Review Article - Interventional Cardiology (2009) Volume 1, Issue 2
Role of percutaneous left ventricular assist devices in preventing cerebral ischemia
- Corresponding Author:
- Vegard Tuseth
Department of Heart Disease
Haukeland University Hospital
N-5021 Bergen, Norway
Tel: +47 5597 5000
Fax: +47 5597 5140
E-mail: vegard.tuseth@helse-bergen.no
Abstract
Keywords
cardiac arrest, cardiogenic shock, cerebral ischemia, metabolism, percutaneous ventricular assist devices, resuscitation, revascularization, tissue perfusion
Left ventricular assist devices (LVADs) provide systemic blood delivery and myocardial unloading in severe heart failure, including the acute setting [1–7]. Recently developed percutaneous ventricular assist devices (PVADs) have the advantage of rapid deployment. Safety and feasibility for short-term use have been established, and PVADs have been assessed in acute myocardial infarction, cardiogenic shock, severe heart failure, in high-risk coronary recascularization, and as a bridge to permanent treatment in decompensated heart failure [8–15]. PVADs unload the heart and improve vital organ perfusion, and recent data indicate the ability of such a device to maintain cerebral and myocardial perfusion during experimental cardiac arrest [16,17].
Contrary to the substantial advances seen in the treatment of chronic heart failure, acute severe heart failure with circulatory insufficiency still has a poor prognosis [18–21]. In cardiogenic shock, tissue hypoperfusion is regularly present, and vital organ dysfunction with tissue damage is frequent [22–24]. In resuscitated patients, cerebral injury is the major limiting factor for clinical outcomes in survivors [25–27].
In this overview, we aim to focus on the potential of PVAD technology to provide protection against acute ischemic cerebral injury in acute hemodynamic collapse.
Current treatment
Treatment strategies for reducing cerebral and myocardial ischemic injury (cardiocerebral resuscitation) make use of urgent hypothermia and coronary revascularization in addition to conventional cardiopulmonary resuscitation (CPR) and have improved clinical outcomes in recent investigations (Figure 1) [28].
Figure 1. Survival and neurological recovery in patients with ST elevation myocardial infarction resuscitated from cardiac arrest. CPR 3 years: 3-year survival without neurological sequelae for cardiac arrest patients treated with conventional CPR. CCR 3 years: 3-year survival without neurological sequelae for cardiac arrest patients treated with CCR (includes urgent hypothermia and coronary revascularization). CCR: Cardiocerebral resuscitation; CPR: Cardio–pulmonary resuscitation.
Reprinted with permission from [28].
Acute hemodynamic interventions in these critically ill patients, including urgent cardiopulmonary bypass, sophisticated resuscitation algorithms and medical therapy, have been studied but have not resulted in better outcomes in clinical practice [29–31].
Moreover, vasopressors and inotropes may have detrimental circulatory and metabolic effects despite the intuitive benefit of increased arterial pressures. Experimentally, both cerebral and coronary perfusion have been shown to be impaired with the use of vasopressor therapy during cardiac arrest [32–34]. Adverse effects have also been indicated on clinical outcomes in resuscitation [35]. Intra-aortic ballon pump (IABP) therapy has been extensively used in cardiogenic shock, but has not been demonstrated to improve mortality. Furthermore, IABP use has been associated with an increased risk of stroke in ST elevation myocardial infarction in a recent meta‑analysis [36].
Mechanical cardiac assist devices are established treatment choices in chronic heart failure and reduce left ventricular workload and oxygen consumption with possible beneficial effects on myocardial metabolism and function [37,38]. In acute cardiac disease with very low cardiac output state, it has been suggested that emergent use of heart assist therapy may unload the left ventricle and may also augment perfusion of the vital organs [39–41] . In particular, brain damage due to cerebral hypoperfusion represents a major limitation for clinical outcomes for patients surviving acute hemodynamic collapse [42,43] and LVAD support may increase cerebral blood flow (CBF) [44,45].
Surgical LVADs have been implanted successfully in acute decompensated heart failure and may be useful as a bridge to permanent treatment or recovery for selected patients after initial stabilization [46,47]. Available cardiac assist systems also include cardiopulmonary bypass (CPB) and extracorporeal membrane oxygenation (ECMO) and extracorporeal life support (ECLS), which have the potential to sustain circulation during cardiac standstill [48–51]. Reports on the use of ECMO, ECLS and CPB in cardiogenic shock and cardiac arrest have shown the feasibility of such interventions, but the potential to improve survival has not been established in these settings [52–57]. Acute implantation has a high risk of complications and requires highly specialized personnel and facilities, thus emergency surgical LVAD support is not routinely available in the treatment of acute heart failure and technical limitations may represent a substantial drawback in a critical setting.
Percutaneous left ventricular assist devices
Ideally, intervention in acute cardiac collapse should sustain sufficient cerebral perfusion to avoid ischemic injury in order to allow for treatment of the underlying disease. Moreover, the intervention should be readily available, easy to establish and any risk of complications should be minimized. Percutaneous assist devices have the potential to meet these requirements.
There are several benefits of percutaneous devices over surgical devices. The generally smaller size and reduced vascular trauma with percutaneous devices can reduce the risk of bleeding and vascular compromise during implantation and use. By permitting rapid hemodynamic support with less complicated implantation procedures, a percutaneous approach seems especially useful for the acute treatment of patients with hemodynamic collapse. Systemic blood delivery may be sufficient to prevent short-term tissue ischemia [58–61]. Depending on the ability to maintain vital organ blood delivery over time, such devices may also be of use during surgical cardioplegia and cardiac arrest.
The two systems in clinical use are the TandemHeart® and the Impella® LP 2.5 (Figure 2). Both of these devices fulfil the criteria for a true LVAD, being able to reduce myocardial workload, and to transport oxygenated blood into the systemic circulation [62]. Hemodynamic and experimental findings indicate both systems may be effective, and clinical data have suggested low complication rates when used for an extended time-period [15,63–66]. For both the devices, bleeding and limb ischemia represent the majority of reported rare but severe complications; both have been studied in unstable and stable patients, and indications for use in clinical practice include high-risk percutaneous coronary intervention and cardiogenic shock [8–15].
The TandemHeart device is a catheter-based left atrial-to-femoral bypass system delivering up to 10 l of blood per minute. Implantation requires large diameter catheters (21 F), and trans-septal–septal puncture. Deployment time is approximately 30 min and complication rates have been reported to be low during clinical use [67]. The Impella LP 2.5 device is a miniature impeller pump that is placed retrogradely into the left ventricle via a 13 F femoral artery sheath with a capacity to pump 2.5 l of blood per minute into the ascending aorta. Placement into the left ventricle may be rapidly performed and the risk of complications has been found to be low [67].
In the presence of femoral or iliac artery disease, access may be obtained with stenting of stenotic vessels prior to implantation of the PVAD. In uncomplicated cases, the Impella LP 2.5 may be deployed within a few minutes, which may be of clinical importance in the treatment of acutely decompensated patients. Transient hemolysis has been reported for the Impella device. This does not regularly represent a clinical problem and measurement of free hemoglobin in serum will reliably differentiate between hemolysis and bleeding if anemia or clinical hematuria is present. Furthermore, potential aortic regurgitation represents a theoretical limitation with the transvalvular device, but hemodynamically significant regurgitation has not been found to be an issue with regular use in patients with intrinsic heart rhythm [8,65].
The TandemHeart device requires a more complicated deployment procedure but has a higher delivery than the Impella LP 2.5. Although both devices deliver a substantially higher output than can be achieved by IABP devices, no clinical benefit has been demonstrated with PVAD compared with IABPs thus far [8,9], and despite intuitively beneficial potential, no PVAD has so far been able to demonstrate clinically beneficial outcomes during temporary use in critically ill patients [68].
The potential of the Impella LP 2.5 for improving blood flow to the head in awake subjects has been indicated [42]. However, the clinical role of PVADs in prevention of cerebral injury is likely to be most relevant for unconscious patients with severely compromised CBF. During cardiac standstill and profound hypotension, the potential for cerebral injury is obvious, and the settings of resuscitated hemodynamic collapse and cardiac arrest represent clinical situations where percutaneous cardiac assist systems may be of particular use. The Impella LP 2.5 has its inlet and outlet proximal to the carotid arteries, whereas only the inlet of the TandemHeart device is situated in the precerebral arterial circulation with the outlet in the femoral artery (Figure 2). The outlet of the Impella device in the ascending aorta can allow for preferential flow to the cerebral and myocardial vessels [16,17,61,69]. Thus, the design of the Impella device could theoretically represent an advantage compared with a left atrial-to-femoral artery bypass system, although the TandemHeart device has a higher maximum output.
According to currently available data, no cerebral embolic complications have been reported with either of the two devices. However, only limited patient data have been published and specific assessment of cerebral injury has, to our knowledge, not been assessed during clinical PVAD support.
Role of percutaneous left ventricular assist devices in preventing cerebral injury
A benefit of PVADs in preventing cerebral injury is unlikely to be found in awake patients where cerebral circulation is adequate to maintain consciousness. Accordingly, in most conscious cardiogenic shock patients, cerebral circulation may be sufficient to avoid short-term ischemic brain damage without additional LVAD therapy.
In cardiac arrest, prognosis remains poor, and even with the immediate start of chest compressions, and urgent percutaneous coronary revascularization and cooling, cerebral injury is still a major problem in patients surviving the acute phase [70]. Thus, in patients with cardiac arrest and pulseless electrical activity requiring resuscitation, PVAD support may have the potential to significantly improve the delivery of oxygenated blood to the brain.
The delivery of oxygenated blood to the arterial circulation depends on adequate filling of the left heart from the pulmonary circulation. Measures that improve venous return can augment the available left-side blood volumes [71]. Intravenous fluid loading may be useful for this purpose [14,16,72]. Furthermore, abdominal compression devices can increase total peripheral resistance and vital organ perfusion pressures without the potential detrimental effect of medical vasopressor substances in this setting [73,74].
The use of PVAD therapy in cardiac arrest patients may be relevant as an adjunct to established resuscitation during concommittant chest compressions in the hyperacute setting, with a possible synergistic effect on vital organ blood delivery.
Further benefits of PVAD therapy may be possible with a device able to obviate the need for chest compressions and vasopressor during ventricular fibrillation (VF), which could allow for more optimal coronary revascularization and medical stabilization of the patient. In order to achieve this, blood delivery from the device should be able to prevent acute cerebral injury and the hemodynamic and clinical effects of the device should not be inferior to established methods for cardiopulmonary resuscitation.
Impella LP 2.5 in cardiac arrest
To our knowledge, the Impella LP 2.5 is the only percutaneous LVAD that has been studied during cardiac arrest. Experimental data from pigs have demonstrated the ability of this device to maintain cerebral and myocardial perfusion for a limited period of VF, and results were improved with intravenous fluid loading [16].
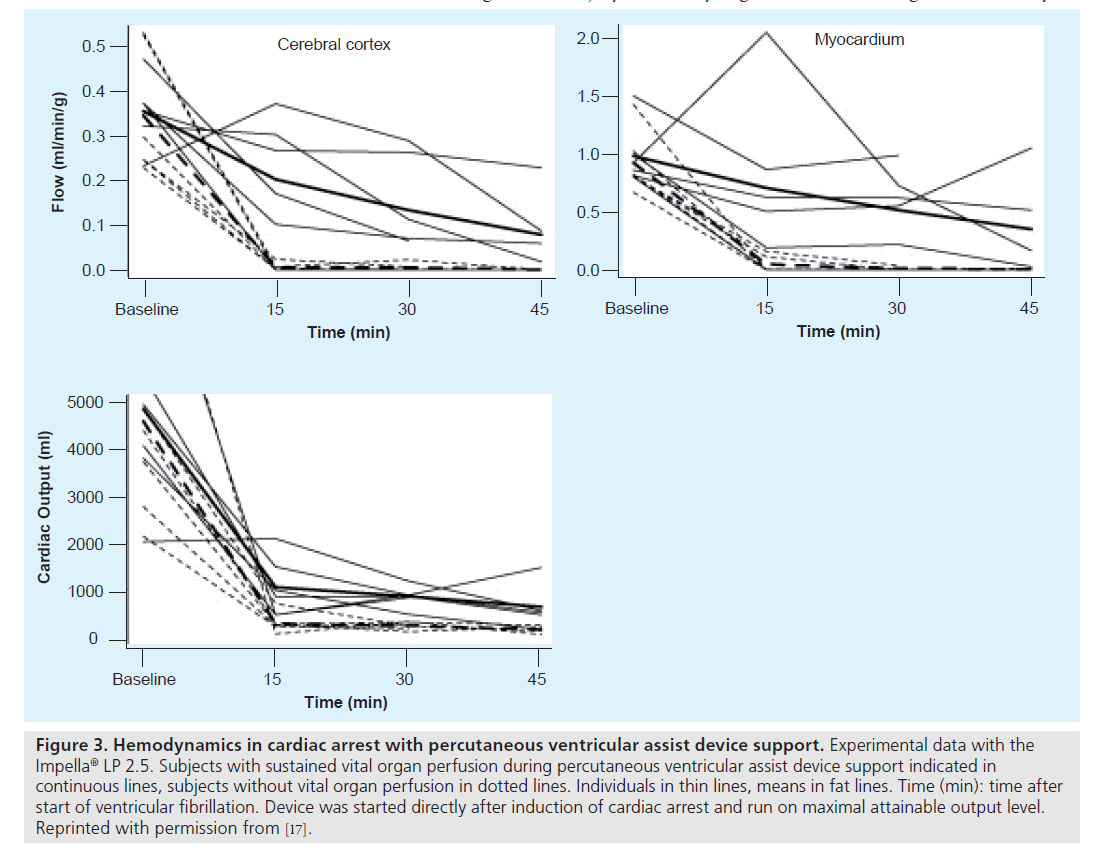
Using the LP 2.5 together with fluid loading, CBF by microspheres could be consistently maintained above 60% of baseline values for a limited period and flow levels above 40% were sustained up to 45 min after onset of VF (Figure 3) [17].
Figure 3. Hemodynamics in cardiac arrest with percutaneous ventricular assist device support. Experimental data with the Impella® LP 2.5. Subjects with sustained vital organ perfusion during percutaneous ventricular assist device support indicated in continuous lines, subjects without vital organ perfusion in dotted lines. Individuals in thin lines, means in fat lines. Time (min): time after start of ventricular fibrillation. Device was started directly after induction of cardiac arrest and run on maximal attainable output level. Reprinted with permission from [17].
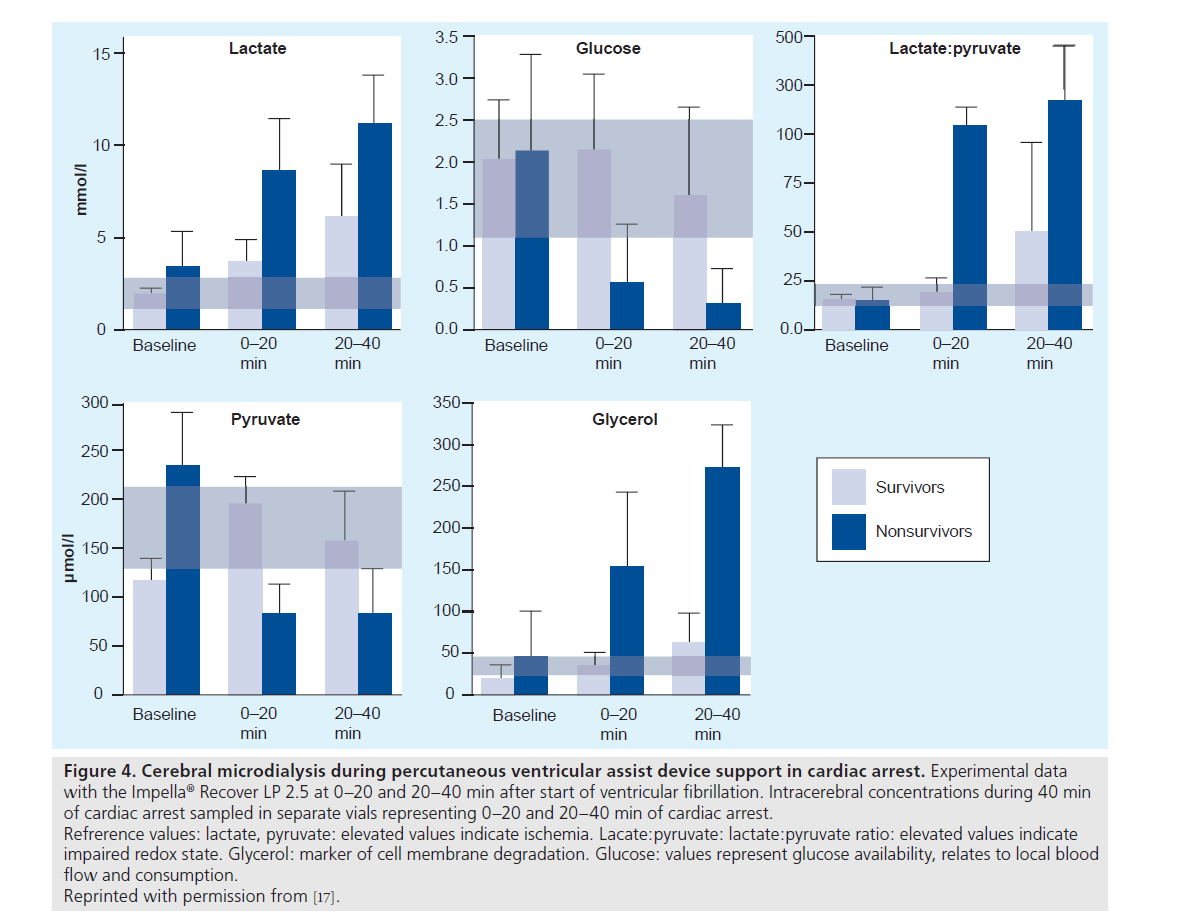
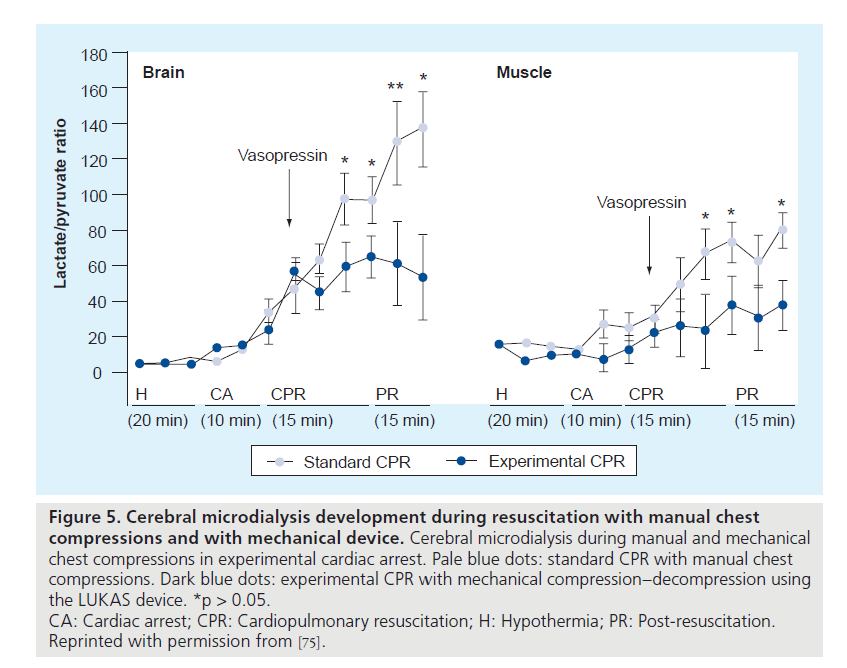
During VF, cerebral ischemia was assessed with cerebral microdialysis. Measurements of metabolic markers of hypoxia and injury in the brain indicated that cerebral injury was avoided for 20 to 40 min of cardiac arrest (Figure 4) [17]. In this study, brain injury and cerebral perfusion were reliably identified by end-tidal CO2 monitoring via the ventilator, allowing for the direct and continuous assessment of cerebral and systemic hemodynamics in a clinical situation with PVAD-assisted cardiac arrest [17]. Moreover, data were favorable when compared with conventional resuscitation with chest compressions and vasopressor therapy (Figure 5) [75].
Figure 4. Cerebral microdialysis during percutaneous ventricular assist device support in cardiac arrest. Experimental data with the Impella® Recover LP 2.5 at 0–20 and 20–40 min after start of ventricular fibrillation. Intracerebral concentrations during 40 min of cardiac arrest sampled in separate vials representing 0–20 and 20–40 min of cardiac arrest. Refrerence values: lactate, pyruvate: elevated values indicate ischemia. Lacate:pyruvate: lactate:pyruvate ratio: elevated values indicate impaired redox state. Glycerol: marker of cell membrane degradation. Glucose: values represent glucose availability, relates to local blood flow and consumption.
Reprinted with permission from [17].
Figure 5. Cerebral microdialysis development during resuscitation with manual chest compressions and with mechanical device. Cerebral microdialysis during manual and mechanical chest compressions in experimental cardiac arrest. Pale blue dots: standard CPR with manual chest compressions. Dark blue dots: experimental CPR with mechanical compression–decompression using the LUKAS device. *p > 0.05.
CA: Cardiac arrest; CPR: Cardiopulmonary resuscitation; H: Hypothermia; PR: Post-resuscitation.
Reprinted with permission from [75].
In addition, in a recent experimental study in a porcine model, cerebral perfusion, hemodynamics and rates of successful defibrillation were found to be comparable to optimal manual resuscitation with open chest cardiac massage, and no added benefit was found with the larger Impella LP 5.0 during 15 min of ischemic VF [69].
Although initial experimental findings suggest that percutaneous LVAD support sustains vital organ perfusion during cardiac arrest, previous studies have indicated only a limited clinical effect of LVAD support, despite initially promising hemodynamic data. Current experimental data from percutaneous LVAD support during VF should obviously be interpreted with caution based on these previous experiences.
In clinical use, the larger Impella LP 5.0, of similar design with maximum output of 5 l per minute, is often preferred to the smaller LP 2.5 owing to its higher delivery and increased mechanical stability. However, the LP 5.0 is more bulky and requires surgical cut-down for arterial access, thus implantation can be somewhat more complicated than for the true percutaneous version. Once inserted, the larger device can achieve higher systemic blood delivery and could have the potential to improve hemodynamics compared with the LP 2.5. However, the device requires sufficient filling from the right side in order to achieve its output potential and efficacy may be limited by poor filling of the left ventricle. Experimental data indicate this may be a particular issue during VF where sufficient circulatory blood volumes cannot be obtained [69].
Cerebral injury during circulatory support
Cerebral hypoperfusion may be present during LVAD support, but severe neurological impairment is not common during long-term use in stabilized patients [43,76,77].
During cardiopulmonary bypass, CBF and cerebral perfusion pressure (CPP) are related to device output [78]. Furthermore, blood oxygenation, hemoglobin levels, arterial microembolization and anesthetic use have been demonstrated to affect the risk of cerebral injury [79–82]. Vasopressor therapy, inotropes and blood transfusions may be useful in preventing cerebral ischemia during controlled cardioplegia and in heart failure, but may be counterproductive in the case of treatment for acute circulatory collapse [32–34,83,84]. Intra-arterial device therapy can theoretically be complicated by the introduction of air and thrombi, which may embolize into the cerebral circulation and contribute to brain injury. The devices may further cause blood clot activation and thrombus dislodgement owing to blood flow turbulence from the pumps. In addition, mechanical fragmentation of aortic plaque material, and also leakage of air via the intravascular catheters, can occur during use. With current surgical heart assist technologies and adequate anticoagulation, the risk of clinically relevant embolization has been minimized [85,86]. It is likely that with cautious implantation and adequate anticoagulation, the risk of cerebral damage caused by PVAD systems may be comparable with conventional cardiac catheterization procedures [87].
Assessment of cerebral injury
Clinical data of cerebral damage during PVAD support have not been reported. Generally, examination of cerebral perfusion and metabolism can involve complicated and invasive procedures and may in some cases be in conflict with guidelines for accepted intervention. The experimental setting allows for controlled hemodynamic intervention, and experimental models make feasible more sophisticated monitoring of cerebral circulation, metabolism and injury. Animal models include the possibility for direct intracardiac and intravascular monitoring and sampling, and also tissue flow measured with labeled microsphere injections, which is considered a gold standard approach for the assessment of tissue perfusion. Analysis requires harvesting of tissue for photometroscopic analysis, which complicates the use of this method in human subjects [88,89].
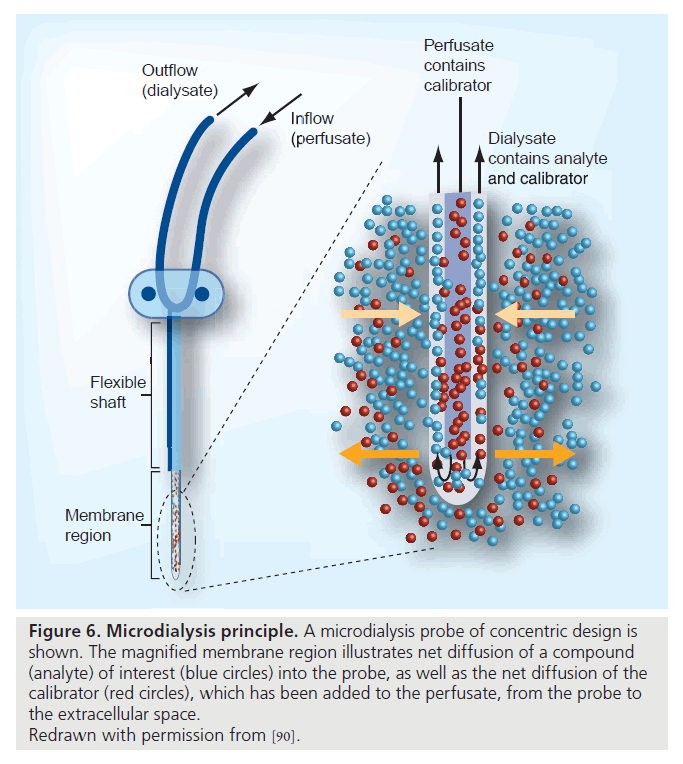
The newly developed cerebral microdialysis represents a method for the direct assessment of cerebral metabolism and redox state and is related to cerebral perfusion and to neurological injury [90–92]. With this technique, a miniature intracerebral dialysis catheter implanted via a cranial burr hole allows for continuous sampling of intracerebral molecules (Figure 6). Intracerebral dialysate can be analyzed for metabolic markers during clinical and experimental cerebral ischemia or injury. The method has been validated clinically and experimentally and is established in clinical use for monitoring of patients with cerebral injury [93–97]. Available data include reports from cerebral trauma, intracranial hemorrhage, cardioplegia and resuscitated cardiac arrest [17,98,99]. During cerebral hypoperfusion, markers of ischemia or injury can be sampled and analyzed continuously, and changes in the metabolic status of the cerebral cortex can be detected with little delay and followed over time. It is likely that this technique will be useful in future studies of cerebral status in hemodynamically compromised patients.
Figure 6. Microdialysis principle. A microdialysis probe of concentric design is shown. The magnified membrane region illustrates net diffusion of a compound (analyte) of interest (blue circles) into the probe, as well as the net diffusion of the calibrator (red circles), which has been added to the perfusate, from the probe to the extracellular space.
Redrawn with permission from [90].
Various other tools for monitoring of cerebral circulation are available in the acute clinical setting. CBF can be estimated at the bedside with transcranial Doppler flow measurements [100,101]. CBF values of 20–40% of normal baseline values have been suggested to be sufficient for prevention of acute cerebral injury [102,103]. CPP can be monitored using intracranial pressure transducers and arterial pressures to guide clinical treatment, although the minimal CPP values indicative of sustained cerebral perfusion have not been definitively resolved [104–106].
End-tidal CO2 values from the ventilator have been demonstrated to be associated with CBF and outcomes after cardiac arrest. In patients where intrinsic cardiac function is insufficient to sustain circulation, end-tidal CO2 values can be used for continuous assessment of the effect of hemodynamic support and of prognosis [107,108]. Generally, higher values are associated with nearnormal systemic and cerebral circulation, whereas very low values can be used to predict death in prolonged resuscitation [109–111]. Similar correlations have been found in experimental studies of percutaneous LVAD support during VF [17].
Recently, brain-specific peptides released into the bloodstream have been demonstrated to correlate with the extent of neurological damage and with prognosis for patients with brain injury, and could be useful as an indirect measure of cerebral circulatory status [112–114]. However, current data assessing neuron-specific enolase during LVAD support without cerebral ischemia found results to be unspecific and interpretation unclear in the presence of concomitant cardiac disease [115].
Blood flow to the head can be indirectly assessed with sidestream dark-field analysis [42], which permits direct and continuous assessment of mirocirculation in the tongue. The method is easy to use and attractive in clinical use but has not been validated with regards to cerebral injury or neurological injury after cerebral hypoperfusion.
Future clinical studies should be designed to assess both cerebral perfusion and injury during the acute treatment phase, and to detect and quantify neurological injury in survivors. The use of transcranial Doppler flow, infrared technology, neuropeptide markers and cereberal microdialysis may all give rapid information with regards to cerebral circulatory and metabolic state. In stabilized patients, neurological testing can be supplemented with CT, MRI and PET-scan imaging for evaluating brain injury and dysfunction [95].
Conclusion
Recently developed PVAD technology improves hemodynamics and tissue perfusion as indicated by clinical and experimental studies, but a mortality benefit has not been found.
Experimental data suggest the Impella LP 2.5 device may be able to sustain cerebral perfusion in cardiac arrest, and that systemic circulation and cerebral perfusion can be monitored via end-tidal CO2. Cerebral injury is a major limitation of outcomes after resuscitation and these recent findings indicate PVAD support may be of benefit in this setting.
The available data indicate a possible role for PVAD therapy for prevention of brain injury in cardiac arrest. Further studies are required to elucidate the potential of PVAD support to prevent cerebral hypoperfusion compared with conventional CPR in the clinical setting.
Future perspective
Percutaneous ventricular assist devices represent a future potential for improving outcomes in patients with critical cerebral hypoperfusion secondary to acute heart failure or cardiac arrest. The use of Impella support during coronary revascularization in cardiac arrest may be of particular advantage as revascularization could be performed without concomitant chest compressions and without the use of vasopressor drugs. If pulse-generating rhythm is restored, the PVAD may have a beneficial effect on myocardial oxygen consumption during the recovery phase. Investigation of the effect of PVAD therapy for preventing cerebral ischemia in different clinical scenarios should be the scope of future clinical studies.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Cerebral ischemia in cardiac collapse
▪ Brain injury limits outcomes after successful resuscitation; novel therapeutic approaches may be warranted.
Percutaneous ventricular assist device therapy in acute cardiac collapse
▪ Allows safe and rapid hemodynamic support in cardiogenic shock and high-risk percutaneous coronary intervention, and may have the potential to improve outcomes compared with more complicated surgical devices.
Cerbral blood flow with percutaneous ventricular assist devices in clinical use
▪ There are no clinical reports on cerebrovascular complications. Percutaneous ventricular assist devices (PVADs) may improve microcirculation in the head in cardiogenic shock.
Prevention of cerebral ischemia with PVADs
▪ The Impella® LP 2.5 device can maintain cerebral perfusion at a clinically relevant level during experimental cardiac arrest. Cerebral ischemia assessed with cerebral microdialysis may be avoided during the initial 20 to 40 min of ventricular fibrillation.
Conclusion
▪ PVAD therapy may represent a promising strategy for improving outcomes after cardiac arrest and severe hemodynamic compromise.
▪ Adjunctive treatment, including fluid loading, may have the potential to further enhance the effect of PVADs.
▪ Clinical trials should be performed to further investigate the potential of PVAD therapy in preventing cerebral ischemia.
References
Papers of special note have been highlighted as:
▪ of interest
- Birks EJ, Tansley PD, Hardy J et al.: Left ventricular assist device and drug therapy for the reversal of heart failure. N. Engl. J. Med. 355, 1873–1884 (2006).
- Rogers JG, Butler J, Lansman SL et al.: INTrEPID Investigators: chronic mechanical circulatory support for inotropedependent heart failure patients who are not transplant candidates: results of the INTrEPID Trial. J. Am. Coll. Cardiol. 50(8), 741–747 (2007).
- DeBakey M: The odyssey of the artificial heart. Artif. Organs 24, 405 (2000).
- Miller LW, Pagani FD, Russell SD et al.: HeartMate II Clinical Investigators. Use of a continuous-flow device in patients awaiting heart transplantation. N. Engl. J. Med. 357(9), 885–896 (2007).
- Dandel M, Weng Y, Siniawski H, Potapov E, Lehmkuhl HB, Hetzer R: Long-term results in patients with idiopathic dilated cardiomyopathy after weaning from left ventricular assist devices. Circulation 12(Suppl. 9), I37–I45 (2005).
- Murphy GS, Hessel EA 2nd, Groom RC: Optimal perfusion during cardiopulmonary bypass: an evidence-based approach. Anesth. Analg. 108(5), 1394–1417 (2009).
- Thiele H, Smalling RW, Schuler GC: Percutaneous left ventricular assist devices in acute myocardial infarction complicated by cardiogenic shock. Eur. Heart J. 28(17), 2057–2063 (2007).
- Seyfarth M, Sibbing D, Bauer I et al.: A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J. Am. Coll. Cardiol. 52(19), 1584–1588 (2008).
- Burkhoff D, Cohen H, Brunckhorst C, O’Neill WW; TandemHeart Investigators Group: A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart® percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am. Heart J. 152(3), 469 (2006).
- Vranckx P, Schultz CJ, Valgimigli M et al.: Assisted circulation using the TandemHeart® during very high-risk percutaneous coronary intervention of the unprotected left main coronary artery in patients declined for CABG. Catheter. Cardiovasc. Interv. 74(2), 302–310 (2009).
- Dixon SR, Henriques JP, Mauri L et al.: A prospective feasibility trial investigating the use of the Impella 2.5 system in patients undergoing high-risk percutaneous coronary intervention (The PROTECT I Trial): initial U.S. experience. JACC Cardiovasc. Interv. 2(2), 91–96 (2009).
- Samoukovic G, Rosu C, Giannetti N, Cecere R: The Impella LP 5.0 as a bridge to long-term circulatory support. Interact. Cardiovasc. Thorac. Surg. 8(6), 682–683 (2009).
- Cheng JM, den Uil CA, Hoeks SE et al.: Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: a meta‑analysis of controlled trials. Eur. Heart J. 30(17), 2102–2108 (2009).
- Henriques JP, Remmelink M, Baan J Jr et al.: Safety and feasibility of elective high-risk percutaneous coronary intervention procedures with left ventricular support of the Impella Recover LP 2.5. Am. J. Cardiol. 97(7), 990–992 (2006).
- Sjauw KD, Remmelink M, Baan J Jr et al.: Left ventricular unloading in acute ST-segment elevation myocardial infarction patients is safe and feasible and provides acute and sustained left ventricular recovery. J. Am. Coll. Cardiol. 51(10), 1044–1046 (2008).
- Tuseth V, Salem M, Pettersen R et al.: Percutaneous left ventricular assist in ischemic cardiac arrest. Crit. Care Med. 37(4), 1365–1372 (2009).
- Tuseth V, Pettersen RJ, Epstein A et al.: Percutaneous left ventricular assist device can prevent acute cerebral ischaemia during ventricular fibrillation. Resuscitation 80(10), 1197–1203 (2009).
- Goldberg RJ, Gore JM, Alpert JS et al.: Cardiogenic shock after acute myocardial infarction. Incidence and mortality from a community-wide perspective, 1975 to 1988. N. Engl. J. Med. 1117–1122 (1991).
- Babaev A, Frederick PD, Pasta DJ, Every N, Sichrovsky T, Hochman JS: Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. J. Am. Med. Assoc. 294, 448–454 (2005).
- Hochman JS, Sleeper LA, Webb JG et al.: Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock? N. Engl. J. Med. 341(9), 625–634 (1999).
- Reynolds HR, Hochman JS: Cardiogenic shock, current concepts and improving outcomes. Circulation 117, 686–697 (2008).
- Den Uil CA, Lagrand WK, Valk SDA, Spronk PE, Simoons ML: Management of cardiogenic shock: focus on tissue perfusion. Curr. Probl. Cardiol. 34, 323–350 (2009).
- Logeart D, Tabet JY, Hittinger L et al.: Transient worsening of renal function during hospitalization for acute heart failure alters outcome. Int. J. Cardiol. 127, 228–232 (2008).
- De Backer D, Creteur J, Dubois MJ et al.: Microvascular alterations in patients with acute severe heart failure and cardiogenic shock. Am. Heart J. 147, 91–99 (2004).
- Schneider A, Böttiger BW, Popp E: Cerebral resuscitation after cardiocirculatory arrest. Anesth. Analg. 108(3), 971–979 (2009).
- Hosmane VR, Mustafa NG, Reddy VK et al.: Survival and neurologic recovery in patients with ST-segment elevation myocardial infarction resuscitated from cardiac arrest. J. Am. Coll. Cardiol. 53(5), 409–415 (2009).
- Davis DP: Cardiocerebral resuscitation: a broader perspective. J. Am. Coll. Cardiol. 53(2), 158–160 (2009).
- Ewy GA, Kern KB: Recent advances in cardiopulmonary resuscitation: cardiocerebral resuscitation. J. Am. Coll. Cardiol. 53(2), 149–157 (2009).
- Martin GB, Rivers EP, Paradis NA, Goetting MG, Morris DC, Nowak RM: Emergency department cardiopulmonary bypass in the treatment of human cardiac arrest. Chest 113(3), 743–751 (1998).
- Gueugniaud PY, David JS, Chanzy E et al.: Vasopressin and epinephrine vs. epinephrine alone in cardiopulmonary resuscitation. N. Engl. J. Med. 359(1), 21–30 (2008).
- Yannopoulos D, Nadkarni VM, McKnite SH et al.: Intrathoracic pressure regulator during continuous-chest-compression advanced cardiac resuscitation improves vital organ perfusion pressures in a porcine model of cardiac arrest. Circulation 112(6), 803–811 (2005).
- Ristagno G, Tang W, Huang L et al.: Epinephrine reduces cerebral perfusion during cardiopulmonary resuscitation. Crit. Care Med. 37, 1408–1415 (2009).
- Tang W, Weil MH, Sun S: Epinephrine increases the severity of postresuscitation myocardial dysfunction. Circulation 92, 3089–3093 (1995).
- Müller S, How OJ, Hermansen SE, Stenberg TA, Sager G, Myrmel T: Vasopressin impairs brain, heart and kidney perfusion: an experimental study in pigs after transient myocardial ischemia. Crit. Care 12(1), R20 (2008).
- Berg RA, Otto CW, Kern KB et al.: High-dose epinephrine results in greater early mortality after resuscitation from prolonged cardiac arrest in pigs: a prospective, randomized study. Crit. Care Med. 22, 282–290 (1994).
- Sjauw KD, Engström AE, Vis MM et al.: A systematic review and meta‑analysis of intra-aortic balloon pump therapy in ST‑elevation myocardial infarction: should we change the guidelines? Eur. Heart J. 30(4), 459–468 (2009).
- Wohlschlaeger J, Schmitz KJ, Schmid C et al.: Reverse remodeling following insertion of left ventricular assist devices (LVAD): a review of the morphological and molecular changes. Cardiovasc. Res. 68(3), 376–386 (2005).
- Goldstein AH, Monreal G, Kambara A et al.: Partial support with a centrifugal left ventricular assist device reduces myocardial oxygen consumption in chronic, ischemic heart failure. J. Card. Fail. 11(2), 142–151 (2005).
- Eya K, Tuzun E, Conger J et al.: Effect of pump flow mode of novel left ventricular assist device upon end organ perfusion in dogs with doxorubicin induced heart failure. ASAIO J. 51(1), 41–49 (2005).
- Konstam MA, Czerska B, Böhm M et al.: Continuous aortic flow augmentation: a pilot study of hemodynamic and renal responses to a novel percutaneous intervention in decompensated heart failure. Circulation 112(20), 3107–3114 (2005).
- Onorati F, Santarpino G, Rubino AS et al.: Body perfusion during adult cardiopulmonary bypass is improved by pulsatile flow with intra-aortic balloon pump. Int. J. Artif. Organs. 32(1), 50–61 (2009).
- Sanui M, King DR, Feinstein AJ, Varon AJ, Cohn SM, Proctor KG: Effects of arginine vasopressin during resuscitation from hemorrhagic hypotension after traumatic brain injury. Crit. Care Med. 34(2), 433–438 (2006).
- Deem S, Hurford WE: Respiratory controversies in the critical care setting: should all patients be treated with hypothermia following cardiac arrest? Respir. Care 52(4), 443–450 (2007).
- Lam K, Sjauw KD, Henriques JP, Ince C, de Mol BA: Improved microcirculation in patients with an acute ST‑elevation myocardial infarction treated with the Impella LP2.5 percutaneous left ventricular assist device. Clin. Res. Cardiol. 98(5), 311–318 (2009).
- Lietz K, Brown K, Ali SS et al.: The role of cerebral hyperperfusion in postoperative neurologic dysfunction after left ventricular assist device implantation for end-stage heart failure. J. Thorac. Cardiovasc. Surg. 137(4), 1012–1019 (2009).
- Samoukovic G, Al-Atassi T, Rosu C, Giannetti N, Cecere R: Successful treatment of heart failure due to acute transplant rejection with the Impella® LP 5.0. Ann. Thorac. Surg. 88(1), 271–273 (2009).
- Maybaum S, Mancini D, Xydas S et al.: Cardiac improvement during mechanical circulatory support: a prospective multicenter study of the LVAD Working Group. Circulation 115(19), 2497–2505 (2007).
- Benedetto U, Luciani R, Goracci M et al.: Miniaturized cardiopulmonary bypass and acute kidney injury in coronary artery bypass graft surgery. Ann. Thorac. Surg. 88(2), 529–535 (2009).
- D’Alessandro C, Aubert S, Golmard JL et al.: Extra-corporeal membrane oxygenation temporary support for early graft failure after cardiac transplantation. Eur. J. Cardiothorac. Surg. (2009) (Epub ahead of print).
- Liu KS, Tsai FC, Huang YK et al.: Extracorporeal life support: a simple and effective weapon for postcardiotomy right ventricular failure. Artif. Organs 33(7), 504–508 (2009).
- Luo XJ, Wang W, Hu SS et al.: Extracorporeal membrane oxygenation for treatment of cardiac failure in adult patients. Interact. Cardiovasc. Thorac. Surg. 9(2), 296–300 (2009).
- Guenther U, Varelmann D, Putensen C, Wrigge H: Extended therapeutic hypothermia for several days during extracorporeal membrane-oxygenation after drowning and cardiac arrest. Two cases of survival with no neurological sequelae. Resuscitation 80(3), 379–381 (2009).
- Thiagarajan RR, Laussen PC, Rycus PT, Bartlett RH, Bratton SL: Extracorporeal membrane oxygenation to aid cardiopulmonary resuscitation in infants and children. Circulation 116(15), 1693–1700 (2007).
- Massetti M, Tasle M, Le Page O et al.: Back from irreversibility: extracorporeal life support for prolonged cardiac arrest. Ann. Thorac. Surg. 79(1), 178–183 (2005).
- Tanno K, Itoh Y, Takeyama Y, Nara S, Mori K, Asai Y: Utstein style study of cardiopulmonary bypass after cardiac arrest. Am. J. Emerg. Med. 26(6), 649–654 (2008).
- Mégarbane B, Leprince P, Deye N et al.: Emergency feasibility in medical intensive care unit of extracorporeal life support for refractory cardiac arrest. Intensive Care Med. 33(5), 758–764 (2007).
- Thiagarajan RR, Brogan TV, Scheurer MA, Laussen PC, Rycus PT, Bratton SL: Extracorporeal membrane oxygenation to support cardiopulmonary resuscitation in adults. Ann. Thorac. Surg. 87(3), 778–785 (2009).
- Idelchik GM, Simpson L, Civitello AB et al.: Use of the percutaneous left ventricular assist device in patients with severe refractory cardiogenic shock as a bridge to long-term left ventricular assist device implantation. J. Heart Lung Transplant 27(1), 106–111 (2008).
- Reitan O, Steen S, Ohlin H: Hemodynamic effects of a new percutaneous circulatory support device in a left ventricular failure model. ASAIO J. 49(6), 731–736 (2003).
- Gregoric ID, Jacob LP, La Francesca S et al.: The TandemHeart® as a bridge to a long-term axial-flow left ventricular assist device (bridge to bridge). Tex. Heart Inst. J. 35(2), 125–129 (2008).
- Remmelink M, Sjauw KD, Henriques JP et al.: Effects of left ventricular unloading by Impella® recover LP2.5 on coronary hemodynamics. Catheter Cardiovasc. Interv. 70(4), 532–537 (2007).
- Cook S, Windecker S: Percutaneous ventricular assist devices for cardiogenic shock. Curr. Heart Fail. Rep. 5(3), 163–169 (2008).
- Meyns B, Stolinski J, Leunens V, Verbeken E, Flameng W: Left ventricular support by catheter-mounted axial flow pump reduces infarct size. Am. Coll. Cardiol. 41(7), 1087–1095 (2003).
- Gimelli G, Wolff MR: Hemodynamically supported percutaneous coronary revascularization improves left ventricular function in patients with ischemic dilated cardiomyopathy at very high risk for surgery, a single-center experience. J. Invasive Cardiol. 20(12), 642–646 (2008).
- Valgimigli M, Steendijk P, Serruys PW et al.: Use of Impella Recover® LP 2.5 left ventricular assist device during high-risk percutaneous coronary interventions; clinical, haemodynamic and biochemical findings. EuroIntervention 2, 91–100 (2006).
- Vranckx P, Meliga E, De Jaegere PP, Van den Ent M, Regar ES, Serruys PW: The TandemHeart®, percutaneous transseptal left ventricular assist device: a safeguard in high-risk percutaneous coronary interventions. The six‑year Rotterdam experience. EuroIntervention 4(3), 331–337 (2008).
- Gregoric ID, Bruckner BA, Jacob L et al.: Techniques and complications of TandemHeart® ventricular assist device insertion during cardiac procedures. ASAIO J. 55(3), 251–254 (2009).
- Cheng JM, den Uil CA, Hoeks SE et al.: Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: a meta‑analysis of controlled trials. Eur. Heart J. 30(17), 2102–2108 (2009).
- Tuseth V, Pettersen RJ, Grong K et al.: Percutaneous left ventricular assist device comparable to open chest cardiac massage in ischemic cardiac arrest. EuroPCR (2009) (Abstract).
- Hosmane VR, Mustafa NG, Reddy VK et al.: Survival and neurologic recovery in patients with ST-segment elevation myocardial infarction resuscitated from cardiac arrest. J. Am. Coll. Cardiol. 53(5), 409–415 (2009).
- Niemann JT, Rosborough JP, Pelikan PC: Hemodynamic determinants of subdiaphragmatic venous return during closed-chest CPR in a canine cardiac arrest model. Ann. Emerg. Med. 19, 1232–1237 (1990).
- Sanders AB, Kern KB, Fonken S, Otto CW, Ewy GA: The role of bicarbonate and fluid loading in improving resuscitation from prolonged cardiac arrest with rapid manual chest compression CPR. Ann. Emerg. Med. 19, 1–7 (1990).
- Lottes AE, Rundell AE, Geddes LA, Kemeny AE, Otlewski MP, Babbs CF: Sustained abdominal compression during CPR raises coronary perfusion pressures as much as vasopressor drugs. Resuscitation 75(3), 515–524 (2007).
- Wenzel V, Lindner KH, Prengel AW, Strohmenger HU: Effect of phased chest and abdominal compressiondecompression cardiopulmonary resuscitation on myocardial and cerebral blood flow in pigs. Crit. Care Med. 28, 1107–1112 (2000).
- Bahlmann L, Klaus S, Baumeier W et al.: Brain metabolism during cardiopulmonary resuscitation assessed with microdialysis. Resuscitation 59(2), 255–260 (2003).
- Hogue CW Jr, Palin CA, Arrowsmith JE: Cardiopulmonary bypass management and neurologic outcomes: an evidence-based appraisal of current practices. Anesth. Analg. 103(1), 21–37 (2006).
- Taylor KM: Brain damage during cardiopulmonary bypass. Ann. Thorac. Surg. 65(Suppl. 4), S20–S26 (1998).
- Newman MF, Croughwell ND, White WD et al.: Effect of perfusion pressure on cerebral blood flow during normothermic cardiopulmonary bypass. Circulation 94(Suppl. 9), II353–II357 (1996).
- Hickey EJ, You X, Kaimaktchiev V, Ungerleider RM: Hypoxemic reperfusion exacerbates the neurological injury sustained during neonatal deep hypothermic circulatory arrest: a model of cyanotic surgical repair. Eur. J. Cardiothorac. Surg. 1(5), 906–914 (2007).
- Halstead JC, Wurm M, Meier DM et al.: Avoidance of hemodilution during selective cerebral perfusion enhances neurobehavioral outcome in a survival porcine model. Eur. J. Cardiothorac. Surg. 32(3), 514–520 (2007).
- Cook DJ, Orszulak TA, Daly RC, MacVeigh I: Minimum hematocrit for normothermic cardiopulmonary bypass in dogs. Circulation 96(Suppl. 9), II200–II204 (1997).
- Thoennissen NH, Allroggen A, Ritter M et al.: Influence of inflammation and pump dynamic on cerebral microembolization in patients with continuous-flow DeBakey LVAD. ASAIO J. 52(3), 243–247 (2006).
- Delle Karth G, Buberl A, Geppert A et al.: Hemodynamic effects of a continuous infusion of levosimendan in critically ill patients with cardiogenic shock requiring catecholamines. Acta Anaesthesiol. Scand. 47(10), 1251–1256 (2003).
- Dasta J, Mody SH, McLaughlin T et al.: Current management of anemia in critically ill patients: analysis of a database of 139 hospitals. Am. J. Ther. 15(5), 423–430 (2008).
- Sandner SE, Zimpfer D, Zrunek P et al.: Low molecular weight heparin as an alternative to unfractionated heparin in the immediate postoperative period after left ventricular assist device implantation. Artif. Organs 32(10), 819–822 (2008).
- John R, Kamdar F, Liao K et al.: Low thromboembolic risk for patients with the Heartmate II left ventricular assist device. Thorac. Cardiovasc. Surg. 136(5), 1318–1323 (2008).
- Hamon M, Gomes S, Oppenheim C et al.: Cerebral microembolism during cardiac catheterization and risk of acute brain injury: a prospective diffusion-weighted magnetic resonance imaging study. Stroke 37(8), 2035–2038 (2006).
- Prinzen FW, Glenny RW: Developments in non-radioactive microsphere techniques for blood flow measurement. Cardiovasc. Res. 28(10), 1467–1475 (1994).
- Kowallik P, Schulz R, Guth BD et al.: Measurement of regional myocardial blood flow with multiple colored microspheres. Circulation 83(3), 974–982 (1991).
- Chaurasia CS, Müller M, Bashaw ED et al.: AAPS-FDA workshop white paper: microdialysis principles, application and regulatory perspectives. J. Clin. Pharmacol. 47(5), 589–603 (2007).
- Frykholm P, Hillered L, Långström B et al.: Increase of interstitial glycerol reflects the degree of ischaemic brain damage: a PET and microdialysis study in a middle cerebral artery occlusion–reperfusion primate model. J. Neurol. Neurosurg. Psychiatry 71, 455–461 (2001).
- Frykholm P, Hillered L, Långström B, Persson L, Valtysson J, Enblad P: Relationship between cerebral blood flow and oxygen metabolism, and extracellular glucose and lactate concentrations during middle cerebral artery occlusion and reperfusion: a microdialysis and positron emission tomography study in nonhuman primates. J. Neurosurg. 102, 1076–1084 (2005).
- Peerdeman SM, Girbes AR, Polderman KH, Vandertop W: Changes in interstitial glycerol concentration in head-injuredpatients: correlation with clinical events. Intensive Care Med. 29, 1825–1828 (2003).
- Vespa PM, McArthur D, O’Phelan K et al.: Persistently low extracellular glucose correlates with poor outcome 6 months after human traumatic brain injury despite a lack of increased lactate: a microdialysis study. J. Cereb. Blood Flow Metab. 23, 865–877 (2003).
- Sarrafzadeh AS, Haux D, Lüdemann L et al.: Cerebral ischaemia in aneurysmal subarachnoid hemorrhage: a correlative microdialysis-PET study. Stroke 35, 638–643 (2004).
- Ståhl N, Ungerstedt U, Nordström CH: Brain energy metabolism during controlled reduction of cerebral perfusion pressure in severe head injuries. Intensive Care Med. 27, 1215–1223 (2001).
- Belli A, Sen J, Petzold A, Russo S, Kitchen N, Smith M: Metabolic failure precedes intracranial pressure rises in traumatic brain injury: a microdialysis study. Acta Neurochir. 150, 461–469 (2008).
- Nordmark J, Rubertsson S, Mörtberg E, Nilsson P, Enblad P: Intracerebral monitoring in comatose patients treated with hypothermia after a cardiac arrest. Acta Anaesthesiol. Scand. 53(3), 289–298 (2009).
- Salazar JD, Coleman RD, Griffith S et al.: Selective cerebral perfusion: real-time evidence of brain oxygen and energy metabolism preservation. Ann. Thorac. Surg. 88(1), 162–169 (2009).
- Schatlo B, Gläsker S, Zauner A, Thompson BG, Oldfield EH, Pluta RM: Continuous neuromonitoring using transcranial Doppler reflects blood flow during carbon dioxide challenge in primates with global cerebral ischemia. Neurosurgery 64(6), 1148–1154 (2009).
- Brauer P, Kochs E, Werner C et al.: Correlation of transcranial Doppler sonography mean flow velocity with cerebral blood flow in patients with intracranial pathology. J. Neurosurg. Anesthesiol. 10(2), 80–85 (1998).
- Baron JC: Perfusion thresholds in human cerebral ischaemia: historical perspective and therapeutic implications. Cerebrovasc. Dis. 11, 2–8 (2003).
- Powers WJ, Grubb RL Jr, Darriet D, Raichle ME: Cerebral blood flow and cerebral metabolic rate of oxygen requirements for cerebral function and viability in humans. J. Cereb. Blood Flow Metab. 5, 600–608 (1985).
- Johnston AJ, Steiner LA, Coles JP: Effect of cerebral perfusion pressure augmentation on regional oxygenation and metabolism after head injury. Crit. Care Med. 33, 189–195 (2005).
- Newman MF, Croughwell ND, White WD et al.: Effect of perfusion pressure on cerebral blood flow during normothermic cardiopulmonary bypass. Circulation 94, II353–II357 (1996).
- Haugen O, Farstad M, Lise Kvalheim V et al.: Mean arterial pressure about 40 mmHg during CPB is associated with cerebral ischaemia in piglets. Scand. Cardiovasc. J. 40, 54–61 (2006).
- Sanders AB, Kern KB, Otto CW, Milander MM, Ewy GA: End-tidal carbon dioxide monitoring during cardiopulmonary resuscitation. A prognostic indicator for survival. JAMA 262, 1347–1351 (1989).
- Gazmuri RJ, Kube E: Capnography during cardiac resuscitation: a clue on mechanisms and a guide to interventions. Crit. Care Med. 7, 411–412 (2003).
- Lewis LM, Stothert J, Standeven J, Chandel B, Kurtz M, Fortney J: Correlation of end-tidal CO2 to cerebral perfusion during CPR. Ann. Emerg. Med. 21, 1131–1134 (1992).
- Weil MH, Bisera J, Trevino RP, Rackow EC: Cardiac output and end-tidal carbon dioxide. Crit. Care Med. 13, 907–909 (1985).
- Wayne MA, Levine RL, Miller CC: Use of end-tidal carbon dioxide to predict outcome in prehospital cardiac arrest. N. Engl. J. Med. 337, 301–306 (1997).
- Chiaretti A, Barone G, Riccardi R et al.: NGF, DCX, and NSE upregulation correlates with severity and outcome of head trauma in children. Neurology 72(7), 609–616 (2009).
- Undén J, Strandberg K, Malm J et al.: Explorative investigation of biomarkers of brain damage and coagulation system activation in clinical stroke differentiation. J. Neurol. 56(1), 72–77 (2009).
- Tiainen M, Roine RO, Pettilä V, Takkunen O: Serum neuron-specific enolase and S-100B protein in cardiac arrest patients treated with hypothermia. Stroke 34(12), 2881–2886 (2003).
- Pfeifer R, Ferrari M, Börner A, Deufel T, Figulla HR: Serum concentration of NSE and S-100b during LVAD in non-resuscitated patients. Resuscitation 79(1), 46–53 (2008).
▪ Clinical assessment of TandemHeart®.
▪ Clinical assessment of Impella® LP 2.5.
▪ Assessment of cerebral ischemia during experimental cardiac arrest with Impella support.
▪ Clinical neurological outcomes post-resuscitation in ST-segment elevation myocardial infarction patients.