Review Article - Interventional Cardiology (2010) Volume 2, Issue 1
Rheolytic thrombectomy: any role left?
- Corresponding Author:
- Marco De Carlo
Cardiothoracic & Vascular Department, University of Pisa, Pisa, Italy
Tel: +39 050 995 325
Fax: +39 050 995 330
E-mail: marcodecarlo@gmail.com
Abstract
Keywords
acute myocardial infarction, ischemic coronary disease, no-reflow, primary percutaneous coronary intervention, reperfusion, thrombectomy
Primary percutaneous coronary intervention (P-PCI) is the preferred treatment for myocardial infarction with ST-segment elevation (STEMI) and is effective in opening the infarctrelated artery [1–3]. However, microvascular obstruction with diminished myocardial perfusion occurs in a large proportion of patients treated with P-PCI despite a patent epicardial vessel, and this event, known as the ‘no-reflow’ phenomenon [4], is associated with increased mortality, increased infarct size and left ventricular remodeling [5–11]. Presence of microvascular damage can be indirectly demonstrated with angiographic parameters such as thrombolysis in myocardial infarction (TIMI) flow grade or myocardial blush grade (MBG), and more precisely with imaging techniques such as contrast ultrasonography [12], MRI [13], myocardial scintigraphy [14] and Doppler flow-wire evaluation [15].
Various mechanisms are implicated in the genesis of the no-ref low phenomenon, such as endothelial dysfunction, plugging of small vessels, compression of small vessels by tissue edema, thrombus and debris dislocation [16]. It is a double-faced process that starts during the ischemic period and increases at the time of reperfusion. Sometimes no-reflow after P-PCI becomes clinically and electrocardiographically evident with persistence of chest pain and incomplete ST-segment elevation resolution (STR). During the ischemic phase, tissue injury induces the production of oxygen reactive species, intracellular calcium overload and microvascular compression by edema. After reperfusion, metabolic events and platelet plugging occur, and they can be further complicated by thrombus and plaque debris mechanical dislocation during P-PCI. In fact, macroscopic distal embolization may occur in up to 16% of patients undergoing P-PCI [17]. However, epicardial vessel flow can be apparently maintained by adenosine-induced hyperemia in the areas surrounding the infarct zone.
Modern therapy of STEMI has to involve control and prevention of microvascular damage mechanisms and distal embolization [18]. Various adjunctive pharmacological therapies have been clinically tested with limited clinical benefits [19–21]. Direct stenting without predilatation may decrease embolization and the incidence of the no-reflow phenomenon [22]. More specific approaches to the problem of embolization during P-PCI include thrombectomy by means of different techniques and the use of embolic protection devices [23–25].
Distal protection devices
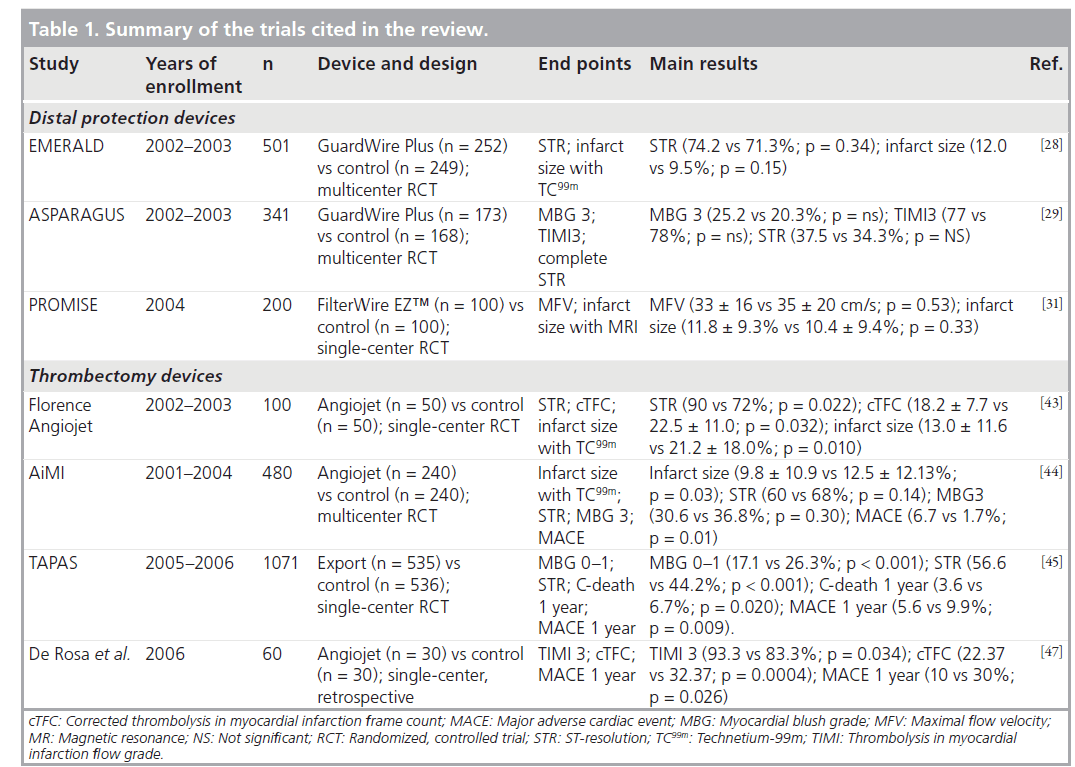
Distal occlusive devices are designed to prevent distal embolization by complete blockage of the antegrade flow by inflation of a balloon distal to the occlusion, followed by aspiration. Distal filters are nonocclusive devices that are advanced in the closed position beyond the target lesion and then opened in order to capture atherothrombotic debris migrating to the distal myocardium during predilatation and stent deployment. Several distal protection devices, either distal occlusive devices or distal filter devices, have proved their beneficial effects in PCI of saphenous vein grafts (SVG) [26,27]. Several randomized trials have also been conducted in primary angioplasty with contradictory findings. In the Enhanced Myocardial Efficacy and Recovery by Aspiration of Liberated Debris (EMERALD) trial [28] with a distal occlusion device (Medtronic Percusurge, Inc., CA, USA), although atherothrombotic debris was found in 78% of patients, there were no benefits in terms of myocardial perfusion in the treatment arm, whereas infarct size was paradoxically increased with the device (Table 1). The Aspiration of Liberated Debris in Acute Myocardial Infarction with Guardwire Plus System (ASPARAGUS) trial [29] obtained similar findings in 341 patients randomized to GuardWire Plus (Percusurge; n = 173) or conventional primary angioplasty (n = 168). There were no device-related procedural complications in either trial.
Despite the promising results of a nonrandomized pilot study conducted at our department [30] with a distal filter (FilterWire, EZ™, Boston Scientific, MA, USA), subsequent randomized trials failed to demonstrate a significant benefit with the use of distal filter devices in P-PCI. In the randomized Protection Devices in PCI Treatment of Myocardial Infarction for Salvage of Endangered Myocardium (PROMISE) trial [31] with FilterWire EZ no benefits were found in terms of myocardial reperfusion (evaluated by Doppler flow-wire) or infarct size (evaluated by MRI) in the device-treated arm.
In a meta-analysis of the randomized trials [32], De Luca demonstrated that distal protection devices were associated with a significant advantage in terms of final myocardial blush grade 3 (51.5 vs 42.2%; OR: 1.73; 95% CI: 1.09–2.75; p = 0.02), although no significant benefit in terms of 30‑day mortality was observed (2.5 vs 2.6%; OR: 0.97; 95% CI: 0.64–1.46; p = 0.88). Safety of adjunctive devices was comparable to standard treatment, as there were no significant differences in procedure-related complications (0.6 vs 0%; OR: 5.15; 95% CI: 0.25–107.9; p = 0.29).
Distal protection devices failed to repeat in the setting of P-PCI the favorable results obtained in the percutaneous treatment of SVGs. Some authors argued that PCI-induced embolic burden is approximately one order of magnitude smaller in STEMI, as compared with vein grafts (1.2 vs 16 mm3 on average) [33], while other authors found comparable embolic burdens [34]. First-generation filter-based devices might have pores that are too large (typically >100 mm) to effectively protect against microembolization. By means of histopathological analysis of the debris retrieved with a distal filter during P-PCI, we demonstrated that angiographic signs of high thrombus burden (cut-off coronary occlusion pattern or large intracoronary minus image) independently predicted the total debris volume [35].
Thrombectomy devices
Thrombectomy devices may not only prevent distal embolization and its metabolic effects on microcirculatory function, but also allow the operator to have a better visualization of the underlying atherosclerotic lesion. Several monorail devices are currently available, both manual and engine-operated. Manual devices consist of a catheter with a large lumen where negative pressure is applied through a simple luer-lock syringe. Mechanical devices are connected to a pressure pump that creates a vortex around the tip that fragments the thrombus and increases suction capability by the Venturi effect (rheolytic thrombectomy).
Over the last few years, several large randomized trials and a great number of small reports of local experiences have been published on thrombectomy during P-PCI [36–42]. The first study that tested the efficacy of rheolytic thrombectomy (RT) was the Florence-AngioJet [43], a singlecenter, randomized trial with AngioJet (Possis Medical, MN USA) (Table 1). The Angiojet system consists of a main unit with a high-pressure infusion pump driving saline solution into a 5 F rapid-exchange catheter. The latter consists of two lumina, a smaller one to run the saline distally, and a bigger one collecting aspirated material. Saline solution exits at the catheter’s tip through microholes oriented proximally and comes back into the collector lumen at approximately 500 km/h, thereby creating a 360° zone of depression around the tip by the Venturi effect, crumbling and aspirating the thrombus. A total of 100 patients with a first acute myocardial infarction (AMI) were enrolled; primary end points were STR, corrected TIMI frame count (cTFC), and infarct size assessed by technetium-99m (TC99m) sestamibi scintigraphy. Results demonstrated benefits of RT in terms of lower cTFC (18.2 ± 7.7 vs 22.5 ± 11.0; p = 0.032), higher incidence of early STR (90 vs 72%; p = 0.022) and smaller infarct size (13.0 ± 11.6% vs 21.2 ± 18.0%; p = 0.010). The 6‑month clinical outcomes were similar in the two arms, with a mortality rate of 2% in both groups, no reinfarctions, and a target vessel revascularization rate of 14 versus 23%, P-PCI versus RT, respectively (p = 0.270). Florence-AngioJet demonstrated the safety and efficacy of RT in P-PCI avoiding pharmacological therapy bias, as nearly all patients received the same IIb/IIIa inhibitor treatment.
The AngioJet Rheolytic Thrombectomy In Patients Undergoing Primary Angioplasty for AMI (AiMI) study [44] was a prospective, multicenter trial that randomized 480 patients with STEMI from 31 contributing sites in the USA and Canada between 2001 and 2004 to receive treatment with RT with the AngioJet device as an adjunct to conventional PCI or conventional PCI alone. The primary end point of the study was final infarct size evaluated by Tc99m sestamibi imaging at 14–28 days after procedure. Secondary end points were final TIMI flow grade 3, MBG 3, STR more than 70%, and the rate of major adverse cardiac events (MACE) at 1 month. The AiMI study failed to demonstrate any advantage of RT in terms of reduction in infarct size and incidence of final TIMI 3 grade flow; on the contrary, it showed a significantly higher MACE rate at 30‑day follow-up with RT. As a matter of fact, as randomization was performed before angiography, intracoronary evident thrombosis was not required as an inclusion criterion.
Manual aspiration devices such as the Export aspiration catheter (Medtronic) have been tested with very positive results in the recent Thrombus Aspiration during Percutaneous Coronary Intervention in Acute Myocardial Infarction Study (TAPAS) [45]. This was a single-center, prospective, randomized trial that enrolled a total of 1071 patients with randomization before diagnostic angiography. The primary end point was the frequency of postprocedural MBG 0 or 1, and occurred in 17.1% in the thrombusaspiration group and in 26.3% in the conventional PCI group (p < 0.001). In addition, the authors observed an unexpected result regarding a significant gradient in the secondary end points of 30‑day mortality and MACE rate in patients with a final MBG of 0 or 1, 2 and 3. Mortality was 5.2, 2.9 and 1.0%, respectively (p = 0.003), and the MACE rate was 14.1, 8.8 and 4.2%, respectively (p < 0.001). These findings were confirmed at 1‑year follow-up; moreover, patients randomized to aspiration versus conventional PCI showed a significantly lower incidence of cardiac death (3.6 vs 6.7%; p = 0.020) and of the composite end point of death and nonfatal reinfarction (5.6 vs 9.9%; p = 0.009).
Matching the results of the AiMI and TAPAS trial is very hard. On the one hand we have a simple, practical, low-cost manual aspiration device, consisting of a relatively flexible and nontraumatic catheter connected to a manual syringe, that showed improvement in terms of myocardial perfusion and 1‑year mortality. On the other hand, there is a complex, expensive, high-pressure pump, with a stiffer catheter, that showed no benefits in terms of reperfusion, infarct size and a higher risk of MACE.
Why are we still talking about rheolytic thrombectomy?
In a Bayesian meta-analysis published in 2008, Grines et al. collected 125 publications, including randomized clinical trials (RCT), all reporting short-term mortality and postprocedural TIMI 3 flow, that enrolled 25,094 subjects with AMI treated with or without RT [46]. The AngioJet experience included 11 studies (two RCT and nine non-RCT) and 1018 patients. The authors concluded that short-term mortality, MACE rate and postprocedural TIMI 3 flow were similar between groups, although the RT group consisted of higher risk patients, with greater thrombus burden, longer symptom-to-balloon time and with a higher proportion of rescue PCI. Interestingly, RT was associated with reduced risk of mortality and increased post-procedural TIMI 3 flow with respect to standard PCI in the subgroup of patients undergoing rescue PCI after failed thrombolysis. However, this meta-analysis highlighted the lack of evidence in favor of RT as an adjunct to primary PCI, with a significant impact on the clinical practice of interventional cardiologists all over the world.
In the AiMI trial most operators were probably not ‘skilled’ in the use of Angiojet; in fact, AiMI was started before the Florence-AngioJet study, but was published later due to slow enrollment in a complex protocol that took 36 months to collect 480 patients from 31 centers. If we look at procedural data, we find a total procedural time significantly longer in the RT group (75.4 ± 30.9 vs 59.2 ± 26.8 min; p < 0.001), probably contributing to a larger infarct size. Furthermore, the device was not activated from proximal to distal to the culprit lesion, but was advanced distally to the lesion before turning on the pump, probably favoring distal embolization. In our experience with AngioJet, we did not experience distal embolization by activating the pump immediately proximal to the lesion and then advancing it in repeated passages. In addition, in the AiMI trial, temporary pacing before PCI was recommended in patients randomized to RT, because of the risk of device-induced asystole. However, more recent studies describe a high rate of Angiojet-induced bradycardia only in right coronary or dominant circumflex STEMI [47]. In our experience, the latest lowprofile version of the RT catheter may cause brief episodes of asystole caused by adenosine release that vanishes in a few seconds and can be solved by asking the patient to cough.
AiMI authors interrogated themselves as to whether randomization after angiography could have introduced a selection bias against enrolling high-risk patients with a large amount of angiographically apparent thrombus. As a matter of fact, the proportion of patients with a totally occluded infarct-related artery was low in both groups, and baseline TIMI 3 flow was more frequent in the control arm (26 vs 19%; p < 0.05). While RT provided no benefit in reducing final infarct size in the overall population, there were differences in specific subsets of patients, in particular those with large thrombus burden. A total of 96 patients (50 treated with adjunct RT and 46 treated with PCI alone) had large or moderate baseline thrombus by angiographic analysis. In this patient subset, mean final infarct size was 10.8% in the RT group versus 8.1% in the PCI-alone group (p = 0.23). In this high thrombus burden group, however, the authors did not include patients with a totally occluded artery with an abrupt cut-off.
In a study focused on the use of drug-eluting stents (DES) and bare-metal stents (BMS) in patients with acute coronary syndromes, Sianos et al. reported the 2‑year clinical outcome and the predictors of stent thrombosis of the infarctrelated artery (IRA) [48]. The authors proposed a new thrombus grading in two categories based on the TIMI 14 trial classification [49], considering as ‘small thrombus burden’ a thrombus less than grade 4, and ‘large thrombus burden’ for thrombus grade 4, with grade 4 being defined as definite thrombus, with the largest dimension more than two vessel diameters. In patients presenting with an occluded infarct-related artery (thrombus grade 5), thrombus burden was reclassified into one of the two categories after flow achievement with either guidewire crossing or a small deflated balloon passage or dilation. Such simple classification of thrombus burden allowed accurate stratification of the risk of stent thrombosis of the IRA.
Intracoronary thrombus appears to be strictly connected to stent underexpansion and malapposition [50], as established by intravascular ultrasound. This condition is one of the strongest predictors of early and late stent thrombosis, both for BMS [51] and DES [52]. Primary stenting during AMI has been recognized as an independent predictor of late stent malapposition both after BMS and DES with an incidence two- to three-fold higher compared with elective stenting [53,54]. Sianos reported a 2‑year cumulative IRA stent thrombosis rate significantly higher in large versus small thrombus burden patients (16 vs six events). Large thrombus burden appeared as the most hazardous independent predictor of stent thrombosis of the IRA. Among patients with large thrombus burden, those treated with RT had a significantly lower 2‑year stent thrombosis rate versus patients not receiving RT (0 vs 11.3%; p < 0.001). The authors concluded that the controversial results of trials involving RT were probably connected to underestimation of importance of thrombus burden, and called for prospective randomized exploration of the potential benefits of thrombectomy devices in high-risk STEMI patients, such as those with large thrombus burden.
Recently De Rosa et al. retrospectively analyzed 60 patients (two groups of 30 consecutive patients) with large thrombus burden defined according to the definitions of the TIMI3 trial: 30 patients were treated with standard P-PCI, and 30 received RT prior to P-PCI [47]. Angiographic analysis showed benefits of RT in terms of final TIMI 3 flow (93.3 vs 83.3%; p = 0.034), and of final cTFC (22.4 vs 32.4; p = 0.0004). The MACE rate at 1‑year follow-up was significantly lower in the RT group (10 vs 30%; p = 0.026), as well as all-cause mortality (3.3 vs 13.3%; p < 0.001) and cardiac mortality (3.3 vs 10.0%; p = 0.007).
Rheolytic thrombectomy: our experience
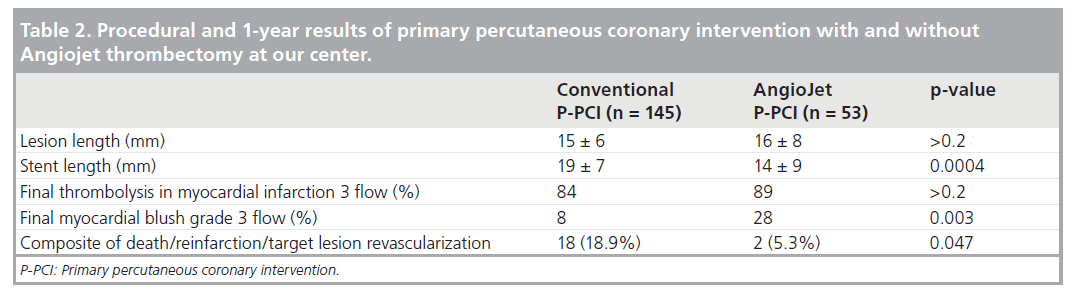
In the Cath Laboratory of the Cardiothoracic and Vascular Department of the University of Pisa, Italy, RT with the AngioJet system has been in use since 2004. Among patients treated with P-PCI within 12 h from symptom onset from August 2004 to October 2007, we retrospectively identified a subgroup of 198 patients with large thrombus burden at angiography, according to Sianos’ classification cited above. A total of 53 of these patients received AngioJet thrombectomy, while 145 did not. Baseline clinical profile was similar between the groups, but a higher rate of rescue P-PCI after failed thrombolysis (21 vs 9%; p = 0.03) appeared in the thrombectomy group. No device-related complications were reported and there was no need for temporary pacing. There was no difference regarding final TIMI 3 flow while final MBG grade 3 was more frequent in the RT group (28 vs 8%; p = 0.003) (Table 2). Moreover, although visual estimation of lesion length after crossing with the guidewire was similar between groups (15 ± 6 vs 16 ± 8 mm; p > 0.2), total length of stent implanted was significantly shorter in the RT group (14 ± 9 vs 19 ± 7 mm; p = 0.0004). In our opinion, clearing the lesion from adherent thrombus by means of RT allowed the operators to choose shorter stents, as they felt more comfortable with precise stent positioning. At 1‑year follow-up, RT was associated with a significantly higher freedom from the composite end point of cardiovascular death, re-infarction and target lesion revascularization (94.6 vs 81.3%; p = 0.047).
These encouraging findings, presented orally in 2009 [55], prompted us to design a multicenter prospective randomized trial to verify whether RT in patients with large thrombus burden may positively affect infarct size and clinical outcome. The Efficacy of Rheolytic Thrombectomy in Patients With High Thrombus Burden During Primary PCI trial started in June 2008 and will enroll 200 STEMI patients presenting within 12 h from symptom onset with an angiographic finding of large thrombus burden according to Sianos’ classification. The primary end points are STR at 60 min after the end of PCI, and infarct size evaluated by means of MRI with delayed enhancement technique at 3 months [56,57]. The secondary end points are final TIMI 3 flow, cTFC and MBG, and freedom from the composite end point of cardiovascular death, reinfarction and target lesion revascularization at 1 year. A substudy within the trial features coronary optical coherence tomography at 1 year to evaluate stent endothelization, malapposition and thrombosis in a high-risk subset of patients suffering from diabetes, chronic kidney disease and coronary multivessel disease.
Conclusion & future perspective
Distal embolization of atherothrombotic debris occurs quite frequently during primary PCI and has a relevant impact on clinical outcome. Both pharmacological and mechanical adjunctive treatments have been tested in the clinical arena, with mixed results. At present, two important points are still to be clarified: which patients benefit from the use of adjunctive devices for thrombus management and how can they be identified by clinical and angiographic characteristics; and which kind of device has the best safety and efficacy profile, and which is also cost effective?
A growing body of evidence supports the concept that patients with STEMI and large fresh thrombus burden may benef it from thrombectomy on top of pharmacological treatment during primary PCI; however, this evidence comes mainly from retrospective analysis and no consistent data are emerging from strong randomized trials. The scientific community calls for clinical trials in order to build evidence-based medicine. However, prospective randomized trials sometimes offer more questions than answers, particularly when trial design is cumbersome, enrollment is slow, patient selection is biased and operator experience with the investigational device is low. On the other hand, single-center studies are often biased by inadequate study design and power, and by conflicts of interest, as pointed out in a recent meta-analysis of the trials involving adjunctive devices to prevent distal embolization during STEMI [58]. Single-center or multicenter design appeared to be the only variable significantly related to different clinical outcome, as an increase by one in the number of centers increased the risk of incomplete STR by 1.4% (95% CI: 0.48–2.28), and the risk of final impaired MBG by 1% (95% CI: 0.02–1.97%). Although the authors of this meta-analysis conclude that the use of thrombectomy devices cannot be recommended based on the results of single-center studies, in our opinion those operators who are familiar with a specific device and who experience a benefit from its use should not abandon thrombectomy on the basis of a multicenter trial with many flaws. An adequately powered, well-designed, multicenter trial performed by skilled operators will contribute to the search for a definitive answer on thrombectomy in the setting of P-PCI.
Executive summary
Introduction
▪ Goals of modern therapy for acute myocardial infarction involve prevention of microvascular damage and of distal embolization, as well as prevention of the ‘no-reflow’ phenomenon. For this purpose, pharmacological approaches and adjunctive devices have been developed.
Distal protection devices
▪ First-generation distal protection devices failed to reproduce the excellent results obtained in saphenous vein graft intervention in the setting of primary percutaneous coronary intervention (PCI).
Thrombectomy devices
▪ Both manual and mechanical devices are available. The thrombectomy literature includes several large randomized trials and a great number of small reports of local experiences. One large multicenter study reported negative results with rheolytic thrombectomy, while a large single-center trial on manual aspiration demonstrated a significant improvement in myocardial reperfusion as well as a reduction in 1‑year cardiac mortality versus conventional primary PCI.
Why are we still talking about rheolytic thrombectomy?
▪ Several small experiences confirm the safety and efficacy of rheolytic thrombectomy, especially in high-risk patients with large thrombus burden at angiography. Furthermore, the negative results of the AngioJet Rheolytic Thrombectomy In Patients Undergoing Primary Angioplasty for Acute Myocardial Infarction trial were probably determined by patient selection bias and limited operator experience with the Angiojet device.
Rheolytic thrombectomy: our experience
▪ In a retrospective analysis of our experience with AngioJet in primary PCI we found positive results in terms of improved myocardial reperfusion and better outcome at 1 year versus conventional PCI. These findings prompted us to design a multicenter, prospective, randomized trial in ST-segment elevation patients with large thrombus burden, to evaluate the impact of rheolytic thrombectomy on infarct size assessed by means of cardiac magnetic resonance imaging.
Conclusion & future prospective
▪ Rheolytic thrombectomy may still play an important role in primary PCI in high-risk patients with large thrombus burden at initial angiography. An adequately powered randomized trial will hopefully prove this concept.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Keeley EC, Boura JA, Grines CL: Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 361(9351), 13–20 (2003).
- Zijlstra F, Hoorntje JC, De Boer MJ et al.: Long-term benefit of primary angioplasty as compared with thrombolytic therapy for acute myocardial infarction. N. Engl. J. Med. 341(19), 1413–1419 (1999).
- Van De Werf F, Bax J, Betriu A et al.: Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the task force on the management of ST-segment elevation acute myocardial infarction of the european society of cardiology. Eur. Heart J. 29(23), 2909–2945 (2008).
- Kloner RA, Ganote CE, Jennings RB: The ‘No-reflow’ phenomenon after temporary coronary occlusion in the dog. J. Clin. Invest. 54(6), 1496–1508 (1974).
- Van Hof A, Liem A, Suryapranata H, Hoorntje J, De Boer M, Zijlstra F: Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Zwolle myocardial infarction study group. Circulation 97(23), 2302–2306 (1998).
- Van’t Hof AW, Liem A, De Boer MJ, Zijlstra F: Clinical value of 12-lead electrocardiogram after successful reperfusion therapy for acute myocardial infarction. Zwolle myocardial infarction study group. Lancet 350(9078), 615–619 (1997).
- Mclaughlin MG, Stone GW, Aymong E et al.: Prognostic utility of comparative methods for assessment of ST-segment resolution after primary angioplasty for acute myocardial infarction: the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) trial. J. Am. Coll. Cardiol. 44(6), 1215–1223 (2004).
- Stone GW, Peterson MA, Lansky AJ, Dangas G, Mehran R, Leon MB: Impact of normalized myocardial perfusion after successful angioplasty in acute myocardial infarction. J. Am. Coll. Cardiol. 39(4), 591–597 (2002).
- Poli A, Fetiveau R, Vandoni P et al.: Integrated analysis of myocardial blush and ST-segment elevation recovery after successful primary angioplasty: real-time grading of microvascular reperfusion and prediction of early and late recovery of left ventricular function. Circulation 106(3), 313–318 (2002).
- Marzilli M, Gliozheni E, Marraccini P, Fedele S: Primary coronary angioplasty in acute myocardial infarction: clinical correlates of the ‘no reflow’ phenomenon. Int. J. Cardiol. 65(Suppl. 1), S23–28 (1998).
- Rezkalla SH, Kloner RA: No-reflow phenomenon. Circulation 105(5), 656–662 (2002).
- Ito H, Maruyama A, Iwakura K et al.: Clinical implications of the ‘no reflow’ phenomenon. A predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation 93(2), 223–228 (1996).
- Wu KC, Zerhouni EA, Judd RM et al.: Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 97(8), 765–772 (1998).
- Koch KC, Vom Dahl J, Kleinhans E et al.: Influence of a platelet gpIIb/IIIa receptor antagonist on myocardial hypoperfusion during rotational atherectomy as assessed by myocardial TC-99m sestamibi scintigraphy. J. Am. Coll. Cardiol. 33(4), 998–1004 (1999).
- Iwakura K, Ito H, Nishikawa N et al.: Early temporal changes in coronary flow velocity patterns in patients with acute myocardial infarction demonstrating the ‘no-reflow’ phenomenon. Am. J. Cardiol. 84(4), 415–419 (1999).
- Jaffe R, Charron T, Puley G, Dick A, Strauss BH: Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation 117(24), 3152–3156 (2008).
- Henriques JP, Zijlstra F, Ottervanger JP et al.: Incidence and clinical significance of distal embolization during primary angioplasty for acute myocardial infarction. Eur. Heart J. 23(14), 1112–1117 (2002).
- Marzilli M, Orsini E, Marraccini P, Testa R: Beneficial effects of intracoronary adenosine as an adjunct to primary angioplasty in acute myocardial infarction. Circulation 101(18), 2154–2159 (2000).
- Gabriel RS, White HD: Extract-TIMI 25 trial: clarifying the role of enoxaparin in patients with ST-elevation myocardial infarction receiving fibrinolysis. Expert Rev. Cardiovasc. Ther. 5(5), 851–857 (2007).
- Sabatine MS, Mccabe CH, Gibson CM, Cannon CP: Design and rationale of Clopidogrel as Adjunctive Reperfusion Therapy–Thrombolysis In Myocardial Infarction (CLARITY-TIMI) 28 trial. Am. Heart J. 149(2), 227–233 (2005).
- Ellis SG, Armstrong P, Betriu A et al.: Facilitated percutaneous coronary intervention versus primary percutaneous coronary intervention: design and rationale of the Facilitated Intervention with Enhanced Reperfusion Speed to Stop Events (FINESSE) trial. Am. Heart J. 147(4), E16 (2004).
- Antoniucci D, Valenti R, Migliorini A et al.: Direct infarct artery stenting without predilation and no-reflow in patients with acute myocardial infarction. Am. Heart J. 142(4), 684–690 (2001).
- Hamburger JN, Serruys PW: Treatment of thrombus containing lesions in diseased native coronary arteries and saphenous vein bypass grafts using the angiojet rapid thrombectomy system. Herz 22(6), 318–321 (1997).
- Ischinger T: Thrombectomy with the x-sizer catheter system in the coronary circulation: initial results from a multi-center study. J. Invasive Cardiol. 13(2), 81–88 (2001).
- Stone GW, Rogers C, Hermiller J et al.: Randomized comparison of distal protection with a filter-based catheter and a balloon occlusion and aspiration system during percutaneous intervention of diseased saphenous vein aorto–coronary bypass grafts. Circulation 108(5), 548–553 (2003).
- Stone GW, Rogers C, Ramee S et al.: Distal filter protection during saphenous vein graft stenting: technical and clinical correlates of efficacy. J. Am. Coll. Cardiol. 40(10), 1882–1888 (2002).
- Carlino M, De Gregorio J, Di Mario C et al.: Prevention of distal embolization during saphenous vein graft lesion angioplasty. Experience with a new temporary occlusion and aspiration system. Circulation 99(25), 3221–3223 (1999).
- Stone GW, Webb J, Cox DA et al.: Distal microcirculatory protection during percutaneous coronary intervention in acute ST-segment elevation myocardial infarction: a randomized controlled trial. JAMA 293(9), 1063–1072 (2005).
- Muramatsu T, Kozuma K, Tsukahara R et al.: Comparison of myocardial perfusion by distal protection before and after primary stenting for acute myocardial infarction: angiographic and clinical results of a randomized controlled trial. Catheter Cardiovasc. Interv. 70(5), 677–682 (2007).
- Limbruno U, Micheli A, De Carlo M et al.: Mechanical prevention of distal embolization during primary angioplasty: safety, feasibility, and impact on myocardial reperfusion. Circulation 108(2), 171–176 (2003).
- Gick M, Jander N, Bestehorn HP et al.: Randomized evaluation of the effects of filter-based distal protection on myocardial perfusion and infarct size after primary percutaneous catheter intervention in myocardial infarction with and without ST-segment elevation. Circulation 112(10), 1462–1469 (2005).
- De Luca G, Suryapranata H, Stone GW, Antoniucci D, Neumann FJ, Chiariello M: Adjunctive mechanical devices to prevent distal embolization in patients undergoing mechanical revascularization for acute myocardial infarction: a meta-analysis of randomized trials. Am. Heart J. 153(3), 343–353 (2007).
- Kuntz RE, Rogers C, Baim DS: Percutaneous coronary intervention-induced emboli during primary PCI for STEMI: too little, too much, or too late? Am. Heart J. 150(1), 4–6 (2005).
- Quan VH, Huynh R, Seifert PA et al.: Morphometric analysis of particulate debris extracted by four different embolic protection devices from coronary arteries, aortocoronary saphenous vein conduits, and carotid arteries. Am. J. Cardiol. 95(12), 1415–1419 (2005).
- Limbruno U, De Carlo M, Pistolesi S et al.: Distal embolization during primary angioplasty: histopathologic features and predictability. Am. Heart J. 150(1), 102–108 (2005).
- Dudek D, Mielecki W, Legutko J et al.: Percutaneous thrombectomy with the rescue system in acute myocardial infarction. Kardiol. Pol. 61(12), 523–533 (2004).
- Burzotta F, Trani C, Romagnoli E et al.: Manual thrombus-aspiration improves myocardial reperfusion: the randomized evaluation of the effect of mechanical Reduction of Distal Embolization by Thrombus – Aspiration in Primary and Rescue Angioplasty (REMEDIA) trial. J. Am. Coll. Cardiol. 46(2), 371–376 (2005).
- Silva-Orrego P, Colombo P, Bigi R et al.: Thrombus aspiration before primary angioplasty improves myocardial reperfusion in acute myocardial infarction: the DEAR-MI (Dethrombosis to Enhance Acute Reperfusion in Myocardial Infarction) study. J. Am. Coll. Cardiol. 48(8), 1552–1559 (2006).
- Beran G, Lang I, Schreiber W et al.: Intracoronary thrombectomy with the x-sizer catheter system improves epicardial flow and accelerates ST-segment resolution in patients with acute coronary syndrome: a prospective, randomized, controlled study. Circulation 105(20), 2355–2360 (2002).
- Ikari Y, Sakurada M, Kozuma K et al.: Upfront thrombus aspiration in primary coronary intevention for patients with ST-segment elevation acute myocardial infarction – report of the vampire trial. J. Am. Coll. Cardiol. Cardiovasc. Intervent. 1(4), 424–431 (2008).
- Sianos G, Papafaklis MI, Vaina S et al.: Rheolytic thrombectomy in patients with ST-elevation myocardial infarction and large thrombus burden: the thoraxcenter experience. J. Invasive Cardiol. 18(Suppl. C), 3C–7C (2006).
- Sardella G, Mancone M, Nguyen BL et al.: The effect of thrombectomy on myocardial blush in primary angioplasty: the Randomized Evaluation of Thrombus Aspiration by Two Thrombectomy Devices in Acute Myocardial Infarction (RETAMI) trial. Catheter Cardiovasc. Interv. 71(1), 84–91 (2008).
- Antoniucci D, Valenti R, Migliorini A et al.: Comparison of rheolytic thrombectomy before direct infarct artery stenting versus direct stenting alone in patients undergoing percutaneous coronary intervention for acute myocardial infarction. Am. J. Cardiol. 93(8), 1033–1035 (2004).
- Ali A, Cox D, Dib N et al.: Rheolytic thrombectomy with percutaneous coronary intervention for infarct size reduction in acute myocardial infarction: 30‑day results from a multicenter randomized study. J. Am. Coll. Cardiol. 48(2), 244–252 (2006).
- Svilaas T, Vlaar PJ, Van Der Horst IC et al.: Thrombus aspiration during primary percutaneous coronary intervention. N. Engl. J. Med. 358(6), 557–567 (2008).
- Grines CL, Nelson TR, Safian RD, Hanzel G, Goldstein JA, Dixon S: A Bayesian meta-analysis comparing angiojet thrombectomy to percutaneous coronary intervention alone in acute myocardial infarction. J. Interv. Cardiol. 21(6), 459–482 (2008).
- De Rosa S, Cirillo P, De Luca G et al.: Rheolytic thrombectomy during percutaneous coronary intervention improves long-term outcome in high-risk patients with acute myocardial infarction. J. Interv. Cardiol. 20(4), 292–298 (2007).
- Sianos G, Papafaklis Mi, Daemen J et al.: Angiographic stent thrombosis after routine use of drug-eluting stents in ST-segment elevation myocardial infarction: the importance of thrombus burden. J. Am. Coll. Cardiol. 50(7), 573–583 (2007).
- Gibson CM, De Lemos JA, Murphy SA et al.: Combination therapy with abciximab reduces angiographically evident thrombus in acute myocardial infarction: a TIMI 14 substudy. Circulation 103(21), 2550–2554 (2001).
- Cutlip DE, Baim DS, Ho KK et al.: Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent clinical trials. Circulation 103(15), 1967–1971 (2001).
- Moussa I, Di Mario C, Reimers B, Akiyama T, Tobis J, Colombo A: Subacute stent thrombosis in the era of intravascular ultrasound-guided coronary stenting without anticoagulation: frequency, predictors and clinical outcome. J. Am. Coll. Cardiol. 29(1), 6–12 (1997).
- Fujii K, Carlier SG, Mintz GS et al.: Stent underexpansion and residual reference segment stenosis are related to stent thrombosis after sirolimus-eluting stent implantation: an intravascular ultrasound study. J. Am. Coll. Cardiol. 45(7), 995–998 (2005).
- Hong MK, Mintz GS, Lee CW et al.: Incidence, mechanism, predictors, and long-term prognosis of late stent malapposition after bare-metal stent implantation. Circulation 109(7), 881–886 (2004).
- Hong MK, Mintz GS, Lee CW et al.: Late stent malapposition after drug-eluting stent implantation: an intravascular ultrasound analysis with long-term follow-up. Circulation 113(3), 414–419 (2006).
- Bellini F, De Carlo M, Gistri R et al.: Efficacy of rheolytic trombectomy in patients with high thrombus burden during primary PCI. EuroIntervention 5(Suppl. E), E10–E121 (2009).
- Van Dijkman PR, Van Der Wall EE, De Roos A et al.: Acute, subacute, and chronic myocardial infarction: quantitative analysis of gadolinium-enhanced MR images. Radiology 180(1), 147–151 (1991).
- Klein C, Nekolla SG, Bengel FM et al.: Assessment of myocardial viability with contrast-enhanced magnetic resonance imaging: comparison with positron emission tomography. Circulation 105(2), 162–167 (2002).
- Inaba Y, Chen JA, Mehta N, Bergmann SR: Impact of single or multicentre study design on the results of trials examining the efficacy of adjunctive devices to prevent distal embolisation during acute myocardial infarction. EuroIntervention 5(3), 375–383 (2009).
▪ Randomized, controlled trial (RCT) fails to prove infarct reduction with distal balloon protection during primary percutaneous coronary intervention (PCI).
▪ RCT fails to prove infarct reduction with filter-based distal protection during primary PCI.
▪▪ Single-center registry proving usefulness of rheolytic thrombectomy in case of large thrombus burden.
▪▪ Single-center trial proving infarct reduction with rheolytic thrombectomy in primary PCI.
▪▪ RCT failing to prove clinical benefit of rheolytic thrombectomy in primary PCI.
▪▪ Large RCT proving reduction in mortality with thrombectomy in primary PCI.
▪ Single-center registry demonstrating the clinical benefit of rheolytic thrombectomy during primary PCI in case of large thrombus burden.
▪ Single-center registry suggesting the role of thrombotic burden in favoring DES thrombosis after primary PCI.
▪ Meta-analysis suggesting that single-center studies may be biased towards more positive findings.