Review Article - Imaging in Medicine (2010) Volume 2, Issue 1
Role of multidetector CT in the evaluation of coronary artery bypass grafts
Adriano Ascarelli, Marco Francone†, David Cannata, Alessandro Cannavale, Iacopo Carbone & Roberto PassarielloDepartment of Radiological Sciences, University ‘La Sapienza’ of Rome, V.le Regina Elena 324, 00161 Rome, Italy
- Corresponding Author:
- Marco Francone
Department of Radiological Sciences
University ‘La Sapienza’ of Rome
V.le Regina Elena 324, 00161 Rome, Italy
E-mail: marco.francone@uniroma1.it
Abstract
Coronary artery bypass graft (CABG) surgery is associated with an improvement of survival and quality of life in patients with multivessel coronary disease and left main stenosis. However CABGs, similarly to the native coronary vessels, may in time, develop stenosis or occlusion. Invasive coronary angiography is considered the gold standard in the evaluation of CABG, but the technique is associated with some complications. Multidetector CT is considered a noninvasive and convenient tool in the study of CABGs in patients with recurrence of chest pains. However, there are limitations related mainly to the presence of vessel calcification, vascular clips, arrhythmias and radiation exposure. The recent introduction of a new generation of multidetector CT scanners with a significant increase in spatial and temporal resolution could allow cardiac CT to have an increasing role in the clinical practice.
Keywords
blooming artifact ▪ calcified plaque ▪ cardiac multidetector computed tomography ▪ coronary angiography ▪ coronary arteries ▪ coronary arteries bypass graft ▪ motion artifact ▪ radiation dose ▪ surgical clips
Coronary artery disease is the main cause of hospitalization and mortality in western countries. For patients with multivessel coronary disease and left main stenosis, surgical placement of coronary artery bypass grafts (CABGs) is an effective and routine treatment that has been associated with an improvement in patient survival and quality of life [1–4]. However, both arterial and venous grafts, as well as the native coronary vessels, are affected by arteriosclerosis and may develop stenosis or occlusion [5–7]. Ischemic symptoms presenting as chest pain/dyspnea recur in 50% of patients in the 10 years following a CABG procedure [8]. A 25-year follow-up angiographic study of CABGs (n = 5065; 91% venous, 9% arterial) reports a 88% patency rate of grafts patency perioperatively, 81% at 1 year and 75% at 5 years, with a further decline to 50% at 15 years or later; moreover, graft closure from thrombosis at 1 month is a recognized complication in 10–15% of cases [5].
Chest pain after CABG surgery can originate from graft occlusion due to early or more tardive thrombosis, malposition or kinking, vasospasm, pleural or pericardial effusion, sternal infection, pulmonary embolism – not common but potentially lethal – and a late complication such as pseudoaneurysm formation [9] and also from advanced atherosclerosis of the native (not grafted) coronary arteries.
In the case of chest pain occuring during the first days after CABG surgery, postoperative assessment allows treatment of graft surgery problems. Reopening the mediastinum at this stage causes less complications considering that severe adhesions have not yet been developed [10,11]. Invasive coronary angiography is considered the gold standard for the direct assessment of bypass graft patency. However, cardiac catheterization is an invasive and expensive examination method, and it is associated with arrhythmia (0.38%), contrast reactions (0.37%), vascular (0.43%) and hemodynamic complications (0.26%) [12].
Multidetector CT (MDCT) may represent a noninvasive and convenient tool in patients with unclear stress test results or recurrence of chest pain after myocardial surgery revascularization [13]. The purpose of this article will be to review the role of MDCT as a noninvasive study of patients that underwent CABGs surgery.
Types of grafts & MDCT appearance
Both arterial and venous conduits are utilized for CABG surgery. Venous grafts are more prone to develop stenosis or occlusion, with an incidence of early thrombosis varying between 15 and 20% within the first year and a total graft occlusion in 55–65% at 10 years. Conversely, patency rates at 10 years for the internal mammary artery graft are much higher than 90% [14–16].
Venous graft
The great saphenous vein represents the most commonly used conduit for CABG surgery owing to its relatively easy harvesting, length of the vein, versatility and spasm resistance. Main drawbacks are varicosity, venous sclerosis and lower patency rates, which is approximately 50% at 10 years (Figure 1) [14]. Graft occlusion can occur in the early phases, most likely as a consequence of vascular damage during surgery (Figure 2), whereas in later stages, exposure to the systemic blood pressure owing to a poor compliance to arterializations may predispose venous conduit to atherosclerosis and occlusion [17–19].
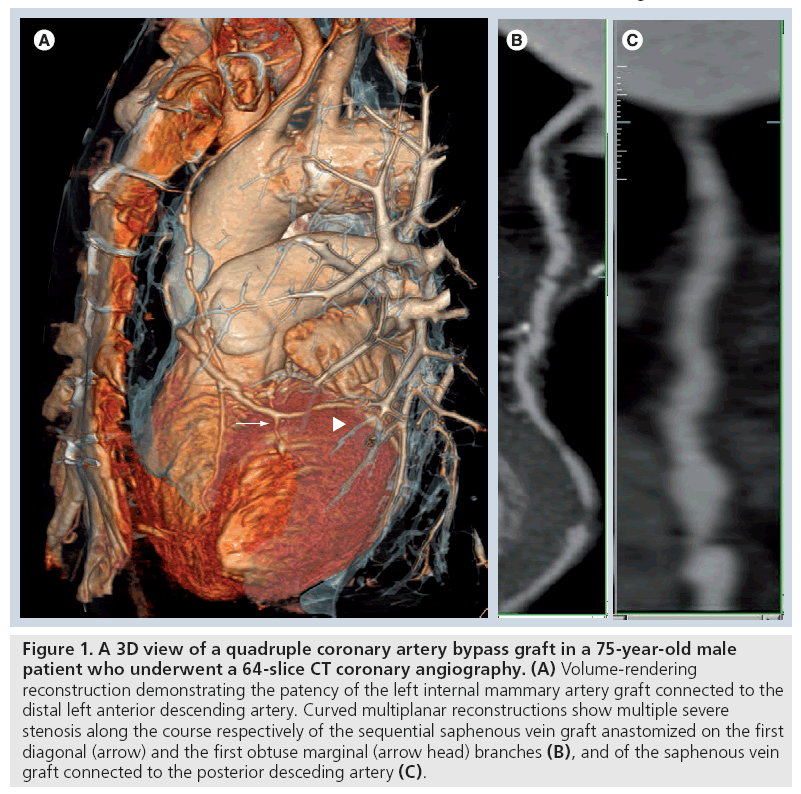
Figure 1: A 3D view of a quadruple coronary artery bypass graft in a 75-year-old male patient who underwent a 64-slice CT coronary angiography. (A) Volume-rendering reconstruction demonstrating the patency of the left internal mammary artery graft connected to the distal left anterior descending artery. Curved multiplanar reconstructions show multiple severe stenosis along the course respectively of the sequential saphenous vein graft anastomized on the first diagonal (arrow) and the first obtuse marginal (arrow head) branches (B), and of the saphenous vein graft connected to the posterior desceding artery (C).
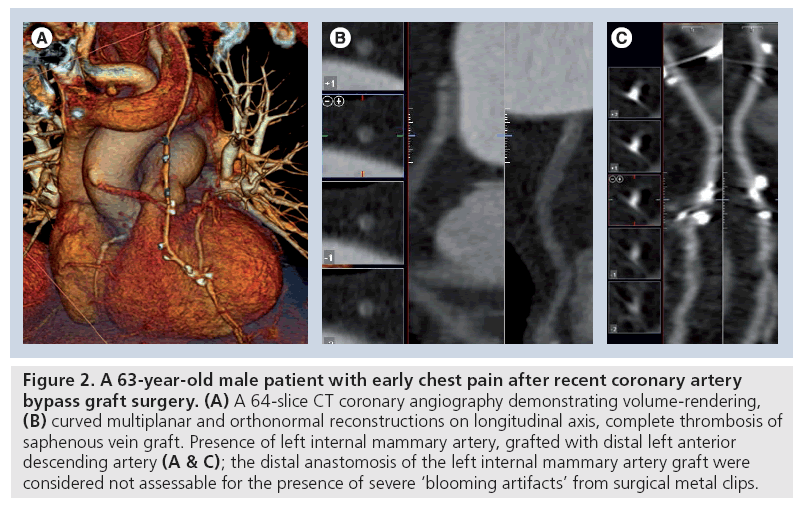
Figure 2: A 63-year-old male patient with early chest pain after recent coronary artery bypass graft surgery. (A) A 64-slice CT coronary angiography demonstrating volume-rendering, (B) curved multiplanar and orthonormal reconstructions on longitudinal axis, complete thrombosis of saphenous vein graft. Presence of left internal mammary artery, grafted with distal left anterior descending artery (A & C); the distal anastomosis of the left internal mammary artery graft were considered not assessable for the presence of severe ‘blooming artifacts’ from surgical metal clips.
Venous grafts are usually proximally anastomosed with the ascending aorta, cranially to the origin of coronary arteries and distally with the portion of coronary artery beyond the obstructive lesion. Left coronary venous CABGs are typically attached to the left side of the aorta, run above the pulmonary artery and distally anastomosed to the left anterior descending artery, diagonal branches, circumflex artery or obtuse marginal branches. By contrast, right coronary artery (RCA) grafts originate from the right or anterior side of the ascending aorta, and are distally connected to the RCA or posterior descending artery; distal anastomosis of the graft may also lie on the phrenic wall of the heart.
Sequential venous CABGs, with multiple lateral–lateral connections and a final terminal– lateral anastomosis can also be used (Figure 1 & 3); the main limitation of a sequential bypass is the potential for multivessel damage in the case of occlusion at the beginning of the graft.
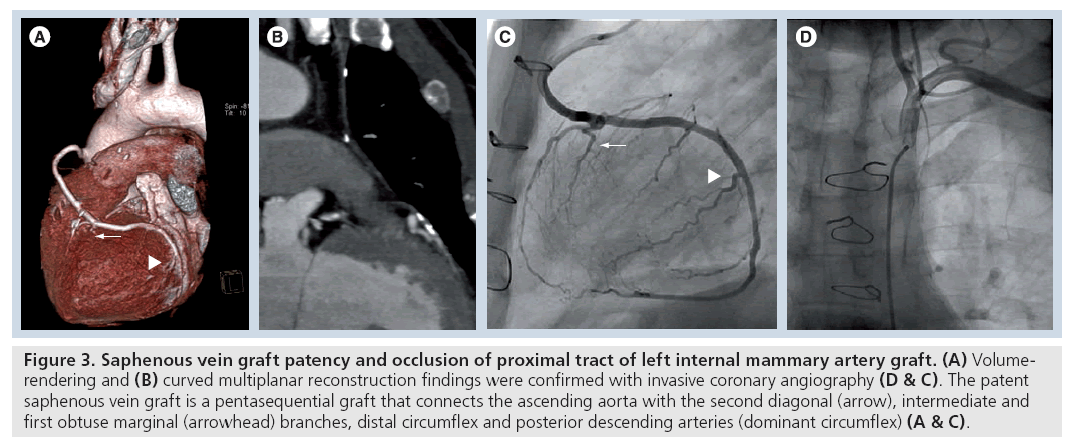
Figure 3: Saphenous vein graft patency and occlusion of proximal tract of left internal mammary artery graft. (A) Volumerendering and (B) curved multiplanar reconstruction findings were confirmed with invasive coronary angiography (D & C). The patent saphenous vein graft is a pentasequential graft that connects the ascending aorta with the second diagonal (arrow), intermediate and first obtuse marginal (arrowhead) branches, distal circumflex and posterior descending arteries (dominant circumflex) (A & C).
Venous CABGs are more easily evaluated with MDCT examination compared with the arterial grafts owing to their larger diameter and the absence of surgical clips along their course [20]. Sometimes a circumferential clip [21] or a metal connector (Symmetry Bypass System aortic connector) [22,23] can be identified at the level of proximal anastomosis with the ascending aorta.
Arterial graft
Resistance to atherosclerosis with a long-term patency rate (above 90% at 10 years) and lower postoperative mortality represent the main advantages of internal mammary CABGs. Therefore, for this arterial graft, the MDCT study requires a better spatial and temporal resolution for the smaller graft calibers, and presence of surgery clips that can determine beam-hardening artifacts [24].
The left internal mammary artery (LIMA) is frequently used to revascularize the anterior cardiac wall. Its origin is not moved from the proximal part of subclavian artery; the graft, repositioned from the left side of the sternum to the anterior mediastinum, along the right ventricle outflow tract, is typically connected with the left anterior descending or with the diagonal branches through a single or multiple sequential anastomosis (Figure 4) [20]. Surgical occlusion of collaterals (intercostals arteries) are visible as numerous metal clips along the graft course.
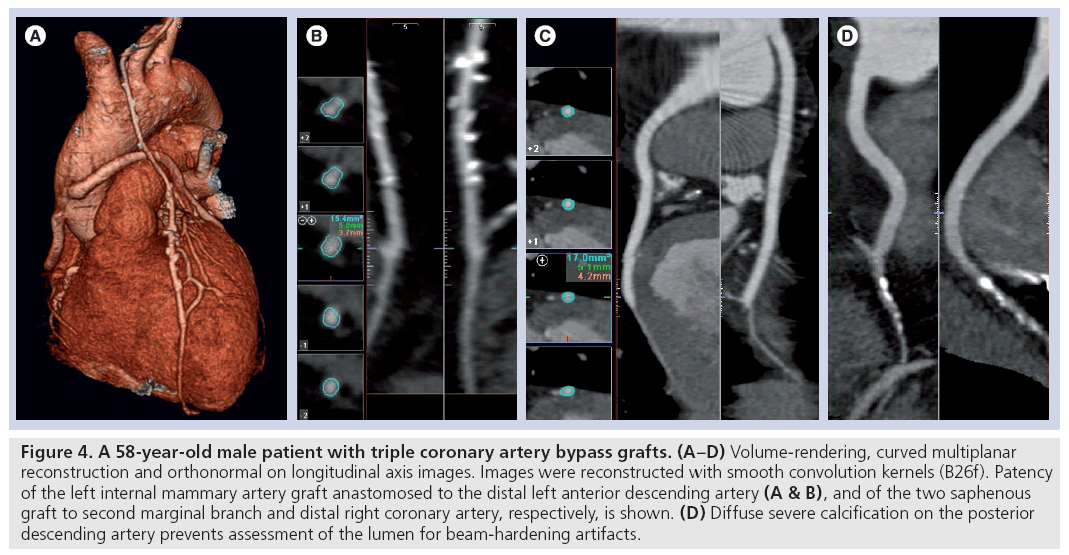
Figure 4: A 58-year-old male patient with triple coronary artery bypass grafts. (A–D) Volume-rendering, curved multiplanar reconstruction and orthonormal on longitudinal axis images. Images were reconstructed with smooth convolution kernels (B26f). Patency of the left internal mammary artery graft anastomosed to the distal left anterior descending artery (A & B), and of the two saphenous graft to second marginal branch and distal right coronary artery, respectively, is shown. (D) Diffuse severe calcification on the posterior descending artery prevents assessment of the lumen for beam-hardening artifacts.
The right internal mammary artery (RIMA) is more commonly used as an ‘arterial free graft’, with the proximal anastomosis removed from the subclavian artery and attached on the ascending aorta. The RIMA is also used for a composite arterial graft, proximally attached to a LIMA graft for a complete arterial bypass graft of two left-sided target vessels (Y shaped). Less frequently, the RIMA can be used as an ‘in situ graft’ for the RCA (Figure 5). Furthermore, metallic surgical clips are used to occlude collaterals vessels and can be recognized on MDCT examination.
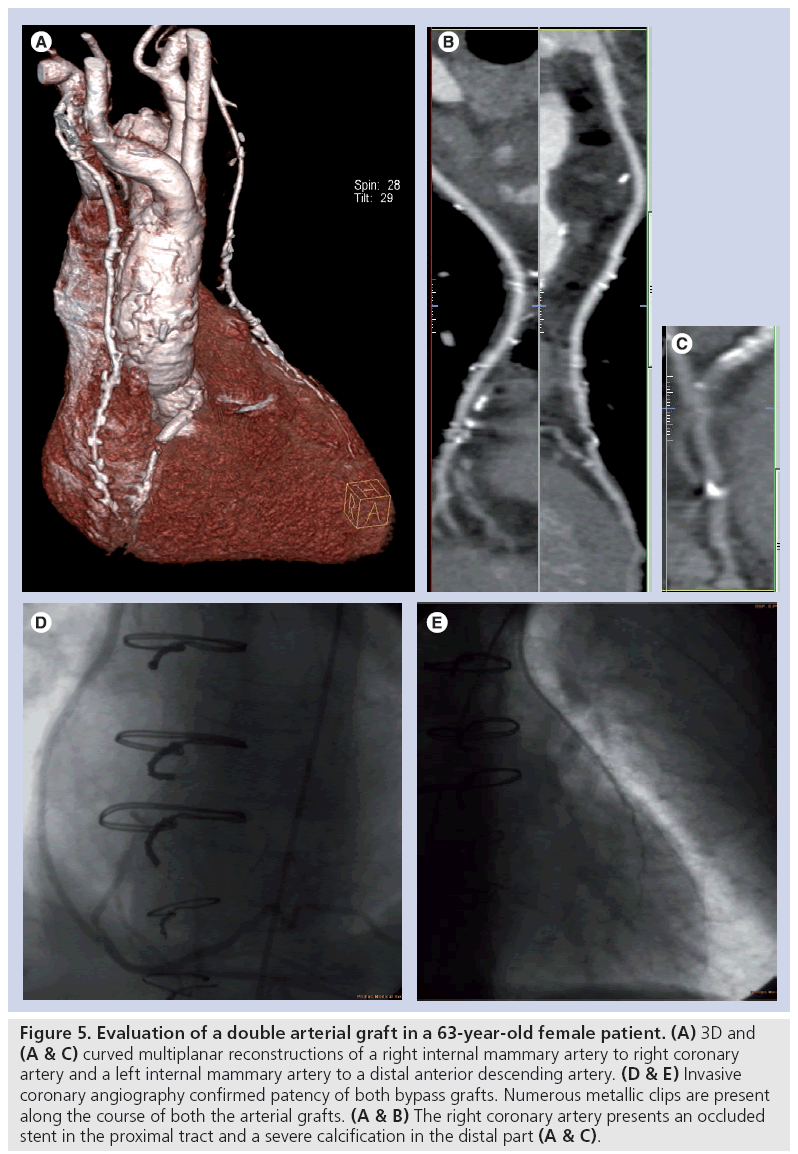
Figure 5: Evaluation of a double arterial graft in a 63-year-old female patient. (A) 3D and (A & C) curved multiplanar reconstructions of a right internal mammary artery to right coronary artery and a left internal mammary artery to a distal anterior descending artery. (D & E) Invasive coronary angiography confirmed patency of both bypass grafts. Numerous metallic clips are present along the course of both the arterial grafts. (A & B) The right coronary artery presents an occluded stent in the proximal tract and a severe calcification in the distal part (A & C).
The radial artery is a muscular artery characterized by a pronounced medial layer and high vasoreactivity. These conduits are often used as a free or composite graft to supply lateral left ventricular wall (circumflex territory) for a larger arterial revascularization. The administration of calcium channel blockers postoperatively to prevent graft spasm and significant improvements in harvesting surgical techniques to avoid endothelial damage led radial artery grafts to be regarded as a valid arterial option with a patency rate ranging from 95.7% at 12 weeks to 91.6% at 10 years, similar to those seen with IMA grafts [25]. MDCT visualization of the radial artery may be hampered by artifacts from the surgical clips used to close the numerous collaterals of this muscular artery.
The right gastroepiploic artery can be used to provide an all-arterial revascularization as a secondary, tertiary or quaternary arterial conduit and sometimes as an ‘in situ’ graft to supply the inferior cardiac wall, anastomosing the posterior descending artery [26]. Right gastroepiploic artery grafts have similar characteristics to other arterial CABGs, however, the need for a combined abdominal and thoracic surgical approach with subsequent increase in surgery time limited the widespread use of this conduit. Numerous vascular clips are visible at MDCT along the course of the right gastroepiploic artery displaced near the small gastric curvature, anterior to the liver, through the diaphragm and in the site of distal anastomosis.
MDCT evaluation: diagnostic performances & limitations
Compared with coronary angiography, MDCT angiography represents a less invasive technique to detect early complication of graft surgery or the more tardive failures. The larger diameter of graft conduits, their relative immobility and a low frequency of calcifications makes the assessment of CABGs less challenging compared with the assessment of native coronary arteries. In addition, CABGs are more often occluded than stenosed, which makes the diagnosis easier [27]. The clinical role of MDCT for the assessment of CABGs was also recognized by the American Heart Association, who reported in a scientific statement that it is “reasonable not only to assess patency of the bypass graft but also the presence of coronary stenoses in the course of the bypass graft or at the anastomotic site, as well as in the native coronary artery system” [28]. In patients who need to repeat cardiac bypass surgery, MDCT angiography allows aquisition of a 3D map of the existing grafts and their relationships with the mediastinal anatomic structures and the anterior thoracic cage (Figure 1). This allows the appropriate surgical approach to be planned and to minimize the risk of injury to the graft vessels during surgical re-entry [29–31]. Furthermore, assessment of CABGs with selective coronary angiography presents some practical disadvantages compared with the evaluation of native vessels, with a longer duration of procedure, more contrast agent used and an increased complication rate [32]. With coronary angiography, it is also sometimes difficult to locate the origin of the grafts and explore them selectively.
Evaluation of patients after CABG surgery has the same common limitations as cardiac MDCT, mainly related to the general MDCT drawbacks (e.g., individual radiation exposure and the use of potentially nephrotoxic contrast media), evaluation of patients with severe arrhythmias and assessment of vessel lumen in the presence of high density structures, such as severely calcified plaques or metal surgery clips. Since the early introduction of MDCT technology, several investigators have examined the potential of methods to detect CABG occlusion and stenoses.
Early-generation scanners, which were able to acquire four slices with a subsecond rotation time, showed high sensitivity and specificity in the detection of venous and arterial occlusions, but the assessment of native vessels was still inadequate (62%), preventing application of this technique in a large clinical setting [33,34]. More recently, 16- and 64-slice MDCT scanners provided a more accurate depiction of coronary vessels with a shorter scan time and fewer cardiac and respiratory artifacts. In particularly, 64-slice MDCT scanners were able to obtain 0.4 mm3 isotropic voxels and 330 ms rotation time, challenging the clinical value of conventional diagnostic x-ray angiography in coronary artery imaging [35–37].
In a large meta-analysis of 15 studies published between 2004 and 2007 and conducted with 16- and 64-slice MDCT scanners, Hamon et al. pooled diagnostic performances of the method for detection of occlusion or significant CABG stenosis [38]. They found a sensitivity and specificity of MDCT compared with selective coronary angiography of 97.6 and 96.7%, respectively, with a positive predictive value of 92.7% and a negative predictive value of 98.9%. These data clearly show how MDCT has already become a powerful and well-established tool for noninvasive assessment of CABGs.
Nevertheless, the recent introduction of a new generation of cardiac CT scanners, such as the dual-source 256- and 320-slice CT, with a significant increment of spatial and temporal resolution owing to a larger detector number, faster gantry rotation time and dual radiation source technology, allows MDCT to obtain an ever-increasing role in clinical cardiological practice [39,40].
Motion artifact
Coronary artery bypass grafts are subject to less cardiac motion and heart rate variability than the native vessels owing to their epicardiac course. Conversely, the assessment of distal anastomosis and the run-off segments may be limited by motion artifact. Heart rate control, multiphase reconstruction and the higher temporal resolution, provided by the new sophisticated scanners (up to 85 ms), minimize motion artifacts in native coronary vessels. In addition, new MDCT (320-slice CT) allows the aquisition of the entire cardiac volume (up to 16 cm thick), during a single gantry rotation and a single cardiac beat, with a heart image free from step artifacts (cardiac rhythm variations). New-generation scanners can obtain a heart image less affected by cardiac movement artifacts with the possibility of diagnostic-quality examinations in patients with a high heart rate [41]
Calcifications & surgical clips
Calcified lesions of the vessel and surgical metal clips in MDCT images appear hyperdense – giving a bright signal, with a shade corona around it. This artifact, also called a ‘blooming artifact’, is due to intrinsic method spatial resolution limitations, the effects of cardiac motion (limitations of temporal resolution) and beam-hardening phenomenon. Severe vessel calcification or surgical clips located close to the graft may overshadow the contrastenhanced lumen, which on occasions, make it impossible to assess vessels patency (Figure 2) [24]. In symptomatic patients in particular, the assessment of native coronary arteries may be clinically relevant allowing the exclusion of new-vessel involvement. However, both native vessels located distally to the anastomosis (‘runoff vessels’) and the non-bypassed arteries are usually affected by severe atherosclerotic disease with multiple severe calcifications, precluding a correct assessment of the lumen (Figure 4) [42]. Alternatively, new CT scanners and appropriate reconstruction protocols using medium-sharp kernels already adopted for the assessment of coronary stent could allow improved visualization of the vessel with calcified lesions or close surgery clips (Figure 6). Increasing the temporal resolution, in case of high density calcified coronary plaques and metallic clips along bypass terminal anastomoses, could reduce the blooming artefact phenomenon, partly caused by cardiac movement [43]. Nevertheless, recent studies report a significant negative impact of coronary calcifications on the diagnostic performance of the latest generation CT too, especially in patients with higher heart rates [39,44].
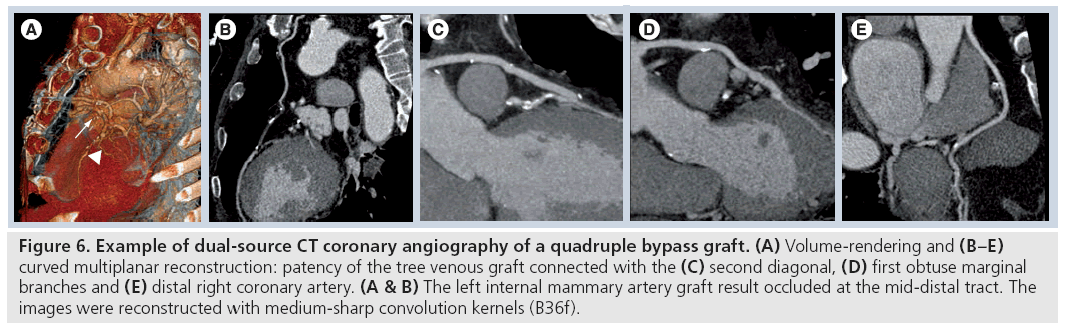
Figure 6: Example of dual-source CT coronary angiography of a quadruple bypass graft. (A) Volume-rendering and (B–E) curved multiplanar reconstruction: patency of the tree venous graft connected with the (C) second diagonal, (D) first obtuse marginal branches and (E) distal right coronary artery. (A & B) The left internal mammary artery graft result occluded at the mid-distal tract. The images were reconstructed with medium-sharp convolution kernels (B36f).
Radiation exposure
Evaluation of CABGs with MDCT, especially in the case of LIMA or RIMA grafts, involves a significantly higher radiation exposure than native coronary artery examination due to a larger cranio–caudal volume covering the entire course of the graft. In the case of venous grafts, it is possible to acquire a shorter scan volume, but it is important to have the correct knowledge of the graft type.
Meyer et al., investigating 64-slice MDCT in CABG assessment, described effective doses of 17.8 ± 5.4 mSv [45]. Divergences in radiation exposure reported in different studies may be explained by different choices of acquisition or the use of different or even no dose modulation strategies. In some studies, for dose reduction, scan volume did not cover the origin and the proximal part of the LIMA or RIMA grafts and the upper level was set at the midlevel of the aortic arch such as a venous graft study [42–45]. However, occlusion at the origin of LIMA or RIMA remains a real possibility [24]. Several dose reduction strategies have been implemented over the last few years that are based on x-ray emission or optimization of scanning parameters (i.e., mAs, kV, pitch and collimation) or which take account of the individual patient’s characteristics (automatic exposure control systems); in particular heart rate control enables low-dose scan protocols, such as, ECG pulsing or prospective ECG-gated acquisition [46].
Recent MDCT coronary artery studies report a consistent dose reduction in the evaluation of low heart rate patients using prospective ECG-triggered protocols with a mean dose of approximately 3–5 mSv and in selected cases below 1 mSv [47,48]. In addition, to evaluate internal mammary artery grafts, new scanners and dose-reduction protocols allow a non- ECG-gated acquisition of the thoracic volume over the aortic bulb and at the same time a cardiosynchronized acquisition of the cardiac volume. Currently, these new strategies, despite the absence of data in literature regarding specific applications in CABG evaluation, promise optimization of image quality while keeping individual exposure at the lowest level.
Conclusion
Examination of asymptomatic patients with inconclusive or positive provocative tests (stress- ECG or stress-SPECT) or with recurrent chest pain complaints after CABG surgery are wellestablished and clinically accepted applications of cardiac MDCT. Bypass grafts can usually be imaged with excellent quality owing to their large caliber and relative lack of cardiac motion. The MDCT ‘roadmap’ provides identification of number, location and types of bypass grafts, as well as the assessment of native coronary arteries. Metal clips are not a significant limitation within the grafts, whereas native arteries may sometimes be more of a challenge in CABG patients owing to an underlying severe and diffuse coronary atherosclerosis with the presence of heavily calcified lesions preventing assessment of the lumen.
Newer generations of CT scanners with improved spatial and temporal resolution may improve our ability to evaluate the often heavily-diseased native coronary arteries and therefore eventually open the generally recommended MDCT application to patients after CABG surgery.
Future perspective
Multidetector CT is an emerging diagnostic technique for coronary artery assessment and has undergone an explosive technological development in the last 10 years. The latest scanner generation, owing to different engineering approaches, have improved the temporal and spatial resolution of the method, producing important results in terms of image quality and radiation exposure. Nevertheless, radiation dose reduction and evaluation of high density coronary lesions remain the main challenges of this diagnostic modality. Over the next few years, further to recent development, it is predictable that an exponential technological improvement in terms of gantry speed rotation, numbers and dimensions of the detectors, acquisition and reconstruction software tools will occur. Further implementation in terms of dose reduction and diagnostic performance will open the indication and clinical role of MDCT in the evalution of CABGs and in the diagnosis of coronary arteries disease.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.

References
- Hlatky MA, Rogers WJ, Johnstone I et al.: Medical care costs and quality of life after randomization to coronary angioplasty or coronary bypass surgery. N. Engl. J. Med. 336, 92–99 (1997).
- BARI investigators: Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. N. Engl. J. Med. 335, 217–225 (1996).
- Hamm CW, Reimers J, Ischinger T et al.: A randomized study of coronary angioplasty compared with bypass surgery in patients with symptomatic multivessel coronary disease. N. Engl. J. Med. 331, 1037–1043 (1994).
- Patel MR, Dehmer DJ, Hirshfield JW, Smith PK, Spertus JA: Appropriateness criteria for coronary revascularization. J. Am. Coll. Cardiol. 53(6), 530–553 (2009).
- Fitzgibbon GM, Kafka HP, Leach AJ et al.: Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J. Am. Coll. Cardiol. 28, 616–626 (1996).
- BARI investigators: Seven-year outcome in the bypass angioplasty revascularization investigation (BARI) by treatment and diabetic status. J. Am. Coll. Cardiol. 35, 1122–1129 (2000).
- Goldman S, Zadina K, Krasnicka B et al.: Predictors of graft patency 3 years after coronary artery bypass graft surgery. J. Am. Coll. Cardiol. 29, 1563–1568 (1997).
- Cameron AA, Davis KB, Rogers WJ: Recurrence of angina after coronary artery bypass surgery: predictors and prognosis (CASS Registry). Coronary artery surgery study. J. Am. Coll. Cardiol. 26(4), 895–899 (1995).
- Aletta AF, Fauzia Q, Read KM et al.: Coronary artery bypass grafts: assessment with multidetector CT in the early and late postoperative settings. Radiographics 25(4), 881–896 (2005).
- Diegeler A, Matin M, Kayser S et al.: Angiographic results after minimally invasive coronary bypass grafting using the minimally invasive direct coronary bypass grafting (MIDCAB) approach. Eur. J. Cardiothorac. Surg. 15, 680–684 (1999).
- Wiklund L, Johansson M, Brandrup-Wognsen G et al.: Difficulties in the interpretation of coronary angiogram early after coronary artery bypass surgery on the beating heart. Eur. J. Cardiothorac. Surg. 17, 46–51 (2000).
- Scanlon PJ, Faxon DP, Audet A-M et al. ACC/AHA guidelines for coronary angiography. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Coronary Angiography). Developed in collaboration with the Society for Cardiac Angiography and Interventions. J. Am. Coll. Cardiol. 33(6), 1756–1824 (1999).
- Hendel RC, Kramer CM, Patel MR et al.: Appropriateness criteria for CCT/CMR. J. Am. Coll. Cardiol. 487, 1475–1497 (2006).
- Campeau L, Enjalbert M, Lesperance J et al.: The relation of risk factors to the development of atherosclerosis in saphenous-vein bypass grafts and the progression of disease in the native circulation. A study 10 years after aortocoronary bypass surgery. N. Engl. J. Med. 311, 1329–1332 (1984).
- Bourassa MG, Enjalbert M, Campeau L et al.: Progression of atherosclerosis in coronary arteries and bypass grafts: the Coronary Artery Surgery Study (CASS) and Montreal Heart Institute experiences. Circulation 72, 71–78 (1984).
- Loop F, Lytle B, Cosgrove D et al.: Influence of the internal mammary artery graft on 10-year survival and other cardiac events. N. Engl. J. Med. 314, 1–6 (1986).
- Bourassa MG, Fisher LD, Campeau L et al.: Long-term fate of bypass grafts: the Coronary Artery Surgery Study (CASS)and Montreal Heart Institute experiences. Circulation 72, V71–V78 (1985).
- Campeau L, Enjalbert M, Lesperance J et al.: Atherosclerosis and late closure of aortocoronary saphenous vein grafts: sequential angiographic studies at 2 weeks, 1 year, 5 to 7 years, and 10 to 12 years after surgery. Circulation 68, II1–II7 (1983).
- Motwani JG, Topol EJ: Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation 97, 916–931 (1998).
- Marano R, Storto ML, Merlino B et al.: Bypass grafts at multidetector row CT. a pictorial review of coronary artery. Chest 127, 1371–1377 (2005).
- Calafiore AM, Bar-El Y, Vitolla G et al.: Early clinical experience with a new sutureless anastomotic device for proximal anastomosis of the saphenous vein to the aorta. J. Thorac. Cardiovasc. Surg. 121, 854–858 (2001).
- Mack MJ, Emery RW, Ley LR et al.: Initial experience with proximal anastomoses performed with a mechanical connector. Ann. Thorac. Surg. 75, 1866–1871 (2003).
- Wiklund L, Bonilla LF, Berglin E et al.: A new mechanical connector for distal coronary artery anastomoses in coronary artery bypass grafting: a randomized, controlled study. J. Thorac. Cardiovasc. Surg. 129, 146–150 (2005).
- Von Kiedrowski H, Wiemer M, Franzke K et al.: Non-invasive coronary angiography: the clinical value of multi-slice computed tomography in the assessment of patients with prior coronary bypass surgery evaluating grafts and native vessels. Int. J. Cardiovasc. Imaging 25, 161–170 (2009).
- Possati G, Gaudino M, Prati F et al.: Long-term results of the radial artery used for myocardial revascularization. Circulation 108, 1350–1354 (2003).
- Buche M, Schoevaerdts JC, Louagie Y et al.: Use of the inferior epigastric artery for coronary bypass. J. Thorac. Cardiovasc. Surg. 103, 665–670 (1992).
- Bourassa MG, Fisher LD, Campeau L et al.: Long-term fate of bypass grafts: the coronary artery surgery study (CASS) and Montreal Heart Institute experiences. Circulation 72(6 Pt 2), V71–V78 (1985).
- Budoff MJ, Achenbach S, Blumenthal RS et al.: Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation 114, 1761–1791 (2006).
- Gilkeson RC, Markowitz AH, Ciancibello L et al.: Multisection CT evaluation of the reoperative cardiac surgery patient. Radiographics 23, S3–S17 (2003).
- Ohtsuka T, Akahane M, Ohtomo K et al.: Three-dimensional computed tomography for reoperative minimally invasive coronary artery bypass. Ann. Thorac. Surg. 70, 1734–1735 (2000).
- Sawamura Y, Takase K, Saito H et al.: Three-dimensional imaging of coronary artery bypass grafts. Ann. Thorac. Surg. 73, 1655 (2002).
- Yamamoto M, Kimura F, Niinami H et al.: Noninvasive assessment of off-pump coronary artery bypass surgery by 16-channel multidetector-row computed tomography. Ann. Thorac. Surg. 81, 820–827 (2006).
- Burgstahler C, Kuettner A, Kopp AF et al.: Non-invasive evaluation of coronary artery bypass grafts using multi-slice computed tomography: initial clinical experience. Int. J. Cardiol. 90, 275–280 (2003).
- Ropers D, Ulzheimer S, Wenkel E et al.: Investigation of aortocoronary artery bypass grafts by multislice spiral computed tomography with electrocardiographic-gated image reconstruction. Am. J. Cardiol. 88, 792–795 (2001).
- Gurevitch J, Gaspar T, Orlov B et al.: Non-invasive evaluation of arterial grafts with newly released multidetector computed tomography. Ann. Thorac. Surg. 76, 1523–1527 (2003).
- Nieman K, Cademartiri F, Lemos PA et al.: Reliable non-invasive coronary angiography with fast submillimeter multislice spiral computed tomography. Circulation 106, 2051–2054 (2002).
- Martuscelli E, Romagnoli A, D’Eliseo A et al.: Accuracy of thin slice computed tomography in the detection of coronary stenoses. Eur. Heart J. 25, 1043–1048 (2004).
- Hamon M, Lepage O, Malagutti P et al.: Diagnostic performance of 16- and 64-section spiral CT for coronary artery bypass graft assessment: meta-analysis. Radiology 247, 679–686 (2008).
- Brodoefel H, Burgstahler C, Tsiflikas I et al.: Dual-source CT: effect of heart rate, heart rate variability, and calcification on image quality and diagnostic accuracy. Radiology 247(2), 346–355 (2008).
- Weustink AC, Neefjes LA, Krestin GP et al.: Impact of heart rate frequency and variability on radiation exposure, image quality, and diagnostic performance in dual-source spiral CT coronary angiography. Radiology 253(3), 672–680 (2009).
- Adler G, Meille L, Rohnean A, Sigal-Cinqualbre A, Capderou A, Paul JF: Robustness of end-systolic reconstructions in coronary dual-source CT angiography for high heart rate patients. Eur. Radiol. DOI: 10.1007/s00330–009–1642–9 (2009) (Epub ahead of print).
- Salm LP, Bax JJ, Jukema JW et al.: Comprehensive assessment of patients after coronary artery bypass grafting by 16-detector-row computed tomography. Am. Heart. J. 150, 775–781 (2005).
- Zhuangli Liang KWC, Do S, Brady T, Pien H: Analysis and mitigation of calcium artifacts in cardiac multidetector CT. Presented at: Biomedical Imaging: From Nano to Macro, 2008. ISBI 2008. 5th IEEE International Symposium. Paris, France, 14–17 May 2008.
- Stolzmann P, Scheffel H, Leschka S et al.: Influence of calcifications on diagnostic accuracy of coronary CT angiography using prospective ECG triggering. AJR Am. J. Roentgenol. 191(6), 1684–1689 (2008).
- Meyer TS, Martinoff S, Hadamitzky M et al.: Improved noninvasive assessment of coronary artery bypass grafts with 64-slice computed tomographic angiography in an unselected patient population. J. Am. Coll. Cardiol. 49, 946–950 (2007).
- Catalano C, Francone M, Ascarelli A, Mangia M, Iacucci I, Passariello R: Optimizing radiation dose and image quality. Eur. Radiol. 17(Suppl. 6), F26–F32 (2007).
- Achenbach S, Marwan M, Ropers D et al.: Coronary computed tomography angiography with a consistent dose below 1 mSv using prospectively electrocardiogram-triggered high-pitch spiral acquisition. Eur. Heart J. doi:10.1093/eurheartj/ehp470 (2009) (Epub ahead of print).
- Rybicki FJ, Otero HJ, Steigner ML et al.: Initial evaluation of coronary images from 320-detector row computed tomography. Int. J. Cardiovasc. Imaging 24(5), 535–546 (2008).