Research Article - Imaging in Medicine (2017) Volume 9, Issue 4
Role of CT volumetry following gastric plication surgery in morbid obesity: Initial experience in correlating postoperative gastric pouch volume with clinical weight loss
Wessam Abdelrahman Elzayat1*, Mona El-Kalioubie1, Mostafa Elshafie2 & Ahmed Abdelaziz Hassan31Diagnostic and Intervention Radiology Department, Cairo University Hospitals, Kasr Al-Ainy, Cairo, Egypt
2Diagnostic and Intervention Radiology Department, Theodor Bilharz Research Institute, Cairo, Egypt
3General Surgery Department, Theodor Bilharz Research Institute, Cairo, Egypt
- Corresponding Author:
- Wessam Abdelrahman
Elzayat
Diagnostic and Intervention Radiology Department
Cairo University Hospitals, Kasr Al-Ainy, Cairo, Egypt
E-mail: ahmedelmaghney@yahoo.com
Abstract
Background: Laparoscopic greater curve gastric plication (LGCP) surgery recently emerged as a new bariatric procedure, currently marketed for weight reduction especially in poor developing countries.
Objective: The objective of this pilot study was to present initial results of correlation between CT volumetric variations in the residual gastric pouch following LGCP with weight loss in morbidly obese Egyptian patients over a one-year follow-up period.
Patients & methods: Forty morbidly obese females (mean age 33.8 +/- 9.1) underwent LGCP. Abdominal multi-slice computed tomography (MSCT) was done, immediately after oral administration of effervescent granules at 1 & 12 months postoperatively. Gastric pouch volumes were measured on 3-dimensional (3-D) masks and correlated with weight loss.
Results: All surgeries were performed safely with significant decrease in the mean weight at 1 year follow-up relative to mean pre-operative weight (p=0.0010) and 1 month post-operative weight (p=0.0091). CT evaluation of gastric volumes following LGCP was successful in all cases. The mean CT volume of the gastric pouch at 1 year followup showed significant increase relative to mean volume at 1 month post-operative (p=0.0024). After 1 year, a non significant weak negative correlation was found between the percentage of gastric pouch volume increase and the percentage of weight loss (r=-0.0656, p=0.86).
Conclusion: LGCP is safe and successful in achieving significant weight loss in obese Egyptian patients at a low cost. 3-D MSCT volumetry allows non-invasive, important anatomical evaluation, helpful in the postoperative follow-up. Further evaluations with greater samples and longer follow-up will help confirm clinical relevance.
Keywords
CT volumetry • gastric plication • morbid obesity • weight-loss • bariatric
Introduction
With morbid obesity and related comorbidities on the rise all over the world, effective, non-invasive and low-cost bariatric procedures for weight reduction are increasingly sought-after, especially in the underdeveloped world [1,2].
Laparoscopic greater curvature plication (LGCP) is a relatively new, reversible, restrictive procedure currently used in multiple countries, which results in creation of a smaller stomach with very minimal incidence of leakage [2,3]. Literature reports have shown encouraging early outcomes after LGCP, while long-term efficacy, safety and durability are still debatable [4-7].
Even though other procedures such as gastric banding or sleeve gastrectomy are most widely used for weight-reduction, the appeal of LGCP, especially to our patients in Egypt is quite clear; the absence of gastrointestinal cutting or implant use as well as the lower cost all pertain to its application [4].
In parallel, comes the need for a noninvasive assessment of the efficacy of LGCP for treatment of morbid obesity in our population. Anatomical and simulation 3D reconstruction techniques such as CT volumetry have enabled exact measurements of residual gastric reservoirs following bariatric surgeries and correlated them to clinical weight loss [8-10].
However, to our knowledge there are no data of CT volumetry assessment following LGCP.
The objective of this prospective, pilot study was to present initial results of correlation between the CT volumetric variations in the residual gastric pouch following LGCP with clinical weight loss in our morbidly obese patient population.
Patients and Methods
This prospective study was approved by the hospital’s ethical committee. Morbidly obese patients admitted in the general surgery department were considered for LGCP surgery. Inclusion criteria were a body mass index (BMI) greater than 36 kg/m2, a history of morbid obesity lasting more than 5 years with failure of conservative weight-loss treatment as well as a good potential and psychological motivation for diet and exercise post-operatively. History of previous gastric surgery, pregnancy, hiatal hernia as well as cardiovascular disease or uncontrolled diabetes putting them at risk for the LGCP procedure was exclusion criteria. A total of 40 patients (all women; mean age 33.8+/-9.1; age range: 25-46 years old) was included and they all underwent LGCP bariatric surgery over the course of the year 2015. Follow-up period for our study purposes was 1 year in all cases.
■ Pre-operative preparation
The procedure was explained to all patients and a written informed consent was obtained. Preoperative weight, height and BMI were recorded and calculated. All 40 women underwent blood testing (including coagulation parameters), liver function, electrolytes and hormonal (thyroid and adrenal glands) tests as well as an upper gastrointestinal (GI) endoscopy, abdominal ultrasound (US) and echocardiography.
■ Surgical technique
Prophylactic low molecular weight heparin administration and antibiotic coverage were done. All patients were operated in a supine anti-Trendelenburg position at 30°. Open pneumoperitoneum (12-15 mm Hg) was achieved by stab incision of 1 cm, one and a half hand breadth from the xyphoid process in the midline, through which a 10 mm trocar was inserted for the 30° laparoscope. The other four trocars were inserted as follows: a 12 mm right hand surgeon’s trocar at the right upper quadrant in mid clavicular line; 5 mm trocar also in the right upper quadrant but below the previous one at the mid axillary line (surgeon’s assistant); a 5 mm trocar below the xiphoid process for liver retraction; and a 5 mm left hand surgeon’s trocar in the left upper quadrant in the mid clavicular line [11].We started by grasping and pulling up the anterior wall of the stomach at the level of the prepyloric area. Then dissection was done in contact to the gastric wall in order to mobilize the greater curvature from the prepyloric area up to a level 2 cm to the angle of His using 10 mm LigaSure Vessel sealing device. The anatomy of the His angle was preserved while both gastroepiploic vessels were sacrificed.
Subsequently, the gastric plication was initiated over the introduction of a 36 F bougie by enfolding the greater curvature and then securing it with a first row of interrupted stitches of 2-0 Ethibond™ (Ethicon, Inc., Somerville, NJ, USA) placed sero-muscular, extra-mucosal (to be protected from gastric acid) which stopped 3 cm short of the pylorus, with another subsequent plication row created with running suture lines of 2-0 Prolene™ (Ethicon, Inc., Somerville, NJ, USA). This led to the creation of a pouch resembling a large sleeve gastrectomy. We tested for leakage using methylene blue then air fluid leak tests. A drain was inserted and removed upon discharge.
■ Post-operative management, CT volumetry and follow-up
Patients were discharged from the hospital as soon as they managed a liquid diet. They were instructed to gradually pass to a soft diet after 2 weeks and then to solid foods after 1 month. Heparin (low-molecular weight) was administered regularly for 2 weeks as well as proton pump inhibitors for 2 months and iron and multivitamin supplements were given to all patients during the first six months following LGCP. All patients were put on a strict dietary and exercise regimen. Follow-up visits were scheduled at 1 week then at 1, 3, 6 and 12 months post-operatively for assessment of weight loss, compliance to the dietary regimen as well as any complications. For our study purposes, we recorded the pre-operative, initial screening (1 month post-operative) and 1-year follow-up weight and BMI. CT volumetry was performed twice: 1 month after surgery then 1 year later.
CT volumetry imaging technique and image analysis.
CT abdominal examination was done using a state-of-the-art 64-channel multislice machine (Toshiba Aquillion) immediately after administration of one packet of effervescent granules (i.e., E-Z Gas) followed by 10 mL of water to achieve gastric pouch distension via rapid release of 300-400 mL of carbon dioxide upon contact with stomach fluid. CT was done with the patient lying supine, from the level of the tracheal carina down to the level of the symphysis pubis. There is a direct proportional relationship between the CT tube current and the patient’s received dose. For effective dose reduction, it is mandatory to keep this parameter as low as possible. Achieving a proper balance between image quality and lowest effective dose is a must. Our scanner, like most modern machines uses automatic exposure control. Gonadal shielding was used in all patients, as it is routine practice in our institution while performing abdominal CT examinations. Neither oral nor intravenous contrast media were used. Using a detector collimation of 1.2 mm and voltage 120 kV, thin slice images were obtained with slice thickness 2 mm, an increase of 1 mm then transferred to an independent workstation (Vitrea version 6.0.1120.5944). 3-D volume-rendering reconstructed images of the gastric pouch containing the entire luminal space in question were automatically generated with automatic calculation of the gastric volume.
■ Statistical analysis
The SPSS software (Statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA), release 21 versions for Mac was used. Data were statistically described in terms of mean +/- standard deviation (SD) and range or frequencies (number of cases) and percentages (%) when appropriate. Comparison of numerical variables was done using Student t test.
Pearson correlation studies were done to examine the relationship between the post- operative gastric pouch volume and weight loss. The p values by Chi-square test were determined: <0.05=significant, <0.01=highly significant and >0.05=non-significant.
Results
■ Weight loss
Following LGCP surgery, all patients lost some weight with variable degrees at the 1 month follow-up evaluation, as well as the 1 year follow-up evaluation, except for one patient that showed stable weight (90 Kg) between the 1 month and 1 year follow-ups. TABLE 1 shows the mean weight of the studied group at the following timings: pre-operative, 1 month post- operative and 1 year follow-up.
| Pre-operative | 1 month post-operative | 1 year follow-up | |
|---|---|---|---|
| Mean weight +/- SD | 121.8 +/- 25 | 108.6 +/- 19.7 | 85.2 +/- 15.9 |
Table 1: Mean weight (kg) +/- standard deviation (SD) of the studied group: pre-operative, 1 month post-operative and 1 year follow-up.
The mean weight 1 month post-operative showed non-statistically significant decrease relative to the mean pre-operative weight (p=0.2062). The mean weight at the 1 year follow-up showed statistically significant decrease relative to the mean pre-operative weight (p=0.0010) as well as relative to the mean 1 month post-operative weight (p=0.0091).
The mean weight loss percentage after 1 month was 6.2% +/- 38.3 and after 1 year 20.9% +/- 12.3.
■ BMI variations
TABLE 2 shows the mean BMI in the patient’s population at the following timings: pre-operative, 1 month post-operative and 1 year follow-up.
| Pre-operative | 1 month post-operative | 1 year follow-up | |
|---|---|---|---|
| Mean BMI +/- SD | 47 +/- 8.2 | 41.7+/- 6.4 | 32.8 +/- 5.5 |
Table 2: Mean body mass index (BMI) (kg/m2) +/- standard deviation (SD) of the studied group: pre-operative, 1 month post-operative and 1 year follow-up.
The mean BMI 1 month post-operative showed non-statistically significant decrease relative to the mean pre-operative BMI (p=0.1245). The mean BMI at the 1 year follow-up showed statistically significant decrease relative to the mean pre-operative BMI (p=0.0002) as well as relative to the mean 1 month post-operative BMI (p=0.0037).
■ CT volumetry results
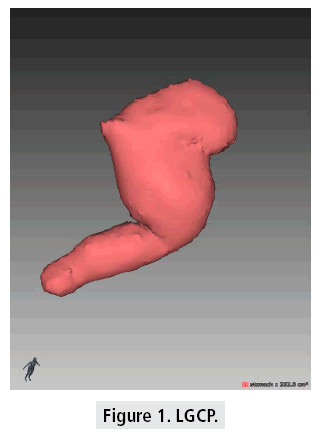
Evaluation of gastric volumes by CT volumetry following LGCP surgery was successful in all 40 cases. The 1st month post-operative gastric pouch volumes ranged between 111 and 341 cm3 in the patient’s population (FIGURE 1)
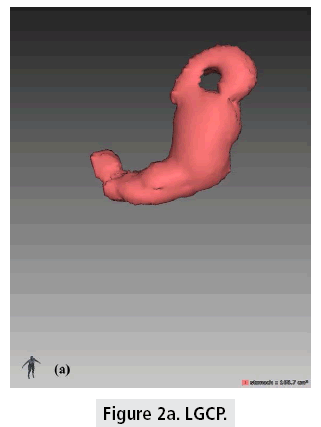
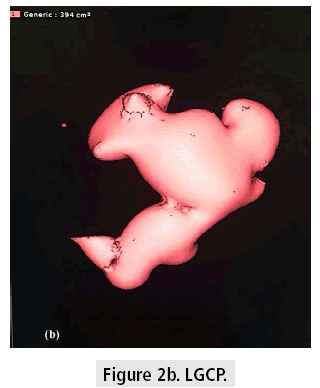
The mean 1rst month postoperative gastric pouch volume was 213.7+/-73.5 cm3 which increased to 355.4+/-103.2 cm3 after 1 year (FIGURES 2a and 2b). The mean gastric pouch volume increase at the end of the 1 year followup was 159+/-64.6 cm3.
The mean CT volume of the gastric pouch at 1 year follow-up showed very statistically significant increase relative to the mean CT volume of the gastric pouch at 1 month postoperative (p=0.0024).
■ Correlation between CT volumetry results and post-operative clinical weight loss
At 1 month follow-up, there was a weak non significant positive correlation between patient’s weight and CT gastric pouch volume (r=0.1548, p=0.67).
At 1 year follow-up, there was a weak non significant positive correlation between patient’s weight and CT gastric pouch volume (r=0.3914, p=0.26).
After 1 year, a non-statistically significant weak negative correlation was found between the percentage of gastric pouch volume increase and the percentage of weight loss (r=-0.0656, p=0.86) indicating somehow that patients with smaller post surgical gastric volumes after 1 year had the highest percentage of weight loss clinically.
Discussion
LGCP is a relatively new restrictive bariatric procedure, in which reduction of gastric volume and expansibility is achieved by vertically suturing the greater curvature in rows, allowing patients to lose weight via early satiety [12]. Previous literature reports have shown that LGCP is safe, cheap with comparable early outcome to other conventional lines of treatment, however there is scarcity of data worldwide especially on the long term [6,7,11].
Even though there is no unanimity on whether post bariatric surgery radiological imaging should be done in all or only selected patients suffering from morbid obesity, imaging in this setting allows early detection of complications as well as assessment of the integrity of the new anatomy and size of the gastric pouch [13-16]. In addition to the patient’s post-operative lifestyle including adequate diet and regular physical exercise, the degree of gastric restriction should be evaluated as part of a revision protocol [9].
A variety of methods have been proposed for measurement of pouch volumes including endoscopic techniques, fluoroscopy with contrast swallows, however invasiveness and inadequate quantitative volumetric measurements accounted for their limited usefulness [9,17-19].
Multi-slice CT-based volumetric assessment of gastric pouches has been used for evaluation of pouch and sleeve volumes and anastomoses in patients after bariatric surgery [9].
LGCP was introduced in Egypt, a couple of years back and this is the first report of postoperative assessment of gastric pouch volume by 3-D MSCT in correlation to post-operative clinical weight loss.
Our initial results demonstrated that patients showed appreciable weight loss following surgery with statistically significant reduction of the mean weight at the 1 year follow-up relative to the mean pre-operative weight (p=0.0010) as well as to the mean 1-month post-operative weight (p=0.0091). The one-year follow-up revealed promising results for the procedure since, the mean weight loss percentage increased from 6.2 % +/- 38.3 after 1 month to 20.9 % +/- 12.3 after 1 year.
In parallel, the mean BMI at the 1 year follow-up showed statistically significant decrease relative to the mean pre-operative BMI (p=0.0002) as well as relative to the mean 1-month post-operative BMI (p=0.0037). Comparable variations were reported by Shen et al, where the mean percentage of excess weight loss increased from 22.9% +/- 6.9% at 1 month to 61.1% +/- 15.9% at 12 months postoperatively [3].
In the 2011, Skrekas et al. research, 135 patients were included and the excess weight loss percentage was reported to be 51.7% at 6 months, 67.1% after 12 months and finally 65.2% at 24 months follow-up [7].
Mean BMI loss was 4.1+/-1.6, 4.8+/- 2.0 and 5.2+/-2.5 kg/m2 at 3, 6 and 12 months respectively in Mui et al study on 23 Chinese patients with morbid obesity following LGCP [12].
The surgical procedure was safe, uneventful and well tolerated by all of our patients. During the postoperative recovery period, one patient presented with vomiting and abdominal pain with spontaneous resolution within a few days. No other minor or major complications were reported. Patient’s satisfaction was noted and patients reported a good eating quality following the surgery. We believe that strict dietary compliance and somehow adequate regular exercise contributed to the 1 year follow-up satisfactory results in our patients, and should be continued in order to achieve longer durable results. These findings were in agreement with Mui et al. study, where no major or minor complications were reported. The mean hospital stay was 2.6 days and LGCP appeared similar in safety to gastric banding or fundoplication [12]. In Ramos et al. study, a few minor complications were observed, including nausea, vomiting and sialorrhea with spontaneous resolution after 2 weeks [5]. Post-operative major complications such as gastric obstruction in Talebpour and Amolie study [20], leaks due to suture line disruption or herniation and gastric fistula formation have rarely been reported [21].
Using MSCT with oral administration of effervescent granules followed by processing of data on a dedicated 3D workstation, accurate calculation of gastric pouch volume was easily feasible in all of our patients. The mean CT volume of the gastric pouch at 1-year follow-up showed statistically significant increase relative to the mean CT volume of the gastric pouch at 1 month post-operative (p=0.0024). During the 1 year follow-up, the gastric pouch volume gradually increased in all patients, which emphasizes the need for previously mentioned continued strict dietary regimen and adequate life style post-operatively.
CT gastric volume correlation with postoperative absolute weight was weakly positive both at 1 month and 1 year follow-up. However, we observed that patients with less post surgical gastric volumes at the 1 year follow-up CT evaluation had the highest percentage of weight loss at that time, indicating that the gastric pouch volume somehow influences clinical weight loss a year after LGCP procedure.
We believe that benefits of CT gastric pouch imaging in a post LGCP setting will outweigh the disadvantage of radiation exposure linked to CT scanning, since it allows the non-invasive acquisition of accurate gastric volumes and like we demonstrated in our results, it can be used somehow as a predictor of clinical weight loss in the follow-up of these patients, since patients with lower percentages of gastric pouch volume expansion were also those who had lost more weight. In addition, CT volumetry may also demonstrate pathological findings like hernias or fistulae that may lead to adverse clinical effects such as recurrent vomiting or abdominal pain post-operatively [9].
Conclusion
In conclusion to our initial experience and results, we expect the LGCP procedure to become popular among morbidly obese patients in Egypt, due to its safety, potential reversibility and in particular it’s low cost which is very important in a developing country with major economic and financial burdens. The MSCT volumetric assessment of the post-operative gastric pouch yielded useful information designating it as a non-invasive, accurate, somehow promising diagnostic and predictor tool for the follow-up of LGCP. In addition, we would like to suggest it as an important corner in the post-surgical revision protocol, especially true for those problematic patients presenting with insufficient loss of weight or complications such as abdominal pain or recurrent vomiting. Further evaluations with greater sample sizes as well as longer follow-up periods are needed to confirm clinical relevance.
References
- Niazi M, Maleki AR, Talebpour M. Short-term outcomes of laparoscopic gastric plication in morbidly obese patients: Importance of post-operative follow-up. Obes. Surg. 23, 87-92 (2013).
- Kim SB, Kim SM. Short-term analysis of food tolerance and quality of life after laparoscopic greater curvature plication. Yonsei. Med. J. 57, 430-440 (2016).
- Shen D, Ye H, Wang Y et al. Comparison of short-term outcomes between laparoscopic greater curvature plication and laparoscopic sleeve gastrectomy. Surg. Endosc. 27, 2768-2774 (2013).
- Shen D, Ye H, Wang Y et al. Laparoscopic greater curvature plication: Surgical techniques and early outcomes of a Chinese experience. Surg. Obes. Relat. Dis. 10, 432-437 (2014).
- Ramos A, Galvao Neto M, Galvao M et al. Laparoscopic greater curvature plication: Initial results of an alternative restrictive bariatric procedure. Obes. Surg. 20, 913-918 (2010).
- Brethauer SA, Harris JL, Kroh M et al. Laparoscopic gastric plication for treatment of severe obesity. Surg Obes Relat Dis. 7:15-22 (2010).
- Skrekas G, Antiochos K, Stafyla VK. Laparoscopic gastric greater curvature plication: Results and complications in a series of 135 patients. Obes. Surg. 21, 1657-1663 (2011).
- Alva S, Eisenberg D, Duffy A et al. Virtual three-dimensional computed tomography assessment of the gastric pouch following laparoscopic Roux-Y gastric bypass. Obes. Surg. 18, 364-366 (2008).
- Karcz WK, Kuesters S, Marjanovic G et al. 3D-MSCT gastric pouch volumetry in bariatric surgery-preliminary clinical results. Obes. Surg. 19, 508-516 (2009).
- Masquiaran HPM, Santamaria VB, Hernandez JA et al. Role of 3D volumetry CT in the correlation between postoperative gastric volume and weight loss in obese patients undergoing gastric sleeve surgery. Poster ECR 2014/C-2145 (2014).
- Ramos AC, Galvao Neto M, Campos JM et al. Laparoscopic greater curve plication: an alternative restrictive bariatric procedure. Bariatric. Times. 7, 8-10 (2010).
- Mui WLM, Lee DWH, Lam KKY et al. Laparoscopic greater curve plication in Asia: Initial experience. Obes. Surg. 23, 179-183 (2013).
- Labrunie E, Marchiori E, Pitombo C. Radiographic evaluation and treatment. Obesity surgery: Principles and practice. New York, McGraw-Hill, Chapter 41 (2008).
- Sims TL, Mullican MA, Hamilton EC et al. Routine upper gastrointestinal gastrografin swallow after laparoscopic Roux-en-Y gastric bypass. Obes. Surg. 13, 66-72 (2003).
- Doraiswamy A, Rasmussen JJ, Pierce J et al. The utility of routine postoperative upper GI series following laparoscopic gastric by-pass. Surg. Endosc. 21, 2159-2162 (2007).
- White S, Han SH, Lewis C et al. Selective approach to use of upper gastroesophageal imaging study after laparoscopic roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. 4, 122-125 (2008)
- Naslund I. A method for measuring the size of the gastric outlet in obesity surgery. Acta. Chir. Scand. 150, 399-404 (1984).
- Nishie A, Brown B, Barloon T et al. Comparison of size of proximal gastric pouch and short-term weight loss following routine upper gastrointestinal contrast study after laparoscopic Roux-en-Y gastric bypass. Obes. Surg. 17, 1183-1188 (2007).
- Madan AK, Tichansky DS, Phillips JC. Does pouch size matter? Obes. Surg. 17, 317-320 (2007).
- Talebpour M, Amoli BS. Laparoscopic total gastric vertical plication in morbid obesity. Journal. of. Laparoendoscopic. and. Advanced. Surgical. Technique. 17, 793-798 (2007).
- Kourkoulos M, Giorgakis E, Kokkinos C et al. Laparoscopic gastric plication for the treatment of morbid obesity: A review. Minimally. Invasive. Surgery. 2012, 1-7 (2012).