Review Article - Interventional Cardiology (2011) Volume 3, Issue 6
Role of atrioventricular nodal ablation and pacemaker therapy in elderly patients with recurrent atrial fibrillation
- Corresponding Author:
- Mackram F Eleid
Division of Cardiovascular Diseases, Department of Internal Medicine
Mayo Clinic, 200 First St. SW
55905 Rochester, MN, USA
E-mail: eleid.mackram@mayo.edu
Abstract
Keywords
atrial fibrillation, atrioventricular nodal ablation, elderly, pacemaker
Atrial fibrillation (AF), an increasingly common condition in the aging population [1], is responsible for a substantial health burden and impairment in patient quality of life [2]. The conduction properties of the atrioventricular (AV) node determine the ventricular response of AF, which is rapid (>100 bpm) in many individuals. Uncontrolled ventricular rates in the setting of AF often result in symptoms including dyspnea, palpitations and fatigue. In some patients, rapid AF can cause hemodynamic instability necessitating urgent medical attention. Chronic tachycardia resulting from AF can also lead to a tachycardia-mediated cardiomyopathy, manifesting in systolic heart failure. AF is associated with increased morbidity and mortality, frequent hospitalizations and increased healthcare costs overall, particularly in the elderly [2].
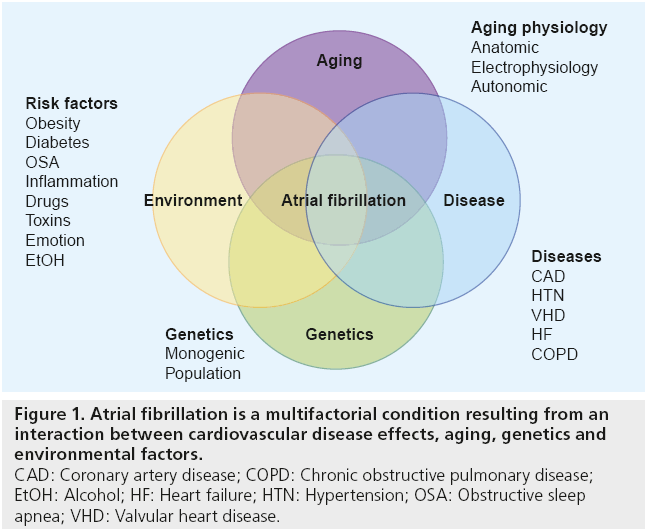
AF is a disease of the elderly, with nearly threequarters of all patients being between the ages of 65 and 85 years. Interplay between advancing age, comorbidities, environmental and genetic factors contribute to the development of AF (Figure 1). The prevalence of AF is currently 1–2% and is expected to increase with the aging population [3,4]. Comorbid medical conditions associated with AF including hypertension, heart failure, valvular heart disease, cardio myopathies, coronary artery disease, obesity, diabetes mellitus, chronic obstructive pulmonary disease, sleep apnea and chronic kidney disease are more frequent in the elderly, play a role in propagating AF, and increase morbidity and mortality [5]. Hospitalizations for AF in the USA have increased dramatically (two- to three-fold) over the last 15 years [6]. As such, the availability of effective and practical therapies for AF in the elderly patient with multiple comorbidities will be highly important in coming years.
Figure 1: Atrial fibrillation is a multifactorial condition resulting from an
interaction between cardiovascular disease effects, aging, genetics and
environmental factors.
CAD: Coronary artery disease; COPD: Chronic obstructive pulmonary disease;
EtOH: Alcohol; HF: Heart failure; HTN: Hypertension; OSA: Obstructive sleep
apnea; VHD: Valvular heart disease.
The two primary approaches to treatment of AF are therapies aimed at restoring sinus rhythm (rhythm control) and therapies that control the ventricular response of AF (rate control) [7]. While pharmacologic agents including b-blockers, non-dihydropyridine calcium channel blockers and digoxin are an often used firstline treatment, there are many AF patients who either do not respond to these therapies or are intolerant of them due to side effects.
In the last decade, radiofrequency catheter ablation of the left atrium has emerged as a relatively successful and commonly used therapy for restoring sinus rhythm in patients with recurrent AF. While AF ablation is moderately effective in preventing recurrence of AF in approximately 70–80% of individuals [8–11], the success rate from long-term follow-up has not been determined. Reporting and comparison of outcomes have been challenging, in part due to the evolving techniques and technologies in AF ablation over the past decade. The type of outcome measured, number of procedures and vigor of follow-up may all impact the apparent success rate. More recent data from longer follow-up studies have shown recurrence of AF, particularly in patients with persistent AF, steadily increases with follow-up time [9]. The efficacy of AF ablation may further decrease with older age, left atrial dilation [12], longer duration of AF, persistent as opposed to paroxysmal AF and underlying heart disease, making its utility in an elderly population limited [13]. For these reasons, combined with an increased risk of AF ablation procedural complications in the elderly, AV nodal ablation provides a more practical approach, which is both definitive and effective in controlling the symptoms and sequelae of AF in this population when standard medical therapy fails.
AV nodal ablation & right ventricular pacing for AF
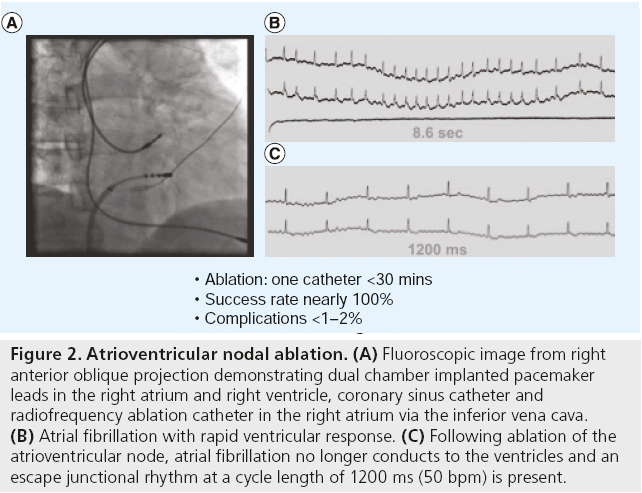
Radiofrequency ablation of the AV node combined with permanent right ventricular (RV) endocardial pacing is a highly effective treatment for controlling the ventricular response of AF. AV nodal ablation is achieved by inserting a steerable ablation catheter via the right or left femoral vein. The tip of the ablation catheter is positioned in the region of the AV junction in the right atrium. The location of the catheter is guided by the fluoroscopic image and by the configuration of the intracardiac electrograms. Mapping and localization of the proximal portion of the AV junction is critically important to ensure a junctional escape rhythm is possible. When ablation is performed during sinus rhythm, an ideal ablation site should display a local electrogram with atrial to ventricular ratio = 1 and a His bundle signal present on the ablation catheter. When ablation is performed during AF, atrial to ventricular ratio is less reliable due to the variable amplitude and cycle lengths of the fibrillation signals. The ideal ablation site is identified by pulling back the ablation catheter from the His bundle location towards the AV junction. The local electrogram should have three components: AF signals, a reproducible His signal and a far-field ventricular signal. In the elderly population, junctional rhythm is present in approximately 70% of patients after successful ablation, when the proximal AV junction can be localized. RF energy is delivered to the AV junction until complete heart block is observed on the intra-cardiac electrogram (Figure 2). Due to its invasive nature and the need for permanent pacing after AV nodal ablation, radiofrequency ablation has generally been reserved as a lastline therapy for individuals with permanent AF who do not respond to or are intolerant of pharmacologic therapy. The American College of Cardiology/American Heart Association/ European Society of Cardiology joint guidelines support this approach for AF rate control therapy as class IIa, or one in which the weight of evidence favors its usefulness [14].
Figure 2: Atrioventricular nodal ablation. (A) Fluoroscopic image from right anterior oblique projection demonstrating dual chamber implanted pacemaker leads in the right atrium and right ventricle, coronary sinus catheter and radiofrequency ablation catheter in the right atrium via the inferior vena cava. (B) Atrial fibrillation with rapid ventricular response. (C) Following ablation of the atrioventricular node, atrial fibrillation no longer conducts to the ventricles and an escape junctional rhythm at a cycle length of 1200 ms (50 bpm) is present.
AV nodal ablation is successful in producing persistent complete heart block in nearly 100% of cases [15]. As patients are left pacemaker-dependent following AV nodal ablation, a permanent pacemaker must be implanted prior to the ablation procedure. Rate adaptive pacing is necessary following AV nodal ablation, to allow for pacing rate increases in response to higher metabolic demands using either an activity or physiologic sensor. The decision to implant single- versus dual-chamber primarily depends on whether the patient has periods of sinus rhythm. The patient with paroxysmal AF and periodic sinus rhythm may benefit from a dual-chamber rateadaptive (DDDR) pacemaker in order to maintain optimal AV synchrony. DDDR pacemakers for this purpose should have automatic modeswitching capability to avoid rapid ventricular pacing during episodes of AF, which is currently available in virtually all pacemakers. However, patients with permanent chronic AF usually do not derive benefit from the addition of an atrial lead (due to lack of sinus rhythm) and require only a single-chamber device with rate-adaptive capabilities.
The benefits of AV nodal ablation and pacemaker implantation for recurrent AF have been shown in several observational studies. AV nodal ablation in patients with uncontrolled AF improves quality of life and exercise tolerance, and decreases both hospital admissions and heart failure episodes [16–19].
Advantages in the elderly
Several characteristics of the elderly population make them particularly suited to AV nodal ablation as a treatment for recurrent AF. Many issues are particularly relevant when drug therapy, either for rate or rhythm control, is considered in elderly patients. Comorbid medical illnesses such as hypertension, underlying heart disease, diabetes, chronic obstructive lung disease, dementia and other conditions are frequently present in the elderly. ‘Polypharmacy’ often leads to increased risk of drug–drug interactions and low compliance to adhering to the prescribed drug regimens. Hepatic and renal insufficiency are not uncommon in the elderly, resulting in complex pharmacokinetic and pharmacodynamics, which often cause an increased risk of side effects and complications. As the AV nodal ablation procedure is relatively simple, safe and effective in preventing tachycardia, it becomes an important treatment option for this group of patients. There is no age limit for nonpharmacologic treatment of AF and decisions regarding patient suitability are best made on an individual basis. Although yet to be shown in randomized control trials, it is also anticipated that the AV nodal ablation/pacemaker approach to treating AF may reduce hospitalization and drug use as well as improve symptoms, health-related quality of life and living independence in the rapidly growing elderly segment of the population.
Safety issues
Patient safety is an important concern in the consideration of AV nodal ablation and pacemaker implantation for treatment of AF. Complications associated with cardiac device implantation are common and include mechanical device problems, hematoma, pneumothorax, pericardial effusion, infection and death. Available data from published studies suggest an estimated peri-implantation risk of death of <0.1% (risk of peri-implantation complications within 30 days of implant), 4.8% (late complications occurring between 1 and 12 months) and 2–3% for radiofrequency ablation and conventional pacing in a population with mean age of 75 years [20,21]. A meta-analysis of cardiac resynchronization therapy (CRT) studies, with a population of mean age 65 years, demonstrated an implantation success rate of 93–95%, peri-implantation death risk of 0.3%, peri-implantation complication risk of 4.3% and late complication risk of 5–6% [22]. A lack of randomized trial data for the AF population has left uncertainty as to whether the benefits of this approach outweigh the risks.
Concerns also exist that ablation of the AV node may be associated with reduced longterm survival. As AV nodal ablation often leaves patients with ventricular rates <40 bpm, patients are often dependent on pacemakers for adequate cardiac output. As a result, failure of the pacemaker could potentially lead to death. In addition, there is concern regarding potential deleterious effects of long-term RV pacing and potential increased risk of ventricular arrhythmia due to AV nodal ablation. These concerns have in part been abated by a study of 350 patients that showed similar 3-year survival rates in patients without underlying heart disease who had undergone either AV node ablation or medical therapy for AF, compared with expected survival of the general population [23]. Due to a lack of randomized trials, there remains a need for further data describing the effect of AV nodal ablation and conventional pacemaker therapy on survival [24].
Potential negative effects of standard RV pacing
RV apical pacing has been used in clinical practice for over four decades. Although the procedure is straightforward, with nearly 100% successful lead placement and a low complication rate, subgroups of patients from post hoc analysis of various largescale trials of pacemakers and implantable cardioverter- defibrillators (ICDs) have been observed to develop heart failure without an identifiable cause as well as a higher incidence of persistent and permanent AF [25–28]. As RV endocardial pacing causes the RV to contract before the left ventricle (LV), termed interventricular dyssynchrony, it has been proposed that it may impair LV systolic function, reduce functional capacity and even increase mortality [29], even though LV ejection fraction is not compromised in the majority of patients [30]. In patients with significant dyssynchrony due to intrinsic conduction disease or pacing, CRT can improve ventricular synchrony and mechanical function, making it a consideration in patients who require long-term pacing. The potential negative impact of RV apical pacing and the potential positive impact of CRT pacing after AV nodal ablation in the elderly population with AF are known.
Interest of pacing in the RV outflow tract (RVOT) or on the His bundle has been developed based on the hypothesis that ventricular contractile synchrony can be better preserved when pacing in these septal areas [31]. Available clinical data, often with inconsistent results, have been from limited, small observation studies using different techniques. A recent small study randomized 33 patients receiving CRT for chronic heart failure to either RVOT or RV apex pacing and found that the site of pacing had no influence on response to CRT [32]. These results may be explained by the fact that pacing merely from the RVOT does not ensure synchronous contraction. Pacing and capturing the His bundle in order to mimic physiologic depolarization requires a detailed mapping. Stability of the ventricular lead could be a limiting factor as well. A better understanding of the mechanisms of RVOT or His bundle pacing and improved clinical tools will be needed to further explore the utility of these pacing methods.
Cardiac resynchronization therapy after AV nodal ablation for AF
Cardiac resynchronization therapy is achieved with the implantation of an additional pacemaker lead on the left lateral ventricle via the coronary sinus and cardiac veins, resulting in improved ventricular synchrony. This therapy has shown benefit by reducing morbidity and mortality in patients in sinus rhythm with symptomatic systolic heart failure and evidence of ventricular dyssynchrony based on ECG QRS-duration. The American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines have made the following recommendations:
▪ CRT is indicated for the treatment of New York Heart Association (NYHA) class III–IV HF patients with persistent symptoms despite optimized medical therapy who have an ejection fraction <35% and a QRS duration ≥120 ms [33];
▪ CRT is reasonable for patients with LV ejection fraction ≤35%, with NYHA functional class III or ambulatory class IV symptoms, who are receiving optimal recommended medical therapy and who have frequent dependence on ventricular pacing [33];
▪ CRT may be considered for patients with LV ejection fraction ≤35% with NYHA functional class I or II symptoms who are receiving optimal recommended medical therapy and who are undergoing implantation of a permanent pacemaker and/or ICD with anticipated frequent ventricular pacing [33].
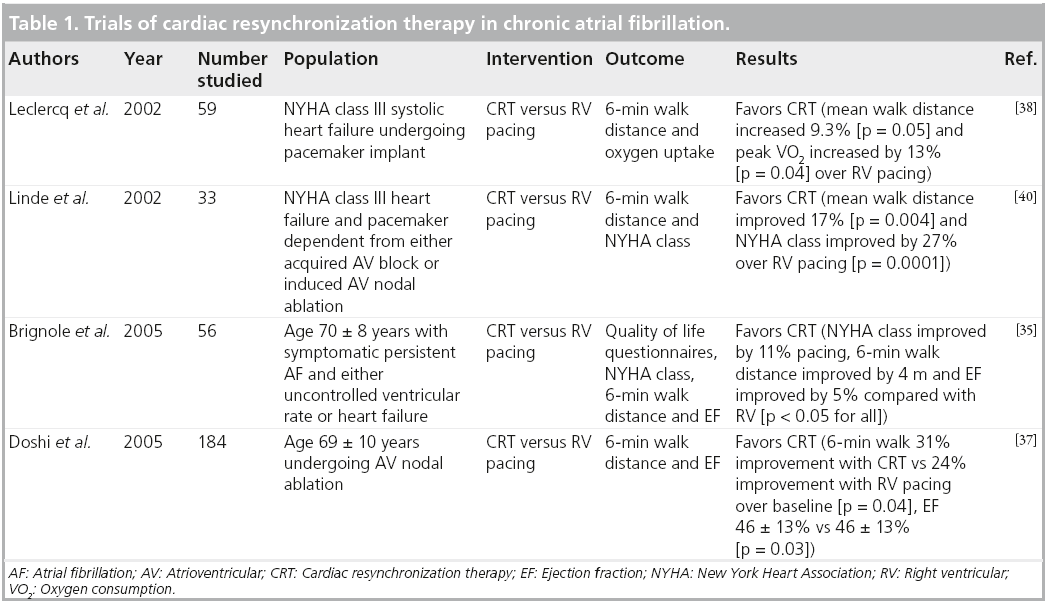
Heart failure often coexists with AF and the prevalence of AF increases with worsening NYHA functional class [34]. Observational studies and small randomized trials support the value of CRT for improving symptoms and left ventricular function in patients with poorly controlled AF who have reduced LV systolic function or heart failure (Table 1) [35,36]. In a randomized controlled trial, patients with symptomatic medically refractory chronic rapid AF were assigned to AV nodal ablation with either RV pacing or CRT. The group with CRT showed greater improvement in exercise tolerance and greater preservation of ejection fraction than the group who received RV pacing [37]. A meta-analysis of three randomized CRT AF trials [35,38–40] showed a trend toward improved survival among patients randomized to CRT but the difference in survival among patients randomized to CRT versus RV pacing was not statistically significant [24].
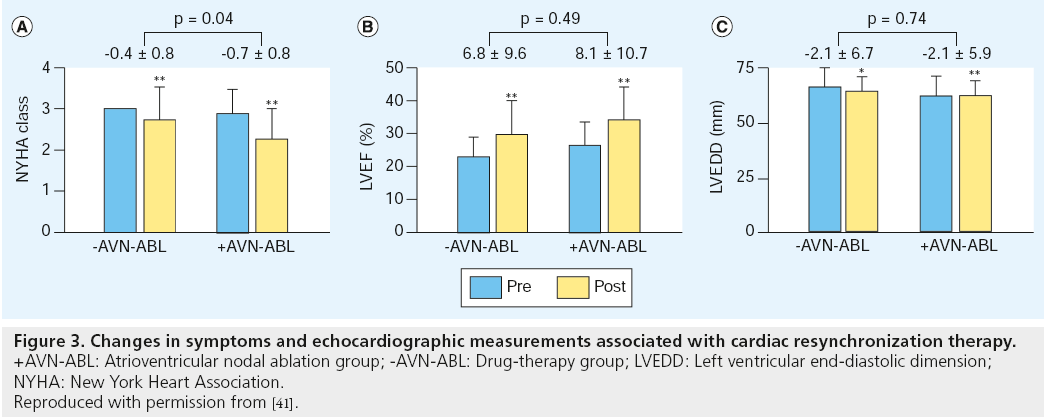
A recent observational cohort study of patients with AF and heart failure who received CRT plus defibrillator therapy, showed that AV nodal ablation to maximize biventricular pacing provided a greater improvement in NYHA class and survival benefit compared with drug therapy for rate control [41]. In 154 patients with a median follow-up of 274 days, the median (Q1, Q3) percentage of biventricular pacing after CRT was 99.0% (95–100%) in the AV nodal ablation group compared with 96.0% (85.5–99.0%) in the drug-treated group (p = 0.05). After CRT, the changes in NYHA functional class and echocardiographic parameters observed in the two groups are shown in Figure 3. Both groups had significant improvements in NYHA class, LV ejection fraction and LV end diastolic dimension. Improvement in NYHA class was significantly greater in the AV nodal ablation group compared with the drug-treated group (0.7 ± 0.8 vs 0.4 ± 0.8; p = 0.04), while improvement in echocardiographic parameters was not significantly different between the two groups.
Figure 3: Changes in symptoms and echocardiographic measurements associated with cardiac resynchronization therapy.
+AVN-ABL: Atrioventricular nodal ablation group; -AVN-ABL: Drug-therapy group; LVEDD: Left ventricular end-diastolic dimension;
NYHA: New York Heart Association.
Reproduced with permission from [41].
In the AF population, current guidelines for device implantation support the use of CRT in patients with AF and reduced ejection fraction ≤35% who are receiving optimal medical therapy and are anticipated to require frequent ventricular pacing, such as with those individuals undergoing AV nodal ablation [33].
While the rationale for treating patients with heart failure and AF with CRT after AV nodal ablation has been established, the role of CRT after AV nodal ablation among patients who do not have advanced HF prior to ablation is not known. At this time it remains unclear as to whether patients with AF and no evidence of heart failure benefit from prophylactic CRT.
Recent CRT data in patients without advanced heart failure
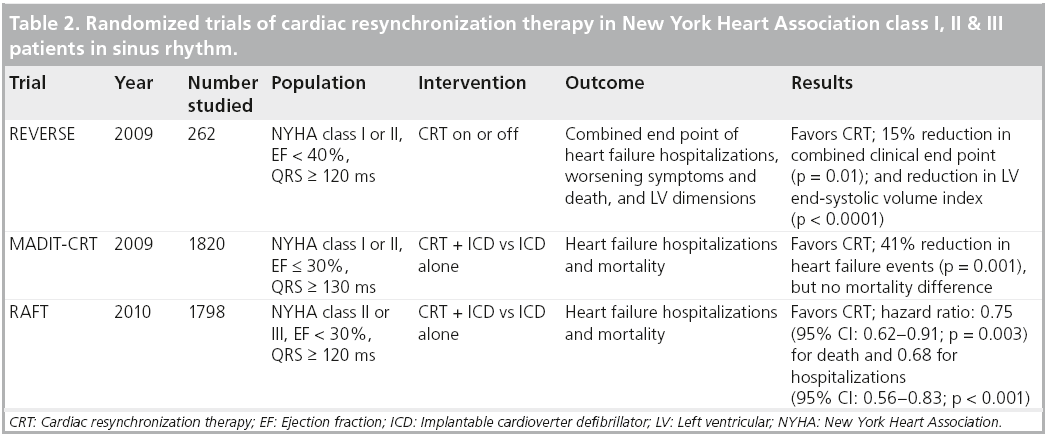
More recent trials of CRT have examined the outcome of CRT implantation in individuals without NYHA class III or IV heart failure (Table 2). The REVERSE trial randomly assigned 262 patients in sinus rhythm, widened QRS and NYHA class I or II symptoms with CRT plus defibrillator implants, to either CRT-on or CRT-off, and after 24 months of follow-up found a statistically significant reduction in clinical end points including death, hospitalizations and worsening symptoms, as well as reduced LV dimensions, with the use of CRT [42]. The RAFT trial reviewed a total of 1800 patients with EF <30% and NYHA class II or III symptoms and randomized them to either ICD alone or ICD plus CRT over 3 years of follow-up, and found a statistically significant reduction in mortality and heart failure hospitalization, but more adverse events with the addition of CRT to ICD therapy [43]. The MADIT-CRT trial randomized a total of 1820 patients with EF ≤ 30%, NYHA class I or II symptoms and QRS ≥ 130 ms to either ICD alone or ICD plus CRT, and found a 41% (p = 0.001) reduction in heart failure events, but no difference in overall death over a mean of 2.4 years follow-up [44].
These results suggest a potential role for CRT in preventing the progression of disease in the population of patients in sinus rhythm with widened QRS, systolic dysfunction and minimal symptoms of heart failure. Given the potential adverse events associated with the CRT pacemaker or defibrillator implantation (mostly related to procedural complexity and associated complications), informed discussion with patients prior to device implantation regarding the potential risks and benefits is of utmost importance. However, considering the morbidity, quality of life impairment and healthcare costs associated with heart failure admissions, a therapy such as CRT that can significantly reduce them is of high value. Whether similar findings can be found in patients with AF after AV nodal ablation remains uncertain, but it is possible that this pacemakerdependent population with similar characteristics to those studied might also benefit from CRT.
Future perspective
Although nonrandomized data has shown an improvement in outcomes with AV nodal ablation for AF, the majority of randomized trials have not found a statistically significant improvement in symptoms, quality of life, exercise capacity, stroke or survival. In addition, randomized trials have not yet definitively shown which type of pacemaker is most beneficial to patients undergoing AV node ablation. Large-scale randomized trials are needed to determine whether AV node ablation is beneficial to patients compared with pharmacologic rate control and to determine whether patients should receive conventional pacemakers versus cardiac resynchronization devices.
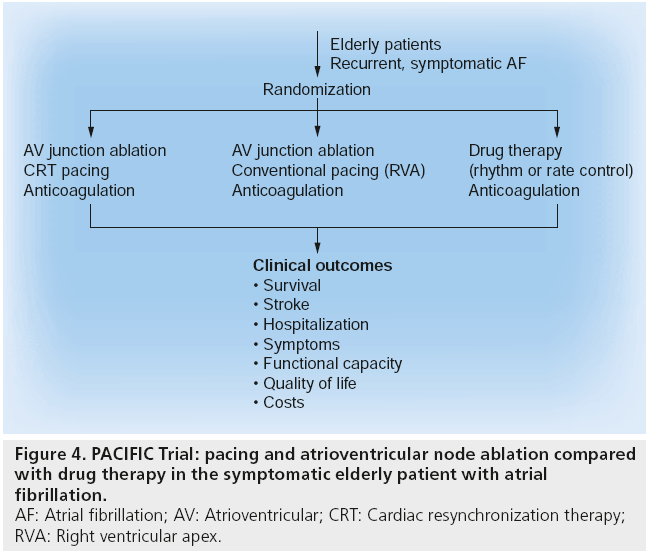
The PACIFIC trial is currently enrolling patients in its pilot study to help answer these questions. The PACIFIC trial randomizes elderly patients with drug refractory symptomatic AF to pharmacologic therapy, AV node ablation with conventional pacemaker implantation, or AV node ablation with cardiac resynchronization therapy pacemaker implantation (Figure 4). By design, the trial will be adequately large enough to measure the end points of survival, quality of life, exercise capacity, stroke and hospitalization. With a rapidly increasing population of elderly patients with symptomatic AF over the next several years [1], results from the PACIFIC trial are expected to be of great help in guiding their management. A future study of left atrial catheter ablation for AF compared with AV nodal ablation may also help inform management decisions in the elderly population.
Conclusion
AF in the rapidly expanding elderly population is a growing epidemic. Therapeutic options are multiple, including medications, primary radio-frequency ablation and AV nodal ablation combined with permanent pacing. AV nodal ablation and pacing holds promise as a safe and effective therapy for elderly patients. Evidence from large randomized trials is needed to determine the clinical benefits and the best method of pacing.
Financial and competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
▪ Atrial fibrillation (AF) in the rapidly expanding elderly population is a growing epidemic.
▪ Therapeutic options include medications, primary radiofrequency ablation and atrioventricular (AV) nodal ablation combined with permanent pacing.
▪ Radiofrequency ablation of the AV node, combined with permanent right ventricular endocardial pacing, is a highly effective treatment for controlling the ventricular response of AF.
▪ The elderly population is particularly suited to AV nodal ablation and permanent pacing for treatment of AF due to higher frequency of comorbidities, risks of medication intolerance and the relative safety and simplicity of the procedure.
▪ Cardiac resynchronization therapy is beneficial for patients with AF and reduced left ventricular systolic function who require frequent pacing.
▪ Large-scale randomized trials are needed to determine whether AV nodal ablation is beneficial in patients, compared with pharmacologic rate control, and to determine the best method of pacing in patients without systolic heart failure.
References
- Miyasaka Y, Barnes ME, Gersh BJ et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 114(2), 119–125 (2006).
- Khoo CW, Lip GY. Burden of atrial fibrillation. Curr. Med. Res. Opin. 25(5), 1261–1263 (2009).
- Go AS, Hylek EM, Phillips KA et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 285(18), 2370–2375 (2001).
- Stewart S, Hart CL, Hole DJ, McMurray JJ. Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart 86(5), 516–521 (2001).
- Camm AJ, Kirchhof P, Lip GY et al. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Europace 12(10), 1360– 1420 (2010).
- Wattigney WA, Mensah GA, Croft JB. Increasing trends in hospitalization for atrial fibrillation in the United States, 1985 through 1999: implications for primary prevention. Circulation 108(6), 711–716 (2003).
- Wyse DG, Waldo AL, DiMarco JP et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N. Engl J. Med. 347(23), 1825–1833 (2002).
- Piccini JP, Lopes RD, Kong MH, Hasselblad V, Jackson K, Al-Khatib SM. Pulmonary vein isolation for the maintenance of sinus rhythm in patients with atrial fibrillation: a meta-analysis of randomized, controlled trials. Circ. Arrhythm. Electrophysiol. 2(6), 626–633 (2009).
- Wokhlu A, Hodge DO, Monahan KH et al. Long-term outcome of atrial fibrillation ablation: impact and predictors of very late recurrence. J. Cardiovasc. Electrophysiol. 21(10), 1071–1078 (2010).
- Cappato R, Calkins H, Chen SA et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ. Arrhythm. Electrophysiol. 3(1), 32–38 (2010).
- Calkins H, Reynolds MR, Spector P et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ. Arrhythm. Electrophysiol. 2(4), 349–361 (2009).
- Berruezo A, Tamborero D, Mont L et al. Pre-procedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. Eur. Heart J. 28(7), 836–841 (2007).
- Marine JE. Catheter ablation therapy for supraventricular arrhythmias. JAMA 298(23), 2768–2778 (2007).
- Fuster V, Ryden LE, Cannom DS et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation 123(10) 269–367 (2011).
- Scheinman MM, Huang S. The 1998 NASPE prospective catheter ablation registry. Pacing Clin. Electrophysiol. 23(6), 1020–1028 (2000).
- Brown CS, Mills RM, Jr., Conti JB, Curtis AB. Clinical improvement after atrioventricular nodal ablation for atrial fibrillation does not correlate with improved ejection fraction. Am. J. Cardiol. 80(8), 1090–1091 (1997).
- Fitzpatrick AP, Kourouyan HD, Siu A et al. Quality of life and outcomes after radiofrequency His-bundle catheter ablation and permanent pacemaker implantation: impact of treatment in paroxysmal and established atrial fibrillation. Am. Heart J. 131(3), 499–507 (1996).
- Weerasooriya R, Davis M, Powell A et al. The Australian Intervention Randomized Control of Rate in Atrial Fibrillation Trial (AIRCRAFT). J. Am. Coll. Cardiol. 41(10), 1697–1702 (2003).
- Wood MA, Brown-Mahoney C, Kay GN, Ellenbogen KA. Clinical outcomes after ablation and pacing therapy for atrial fibrillation: a meta-analysis. Circulation 101(10), 1138–1144 (2000).
- Connolly SJ, Kerr CR, Gent M et al. Effects of physiologic pacing versus ventricular pacing on the risk of stroke and death due to cardiovascular causes. Canadian Trial of Physiologic Pacing Investigators. N. Engl. J. Med. 342(19), 1385–1391 (2000).
- Lamas GA, Lee KL, Sweeney MO et al. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N. Engl. J. Med. 346(24), 1854–1862 (2002).
- McAlister FA, Ezekowitz J, Hooton N et al. Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction: a systematic review. JAMA 297(22), 2502–2514 (2007).
- Ozcan C, Jahangir A, Friedman PA et al. Long-term survival after ablation of the atrioventricular node and implantation of a permanent pacemaker in patients with atrial fibrillation. N. Engl. J. Med. 344(14), 1043–1051 (2001).
- Bradley DJ, Shen WK. Atrioventricular junction ablation combined with either right ventricular pacing or cardiac resynchronization therapy for atrial fibrillation: the need for large-scale randomized trials. Heart Rhythm 4(2), 224–232 (2007).
- Steinberg JS, Fischer A, Wang P et al. The clinical implications of cumulative right ventricular pacing in the multicenter automatic defibrillator trial II. J. Cardiovasc. Electrophysiol. 16(4), 359–365 (2005).
- Sweeney MO, Hellkamp AS. Heart failure during cardiac pacing. Circulation 113(17), 2082–2088 (2006).
- Sweeney MO, Ruetz LL, Belk P, Mullen TJ, Johnson JW, Sheldon T. Bradycardia pacing-induced short-long-short sequences at the onset of ventricular tachyarrhythmias: a possible mechanism of proarrhythmia? J. Am. Coll. Cardiol. 50(7), 614–622 (2007).
- Wilkoff BL, Cook JR, Epstein AE et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA 288(24), 3115–3123 (2002).
- Tops LF, Schalij MJ, Holman ER, van Erven L, van der Wall EE, Bax JJ. Right ventricular pacing can induce ventricular dyssynchrony in patients with atrial fibrillation after atrioventricular node ablation. J. Am. Coll. Cardiol. 48(8), 1642–1648 (2006).
- Chen L, Hodge D, Jahangir A et al. Preserved left ventricular ejection fraction following atrioventricular junction ablation and pacing for atrial fibrillation. J. Cardiovasc. Electrophysiol. 19(1), 19–27 (2008).
- Mond HG, Hillock RJ, Stevenson IH, McGavigan AD. The right ventricular outflow tract: the road to septal pacing. Pacing Clin. Electrophysiol. 30(4), 482–491 (2007).
- Ronn F, Kesek M, Karp K, Henein M, Jensen SM. Right ventricular lead positioning does not influence the benefits of cardiac resynchronization therapy in patients with heart failure and atrial fibrillation. Europace doi:10.1093/ europace/eur193 (2011) (Epub ahead of print).
- Epstein AE, DiMarco JP, Ellenbogen KA et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation 117(21), e350–e408 (2008).
- Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am. J. Cardiol. 91(6A), 2D–8D (2003).
- Brignole M, Gammage M, Puggioni E et al. Comparative assessment of right, left, and biventricular pacing in patients with permanent atrial fibrillation. Eur. Heart J. 26(7), 712–722 (2005).
- Leon AR, Greenberg JM, Kanuru N et al. Cardiac resynchronization in patients with congestive heart failure and chronic atrial fibrillation: effect of upgrading to biventricular pacing after chronic right ventricular pacing. J. Am. Coll. Cardiol.39(8), 1258–1263 (2002).
- Doshi RN, Daoud EG, Fellows C et al. Left ventricular-based cardiac stimulation post AV nodal ablation evaluation (the PAVE study). J. Cardiovasc. Electrophysiol. 16(11), 1160–1165 (2005).
- Leclercq C, Walker S, Linde C et al. Comparative effects of permanent biventricular and right-univentricular pacing in heart failure patients with chronic atrial fibrillation. Eur. Heart J. 23(22), 1780–1787 (2002).
- Linde C, Braunschweig F, Gadler F, Bailleul C, Daubert JC. Long-term improvements in quality of life by biventricular pacing in patients with chronic heart failure: results from the Multisite Stimulation in Cardiomyopathy study (MUSTIC). Am. J. Cardiol. 91(9), 1090–1095 (2003).
- Linde C, Leclercq C, Rex S et al. Long-term benefits of biventricular pacing in congestive heart failure: results from the Multisite Stimulation in Cardiomyopathy (MUSTIC) study. J. Am. Coll. Cardiol. 40(1), 111–118 (2002).
- Dong K, Shen WK, Powell BD et al. Atrioventricular nodal ablation predicts survival benefit in patients with atrial fibrillation receiving cardiac resynchronization therapy. Heart Rhythm 7(9), 1240–1245 (2010).
- Daubert C, Gold MR, Abraham WT et al. Prevention of disease progression by cardiac resynchronization therapy in patients with asymptomatic or mildly symptomatic left ventricular dysfunction: insights from the European cohort of the REVERSE (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) trial. J. Am. Coll. Cardiol. 54(20), 1837–1846 (2009).
- Tang AS, Wells GA, Talajic M et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N. Engl. J. Med. 363, 2385–2395 (2010).
- Moss AJ, Hall WJ, Cannom DS et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N. Engl. J. Med. 361(14), 1329–1338 (2009).
- Miyasaka Y, Barnes ME, Gersh BJ et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 114(2), 119–125 (2006).
- Khoo CW, Lip GY. Burden of atrial fibrillation. Curr. Med. Res. Opin. 25(5), 1261–1263 (2009).
- Go AS, Hylek EM, Phillips KA et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 285(18), 2370–2375 (2001).
- Stewart S, Hart CL, Hole DJ, McMurray JJ. Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart 86(5), 516–521 (2001).
- Camm AJ, Kirchhof P, Lip GY et al. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Europace 12(10), 1360– 1420 (2010).
- Wattigney WA, Mensah GA, Croft JB. Increasing trends in hospitalization for atrial fibrillation in the United States, 1985 through 1999: implications for primary prevention. Circulation 108(6), 711–716 (2003).
- Wyse DG, Waldo AL, DiMarco JP et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N. Engl J. Med. 347(23), 1825–1833 (2002).
- Piccini JP, Lopes RD, Kong MH, Hasselblad V, Jackson K, Al-Khatib SM. Pulmonary vein isolation for the maintenance of sinus rhythm in patients with atrial fibrillation: a meta-analysis of randomized, controlled trials. Circ. Arrhythm. Electrophysiol. 2(6), 626–633 (2009).
- Wokhlu A, Hodge DO, Monahan KH et al. Long-term outcome of atrial fibrillation ablation: impact and predictors of very late recurrence. J. Cardiovasc. Electrophysiol. 21(10), 1071–1078 (2010).
- Cappato R, Calkins H, Chen SA et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ. Arrhythm. Electrophysiol. 3(1), 32–38 (2010).
- Calkins H, Reynolds MR, Spector P et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ. Arrhythm. Electrophysiol. 2(4), 349–361 (2009).
- Berruezo A, Tamborero D, Mont L et al. Pre-procedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. Eur. Heart J. 28(7), 836–841 (2007).
- Marine JE. Catheter ablation therapy for supraventricular arrhythmias. JAMA 298(23), 2768–2778 (2007).
- Fuster V, Ryden LE, Cannom DS et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation 123(10) 269–367 (2011).
- Scheinman MM, Huang S. The 1998 NASPE prospective catheter ablation registry. Pacing Clin. Electrophysiol. 23(6), 1020–1028 (2000).
- Brown CS, Mills RM, Jr., Conti JB, Curtis AB. Clinical improvement after atrioventricular nodal ablation for atrial fibrillation does not correlate with improved ejection fraction. Am. J. Cardiol. 80(8), 1090–1091 (1997).
- Fitzpatrick AP, Kourouyan HD, Siu A et al. Quality of life and outcomes after radiofrequency His-bundle catheter ablation and permanent pacemaker implantation: impact of treatment in paroxysmal and established atrial fibrillation. Am. Heart J. 131(3), 499–507 (1996).
- Weerasooriya R, Davis M, Powell A et al. The Australian Intervention Randomized Control of Rate in Atrial Fibrillation Trial (AIRCRAFT). J. Am. Coll. Cardiol. 41(10), 1697–1702 (2003).
- Wood MA, Brown-Mahoney C, Kay GN, Ellenbogen KA. Clinical outcomes after ablation and pacing therapy for atrial fibrillation: a meta-analysis. Circulation 101(10), 1138–1144 (2000).
- Connolly SJ, Kerr CR, Gent M et al. Effects of physiologic pacing versus ventricular pacing on the risk of stroke and death due to cardiovascular causes. Canadian Trial of Physiologic Pacing Investigators. N. Engl. J. Med. 342(19), 1385–1391 (2000).
- Lamas GA, Lee KL, Sweeney MO et al. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N. Engl. J. Med. 346(24), 1854–1862 (2002).
- McAlister FA, Ezekowitz J, Hooton N et al. Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction: a systematic review. JAMA 297(22), 2502–2514 (2007).
- Ozcan C, Jahangir A, Friedman PA et al. Long-term survival after ablation of the atrioventricular node and implantation of a permanent pacemaker in patients with atrial fibrillation. N. Engl. J. Med. 344(14), 1043–1051 (2001).
- Bradley DJ, Shen WK. Atrioventricular junction ablation combined with either right ventricular pacing or cardiac resynchronization therapy for atrial fibrillation: the need for large-scale randomized trials. Heart Rhythm 4(2), 224–232 (2007).
- Steinberg JS, Fischer A, Wang P et al. The clinical implications of cumulative right ventricular pacing in the multicenter automatic defibrillator trial II. J. Cardiovasc. Electrophysiol. 16(4), 359–365 (2005).
- Sweeney MO, Hellkamp AS. Heart failure during cardiac pacing. Circulation 113(17), 2082–2088 (2006).
- Sweeney MO, Ruetz LL, Belk P, Mullen TJ, Johnson JW, Sheldon T. Bradycardia pacing-induced short-long-short sequences at the onset of ventricular tachyarrhythmias: a possible mechanism of proarrhythmia? J. Am. Coll. Cardiol. 50(7), 614–622 (2007).
- Wilkoff BL, Cook JR, Epstein AE et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA 288(24), 3115–3123 (2002).
- Tops LF, Schalij MJ, Holman ER, van Erven L, van der Wall EE, Bax JJ. Right ventricular pacing can induce ventricular dyssynchrony in patients with atrial fibrillation after atrioventricular node ablation. J. Am. Coll. Cardiol. 48(8), 1642–1648 (2006).
- Chen L, Hodge D, Jahangir A et al. Preserved left ventricular ejection fraction following atrioventricular junction ablation and pacing for atrial fibrillation. J. Cardiovasc. Electrophysiol. 19(1), 19–27 (2008).
- Mond HG, Hillock RJ, Stevenson IH, McGavigan AD. The right ventricular outflow tract: the road to septal pacing. Pacing Clin. Electrophysiol. 30(4), 482–491 (2007).
- Ronn F, Kesek M, Karp K, Henein M, Jensen SM. Right ventricular lead positioning does not influence the benefits of cardiac resynchronization therapy in patients with heart failure and atrial fibrillation. Europace doi:10.1093/ europace/eur193 (2011) (Epub ahead of print).
- Epstein AE, DiMarco JP, Ellenbogen KA et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation 117(21), e350–e408 (2008).
- Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am. J. Cardiol. 91(6A), 2D–8D (2003).
- Brignole M, Gammage M, Puggioni E et al. Comparative assessment of right, left, and biventricular pacing in patients with permanent atrial fibrillation. Eur. Heart J. 26(7), 712–722 (2005).
- Leon AR, Greenberg JM, Kanuru N et al. Cardiac resynchronization in patients with congestive heart failure and chronic atrial fibrillation: effect of upgrading to biventricular pacing after chronic right ventricular pacing. J. Am. Coll. Cardiol.39(8), 1258–1263 (2002).
- Doshi RN, Daoud EG, Fellows C et al. Left ventricular-based cardiac stimulation post AV nodal ablation evaluation (the PAVE study). J. Cardiovasc. Electrophysiol. 16(11), 1160–1165 (2005).
- Leclercq C, Walker S, Linde C et al. Comparative effects of permanent biventricular and right-univentricular pacing in heart failure patients with chronic atrial fibrillation. Eur. Heart J. 23(22), 1780–1787 (2002).
- Linde C, Braunschweig F, Gadler F, Bailleul C, Daubert JC. Long-term improvements in quality of life by biventricular pacing in patients with chronic heart failure: results from the Multisite Stimulation in Cardiomyopathy study (MUSTIC). Am. J. Cardiol. 91(9), 1090–1095 (2003).
- Linde C, Leclercq C, Rex S et al. Long-term benefits of biventricular pacing in congestive heart failure: results from the Multisite Stimulation in Cardiomyopathy (MUSTIC) study. J. Am. Coll. Cardiol. 40(1), 111–118 (2002).
- Dong K, Shen WK, Powell BD et al. Atrioventricular nodal ablation predicts survival benefit in patients with atrial fibrillation receiving cardiac resynchronization therapy. Heart Rhythm 7(9), 1240–1245 (2010).
- Daubert C, Gold MR, Abraham WT et al. Prevention of disease progression by cardiac resynchronization therapy in patients with asymptomatic or mildly symptomatic left ventricular dysfunction: insights from the European cohort of the REVERSE (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) trial. J. Am. Coll. Cardiol. 54(20), 1837–1846 (2009).
- Tang AS, Wells GA, Talajic M et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N. Engl. J. Med. 363, 2385–2395 (2010).
- Moss AJ, Hall WJ, Cannom DS et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N. Engl. J. Med. 361(14), 1329–1338 (2009).