Device Evaluations - Interventional Cardiology (2011) Volume 3, Issue 3
Review of the everolimus-eluting coronary stent system
- Corresponding Author:
- Masato Nakamura
Division of Cardiovascular Medicine, Toho University Ohashi Medical Center, 2-17-6 Ohashi, Meguro-ku, Tokyo 153-8515, Japan
Tel: +81 833 468 1251
Fax: +81 833 468 1269
E-mail: masato@oha.toho-u.ac.jp
Abstract
Keywords
coronary artery disease,drug-eluting stent,everolimus,percutaneous coronary intervention
Since balloon angioplasty was first introduced in a clinical setting, the Achilles’ heel of percutaneous coronary intervention (PCI) has been restenosis. To overcome this problem, technical considerations, such as spot stenting and stent deployment after debulking plaque, have been introduced with some success [1,2]. In addition, technical advancements have been made with the devices themselves, including the release of antirestenotic drugs from the stents. Trials using sirolimus-eluting stents (SESs) and paclitaxeleluting stents (PESs) have clearly demonstrated that drug-eluting stents (DESs) result in a marked reduction of neointimal proliferation and target lesion revascularization compared with bare-metal stents (BMSs) [3–6]. Soon after the introduction of DESs, they became a mainstay in PCI and have led to the treatment of more complex lesion morphology in varied clinical settings. However, concern has been raised regarding the risk of late and very late stent thrombosis [7,8]. Pooled data analyses of randomized clinical trials and registry studies provide evidence supporting this safety issue in first-generation DESs [9–11]. The increase in very late stent thrombosis with DESs also remains a concern because of the required long duration of treatment with dual antiplatelet drugs [12].
With the goal of further enhancing the safety and efficacy of DESs, a next-generation DES was developed. The everolimus-eluting stent (EES; Xience V® [Abbott Laboratories, IL, USA]/Promus® [Boston Scientific, MA, USA]) is a newer-generation DES that was approved in Japan in March 2010 as the fourth DES following the SES, the PES and the zotarolimus-eluting stent (ZES; Endeavor®, [Medtronic, MN, USA]). The efficacy and safety of EES have been demonstrated in the SPIRIT trial series [13–15]. However, the current body of real-world clinical practice data is currently not sufficient to confirm the promising randomized trial data. Therefore, outcomes from such ongoing trials as the EXECUTIVE trial are anticipated, which is assessing the efficacy of the EES compared with the PES in 200 patients, to substantiate the efficacy and safety of the EES. Simultaneously, more attention should be paid to the characteristics of DES components. To improve the efficacy of EESs, they must be used optimally. Each DES has a different stent delivery system and, although procedural technique has been underestimated, it has been directly linked to outcomes of safety and efficacy, having a marked impact on the prevention of intimal proliferation [16,17]. In this article, we comprehensively review EESs, with a special focus on technical aspects.
Everolimus-eluting stent overview
▪ Stent design
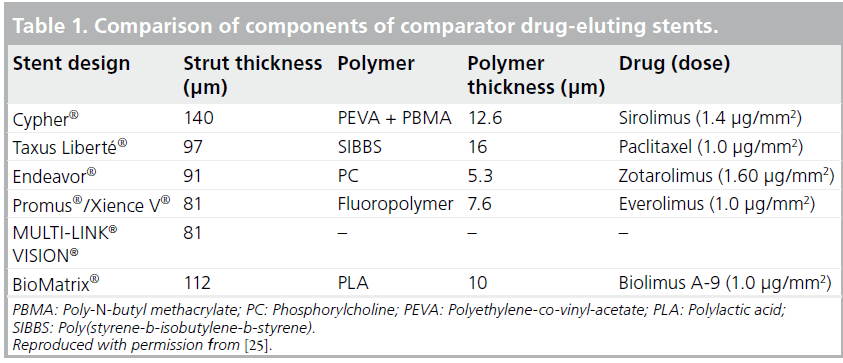
The rationale behind the design and choice of polymer for the EES is beyond the scope of this article. In brief, the EES consists of a stent platform, polymer and drug, in a similar manner to the SES and the PES. The lipophilic antiproliferative drug, everolimus, is released from a thin, durable polymer applied to a low-profile stent. The EES platform used is the MULTI-LINK® VISION® (Abbott Laboratories, IL, USA) coronary stent, which is characterized by an open-cell, nonlinear link design made from an L-605 cobalt–chromium alloy. This material and stent design have resulted in a thinner and more flexible DES compared with first-generation DESs made of stainless steel (Table 1). These qualities of the stent platform have resulted in improved deliverability and conformability of the stent. The performance of the MULTI-LINK VISION stent has previously been demonstrated in clinical practice and this stent is among the most commonly used worldwide [18–20].
Everolimus is a semisynthetic macrolide immunosuppressant drug, synthesized by chemical modification of rapamycin. This drug has been demonstrated to inhibit vascular smooth muscle cell proliferation and to function through the formation of a complex with the cytoplasmic FK 506 binding protein. This complex binds and interferes with the mTOR protein, resulting in the inhibition of cell metabolism, growth and proliferation by arresting growth at the G1 stage of the cell cycle. Everolimus has increased solubility in organic solvents compared with sirolimus, in conjunction with a similar ability to inhibit smooth muscle cell proliferation, despite having a two- to three-fold lower affinity for FK 506 binding protein [21]. To minimize polymer loading in the vessel, an absorbable composite coating is used on the EES, consisting of two durable layers: a primary and reservoir layer. The primary layer is a nonerodable polymer made of poly-N-butyl methacrylate (PBMA) and plays a role in adhering the drug coating to the stent. The reservoir layer, made of vinylidine fluoride and hexafluoropropylene monomers, contains and releases everolimus. The drug load is 100 μg/cm2 for all stent sizes. The formulation of drug and polymer results in controlled elution of 80% of the drug within 1 month and the remainder within 4 months [22]. The integrity of the EES polymer has been shown in scanning electron microscope studies evaluating the coating integrity of DESs after failed implantation in calcified and/or tortuous vessels [23], and of the expanded stent at 14 atm in a water bath [24]. Although the extensive methodological limitations of the process of harvesting DESs hinder the ability to draw conclusions, these studies demonstrate the continued integrity of the EES as well as differences in the degree of coating integrity among DESs.
Preclinical studies
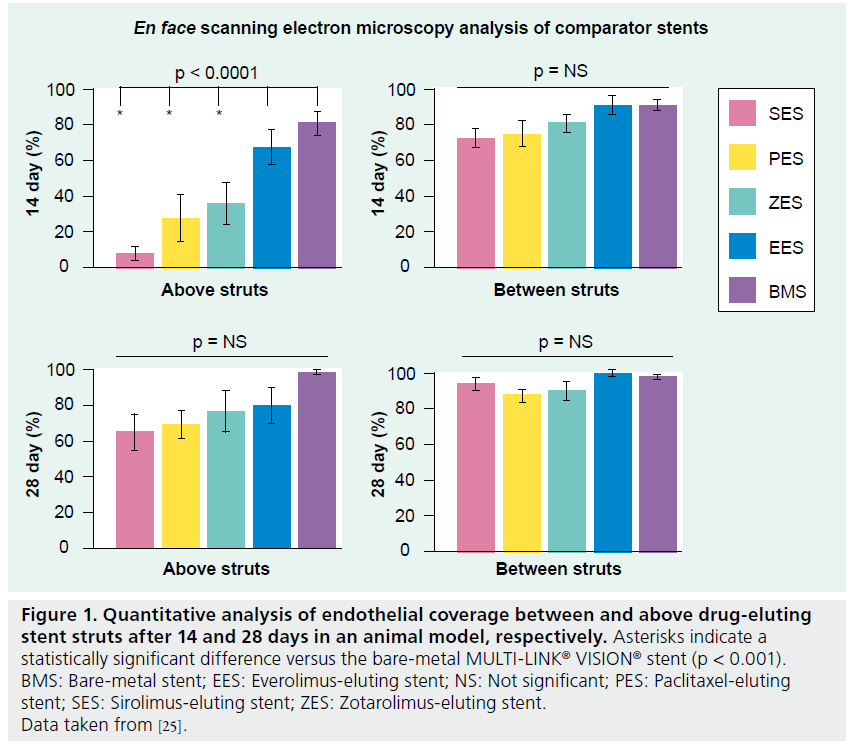
The polymer coating of a stent system plays an important role in drug delivery to the vessel wall. However, the coating itself may also increase the risk of inducing inflammation and promoting thrombus formation. Furthermore, incomplete endothelialization of stent strut surfaces and endothelial dysfunction may be essential components of stent thrombosis formation, although the exact mechanisms through which thromboses form are still poorly understood. Therefore, the properties of an ideal coating would include biocompatibility, healing of stent implants and continued integrity of the coating. Joner et al. addressed the recovery of endothelialization after stent implantation in a rabbit iliofemoral artery stenting model [25]. They focused on between- and above-stent strut endothelial coverage and relevant mitogenic and antithrombotic properties among leading polymer- based stent platforms. Notable differences among the stents were observed after 14 days. Endothelial coverage occurred more rapidly in the EES (>70% above-stent strut) relative to the SES, PES and ZES at 14 days (Figure 1). Reduced expression of platelet endothelial cell-adhesion molecule-1 in conjunction with increased mRNA levels and secretion of VEGF 14 days after stent implantation were found in those arteries treated with the EES compared with first-generation SESs or PESs. Nonetheless, by 28 days, endothelial function had recovered – as represented by the weak or absent expression of the antithrombotic cofactor – in all DESs tested.
Figure 1: Quantitative analysis of endothelial coverage between and above drug-eluting stent struts after 14 and 28 days in an animal model, respectively. Asterisks indicate a statistically significant difference versus the bare-metal MULTI-LINK® VISION® stent (p < 0.001). BMS: Bare-metal stent; EES: Everolimus-eluting stent; NS: Not significant; PES: Paclitaxel-eluting stent; SES: Sirolimus-eluting stent; ZES: Zotarolimus-eluting stent. Data taken from [25].
Clinical review
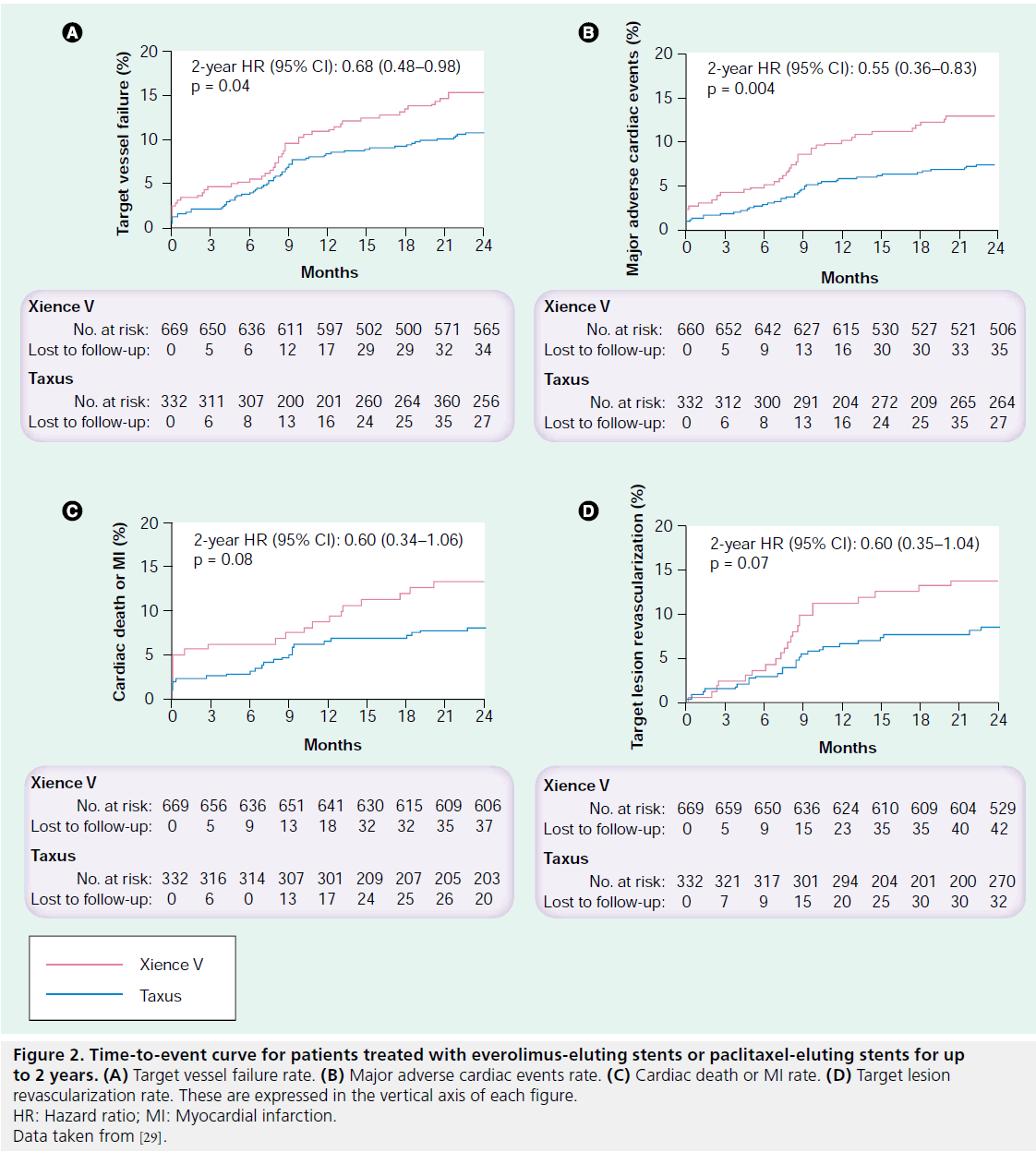
The efficacy and safety of the EES has been mainly demonstrated in the SPIRIT I, II, III, IV and V clinical trials. SPIRIT I consisted of 60 patients in Europe who were randomized to receive either a bare-metal MULTI-LINK VISION stent or an EES. Mean in-stent late loss (EES: 0.10 vs BMS: 0.87 mm; p < 0.001) [13] and major adverse cardiac event (MACE) rates (EES: 15.4% vs BMS: 21.4%; p = 0.59) were lower in the EES group at 1 year compared with the BMS group [26]. The SPIRIT II trial was a 300-patient randomized clinical trial enrolling patients in the26 Asia–Pacific region and Europe. Patients were randomized 3:1 to receive the EES (n = 223) or the first-generation Taxus® Express2™ stent (Boston Scientific; n = 77). Even though there were no significant differences in MACE rates (EES: 2.7% vs PES: 6.5%), angiographic late loss and intimal hyperplasia, as evaluated by intravascular ultrasound (IVUS), were significantly smaller in the EES group versus the PES group at 6 months [14]. However, at 1 year, clinical outcomes were numerically higher in the EES arm compared with the PES group [27], and a catchup phenomenon was noted as similar rates of angiographic late loss and similar rates of volume of intimal hyperplasia were observed between the EES and the PES after a 2-year follow-up [28]. SPIRIT III was a randomized trial that also compared the EES and Taxus Express2 stent. A total of 1002 patients were randomized 2:1 to an EES (n = 669) or a PES (n = 333) at centers in the USA. In this trial, significantly lower late loss and binary restenosis rates were observed in the EES group at 8 months [15]. There was no difference in target vessel revascularization but the rate of MACE was reduced in EES-treated patients at 9 months and, in contrast to the outcomes of SPIRIT II, this comparative reduction was sustained at 2 years (Figure 2) [29]. The reduction in procedure-related myocardial necrosis observed with EESs may be the result of less side branch compromise due to the thinner polymer and stent strut width compared with the firstgeneration PES. Discontinuation of dual antiplatelet therapy after 6 months did not result in increased stent thrombosis, thus supporting the safety of the EES. SPIRIT IV was a 3687-patient trial evaluating the safety and efficacy of the EES compared with the PES in patients with more complex lesion morphology. Target lesion revascularization and stent thrombosis rates were significantly lower in the EES arm at 1 year compared with the PES arm [30]; however, in the subset of patients with diabetes mellitus (DM), no differences in MACE or target vessel failure were observed between the treatment arms [30]. These data support the clinical benefit of the EES compared with the Taxus Express2 stent in high-risk patients and lesions [30]. However, these benefits do not appear to extend to patients with DM [30]. The safety and efficacy of the EES in real-world practice has recently been reported. In the single- center Xience Stent Evaluated at Rotterdam Cardiology Hospital (X-SEARCH) registry [31], Onuma et al. demonstrated the outcomes of the EES in an all-comers population [31]. A total of 649 patients were enrolled and outcomes were analyzed at 6 months [31].
Figure 2: Time-to-event curve for patients treated with everolimus-eluting stents or paclitaxel-eluting stents for up to 2 years. (A) Target vessel failure rate. (B) Major adverse cardiac events rate. (C) Cardiac death or MI rate. (D) Target lesion revascularization rate. These are expressed in the vertical axis of each figure. HR: Hazard ratio; MI: Myocardial infarction. Data taken from [29].
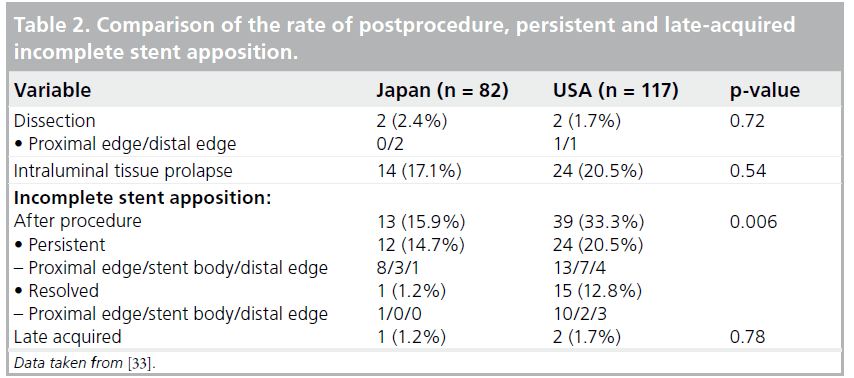
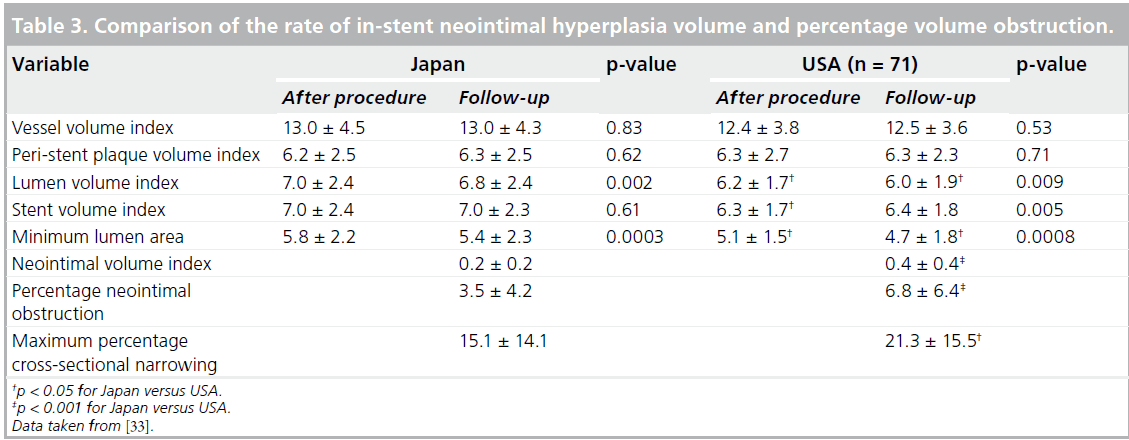
The EES was compared with three historical controls (BMS: n = 450; SES: n = 508; PES: n = 576). Even though EESs were implanted in a patient population with a more complex clinical background, with older patients and those suffering more frequent acute myocardial infarction, the EES demonstrated a comparable safety profile with the other stents and revealed improved efficacy compared with BMSs and PESs [31]. Latib et al. published clinical outcomes with the EES in an unrestricted population that included treatment for off-label indications [32]. In this study, 573 lesions in 345 patients treated with the EES were analyzed [32]. The majority of patients (71.9%) were treated for one or more off-label indications [32]. After a mean followup period of 378 days, MACEs had occurred in 10.6% of patients overall and target lesion revascularization (TLR) in 7.9% of patients [32]. Off-label EES deployment was associated with slightly higher MACE and TLR rates than were seen in the SPIRIT trials, but not with a statistically increased risk of MACE compared with on-label EES usage within this registry [32]. Previous bypass surgery and DM were independent predictors of MACE in a multivariate analysis of these registry patients [32]. This study was underpowered to detect differences in TLR rates [32]; therefore, the TLR rate may be higher in patients treated for off-label compared with on-label indications [32]. An important point to mention is that the mortality and myocardial infarction rates were similar in patients receiving off-label compared with on-label EES implantation [32]. Further evaluation, including long-term follow-up data, will be mandatory in order to draw any final conclusions regarding the safety and efficacy of the EES. In the SPIRIT III trial, IVUS data showed no significant difference in the rate of incomplete stent apposition either at the completion of procedure or after 8 months follow-up when comparing EESs and PESs; however, the outcome was somewhat different in the SPIRIT Japan registry [33]. The SPIRIT Japan registry consisted of 82 lesions; all target lesions were followed up by means of coronary angiography at 8 months [33]. A subset of lesions was evaluated by IVUS at 8 months [33], and follow-up data were analyzed by the same core laboratory as in the SPIRIT III randomized trial. Comparing the SPIRIT III randomized arm (in the USA) with SPIRIT III Japan provided interesting information about differences in stenting procedures and patient populations between the USA and Japan. The Japanese cohort consisted of an older population with a reduced prevalence of obesity (defined as BMI >30) and dyslipidemia compared with the US cohort (EES arm of the randomized cohort) [33]. There were no significant differences in the prevalence of DM or hypertension [33]. Angiographic parameters were similar between the Japanese and US arms; however, procedural characteristics were different [33]. Maximal dilatation balloon pressure was greater (Japan: 17.9 atm vs USA: 15.7 atm; p = 0.0003) and postprocedure dilatation was more frequently performed in the Japanese arm (65.9 vs 48.7%; p = 0.02) [33]. These procedural differences are likely to influence the underlying differences in IVUS findings. For example, postprocedural incomplete stent apposition was significantly more frequently observed in the US arm (Table 2) and the volume of in-stent neointimal hyperplasia was also significantly reduced in the Japanese arm (Table 3) [33]. During clinical follow-up over 2 years, stent thrombosis was not observed in any Japanese patient. It should be noted that owing to the small sample size, drawing conclusions about the relationship between perceived safety issues and procedural characteristics is difficult. Nonetheless, it seems reasonable to consider that stent apposition is important, not only for efficacy but also for safety. The findings of the SPIRIT III Japan registry data seem to be consistent with those reported by Latib et al., which were previously mentioned [32].
A technical note & discussion
Optimization of stent expansion is considered to be an important element in accomplishing favorable short- and long-term outcomes. It is important to expand the stent optimally in order to prevent suboptimal stent deployment and to reduce the likelihood of clinical adverse events. How can one obtain optimal stent apposition? The possible underlying reasons for suboptimal deployment are multifactorial; for example, one cause is underexpansion. In addition, one must take into account the mechanical characteristics of the DES. As with BMSs, the main function of the stent is to support the vessel wall and prevent elastic recoil. In previous studies, acute stent recoil ranged between 3 and 15% [34–36]. These variable data were based, in part, on the difference in stent material and design and, in part, on differences in the definition of recoil. Tanimoto et al. found that acute absolute stent recoil of the EES was 0.13 ± 0.21 mm and the acute percentage recoil was 4.3 ± 7.1% [37], suggesting that acute recoil is still encountered with second-generation DESs. Tanimoto et al. also reported that smaller reference diameter and lower maximum balloon pressure led to a higher percentage recoil [37]. Another important mechanical consideration is understanding the characteristics of the dilatation balloon and the initial dilatation pressure of the EES. The stent delivery system of the EES is designed with a semicompliant material (polyether block amide) with short tapers to prevent endothelial and vessel injury, which are adjacent to the stented segment. According to the directions for use and our personal examination, this delivery balloon is able to dilate the EES more quickly and begins to dilate at a lower pressure compared with other DES delivery systems. However, the semicompliant nature of the balloon, especially that of the 2.5-mm size, has higher compliance compared with other DESs, which may lead to an increased risk of severe complications such as a dissection at the stent edge. Such behavior may be due, to some extent, to the balloon material, stent strut thickness and stent material.
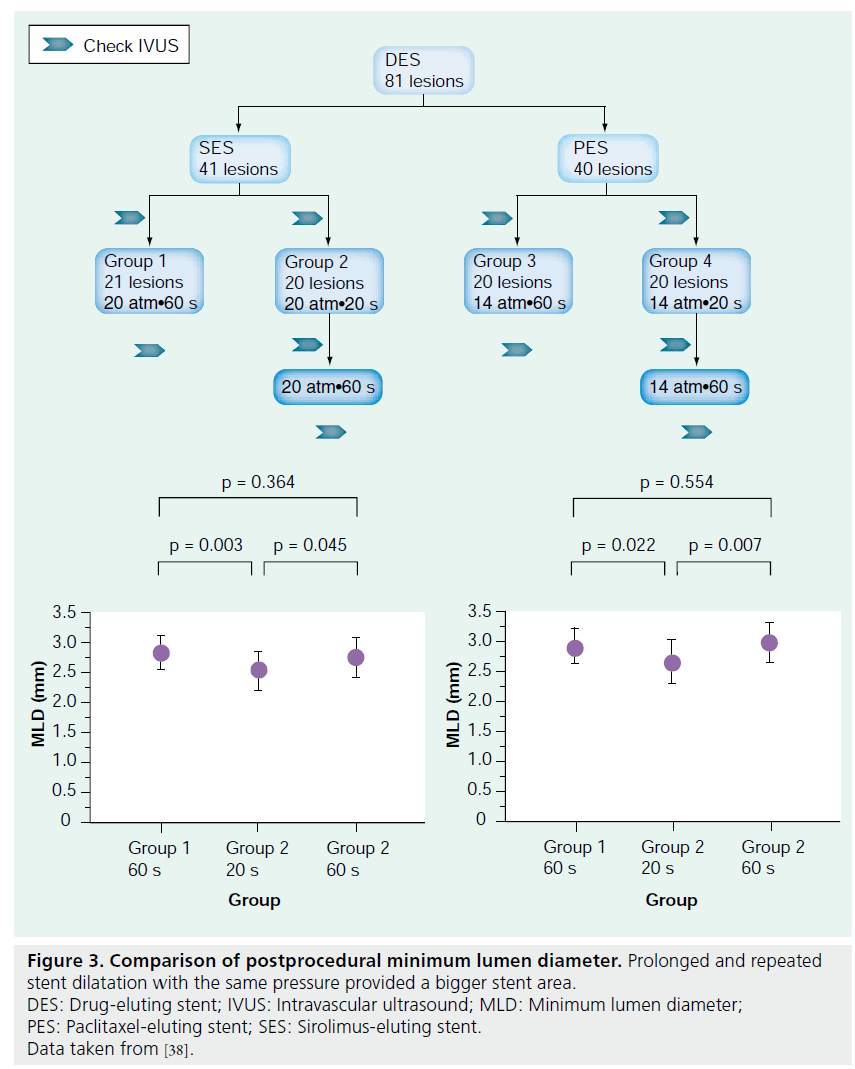
To overcome these problems and to achieve optimal stent deployment, not only for the EES but for all DESs, there are some measures that physicians are able to take. As suggested by the SPIRIT III Japan registry, postdilatation with an adequate high-pressure atmosphere may be one option for obtaining optimal EES expansion. The use of an IVUS modality may also help in confirming proper stent expansion. IVUS guidance during PCI has been suggested to potentially reduce adverse clinical events. Both Tanimoto et al. and Latib et al. pointed out the usefulness of IVUS [32,37]. Another interesting finding was recently published by Kawasaki et al. The authors investigated the degree of stent expansion with different stentdeployment methods [38]. The authors concluded that prolonged stent dilatation is an effective method to optimally expand a stent [38]. Stent expansion was significantly greater after 60-s dilatation compared with 20-s dilatation [38]. Furthermore, repeated dilatation with the same pressure resulted in better expansion (Figure 3) [38]. Therefore, to obtain optimal EES expansion, one has to keep in mind the importance of dilatation duration and repetition. In addition, to prevent severe complications such as stent dissection at the edge, maintaining a low pressure over a long duration is a useful strategy, as this leads to gradual EES expansion.
Figure 3: Comparison of postprocedural minimum lumen diameter. Prolonged and repeated stent dilatation with the same pressure provided a bigger stent area. DES: Drug-eluting stent; IVUS: Intravascular ultrasound; MLD: Minimum lumen diameter; PES: Paclitaxel-eluting stent; SES: Sirolimus-eluting stent. Data taken from [38].
Taken together, our recommended EES dilatation procedure can be summarized as follows: first, start dilatation from a low pressure (<10 atm) for a sufficient duration (20 s); second, incrementally increase the dilatation pressure; third, repeated dilatation with long inflation times (at least three times); fourth, use a noncompliant balloon if this is not sufficient; and, fifth, use IVUS modality during complex procedures. Although these practices need to be further investigated relative to clinical outcomes, these options may contribute to improved EES clinical outcomes.
Alternative devices
Currently, the first-generation Cypher® SES (Cordis Corporation, NJ, USA), and the second- generation Taxus® Liberté® PES System (Boston Scientific Corporation, MA, USA) and Endeavor® ZES (Medtronic, USA) are available in the Japanese market. Promus/Xience V is the fourth regulatory-approved DES in Japan, which has been available in the rest of the world for several years.
Conclusion & future perspective
The everolimus-eluting Promus/Xience V stent consists of three DES components: everolimus as the drug, vinylidine fluoride and hexafluoropropylene monomers with a primary PBMA polymer coating and the MULTI-LINK VISION stent platform. Its thinner stent platform provides enhanced stent deliverability and stent flexibility. Recent clinical trial outcomes with the EES raise expectations regarding the safety and efficacy of this DES. Meanwhile, given that real-world data are currently limited, EES safety and efficacy should be reinforced by more evidence both from randomized trials and registries. Procedural and mechanical issues are also important elements to be considered. In order to achieve optimal EES expansion, adequate stenting strategies should be used, hopefully resulting in improved DES outcomes for the future.
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
▪▪ The everolimus-eluting stent (EES) consists of everolimus as a drug, MULTI-LINK® VISION® as a stent platform and a thin polymer made of two layers.The MULTI-LINK VISION stent is made from an L-605 cobalt–chromium alloy and resulted in a thinner and more flexible platform compared with earlier-generation stents.
▪▪ Everolimus is a semisynthetic macrolide immunosuppressant drug synthesized by chemical modification of rapamycin, which has increased solubility in organic solvents.
▪▪ The EES polymer coating showed the recovery of endothelial function in a preclinical study.
▪▪ The efficacy and safety of the EES has been demonstrated mainly in the SPIRIT trial series, suggesting promising clinical outcomes, although long-term follow-up data need to be evaluated.
▪▪ The mechanical characteristics of the drug-eluting stent should be taken into account in order to obtain optimal stent apposition
▪▪ Dilatation pressure and duration, noncompliant balloon usage and the use of an imaging modality are recommended to optimize EES implantation.
References
- Colombo A, De Gregorio J, Moussa I et al.: Intravascular ultrasound-guided percutaneous transluminal coronary angioplasty with provisional spot stenting for treatment of long coronary lesions. Am. Coll. Cardiol. 38(5), 1427–1433(2001).
- Bramucci E, Angoli L, Merlini PA et al.: Adjunctive stent implantation following directional coronary atherectomy in patients with coronary artery disease. J. Am. Coll. Cardiol. 32(7), 1855–1860 (1998).
- Moses JW, Leon MB, Popma JJ et al.: Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med. 349, 1315–1323 (2003).
- Morice MC, Serruys PW, Sousa JE et al.: randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N. Engl. J. Med. 346, 1773–1780 (2002).
- Stone GW, Ellis SG, Cannon L et al.: Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease; a randomized controlled trial. JAMA 294, 1215–1223 (2005).
- Stone GW, Ellis SG, Cox DA et al.: polymer-based paclitaxel-eluting stent in patients with coronary artery disease. N. Engl. J. Med. 350, 221–231 (2004).
- Ong AT, McFadden EP, Regar E, de Jaegere PP, van Domburg RT, Serruys PW: Late angiographic stent thrombosis (LAST) events with drug-eluting stents.Am. Coll. Cardiol. 45, 2088–2092 (2005).
- Iakovou I, Schmidt T, Bonizzoni E et al.: Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 293, 2126–2130 (2005).
- Stone GW, Moses JW, Ellis SG et al.: Safety and efficacy of sirolimus and paclitaxel-eluting coronary stents. N. Engl. J. Med. 356, 998–1008 (2007).
- Abbott JD, Voss MR, Nakamura M et al.: Unrestricted use of drug eluting stents compared with bare metal stents in routine clinical practice: findings from National Heart, Lung and Blood Institute Dynamic registry. J. Am. Coll. Cardiol. 50, 2029– 2036 (2007).
- Marroquin OC, Seizer F, Mulukutla SR et al.: A comparison of bare-metal anddrug-eluting stents for off-label indications. Engl. J. Med. 358, 342–352 (2008).
- Kandzari DE, Angiolillo DJ, Price MJ et al.: Identifying the ‘optimal’ duration of dual antiplatelet therapy after drug-eluting stent revascularization. JACC Cardiovasc. Interv. 2, 1279–1285 (2009).
- Serruys PW, Ong AT, Piek JJ et al.: A randomized comparison of a durable polymer Everolimus-eluting stent with a bare metal coronary stent: the first SPIRIT first trial. EuroIntervention 1, 58–65(2005).
- Serruys PW, Ruygrok P, Neuzner J et al.: A randomized comparison of an everolimus-eluting coronary stent with a paclitaxel-eluting coronary stent: the SPIRIT II trial. EuroIntervention 2, 286–294 (2006).
- Stone GW, Midei M, Newman W et al.: Comparison of everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease. A randomized trial. JAMA 299, 1903–1913 (2008).
- Fujii K, Carlier SG, Mintz GS et al.: Stent underexpansion and residual reference segment stenosis are related to stent thrombosis after sirolimus-eluting stent implantation: an intravascular ultrasound study. J. Am. Coll. Cardiol. 45, 995–998 (2005).
- Sonoda S, Morino Y, Ako J et al.: Impact of final stent dimensions on long-term results following sirolimus-eluting stent implantation: serial intravascular ultrasound analysis from the SIRIUS trial. J. Am. Coll. Cardiol. 43, 1959–1963 (2004).
- Kaiser C, Brunner-La Rocca HP, Buser PT et al.: Incremental cost–effectiveness ofdrug-eluting stents compared with a third-generation bare-metal stent in a real-world setting: randomized Basel Stent Kosten Effektivitats Trial (BASKET). Lancet 366, 921–929 (2005).
- Ortolani P, Marzocchi A, Marrozzini C et al.: Randomized comparative trial of a thin-strut bare metal cobalt-chromium stent versus a sirolimus-eluting stent for coronary revascularization. Cathether Cardiovasc. Interv. 69, 790–798 (2007).
- Xu YW, Wei YD, Tang K et al.: Multi-link Vision and MiniVision stent registry in Asian patients with coronary artery disease: a prospective, multi-center study. Chin. Med. J. (Engl.) 120, 1093–1096 (2007).
- Schuler PW, Sedrani R, Cottens S et al.: SDZ RAD, a new rapamycin derivative: pharmacological properties in vitro and in vivo. Transplantation 64, 36–42 (1997).
- Ding N, Pacetti SD, Tang FW et al.: XIENCE V stent design and rationale. J. Interv. Cardiol. 22, S18–S27 (2009).
- Wiemer M, Butz T, Schmidt W, Horstkotte D, Langer C: Scanning electron microscopic analysis of different drug eluting stents after failed implantation: from nearly undamaged to major damaged polymers. Catheter Cardiovasc. Interv. 75, 905–911 (2010).
- Basalus MW, Ankone MJ, van Houwelingen GK, de Man FH, von Birgelen C: Coating irregularities of durable polymer-based drug-eluting stents as assessed by scanning electron microscopy.
EuroIntervention 5, 157–165 (2009). - Joner M, Nakazawa G, Finn AV et al.: Endothelial cell recovery between comparator polymer-based drug-eluting stents. J. Am. Coll. Cardiol. 52, 333–342 (2008).
- Tsuchida K, Piek JJ, Neumann FJ et al.: One-year results of a durable polymer everolimus-eluting stent in de novo coronary narrowings (the SPIRIT FIRST trial). EuroIntervention 1, 266–272 (2005).
- Ruygrok PN, Desaga M, Van Den Branden F et al.: One year clinical follow-up of the XIENCE V everolimus-eluting stent system in the treatment of patients withde novo native coronary artery lesions: theSPIRIT II study. EuroIntervention 3, 315–320 (2007).
- Claessen BE, Beijk MA, Legrand V et al.: Two-year clinical, angiographic, and intravascular ultrasound follow-up of the XIENCE V everolimus-eluting stent in the treatment of patients with de novo native coronary artery lesions: the SPIRIT II trial. Circ. Cardiovasc. Interv. 2, 339–347 (2009).
- Stone GW, Midei M, Newman W et al.: Randomized comparison of everolimus-eluting and paclitaxel-eluting stents: two-year clinical follow-up from the clinical evaluation of the XIENCE V everolimus eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions (SPIRIT) III trial. Circulation 119, 680–686 (2009).
- Stone GW, Rizvi A, Newman W et al.: Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N. Engl.Med. 362, 1663–1674 (2010).
- Onuma Y, Kukreja N, Piazza N et al.; Interventional Cardiologists of the Thoraxcenter (2000 to 2007): The everolimus-eluting stent in real world patients: 6 month follow-up of the X-SEARCH (XIENCE V stent evaluated at Rotterdam Cardiac Hospital) registry. Am. Coll. Cardiol. 54, 269–276(2009).
- Latib A, Ferri L, Ielasi A et al.: Clinical outcomes after unrestricted implantation of everolimus-eluting stents. JACC Cardiovasc. Interv. 2, 1219–1226 (2009).
- Shimohama T, Ako J, Yamasaki M et al.: SPIRIT III JAPAN versus SPIRIT III USA: a comparative intravascular ultrasound analysis of the everolimus-eluting stent. Am. J. Cardiol. 106, 13–17 (2010).
- Haude M, Erbel R, Meyer J et al.: Quantitative analysis of elastic recoil after balloon angioplasty and after intracoronary implantation of balloon-expandable Palmaz-Schatz stents. J. Am. Coll. Cardiol. 21, 26–34 (1993).
- Bermejo J, Botas J, Garcia E et al.: Mechanisms of residual lumen stenosis after high-pressure stent implantation: a quantitative coronary angiography and intravascular ultrasound study. Circulation 98, 112–118 (1998).
- Rechavia E, Litvack F, Macko G, Eigler NL: Influence of expanded balloon diameter on Palmaz-Schatz recoil. Catheter Cardiovasc. Diagn. 36, 11–16 (1995).
- Tanimoto S, Serruys PW, Thuesen L et al.: Comparison of in vivo stent recoil between the Bioabsorbable everolimus-eluting coronary stent and the everolimus-eluting coronary chromium coronary stent: insights from the ABSORB and SPIRIT trials. Catheter Cardiovasc. Interv. 70, 515–523(2007).
- Kawasaki T, Koga H, Serikawa T et al.: Impact of a prolonged delivery inflation time for optimal drug-eluting stent expansion. Catheter Cardiovasc. Interv. 73, 205–211(2009).