Research Article - Neuropsychiatry (2018) Volume 8, Issue 5
Risperidone Exposure and Breast Cancer Risk: A Cohort Study Using the Taiwan National Health Insurance Research Database
- Corresponding Author:
- Dr. Frank Huang-Chih Chou
Department of Community Psychiatry, Kaohsiung Municipal Kai-Syuan Psychiatric Hospital, 130, Kai-Syuan 2nd Rd, Lingya District, Kaohsiung City, Taiwan
Tel: 886-7-7513171-2232
Fax: 886-7-5373299
Abstract
Background
Conventional (typical) antipsychotics are thought to be associated with the development of breast cancer (BC) due to their dopamine-antagonistic effects that lead to increased circulating prolactin. Atypical antipsychotics, particularly risperidone and paliperidone may also increase prolactin secretion. We investigated whether risperidone use was associated with an increased BC risk by estimating the incidence of BC in users of risperidone, other atypical antipsychotics, and conventional antipsychotics.
Methods
This retrospective cohort study used data from Taiwan’s Nation Health Insurance Research Database (NHIRD) which captures claims from mandatory universal health insurance in Taiwan. All women aged ≥18 years who initiated treatment with any antipsychotic between July 2000-December 2011 were identified in the database. BC was identified from the NHIRD Registry of Catastrophic Illness and Taiwan Cancer Registry. Cox proportional hazards models were used to adjust for potential confounders and compare BC incidence among the three antipsychotic exposure groups.
Results
There were 233,237 women included in the total cohort analysis. The mean follow-up period was 3.34-5.56 years. Crude incidence rates of BC were 123.1 per 100,000 person-years in the risperidone group, 135.7 per 100,000 person-years in the other atypical group and 149.8 per 100,000 person-years in the typical group. The adjusted hazard ratio was 1.13 (95%CI 0.98-1.29) and 1.07 (95%CI 0.95-1.22) comparing the other atypical and the typical groups, respectively with the risperidone group.
Conclusion
There is no evidence of an increased risk of BC associated with risperidone compared to other atypical or conventional antipsychotics.
Keywords
Risperidone, Taiwan National Health Insurance Database (NHIRD), Breast Cancer, Conventional (Typical) Antipsychotics, Atypical Antipsychotics
Introduction
Breast cancer (BC) is the most commonly diagnosed cancer in women and accounts for approximately 25% of all female cancers [1]. In Taiwan, BC is also one of the most common cancers and the fourth leading cause of cancer death in women [2]. Between 1997 and 2013, the incidence of BC in Taiwan increased from 52.3 to 93.0 per 100,000 person-years, and the median age at diagnosis was 52.6 years [3].
More than 95% of BC display overexpression of the prolactin receptor, and genes that are activated by this receptor are associated with tumorigenesis and cancer cell proliferation. Nevertheless, while some epidemiological studies suggest a possible link between circulating prolactin levels and increased BC risk [4-9], such results have not been consistently observed and the link between prolactin and the pathogenesis of BC remains controversial [10].
Antipsychotic drugs are the most common cause of pharmacologically-induced hyperprolactinemia. Most antipsychotic drugs are dopamine type 2 receptor antagonists which may result in raised circulating prolactin levels [11]. Retrospective cohort studies exploring the effect of antipsychotic drugs on BC risk have produced disparate results. Studies conducted in Sweden and the United Kingdom (UK) showed no increased risk of BC in women using risperidone compared to other antipsychotics [12,13], whereas a study in the United States (US) showed a 16% increase in BC in users compared to non-users of dopamine antagonists including antipsychotics, with a dose-response relationship between larger cumulative dosages and greater risk [14]. Risperidone is known to elevate prolactin more often and to a greater degree than other atypical antipsychotic drugs [15,16]. We applied the same research protocol employed by Reutfors et al. [13] in Sweden to study long-term risperidone exposure and the risk of BC in Taiwan.
Methods
▪ Data source
The NHIRD is a large, representative, population-based claims database provided by the Taiwan National Health Research Institute [17]. The National Health Insurance program was implemented in March 1995, and provides mandatory universal health insurance for approximately 99% of >23 million people in Taiwan. The NHIRD offers a comprehensive set of patient and clinical information, including demographic data, diagnostic codes, dates and types and procedures, dispensed prescription drugs, of expenditures. The National Health Insurance Bureau of Taiwan randomly reviews the charts of one per 100 ambulatory, and one per 20 inpatient claim cases, and interviews patients to verify the accuracy of the diagnosis [17-19]. All personally identifiable information is encrypted to protect patient privacy. A separate subset of the NHIRD is the Registry of Catastrophic Illness (NHIRD-RCI). Insured patients who suffer from certain major diseases can apply for a catastrophic illness certificate which grants exemption from all copayments. Cancers are statutorily included in the catastrophic illness category. To apply for a cancer catastrophic illness certificate, cytological or pathological reports or evidence supporting the diagnosis of malignancy is required. Records from this database were used to identify cancer diagnoses.
▪ Study population
Individuals with exposure to an antipsychotic between July 2000 and December 2011 were identified by the presence of an antipsychotic prescription/dispensing record in the NHIRD classified as risperidone, any other atypical antipsychotic (except paliperidone), or a conventional antipsychotic using the Anatomical Therapeutic Chemical classification (Appendix). Exposure index date was defined as the first (i.e. the earliest) prescription followed by at least another prescription of the same antipsychotic drug within 90 days, and with no other antipsychotics between the two dispensings. The date of the second dispensing among the earliest pair of consecutive prescriptions in the databases was set as the exposure index date. Eligible study subjects were women ≤ 18 years of age on the exposure index date. Study follow-up started 365 days after the exposure index date. To exclude prevalent BC cases, patients with a diagnosis of BC prior to the exposure index date or within 365 days after the exposure index date were excluded from the study population. Additional inclusion criteria were having lived or immigrated to Taiwan for at least 6 months prior to the start of exposure (i.e. the first dispensing of the qualifying antipsychotic pair), and having remained in the database for at least 365 days after the exposure index date. Individuals were excluded if they had a record of malignancy (other than non-melanoma skin cancer) any time prior to the start of follow-up (365 days after the exposure index date), or if they had ever received a prescription for paliperidone.
▪ Exposure group assignment
We divided exposed subjects into risperidone, other atypical and typical antipsychotic drug groups. The exposure groups were assigned in the following hierarchical order: women with risperidone prescriptions were assessed for eligibility to be included in the risperidone cohort; if not eligible (due to no records of risperidone prescriptions or due to failing other eligibility criteria), women with any other atypical antipsychotic drug prescriptions were assessed for eligibility for the other atypical antipsychotic cohort. If not eligible, remaining women were assessed for eligibility for inclusion in the typical antipsychotic cohort. Exposed individuals had no previous prescription record for the specified antipsychotic prior to the first dispensation.
▪ Breast cancer case ascertainment
The Taiwan NHIRD-RCI and the Taiwan Cancer Registry (TCR) are considered accurate and specific for cancer diagnosis given that both sources require pathologic/histology verification. The primary study endpoint was a BC diagnosis (the earliest date of record) identified from the TCR annual report, and/or the NHIRD-RCI.
▪ Study follow-up
All women included in the study were followed over time for the occurrence of incident BC. There were two follow-up approaches: the total cohort follow-up period extended from 365 days following the exposure index date until occurrence of BC, emigration, death, or end of the study period (31st December 2012), whichever occurred first. The active treatment follow-up time extended from 365 days following the exposure index date until discontinuation of the initial treatment regimen + 365 days, occurrence of BC, disenrollment from the database, or end of the study period, whichever occurred first. A gap of 1 year or more was considered as discontinuation of a treatment regimen, with the actual date of treatment discontinuation defined as the date of the last received prescription plus days of drug supply.
▪ Comorbidity profile
The presence of comorbidities prior to the exposure index date was investigated based on all available history records in the NHIRD using International Classification of Diseases, 9th Revision Clinical Modification and 10th Revision codes (ICD-9-CM/ICD-10). The prevalence of benign breast disorders as well as the following potentially prolactin-elevating somatic conditions (within 6 months prior to exposure index date) was also assessed: acromegaly, pituitary hyperfunction (including druginduced hyperprolactinemia), hypothyroidism, sarcoidosis, liver cirrhosis, chronic renal failure, Cushing’s disease, and polycystic ovary syndrome.
▪ Statistical analyses
BC incidence rates were estimated using persontime of the total cohort follow-up, as well as the person-time of active treatment exposure followup, for each of the three antipsychotic drug exposure cohorts. Incidence rates were reported as number of cases per 100,000 person-years. BC incidence rates were compared between other atypical antipsychotics and risperidone groups or conventional antipsychotics and risperidone groups using Cox proportional hazards regression models. Covariates (including age, index year, psychiatric diagnosis, medical service use situation, BC-associated physical illnesses, prolactin-elevating medications, mammography) were only retained in the final Cox regression model if their inclusion in a model containing the single covariate and the antipsychotic exposure variable changed the hazard ratio (HR) for the antipsychotic exposure variable by 10% or more, relative to the unadjusted HR for antipsychotic exposure (i.e., adjusted HR/unadjusted HR is either >1.10 or <0.90) [20].
To account for cases of BC possibly missed in either the TCR or NHIRD-RCI, we conducted a sensitivity analysis to additionally include BC diagnosed from NHIRD inpatient records. That is, the sensitivity analysis identified BC cases from any one or more of the following three sources: TCR, NHIRD-RCI, or NHIRD inpatient diagnoses.
We also estimated BC incidence rates for newly treated patients, defined as those patients having no prescription record for any antipsychotic in the six months prior to the first identified index treatment of each exposure group.
Data management and analyses were conducted using SAS software, Version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
▪ Characteristics of study subjects
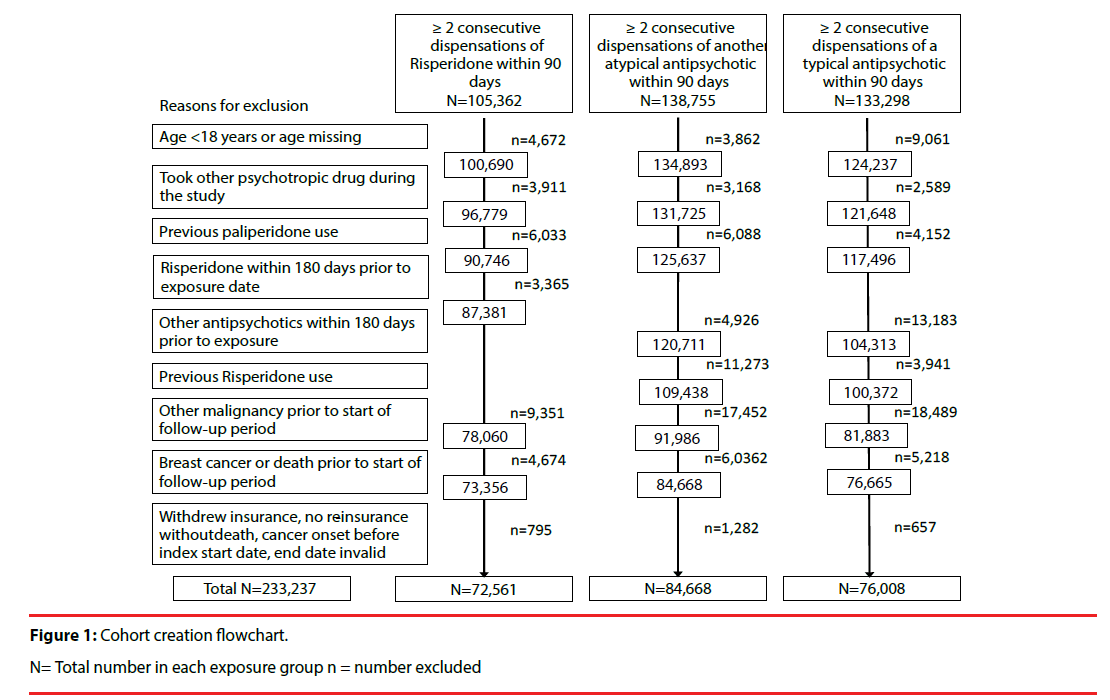
There were approximately 9.3 million women aged ≥ 18 years in Taiwan over the study period [21]. During the 11.5-year study period, 233,237 women were included in the total study cohort: there were 72,561 (0.8%) eligible women included in the risperidone group, 84,668 (0.9%) included in the other atypical antipsychotics group, and 76,008 (0.8%) included in the typical antipsychotics group (Figure 1). The mean age of women was 54.4 years in the risperidone group, 57.0 years in the other atypical group, and 53.0 years in the typical group (Table 1). The risperidone group appeared to have a higher proportion of younger women (30.4% were aged 18-39 years versus 26.4% and 27.1% in the other atypical and typical groups, respectively), whereas the other atypical group appeared to have a higher proportion of older women (36.9% were aged ≥ 70 years versus 31.9% in the risperidone group and 23.7% in the typical group).
| Risperidone | Other atypical | Typical | ||||
|---|---|---|---|---|---|---|
| Total number included | 72,561 | 84,668 | 76,008 | |||
| n | % | n | % | n | % | |
| Age at inclusion | ||||||
| Mean (SD) | 54.4 | (21.2) | 57.0 | (21.2) | 53.0 | (18.7) |
| Age group | ||||||
| 18-39 | 22,031 | 30.4 | 22,387 | 26.4 | 20,623 | 27.1 |
| 40-49 | 10,905 | 15.0 | 12,258 | 14.5 | 14,000 | 18.4 |
| 50-59 | 8,852 | 12.2 | 10,172 | 12.0 | 13,061 | 17.2 |
| 60-69 | 7,663 | 10.6 | 8,635 | 10.2 | 10,334 | 13.6 |
| ≥ 70 | 23,110 | 31.9 | 31,216 | 36.9 | 17,990 | 23.7 |
| Index year | ||||||
| 2000 | 1,480 | 2.0 | 413 | 0.5 | 4,777 | 6.3 |
| 2001 | 5,474 | 7.5 | 1,584 | 1.9 | 10,198 | 13.4 |
| 2002 | 6,309 | 8.7 | 2,450 | 2.9 | 7,945 | 10.5 |
| 2003 | 7,535 | 10.4 | 4,265 | 5.0 | 7,557 | 9.9 |
| 2004 | 7,555 | 10.4 | 5,548 | 6.6 | 7,434 | 9.8 |
| 2005 | 6,519 | 9.0 | 6,910 | 8.2 | 6,463 | 8.5 |
| 2006 | 6,857 | 9.5 | 7,401 | 8.7 | 5,216 | 6.9 |
| 2007 | 7,040 | 9.7 | 7,975 | 9.4 | 4,800 | 6.3 |
| 2008 | 6,494 | 9.0 | 9,617 | 11.4 | 4,598 | 6.1 |
| 2009 | 5,918 | 8.2 | 11,208 | 13.2 | 5,854 | 7.7 |
| 2010 | 5,811 | 8.0 | 13,034 | 15.4 | 5,444 | 7.2 |
| 2011 | 5,569 | 7.7 | 14,263 | 16.9 | 5,722 | 7.5 |
| Psychiatric diagnosis | ||||||
| Any psychiatric diagnosis, ICD: 290-319 | 71,951 | 99.2 | 83,295 | 98.4 | 62,393 | 82.1 |
| Schizophrenia | 35,084 | 48.4 | 13,892 | 16.4 | 7,931 | 10.4 |
| Bipolar disorder | 15,365 | 21.2 | 20,946 | 24.7 | 6,292 | 8.3 |
| Dementia | 23,901 | 32.9 | 27,728 | 32.8 | 10,313 | 13.6 |
| Major depression | 19,173 | 26.4 | 33,649 | 39.7 | 12,686 | 16.7 |
| Other organic psychiatric disorder | 21,299 | 29.4 | 20,802 | 24.7 | 8,204 | 10.8 |
| Alcohol use disorder | 2,201 | 3.0 | 4,740 | 5.6 | 2,618 | 3.4 |
| Other substance use disorder | 2,980 | 4.1 | 5,449 | 6.4 | 2,448 | 3.2 |
| Other psychosis | 23,073 | 31.8 | 13,968 | 16.5 | 6,255 | 8.2 |
| Neurotic stress related or somatoform disorder | 47,445 | 65.4 | 64,470 | 76.1 | 51,364 | 67.6 |
| Personality disorder | 3,419 | 4.7 | 5,958 | 7.0 | 1,924 | 2.5 |
| Mental retardation and autism | 3,754 | 5.2 | 1,837 | 2.2 | 1,126 | 1.5 |
| Suicide attempt | 815 | 1.1 | 1,621 | 1.9 | 728 | 1.0 |
| Psychiatric conditions, other than the above | 340 | 0.5 | 1,003 | 1.2 | 2,182 | 2.9 |
| No psychiatric diagnosis | 610 | 0.8 | 1,373 | 1.6 | 13,615 | 17.9 |
Index year: the year of the exposure index date (the earliest prescription followed by at least another prescription of the same antipsychotic drug within 90 days).
Table 1: Demographic data of included women by index antipsychotic drug exposure (N= 233,237)
‘Neurotic stress related or somatoform disorder’ was the most common psychiatric diagnosis for all three groups (65%-75%) based on all available history in the database (Table 1). The second most common diagnoses were ‘schizophrenia’ for the risperidone group (48.4% of women), and ‘major depression’ for the other atypical and typical groups (39.7% and 16.7%, respectively).
The highest proportion of women who had received inpatient psychiatric care within 180 days prior to the first prescription was in the risperidone group (16.0% versus 8.1% in the other atypical group and 3.4% in the typical group) (Table 2). The other atypical group had the highest proportion of women who had received inpatient care for somatic symptoms within 180 days prior to the first prescription (25.1% vs. 19.2% in the risperidone group and 17.1% in the typical group. Most of the risperidone and other atypical subjects had a psychiatric diagnosis (92.2% and 89.9% respectively), compared with 48.9% of those in the typical group. Higher rates of benign breast neoplasm were observed in the other atypical and typical groups (0.7% in each cohort) than the risperidone group (0.4%). Although medical and medication use 180 days prior to the index exposure differed between the three groups, most of these variables were not confounders for BC risk. Of the covariates tested, all factors except age were excluded from the Cox regression model using the 10% change-in-estimate rule of confounder screening.
| Risperidone | Other atypical | Typical | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Psychiatric inpatient care within 180 days prior to the first prescription (of the index exposure) | ||||||
| Yes | 11,586 | 16.0 | 6,870 | 8.1 | 2,563 | 3.4 |
| Somatic inpatient care within180 days prior to the index exposure | ||||||
| Yes | 13,961 | 19.2 | 21,237 | 25.1 | 12,978 | 17.1 |
| Inpatient and outpatient diagnosis within 180 days prior to the index exposure | ||||||
| Psychiatric diagnosis, ICD: 290-319 | 66,877 | 92.2 | 76,084 | 89.9 | 37,149 | 48.9 |
| Schizophrenia | 24,959 | 34.4 | 7,886 | 9.3 | 4,939 | 6.5 |
| Bipolar disorder | 7,310 | 10.1 | 10,303 | 12.2 | 2,356 | 3.10 |
| Dementia | 16,215 | 22.4 | 18,540 | 21.9 | 4,164 | 5.5 |
| Major depression | 8,847 | 12.2 | 20,634 | 24.4 | 5,049 | 6.6 |
| Other organic psychiatric disorder | 8,859 | 12.2 | 8,770 | 10.4 | 2,601 | 3.4 |
| Alcohol use disorder | 631 | 0.9 | 1,533 | 1.8 | 824 | 1.1 |
| Other substance use disorder | 770 | 1.1 | 1,535 | 1.8 | 729 | 1.0 |
| Other psychosis | 12,033 | 16.6 | 6,209 | 7.3 | 2,656 | 3.5 |
| Neurotic stress related or somatoform disorder | 16,720 | 23.0 | 29,310 | 34.6 | 19,553 | 25.7 |
| Personality disorder | 1,008 | 1.4 | 2,129 | 2.5 | 584 | 0.8 |
| Mental retardation and autism | 1,906 | 2.6 | 978 | 1.2 | 492 | 0.7 |
| Suicide attempt | 113 | 0.2 | 244 | 0.3 | 87 | 0.1 |
| Autism | 87 | 0.1 | 39 | 0.1 | 18 | 0.0 |
| Disruptive behavior | 25 | 0.0 | 19 | 0.0 | 9 | 0.0 |
| Psychiatric conditions, other than the above | 2,230 | 3.1 | 6,494 | 7.7 | 3,944 | 5.2 |
| None of the above | 5,684 | 7.8 | 8,584 | 10.1 | 38,859 | 51.1 |
| Patients with no psychiatric diagnosis recorded | ||||||
| Autoimmune disorders | 72 | 0.1 | 119 | 0.1 | 53 | 0.1 |
| Seizures | 2,082 | 2.9 | 1,899 | 2.2 | 1,228 | 1.6 |
| Herpes zoster | 535 | 0.7 | 808 | 1.0 | 693 | 0.9 |
| Polycystic ovary syndrome | 116 | 0.2 | 200 | 0.2 | 97 | 0.1 |
| Pseudocyesis | 70 | 0.1 | 95 | 0.1 | 58 | 0.1 |
| Renal dysfunction | 1,806 | 2.5 | 3,100 | 3.7 | 1,670 | 2.2 |
| Inpatient and outpatient diagnosis (prolactin related) within 180 days prior to the index exposure | ||||||
| Benign neoplasm of female breast | 296 | 0.4 | 586 | 0.7 | 527 | 0.7 |
| Hyperprolactinemia | 208 | 0.3 | 276 | 0.3 | 117 | 0.2 |
| Hypothyroidism | 644 | 0.9 | 995 | 1.2 | 493 | 0.7 |
| Liver cirrhosis | 40 | 0.1 | 56 | 0.1 | 61 | 0.1 |
| Chronic renal failure | 317 | 0.4 | 377 | 0.5 | 319 | 0.4 |
| Cushing’s disease | 86 | 0.1 | 153 | 0.2 | 114 | 0.2 |
| Empty sella syndrome | 44 | 0.1 | 60 | 0.1 | 31 | 0.0 |
| Lymphoid hypophysis | 19 | 0.0 | 23 | 0.0 | 14 | 0.0 |
| Chest wall trauma | 20 | 0.0 | 32 | 0.0 | 22 | 0.0 |
| Cirrhosis | 368 | 0.5 | 494 | 0.6 | 434 | 0.6 |
| Chronic liver disease and cirrhosis | 2,803 | 3.9 | 4,167 | 4.9 | 4,299 | 5.7 |
| Alcoholic cirrhosis of liver | 40 | 0.1 | 56 | 0.1 | 61 | 0.1 |
| Cirrhosis of liver without mention of alcohol | 344 | 0.5 | 458 | 0.5 | 406 | 0.5 |
| Biliary cirrhosis | 3 | 0.0 | 17 | 0.0 | 7 | 0.0 |
| Prolactin-elevating medications (common) | ||||||
| Gastrointestinal medications | 18,559 | 25.6 | 28,412 | 33.6 | 28,542 | 37.6 |
| Methyldopa | 39 | 0.1 | 74 | 0.1 | 56 | 0.1 |
| Prolactin-elevating medications (possible) | ||||||
| Selective serotonin reuptake inhibitors | 16,647 | 22.9 | 30,583 | 36.1 | 8,500 | 11.2 |
| Opiates | 16,221 | 22.4 | 25,145 | 29.7 | 22,042 | 29.0 |
| Prolactin-elevating medications (rare) | ||||||
| Antihypertensive medications | 1,692 | 2.3 | 2,090 | 2.5 | 3,510 | 4.6 |
| Tricyclic and tetracyclic antidepressants | 4,697 | 6.5 | 8,031 | 9.5 | 6,116 | 8.1 |
| Other psychotropics | 17,005 | 23.4 | 29,577 | 34.9 | 16,505 | 21.7 |
| Estrogens | 2,623 | 3.6 | 3,593 | 4.2 | 4,516 | 5.9 |
| Mammogram exams | ||||||
| Within 1 year prior to index drug exposure | 624 | 0.9 | 1,211 | 1.4 | 1,142 | 1.5 |
| Within 1 year after index drug exposure | 524 | 0.7 | 1,015 | 1.2 | 1,109 | 1.5 |
| Ultrasound breast exams | ||||||
| Within 1 year prior to index drug exposure | 802 | 1.1 | 2,143 | 2.5 | 1,266 | 1.7 |
| Within 1 year after index drug exposure | 962 | 1.3 | 2,069 | 2.4 | 1,455 | 1.9 |
Table 2: Medical history and use of medications of the study cohort.
Women in the typical group were more likely to have index antipsychotic drug exposure in the early years of the study (mean total cohort follow-up period of 5.56 years), whereas women in the other atypical cohort were more likely to be included in the final years of the study (mean total cohort follow-up of 3.34 years) (Tables 1 and 3).
| Risperidone | Other atypical | Typical | |
|---|---|---|---|
| Total number | 72,561 | 84,668 | 76,008 |
| Number Censored | |||
| End of follow-up | |||
| Death | 15,356 | 14,303 | 12,875 |
| Emigration | 1,336 | 1,315 | 1,424 |
| Number of breast cancer cases | |||
| Total cohort follow-up | 432 | 384 | 633 |
| Active treatment follow-up | 151 | 161 | 110 |
| Person-years of follow-up | |||
| Total cohort follow-up (person-years) | 351,012 | 282,902 | 422,554 |
| mean (SD) (years) | 4.84 (3.14) | 3.34 (2.64) | 5.56 (3.48) |
| Active treatment follow-up (person-years) | 114,315 | 116,169 | 66,603 |
| Mean (SD) (years) | 1.58 (2.22) | 1.37 (1.87) | 0.88 (1.63) |
Table 3: Demographic data of patients with breast cancer identified from the Taiwan Cancer Registry annual report and/ or NHIRD Registry of Catastrophic Illness.
▪ BC incidence and risk estimates
During the total cohort follow-up period, there were a total of 1,449 cases of BC identified in the study cohort from the TCR annual report and/ or NHIRD-RCI (Table 3). The rate of incident BC (total cohort follow-up period) was 123.1 per 100,000 person-years in the risperidone group, 135.7 per 100,000 person-years in the other atypical group and 149.8 per 100,000 personyears in the typical group (Table 4). From the crude (i.e., unadjusted) Cox regression model, the typical group had a significantly higher BC incidence rate than the risperidone group (HR 1.19, 95% confidence interval [95% CI] 1.06- 1.35). The crude HR for development of BC was 1.14, 95% CI (0.99-1.31) for the other atypical group compared with the risperidone group.
| PYR | Number of cases | Cases/ 100,000 PYR | Crude HR | Crude 95% CI |
Adjusted* HR | Adjusted* 95% CI | |
|---|---|---|---|---|---|---|---|
| Total cohort follow-up | |||||||
| Risperidone | 351,012 | 432 | 123.1 | Ref=1 | - | Ref=1 | - |
| Other atypical | 282,902 | 384 | 135.7 | 1.14 | (0.99-1.31) | 1.13 | (0.98-1.29) |
| Typical | 422,554 | 633 | 149.8 | 1.19 | (1.06-1.35) | 1.07 | (0.95-1.22) |
| Active treatment follow-up | |||||||
| Risperidone | 124,822 | 151 | 121.0 | Ref=1 | - | Ref=1 | - |
| Other atypical | 137,562 | 161 | 117.0 | 1.09 | (0.87-1.36) | 1.10 | (0.88-1.38) |
| Typical | 69,912 | 110 | 157.3 | 1.30 | (1.02-1.67) | 1.19 | (0.93-1.52) |
| Sensitivity analyses | |||||||
| Including additional 1,526 BC cases from inpatient records. | |||||||
| Risperidone | 350,955 | 453 | 12.91 | Ref=1 | -- | Ref=1 | - |
| Other atypical | 282,858 | 415 | 14.67 | 1.17 | (1.02-1.34) | 1.15 | (1.01-1.32) |
| Typical | 422483 | 658 | 15.57 | 1.18 | (1.05-1.34) | 1.07 | (0.95-1.21) |
| Treatment-naïve total cohort follow-up | |||||||
| Risperidone | 225,568 | 278 | 123.2 | Ref=1 | - | Ref=1 | - |
| Other atypical | 220,594 | 299 | 135.5 | 1.13 | (0.96-1.33) | 1.11 | (0.94-1.31) |
| Typical | 1,357,727 | 2,079 | 153.1 | 1.19 | (1.05-1.35) | 1.11 | (0.98-1.26) |
* Adjusted by age group
Table 4: Breast cancer incidence rates (breast cancer cases identified by Taiwan Cancer Registry annual report and/or NHIRD Registry of Catastrophic Illness).
After adjustment for age, the HR comparing the typical antipsychotic group to the risperidone group was no longer significant (HR 1.07, 95% CI =0.95-1.22). The adjusted HR for the other atypical antipsychotic group versus the risperidone group was 1.13, (95% CI=0.98-1.29).
Results from the active treatment cohort followup period (time on drug) were similar to those of the total cohort follow-up (Table 4). In the active treatment analysis, the rate of incident BC was 121.0 per 10,000 person-years in the risperidone group, 117.0 per 100,000 personyears in the other atypical group, and 157.3 per 10,000 person-years in the typical group. There were no significant differences among the three groups based on the HR estimates after adjustment for age.
▪ Sensitivity analyses
Among the subgroup of women who were newly treated with antipsychotic drugs (i.e., no prescriptions for any antipsychotic during the 6 months prior to index exposure), the rates of incident BC were 123.2 per 100,000 personyears, 135.5 per 100,000 person-years, and 153.1 per 100,000 person-years, for the risperidone, other atypical and typical groups, respectively. There were no significant differences among the three groups based on the HR estimates after adjustment for age (Table 4).
The results adjusted for age remained similar when we included BC cases identified by inpatient diagnosis (n=1526) in addition to the 1,449 cases identified by the TCR and/or NHIRD-RCI (Table 4).
Discussion
Using a population-based retrospective cohort approach, we found no increased risk of BC among women using risperidone compared to women using other antipsychotic drugs in Taiwan. While the unadjusted rate of incident BC was observed to be higher in women using typical antipsychotics, there was no statistically significant increased risk after adjustment for age. The consistency of the results across the different sensitivity analyses confirms the robustness of the study results.
Age is a well-recognized risk factor for BC [22], and we observed differences in the age distribution of women included in each of the three groups, with almost half of all women in the typical group (49.20%) aged between 40-69 years, versus 37.79% and 36.69% in the risperidone and other atypical groups. In Taiwan, BC incidence rates increase markedly after 49 years of age [3], and the disparity in age distribution between groups may have caused the difference in BC incidence rates in the crude analysis.
A higher percentage of women in the risperidone group had a recent mammogram and a diagnosis of schizophrenia, which may be associated with a lower risk of cancer [23], and a lower proportion had benign breast neoplasms, baseline hyperprolactinemia, or were taking prolactin-elevating medications: all of which may have influenced the risk of BC in this group. However, none of these factors were retained in the Cox regression model for BC risk based on the 10% change-in-estimate rule of confounder screening.
Risperidone has been marketed in Taiwan since 2000, other atypical antipsychotics since 2008, whereas typical antipsychotic drugs have been available for decades. In our study, the typical group had the longest total person-years of follow-up time, but the shortest active treatment follow-up time. This may reflect the higher percentage of women in this group who had no psychiatric diagnosis, reflecting widespread use of sulpiride, an antipsychotic commonly used to treat gastrointestinal conditions in Taiwan. Alternatively, patients who were initiated on typical antipsychotics may have been more likely to have had their medication changed during the study. By contrast, the risperidone group had the longest active treatment follow-up time, possibly reflecting a positive benefit and more acceptable side-effect profile than other antipsychotics.
In 2013, the estimated incidence of BC in Taiwan was 93 per 100,000 person-years [3]. Our study found that the incidence of BC among women having antipsychotics was 30%-70% higher than that reported in the general population. This finding should be interpreted with caution given that patients exposed to antipsychotics receive more healthcare services and BC is more likely to be detected earlier than in the general population. This detection bias may contribute to the higher incidence rate of BC; however, a similar trend was observed in a large retrospective US study which found a 16% increase in BC among users of antipsychotics [14]. On the other hand, there have been numerous studies that have not shown associations between BC risk and antipsychotic use, and no causal association has been identified [10].
Consistent with our results, cohort studies conducted in the UK and Sweden observed no increased BC risk in risperidone users, and an apparent increased risk associated with typical antipsychotic use that was no longer present after adjustment for age [12,13]. Our study had a longer follow-up period compared to the Swedish study that used the same protocol (2.4-2.8 years versus 3.3-5.6 years), and we were able to include a substantially larger sample size compared to either the UK or Swedish studies. Our results build on these previous studies and provide additional evidence supporting that risperidone use is not associated with an increased risk of BC.
Strengths of the study include the use of large, nationally-representative data from the NHIRD, in which more than 98% of Taiwan’s population is enrolled. While previous studies comparing the risk of BC between users versus non-users of antipsychotics may have been confounded by the presence of underlying disease, the NHIRD made it possible to compare the BC risk accounting for other medical conditions or medications that are associated with elevated prolactin.
A potential study limitation is that women who were included in one exposure group may have switched their antipsychotic drug during the follow-up period. We accounted for possible changes in treatment by evaluating the active treatment follow-up period as well as the total follow-up period. Similar results in both analyses suggest that changes to antipsychotic treatment had little impact on the results, and support the robustness of the conclusion.
Another study limitation is that several BC risk factors, such as excessive alcohol use, obesity, the presence of non-alcoholic fatty liver disease, and smoking status, were not included in this study, and potential differences between the study groups in terms of these risk factors may have influenced cancer risk. Finally, we did not assess the impact of dose or exposure time on BC risk, although previous studies of potential dose-relationships between antipsychotic use and BC risk have given conflicting results [12,14].
Conclusion
In this large retrospective cohort study, we found no evidence for an increased risk of BC among women using risperidone versus other antipsychotics. These results are consistent with the findings of previous studies, and provide reassurance for women requiring antipsychotic medication and prescribers of these drugs.
Ethics Approval
The study was approved by the Ethics Review Board of Kaohsiung Municipal Kai-Syuan Psychiatric Hospital (KSPH-2013-34).
FHCC, KYT and SPS received grants from Janssen Research & Development for the submitted work. HCW and YMC declare no conflicts of interest.
Acknowledgments
This study used data obtained from the Taiwan’s Nation Health Insurance Research Database, which is managed by the Bureau of National Health Insurance, Department of Health and the National Health Research Institutes, Taiwan. Editorial assistance was provided by Joanne Wolter, MBBS, PhD (independent on behalf of Johnson & Johnson Pte Ltd). Dr Wolter declares no conflict of interest.
The study was supported by Janssen Research & Development, LLC (Titusville, New Jersey, United States), which manufactures and markets risperidone. The sponsor planned the study and reviewed the manuscript. The creation of the data sets and analysis were conducted by the Kaohsiung Municipal Kai-Syuan Psychiatric Hospital and National Health Research Institute under contracts with Janssen Research and Development. Both Kaohsiung Municipal Kai-Syuan Psychiatric Hospital and National Health Research Institute had completed control over the data, and the analyses were performed at the sites of Kaohsiung Municipal Kai-Syuan Psychiatric Hospital and Taipei Information Center, respectively.
Declaration of Competing Interests
The authors declare the following conflicts of interest:
FHCC, KYT and SPS received grants from Janssen Research & Development for the submitted work.
HCW and YMC declare no conflicts of interest. HQ, YW and HP are employees of Janssen Research & Development LLC which manufactures and markets risperidone. HP reports stock ownership in Johnson & Johnson Pte Ltd. The contents do not represent the views of the Kaohsiung Municipal Kai-Syuan Psychiatric Hospital or the Taiwan Government.
References
- Globocan 2012. Estimated cancer incidence, mortality and prevalence worldwide in 2012: Cancer Fact Sheets: Breast cancer (2012).
- Ministry of Health and Welfare. Statistical results on causes of death in Taiwan (2013).
- Liu FC, Lin HT, Kuo CF, et al. Epidemiology and survival outcome of breast cancer in a nationwide study. Oncotarget 8(10), 16939-16950 (2017).
- Hankinson SE, Willett WC, Michaud DS, et al. Plasma prolactin levels and subsequent risk of breast cancer in postmenopausal women. J. Natl. Cancer. Inst 91(7), 629-634 (1999).
- Tworoger SS, Hankinson SE. Prolactin and breast cancer risk. Cancer. Lett 243(2), 160-169 (2006).
- Tworoger SS, Sluss P, Hankinson SE. Association between plasma prolactin concentrations and risk of breast cancer among predominately premenopausal women. Cancer. Res 66(4), 2476-2482 (2006).
- Maguire GA. Prolactin elevation with antipsychotic medications: mechanisms of action and clinical consequences. J. Clin. Psychiatry 63(Suppl 4), 56-62 (2002).
- Manjer J, Johansson R, Berglund G, et al. Postmenopausal breast cancer risk in relation to sex steroid hormones, prolactin and SHBG (Sweden). Cancer. Caus. Cont 14(7), 599-607 (2003).
- Tworoger SS, Eliassen AH, Sluss P, et al. A prospective study of plasma prolactin concentrations and risk of premenopausal and postmenopausal breast cancer. J. Clin. Oncol 25(12), 1482-1488 (2007).
- De Hert M, Vancampfort D, Stubbs B, et al. Antipsychotic treatment, prolactin, and breast tumorigenesis. Psychiatr. Danub 28(3), 243-254 (2016).
- David SR, Taylor CC, Kinon BJ, et al. The effects of olanzapine, risperidone, and haloperidol on plasma prolactin levels in patients with schizophrenia. Clin. Ther 22(9), 1085-1096 (2000).
- Azoulay L, Yin H, Renoux C, et al. The use of atypical antipsychotics and the risk of breast cancer. Breast. Cancer. Res. Treat 129(2), 541-548 (2011).
- Reutfors J, Wingard L, Brandt L, et al. Risk of breast cancer in risperidone users: A nationwide cohort study. Schizophr. Res 182, 98-103 (2017).
- Wang PS, Walker AM, Tsuang MT, et al. Dopamine antagonists and the development of breast cancer. Arch. Gen. Psychiatry 59(12), 1147-1154 (2002).
- Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am. J. Psychiatry 171(6), 616-621 (2014).
- Bushe C, Shaw M, Peveler RC. A review of the association between antipsychotic use and hyperprolactinaemia. J. Psychopharmacol 22(2 Suppl), 46-55 (2008).
- National Health Insurance Research Database, Taiwan (2013).
- Chou FH, Tsai KY, Chou YM. The incidence and all-cause mortality of pneumonia in patients with schizophrenia: a nine-year follow-up study. J. Psychiatr. Res 47(4), 460-466 (2013).
- Tsai KY, Lee CC, Chou YM, et al. The risks of major osteoporotic fractures in patients with schizophrenia: a population-based 10-year follow-up study. Schizophr. Res 159(2-3), 322-328 (2014).
- Walter S, Tiemeier H. Variable selection: current practice in epidemiological studies. Eur. J. Epidemiol 24(12), 733-736 (2009).
- Ministry of the Interior: 2015 Statistical Yearbook of Interior: Population (2017). .
- McGuire A, Brown JA, Malone C, et al. Effects of age on the detection and management of breast cancer. Cancers. (Basel) 7(2), 908-929 (2015).
- Hodgson R, Wildgust HJ, Bushe CJ. Cancer and schizophrenia: is there a paradox? J. Psychopharmacol 24(4 Suppl), 51-60 (2010).