‘pre-grafting’ | ||
| Status of native TVECA flow: | Angiography | No |
| – Arterial inflow phase evaluation | No | |
| – Does the graft/anastomosis compromise native flow? | No | |
| Status of native TVECA RMP: | Perfusion | No |
| – Microvascular perfusion phase | No | |
| – Does the graft/anastomosis compromise RMP? | No | |
| Status of graft reflux at anastomosis: | Angiography | No |
| – Pulsatile for in situ arterial grafts | No | |
| – Static for saphenous vein grafts, radial grafts | No | |
| Open: post-grafting | ||
| Status of simultaneous native TVECA and graft flows: | Angiography | Yes (visual) |
| – Inflow timing | Yes | |
| – Inflow delay in in situ IMA vs aortocoronary grafts | Yes | |
| Status of competitive flow in arterial grafts: | Angiography | Yes (visual) |
| – Beats (>3–4 significant) | Yes | |
| – Location (anastomosis vs proximal TVECA) | Yes | |
| Status of change in RMP: | Perfusion | Yes (CAPA) |
| – Change per TVECA | Yes (CAPA) | |
| – Change in TVECAs into global construct | Yes (CAPA) | |
| – Change in myocardial substrate collateral flow | Yes (CAPA) | |
Table 3: New information about bypass grafts determined at coronary artery bypass grafting by qualitative and quantitative near-infrared fluorescence angiography.
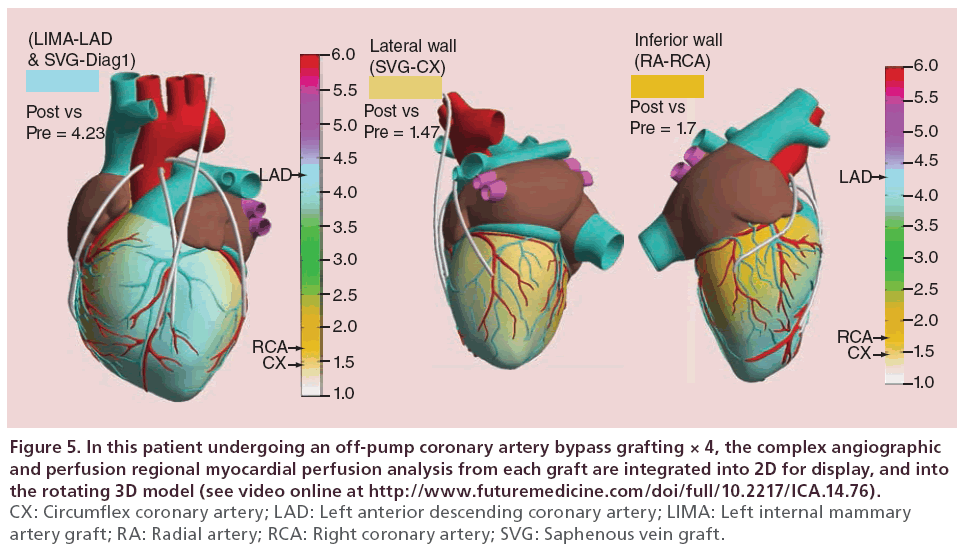
• Individual TVECA graft outcomes can be aggregated together to yield a global documentation of the change in myocardial perfusion as a result of bypass grafting (Figure 5). Since CABG as a revascularization strategy fundamentally is about increasing blood flow and perfusion to the myocardium (and not the coronary vasculature alone), this real-time image-based assessment of grafting results further documents that the importance of the functionality of revascularization as seen in PCI is also applicable to CABG;
Figure 5: In this patient undergoing an off-pump coronary artery bypass grafting × 4, the complex angiographic and perfusion regional myocardial perfusion analysis from each graft are integrated into 2D for display, and into the rotating 3D model (see video online at http://www.futuremedicine.com/doi/full/10.2217/ICA.14.76). CX: Circumflex coronary artery; LAD: Left anterior descending coronary artery; LIMA: Left internal mammary artery graft; RA: Radial artery; RCA: Right coronary artery; SVG: Saphenous vein graft.
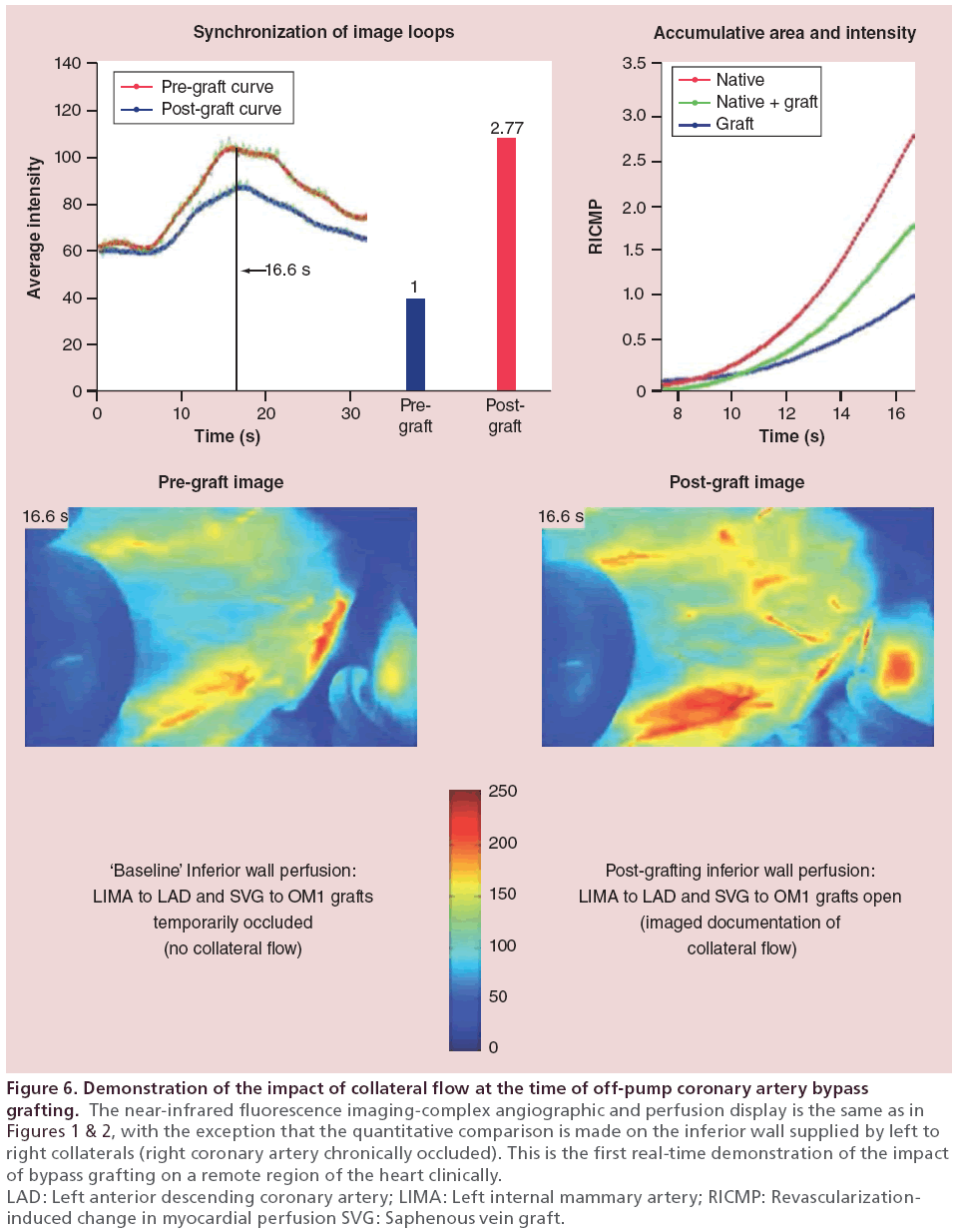
• As an example of the influence of RMP status on potential long-term outcomes, Figure 6 illustrates the CAPA analysis from the inferior wall of a patient with a chronic RCA occlusion. The comparison analysis on the ungrafted inferior wall after grafting the LAD and circumflex marginal artery (CMA) arteries. As illustrated, there was a 2.5-fold increase in perfusion to the inferior wall after grafting the anterior and lateral territories, through collaterals.
Figure 6: Demonstration of the impact of collateral flow at the time of off-pump coronary artery bypass
grafting. The near-infrared fluorescence imaging-complex angiographic and perfusion display is the same as in
Figures 1 & 2, with the exception that the quantitative comparison is made on the inferior wall supplied by left to
right collaterals (right coronary artery chronically occluded). This is the first real-time demonstration of the impact
of bypass grafting on a remote region of the heart clinically.
LAD: Left anterior descending coronary artery; LIMA: Left internal mammary artery; RICMP: Revascularizationinduced
change in myocardial perfusion SVG: Saphenous vein graft.
Evidence to suggest that this documented change in regional/global myocardial perfusion is associated with stenosis functionality
Within the context of our Heart Team collaborations, we have become increasingly interested in the possible correlation between the preoperative documentation of a perfusion deficit and/or regional ischemia using FFR or IFR, and the change in RMP that occurs at the time of surgical revascularization, documented by the NIRF-CAPA platform.
We have analyzed this using a surrogate for regional or global ischemia because of the absence of prospective documentation of intermediate lesion functionality at the time of cardiac catheterization. As such, we have correlated regional ischemia according to the clinical scale shown in Table 4.
| Ischemia level | Condition | Ischemia score |
|---|---|---|
| High | ST-elevation myocardial infarction, IABP, non-ST-elevation myocardial infarction, with congestive heart failure Non-ST-elevation myocardial infarction, clinically stable; non-ST-elevation myocardial infarction + fractional flow reserve |
1 0.75 |
| Moderate | CSA with documented ischemic region (MRI, nuclear); CSA with + fractional flow reserve CSA with presumed ischemia, localized (anterior, lateral, inferior) |
0.5 0.25 |
| Low | CSA with minimal ischemia (non-specific ECG changes only) | 0 |
Table 4: Clinical ischemia scale.
All anatomic lesions were characterized by an independent interventional cardiology analysis using the SYNTAX score, both individually for TVECA arteries and then globally. For this analysis, a ‘functional SYNTAX score’ was also determined by combining the regional ischemia data using the scale in Table 4 with the SYNTAX lesion characterization. For the NIRFCAPA analysis on a per graft basis, we classified the documented change in RMP as minimal (<10%), moderate (>10 but <20%), or significant (>20%).
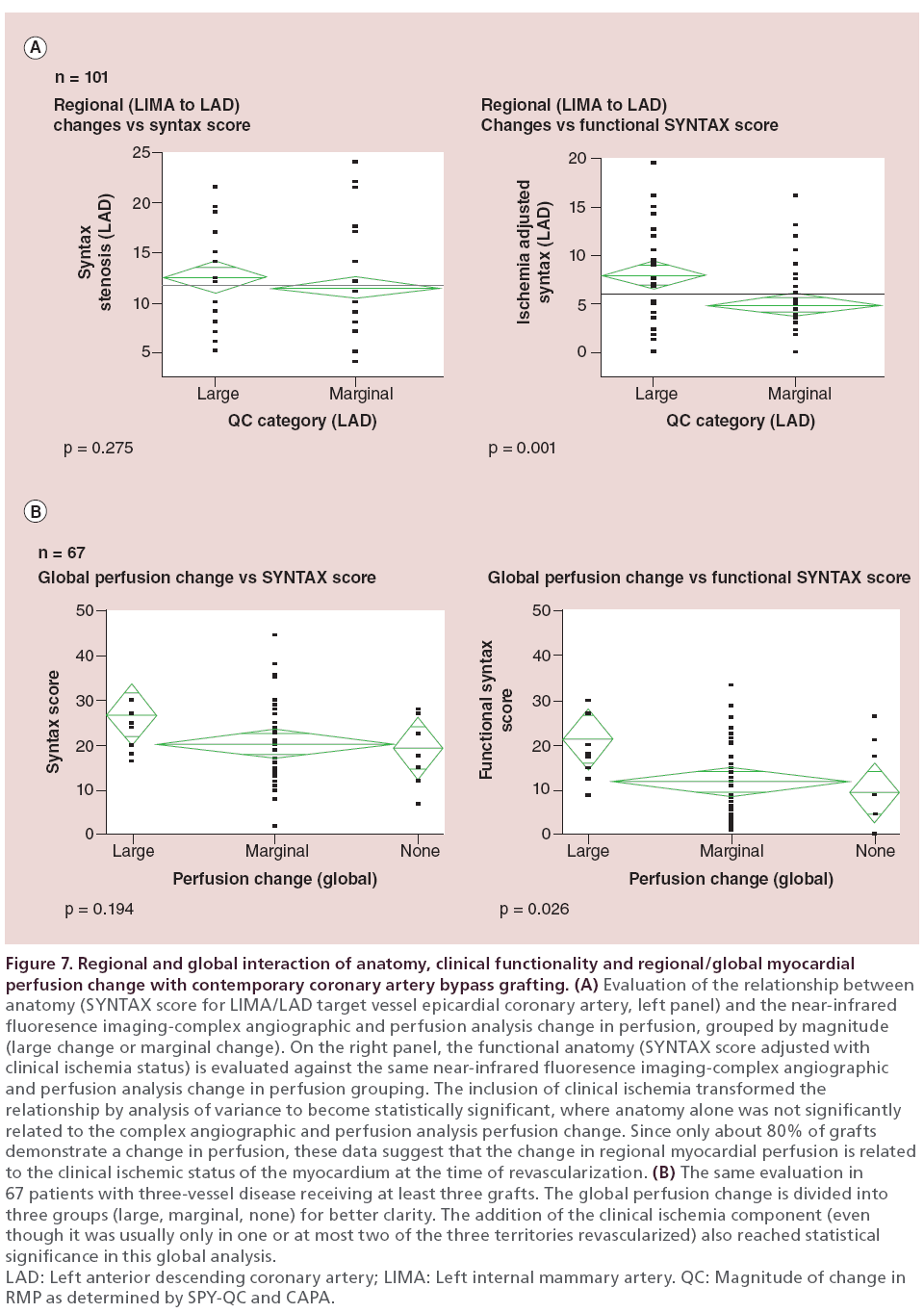
If the documented change in RMP was a response to the presence of ischemia–perfusion deficit in the myocardium surrounding the TVECA, then there should be a correlation between the change in RMP and the ‘functional SYNTAX score’, but not necessarily with the anatomic SYNTAX score. We tested this on a regional basis (LIMA to LAD with perfusion to the anterior wall (n = 101 grafts), and globally in 86 patients with RMP measured in three or more grafts.
In the regional analysis (Figure 7A), there was no correlation by ANOVA between left main/LAD stenosis SYNTAX score and the categories of RMP change. However, when the clinical ischemia data were added to the anatomic data to create the ‘functional SYNTAX score’, there was a statistically significant correlation between the magnitude of change in RMP and the combination of anatomy and functionality (p < 0.005).
Figure 7: Regional and global interaction of anatomy, clinical functionality and regional/global myocardial
perfusion change with contemporary coronary artery bypass grafting. (A) Evaluation of the relationship between
anatomy (SYNTAX score for LIMA/LAD target vessel epicardial coronary artery, left panel) and the near-infrared
fluoresence imaging-complex angiographic and perfusion analysis change in perfusion, grouped by magnitude
(large change or marginal change). On the right panel, the functional anatomy (SYNTAX score adjusted with
clinical ischemia status) is evaluated against the same near-infrared fluoresence imaging-complex angiographic
and perfusion analysis change in perfusion grouping. The inclusion of clinical ischemia transformed the
relationship by analysis of variance to become statistically significant, where anatomy alone was not significantly
related to the complex angiographic and perfusion analysis perfusion change. Since only about 80% of grafts
demonstrate a change in perfusion, these data suggest that the change in regional myocardial perfusion is related
to the clinical ischemic status of the myocardium at the time of revascularization. (B) The same evaluation in
67 patients with three-vessel disease receiving at least three grafts. The global perfusion change is divided into
three groups (large, marginal, none) for better clarity. The addition of the clinical ischemia component (even
though it was usually only in one or at most two of the three territories revascularized) also reached statistical
significance in this global analysis.
LAD: Left anterior descending coronary artery; LIMA: Left internal mammary artery. QC: Magnitude of change in
RMP as determined by SPY-QC and CAPA.
Similarly, in the global analysis (Figure 7B), there was no correlation between these same categories of RMP change and global anatomy by the full SYNTAX characterization. However, when the clinical ischemia characterization was added to the anatomy, the relationship between the ‘functional SYNTAX score’ and the RMP change was significant at the global level as well.
These are the first objective, intraoperative clinical data suggesting that the importance of functional anatomy and RMP status in the setting of PCI might also be important in surgical revascularization. We are embarking on a prospective study where all intermediate lesions will be evaluated by combined pressure and coronary flow velocity measurements to derive FFR, iFR, coronary flow reserve and microvascular resistance data in patients with two- or three-vessel coronary disease where by SYNTAX criteria they should undergo surgical revascularization. FFR and iFR could be affected by transiently elevated microvascular resistance during acute coronary syndromes, leading to potentially inaccurate results, and thus SIHD and NSTEMI patients with creatinine kinase elevations <1000 IU will be studied. Except where the functional data are important in determining the surgical strategy, a blinded comparison of the functional anatomy and the quantified perfusion change results of surgical revascularization will be performed. This study, termed PERSEUS (NCT02138305), will allow for objective correlation of the change in regional perfusion with documented functional anatomy using these new catheter-based technologies. In addition, we hypothesize that the relative increase in perfusion seen with the CAPA analysis is a result of a reduction in myocardial resistance elevated pre-grafting because of the regional ichemia–perfusion deficit [68].
The mortality benefit documented with CABG – revisited
In the PREVENT IV study, patients were stratified based on angiographic findings of graft occlusion or no graft occlusion at 1 year, and these patients were followed for a total of 5 years postoperatively [26]. It was anticipated that the patients with occluded grafts would have a worse clinical outcome in terms of mortality and myocardial infarction. Surprisingly, the patients had more recurrence of angina but there was no difference in mortality and no difference in incidents of myocardial infarction. That is, the graft failure had no impact on the development of significant amounts of myocardial ischemia in subsequent followup. By these criteria, then, the graft that went down was not functionally necessary. This raises the question of prospective determination of lesion functionality in order to avoid placement of unnecessary grafts, that is, FFR or iFR-guided CABG. The FAME III study is just underway to evaluate this surgical hypothesis, but without intraoperative image-based confirmation of the physiologic consequences of revascularization (NCT021100722).
The physiologic basis for the late mortality benefit of coronary bypass surgery is likely related to the RMP status discussed and imaged here. Factors include the coupling of arterial grafts with the regional myocardium, the presence of pre-existing collaterals and the presence of regional ichemia–perfusion deficit that drives the need for more blood flow. Our quantified imaging data documented now in >1000 CABG patients illustrates that the augmentation of myocardial perfusion is the most important consequence of surgical revascularization, and not which vessels were bypassed.
If indeed the quantified change in regional perfusion is a response to TVECA perfusion deficit or ischemia pre-grafting, as is suggested by our findings, then the physiologic explanation for the improved survival benefit might be as follows: the aggregate increase in myocardial perfusion from bypass grafts that are functionally beneficial creates a ‘safety net’ of excess perfusion that provides long-term protection from death and myocardial infarction, as suggested by the PREVENT IV 5-year data, and the 4- and 5-year data from SYNTAX and FREEDOM. This ‘safety-net’ perfusion benefit would be augmented in patients with preexisting development of collaterals and more extensive myocardial disease, where the heart was then able to move blood around where it needed to be, regardless of the native coronary or bypass graft source.
Thus in SIHD the pre-intervention evaluation of functional anatomy might not only predict the efficacy of targeted PCI, but might also assess patients for the likelihood that they will improved long-term survival and wellbeing from CABG. This might then become the strongest indication for adopting a functional anatomy revascularization strategy for both PCI and CABG in the future.
Conclusion
Depending upon the severity of the combination of anatomic, functional and RMP status, the most beneficial reasonable IR will be the one that provides the maximal achievable increase in perfusion to the myocardium, sustained over time for as long as possible. With less severe anatomy, functional and RMP status, functionally driven PCI intervention is the most effective and cost-effective intervention, in part because the overall severity of disease usually is not impacting on mortality (except from inappropriate intervention, or infrequence circumstances of anatomically simple but functionally critical lesions). However, as the overall anatomic, functional and RMP status severity increases, so does the opportunity for surgery to provide the ‘perfusion excess’, ‘safety-net’ benefit needed to impact long-term mortality by providing the maximal achievable increase in perfusion sustained over time.
Our intraoperative imaging data provide important support for these concepts. They also highlight that preoperative and intraoperative documentation of the physiologic substrate for revascularization, and the impact of revascularization on that physiologic substrate, is a more promising framework for improving revascularization outcomes from both PCI and CABG. We believe that, in similar fashion to the impact of FFR and iFR on PCI, the addition of preoperative and intraoperative physiologic determination to the traditional framework of anatomy-based surgical revascularization will similarly improve outcomes, reduce costs, and dramatically increase our knowledge about CABG and its benefits.
Future perspective
These important advances in stable ischemic heart disease will continue in the future. Understanding of the full implications of physiologic assessment in the cardiac catheterization laboratory continues to evolve. While there are still controversies and on answered questions, these physiologic data lead to better quality of care, better outcomes and lower healthcare costs.
Given the importance of these findings in determining optimal care with percutaneous revascularization, it is inevitable that these findings will also impact on surgical revascularization going forward. The availability and utility of real-time, intraoperative assessment of the physiologic consequences of CABG will be facilitative in this impact on surgical revascularization. The ability to provide real-time physiologic data will dramatically improve our understanding of the interaction between the bypass graft and the perfused myocardium, graft patency, competitive flow, graft failure, and the optimal strategy for surgical revascularization for symptom relief and long-term mortality benefit. Incorporation of these preoperative and intraoperative physiologic data into CABG is expected to yield quality, outcomes and cost benefits similar to those seen in PCI.
Financial & competing interests disclosure
TB Ferguson and C Chen are the copyright and patent holders of the analysis software CAPA, along with Novadaq Technologies, Inc. AN Buch is a consultant for Volcano, Inc., the Sponsor of the PERSEUS study. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
• Physiologic evaluation in the catheterization laboratory with fractional flow reserve, instantaneous diastolic pressure ratio, coronary flow reserve and microvascular resistance measurement is transforming the percutaneous cardiovascular interventional (PCI) treatment of patients with stable ischemic heart disease.
• This evolving transformation is changing the traditional anatomic construct for PCI into a construct based on the physiology of myocardial ichemia–perfusion deficit, and the relief of ichemia–perfusion deficit.
• The anatomic basis for revascularization still persists in coronary artery bypass grafting (CABG), but newer information related to graft patency suggests that, in similar fashion to PCI, this anatomy-only construct is incomplete.
• Real-time, intraoperative near-infrared fluorescence imaging and quantification of changes in perfusion in the target vessel epicardial coronary artery (TVECA) regional myocardial perfusion (RMP) have documented the importance of the RMP in determining this physiologic response to grafting.
• This imaging data suggest that the RMP physiology influences competitive flow between the graft and the TVECA, graft patency and possibly the choice of graft conduit.
• We present preliminary data to suggest that the preoperative physiologic evaluation of the RMP in the TVECA is linked to the RMP physiologic response to grafting in CABG.
• This RMP physiologic response to surgical revascularization may be the physiologic substrate for the improved survival benefit with CABG versus PCI documented in recent randomized controlled trials.
• The incorporation of these preoperative and intraoperative physiologic data into CABG is expected to yield quality, outcomes and cost benefits similar to those seen in PCI.
References
Papers of special note have been highlighted as:
• of interest
- Sones FM Jr, Shirley EK. Cine coronary arteriography. Mod. Concepts Cardiovasc. Dis. 31, 735–738 (1962).
- Bruschke AVG, Proudfit WL, Sones FM. Progress study of 590 consecutive nonsurgical cases of coronary disease followed 5–9 years: I. Arterographic correlations. Circulation 47(6), 1147–1153 (1973).
- Gould KL, Lipscomb K. Effects of coronary stenoses on coronary flow reserve and resistance. Am. J. Cardiol. 34, 48–55 (1974).
- Favaloro RG. Critical analysis of coronary artery bypass graft surgery: a 30-year journey. J. Am. Coll. Cardiol. 31(4, Suppl. B), 1B–63B (1998).
- Gruentzig AR, Senning A, Siegenthaler WE. Nonoperative dilitation of coronary-artery stenosis. N. Engl. J. Med. 301(2), 61–68 (1979).
- Gruentzig AR, King S, Schlumpf A, Siegenthaler W. Long-term follow-up after percutaneous transluminal coronary angioplasty. N. Engl. J. Med. 316(18), 1127–1132 (1987).
- Taggart DP. Thomas B. Ferguson Lecture. Coronary artery bypass grafting is still the best treatment for multivessel and left main disease, but patients need to know. Ann. Thorac.Surg. 82(6), 1966–1975 (2006).
- Ferguson TB Jr, Dziuban S, Edwards FH et al. The Society of Thoracic Surgeons National Database: current changes and challenges for the new millennium. Ann. Thorac. Surg. 69, 680–691 (2000).
- Ferguson TB, Jr., Hammill BG, Peterson ED, Delong ER, Grover FL. A decade of change – risk profiles and outcomes for isolated coronary artery bypass grafting procedures, 1990–1999: a report from the STS National Database Committee and the Duke Clinical Research Institute. Society of Thoracic Surgeons. Ann. Thorac. Surg. 73(2), 480–489; discussion 489–490 (2002).
- Elbardissi AW, Aranki SF, Sheng S, O’brien SM, Greenberg CC, Gammie JS. Trends in isolated coronary artery bypass grafting: an analysis of the Society of Thoracic Surgeons adult cardiac surgery database. J. Thorac. Cardiovasc. Surg. 143(2), 273–281 (2012).
- Puskas JD, Lazar HL, Mack MJ, Sabik JF 3rd, Taggart DP. State-of-the-art coronary artery bypass graft. Semin. Thorac. Cardiovasc. Surg. 26(1), 76–94 (2014).
- Shahian DM, O’brien SM, Sheng S et al. Predictors of long-term survival after coronary artery bypass grafting surgery: results from the Society of Thoracic Surgeons Adult Cardiac Surgery Database (the ASCERT study). Circulation 125(12), 1491–1500 (2012).
- Mohr FW, Rastan AJ, Serruys PW et al. Complex coronary anatomy in coronary artery bypass graft surgery: impact
- Pagni S, Storey J, Ballen J et al. Factors affecting internal mammary artery graft survival: How is competitive flow from a patent native coronary vessel a risk factor? J. Surg. Res. 71, 172–178 (1997).
- Kitamura S, Seki T, Kawachi K, Morita R et al. Excellent patency and growth potential of internal mammary artery grafts in pediatric coronary artery bypass surgery: new evidence for a ‘live’ conduit. Circulation 78(Suppl. I), I129–I139 (1988).
- Sabik JF, 3rd, Blackstone EH. Coronary artery bypass graft patency and competitive flow. J. Am. Coll. Cardiol. 51(2), 126–128 (2008).
- Pevni D, Hertz I, Medalion B et al. Angiographic evidence for reduced graft patency due to competitive flow in composite arterial T-grafts. J. Thorac. Cardiovasc. Surg. 133(5), 1220–1225 (2007).
- Ferguson TB Jr, Chen C, Babb JD, Efird JT, Daggubati R, Cahill JM. Fractional flow reserve-guided coronary artery bypass grafting: can intraoperative physiologic imaging guide decision making? J. Thorac. Cardiovasc. Surg. 146(4), 824–835 e821 (2013).
- Alexander JH, Hafley G, Harrington RA et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA 294(19), 2446–2454 (2005).
- Shroyer A, Grover FL, Hattler B et al. For the Veterans Affairs Randomized on/Off Bypass (Rooby) Study Group. On-pump versus off-pump coronary-artery bypass surgery. N. Engl. J. Med. 361(19), 1827–1837 (2009).
- Asa M. Medistim ASA First Quarter 2013. http://medistim.com/investor/annual-quarterly-reports/
- Balacumaraswami L, Abu-Omar Y, Choudhary B, Pigott D, Taggart DP. A comparison of transit-time flowmetry and intraoperative fluorescence imaging for assessing coronary artery bypass graft patency. J. Thorac. Cardiovasc. Surg. 130(2), 315–320 (2005).
- Singh SK, Desai ND, Chikazawa G et al. The Graft Imaging to Improve Patency (GRIIP) clinical trial results. J. Thorac. Cardiovasc. Surg. 139(2), 294–301, 301 e291 (2010).
- Desai ND, Miwa S, Kodama D et al. Improving the quality of coronary bypass surgery with intraoperative angiography: validation of a new technique. J. Am. Coll. Cardiol. 46(8), 1521–1525 (2005).
- Lopes RD, Williams JB, Mehta RH et al. Edifoligide and long-term outcomes after coronary artery bypass grafting: PRoject of Ex-vivo Vein graft ENgineering via Transfection IV (PREVENT IV) 5-year results. Am. Heart J. 164(3), 379–386 e371 (2012).
- Koshizaka M, Hafley G, Lopes R et al. Diabetic patients have similar vein graft failure at 1 year, but higher 5-year mortality than non-diabetic patients undergoing coronary artery bypass surgery: insights from the PREVENTIV Trial. J. Am. Coll. Cardiol. 59(13), E1446 (2012).
- Hess CN, Lopes RD, Gibson CM et al. Saphenous vein graft failure after coronary artery bypass surgery: insights from PREVENT IV. Circulation 130(17), 1445–1451 (2014).
- Buxton BF, Shi WY, Tatoulis J, Fuller JA, Rosalion A, Hayward PA. Total arterial revascularization with internal thoracic and radial artery grafts in triple-vessel coronary artery disease is associated with improved survival. J. Thorac. Cardiovasc. Surg. 148(4), 1238–1244 (2014).
- Raza S, Sabik JF 3rd, Masabni K, Ainkaran P, Lytle BW, Blackstone EH. Surgical revascularization techniques that minimize surgical risk and maximize late survival after coronary artery bypass grafting in patients with diabetes mellitus. J. Thorac. Cardiovasc. Surg. 148(4), 1257–1266, e1259 (2014).
- Zishiri ET, Williams S, Cronin EM et al. Early risk of mortality after coronary artery revascularization in patients with left ventricular dysfunction and potential role of the wearable cardioverter defibrillator. Circ. Arrhythm. Electrophysiol. 6(1), 117–128 (2013).
- Halkin A, Singh M, Nikolsky E et al. Prediction of mortality after primary percutaneous coronary intervention for acute myocardial infarction: the CADILLAC risk score. J. Am. Coll. Cardiol. 45(9), 1397–1405 (2005).
- Weintraub WS, Grau-Sepulveda MV, Weiss JM et al. Prediction of long-term mortality after percutaneous coronary intervention in older adults: results from the National Cardiovascular Data Registry. Circulation 125(12), 1501–1510 (2012).
- Kurlansky PA. Arterial grafting and the challenge of the patient with diabetes. J. Thorac. Cardiovasc. Surg. 148(4), 1253–1256 (2014).
- Taggart DP. Incomplete revascularization: appropriate and inappropriate. Eur. J. Cardiothorac. Surg. 41(3), 542–543 (2012).
- Garcia S, Sandoval Y, Roukoz H et al. Outcomes after complete versus incomplete revascularization of patients with multivessel coronary artery disease: a meta-analysis of 89,883 patients enrolled in randomized clinical trials and observational studies. J. Am. Coll. Cardiol. 62(16), 1421–1431 (2013).
- Dauerman HL. Reasonable incomplete revascularization. Circulation 123(21), 2337–2340 (2011).
- Serruys Pw, Morice M-C, Kappetein AP, Colombo A et al. for the Syntax Investigators. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N. Engl. J. Med. 360, 961–972 (2009).
- Mohr FW, Morice M-C, Kappetein AP et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 381(9867), 629–638 (2013).
- Kereiakes DJ. Complete revascularization: a quality-performance metric? J. Am. Coll. Cardiol. 62(16), 1432–1435 (2013).
- De Bruyne B. Multivessel disease: from reasonably incomplete to functionally complete revascularization. Circulation 125(21), 2557–2559 (2012).
- Pijls NHJ. Fractional flow reserve to guide coronary revascularization. Circ. J. 77(3), 561–569 (2013).
- Tonino PAL, De Bruyne B, Pijls NHJ et al., for the Fame Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N. Engl. J. Med. 360(3), 213–224 (2009).
- Tonino PA, Fearon WF, De Bruyne B et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J. Am. Coll. Cardiol. 55(25), 2816–2821 (2010).
- Pijls NH, Van Schaardenburgh P, Manoharan G et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J. Am. Coll. Cardiol. 49(21), 2105–2111 (2007).
- De Bruyne B, Pijls NH, Kalesan B et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N. Engl. J. Med. 367(11), 991–1001 (2012).
- Berry C, Layland J, Sood A et al. Fractional flow reserve versus angiography in guiding management to optimize outcomes in non-ST-elevation myocardial infarction (FAMOUS-NSTEMI): rationale and design of a randomized controlled clinical trial. Am. Heart J. 166(4), 662–668, e663 (2013).
- Carrick D, Behan M, Foo F et al. Usefulness of fractional flow reserve to improve diagnostic efficiency in patients with non-ST elevation myocardial infarction. Am. J. Cardiol. 111(1), 45–50 (2013).
- Hennigan B, Layland J, Fearon WF, Oldroyd KG. Fractional flow reserve and the index of microvascular resistance in patients with acute coronary syndromes. EuroIntervention 10(Suppl. T), T55–T63 (2014).
- Layland J, Carrick D, McEntegart M et al. Vasodilatory capacity of the coronary microcirculation is preserved in selected patients with non-ST-segment-elevation myocardial infarction. Circ. Cardiovasc. Interv. 6(3), 231–236 (2013).
- Layland J, Oldroyd KG, Curzen N et al. Fractional flow reserve vs. angiography in guiding management to optimize outcomes in non-ST-segment elevation myocardial infarction: the British Heart Foundation FAMOUS-NSTEMI randomized trial. Eur. Heart J. 36(2), 100–111 (2014).
- Fihn SD, Blankenship JC, Alexander KP et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 64(18), 1929–1949 (2014).
- Siebes M. Coronary flow and physiology beyond the stenosis. Heart Metabol. 58, 4–9 (2013).
- Botman CJ, Schonberger J, Koolen S et al. Does stenosis severity of native vessels influence bypass graft patency? A prospective fractional flow reserve-guided study. Ann. Thorac. Surg. 83(6), 2093–2097 (2007).
- Frohlich GM, Redwood S, Rakhit R et al. Long-term survival in patients undergoing percutaneous interventions with or without intracoronary pressure wire guidance or intracoronary ultrasonographic imaging: a large cohort study. JAMA Intern. Med. 174(8), 1360–1366 (2014).
- Malhotra A. The whole truth about coronary stents: the elephant in the room. JAMA Intern. Med. 174(8), 1367–1368 (2014).
- Weintraub WS, Grau-Sepulveda MV, Weiss JM, O’brien SM et al. Comparative effectiveness of revascularization strategies. Engl. J. Med. 366(16), 1467–1476 (2012).
- Farkouh ME, Domanski M, Sleeper LA et al. Strategies for multivessel revascularization in patients with diabetes. N. Engl.Med. 367, 2375–2384 (2012).
- Gersh BJ, Frye RL. Methods of coronary revascularization – things may not be as they seem. N. Engl. J. Med. 352(21), 2235–2236 (2005).
- Van De Hoef TP, Nolte F, Rolandi MC et al. Coronary pressure-flow relations as basis for the understanding of coronary physiology. J. Mol. Cell. Cardiol. 52(4), 786–793 (2012).
- Van De Hoef T, Nolte F, Damman P et al. Diagnostic accuracy of basal stenosis resistance index for myocardial ischemia identified by fractional flow reserve and implications of stenosis resistance index determined at sub-maximally elevated coronary flow rates. J. Am. Coll. Cardiol. 61(10), E1760 (2013).
- Verhoeff BJ, Van De Hoef TP, Spaan JA, Piek JJ, Siebes M. Minimal effect of collateral flow on coronary microvascular resistance in the presence of intermediate and noncritical coronary stenoses. Am. J. Physiol. Heart Circ. Physiol.303(4), H422–H428 (2012).
- Gregorini L, Marco J, Heusch G. Peri-interventional coronary vasomotion. J. Mol. Cell. Cardiol. 52(4), 883–889 (2012).
- Seiler C. The human myocardial stain as mitigated by coronary collaterals. Circulation 127(6), 670–672 (2013).
- Meier P, Gloekler S, Zbinden R et al. Beneficial effect of recruitable collaterals: a 10-year follow-up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation 116(9), 975–983 (2007).
- Meier P, Hemingway H, Lansky Aj, Knapp G, Pitt B, Seiler C. The impact of the coronary collateral circulation on mortality: a meta-analysis. Eur. Heart J. 33, 614–621 (2012).
- Ferguson TB, Chen C. Does the Concept of functional stenosis exist in surgical revascularization with CABG? J. Am. Coll. Cardiol. 59(13), E1453 (2012).
- Ferguson Tb Jr, , CC, Buch An. Fractional Flow Reserve-guided Coronary Bypass Surgery: should surgeons use it? Curr. Opin. Cardiol. 28(6), 654–660 (2013).
- Verhoeff BJ, Siebes M, Meuwissen M et al. Influence of percutaneous coronary intervention on coronary microvascular resistance index. Circulation 111(1), 76–82 (2005).
• Seminal experimental study laying the groundwork for clinical physiologic assessment and understanding of flow reserve.
• Interesting panel discussion about contemporary coronary artery bypass grafting (CABG) from among senior surgeons.
• Excellent experimental study documenting physiology of competitive flow; results recently validated clinically with intraoperative real-time fluorescence imaging and quantitative analysis.
• First report of quantitative documentation of the effect of coronary bypass grafting on regional myocardial perfusion at CABG.
• Important editorial introducing additional aspects of this evolving concept.
• Critical randomized controlled trial 5-year data on survival and freedom from MI advantage with CABG from the SYNTAX trial in patients with increasingly complex coronary artery disease.
• Excellent flow and physiology analysis of target vessel epicardial coronary artery as applies to CABG.
• Oft-cited surgical paper linking for the first time preoperative fractional flow reserve and 1-year graft patency outcomes.
• Important study linking collateral circulation with mortality, with direct implications for contemporary CABG.