Review Article - Interventional Cardiology (2011) Volume 3, Issue 1
Safety of coronary angiography and percutaneous coronary intervention in patients on uninterrupted warfarin therapy: a meta-analysis
- Corresponding Author:
- Dushad Ram
Regions Hospital and the University of Minnesota Medical School, MN, USA
E-mail: dennis.w.zhu@healthpartners.com
Abstract
Keywords
angiography, bleeding, hematoma, warfarin
The optimal periprocedural management of anticoagulation in patients on chronic warfarin therapy undergoing coronary angiography (CA) and percutaneous coronary intervention (PCI) remains undetermined. Physicians must balance the periprocedural risks of bleeding and thromboembolism. Current practice involves the discontinuation of warfarin therapy prior to invasive cardiac procedures until the preprocedural international normalized ratio (INR) is less than 1.5 [1–3]. It has been demonstrated that the incidence of thromboembolism was higher in patients with prosthetic mechanical heart valve(s) or atrial fibrillation with moderate- or high-risk factors when oral anticoagulation was discontinued [3,4]. Bridging therapy with unfractionated heparin (UFH) or lowmolecular- weight heparin (LMWH) is often administered to reduce thromboembolic risk while oral anticoagulation is withheld. However, the safety of bridging therapy has been recently questioned, as it may result in an increased incidence of bleeding complications and prolonged hospitalizations [5–7]. An alternative approach for these patients, is to continue warfarin therapy without interruption in the periprocedural period. Current data regarding this approach are limited. We conducted a systematic review of the literature relating to continuation of warfarin therapy at the time of CA and PCI, in order to determine the bleeding risk of these patients.
Methods
▪ Literature search strategy
A search of a major electronic database was independently conducted by two reviewers (Imdad Ahmed and Chad M House). Reference lists and abstracts were searched by one reviewer (Imdad Ahmed). Medline (1950 to December 2009 week 4) and PubMed (1960 to December 2009 week 4) were searched using the following MeSH terms: warfarin, anticoagulants, coronary angiography, catheterization, bleeding, perioperative anticoagulation, heparin, thromboembolism, PTCA, coronary stenting and hematoma. Only English language literatures were searched. The ‘related articles’ feature was also used within PubMed to identify additional articles. Embase (1980–2009 week 4) was searched using warfarin, anticoagulants, coronary angiography, catheterization, bleeding, perioperative anticoagulation, heparin, thromboembolism, PTCA, coronary stenting and hematoma as MeSH terms. A search of the Cochrane library using ‘anticoagulants’ AND ‘angiography’/‘ca theterizations’/‘stenting’ as keywords was performed. ClinicalTrials.gov was searched using ‘anticoagulant’ as a keyword. Reference lists of relevant articles were manually searched.
▪ Study eligibility
Studies were included if they met the following criteria: were original articles; included patients who were undergoing coronary angiography/ catheterization with or without angioplasty/stent placement and were on uninterrupted warfarin therapy throughout the periprocedural period; and they reported the incidence of bleeding events and procedure-related complications.
The aim of the meta-analysis was to assess procedural-related complications, which are defined as access site bleeding delaying discharge, pseudoaneurysm, the need for corrective surgery and transfusion of blood products due to access site complications. We also evaluated combined major bleeding from other sites and procedural-related complications in all patients. Studies were excluded if proceduralrelated complications were not clearly described. Studies were also excluded if warfarin therapy was discontinued prior to the procedure or if warfarin was started after the procedure in any patients. Studies were excluded if complications between the continued warfarin groups and control groups (nonanticoagulated) were not clearly defined or distinctions were unclear. Reviews, comments, surveys, clinical guidelines, single case reports and letters to the editor were also excluded. Case series which met the above criteria, were included to calculate the bleeding event.
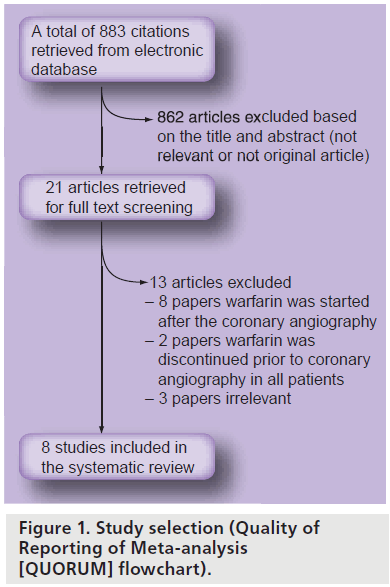
Studies selected for a full test review were evaluated by two independent reviewers (Imdad Ahmed and Chad M House) with disagreements resolved by consensus. A total of 883 citations were retrieved with the aforementioned search strategy. Based on the inclusion and exclusion criteria, a total of eight studies (one randomized, four cohort and three case series) were included for analysis.
▪ Study outcomes
Odds ratio (OR) was the measured outcome in studies with a comparison group and was expressed with a 95% CI. An OR less than 1.0 favors the warfarin uninterrupted group and an OR greater than 1.0 favors the control group. In the case series, the outcomes were expressed using a proportion with a 95% CI. An overall bleeding event rate was calculated using the rates from the warfarin groups from the cohort studies and the rates from case series. Although the OR is a better estimate of the effect of continued warfarin among cohort studies, we calculated the overall bleeding rate to get an overall estimate of bleeding event rate by including all studies. A heterogeneity test was also performed.
▪ Study quality assessment
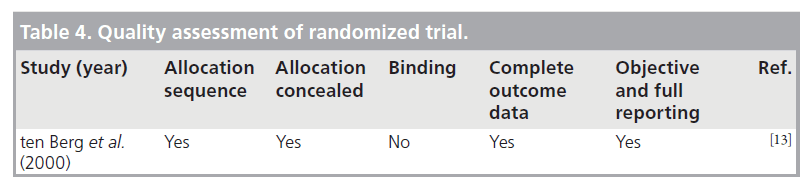
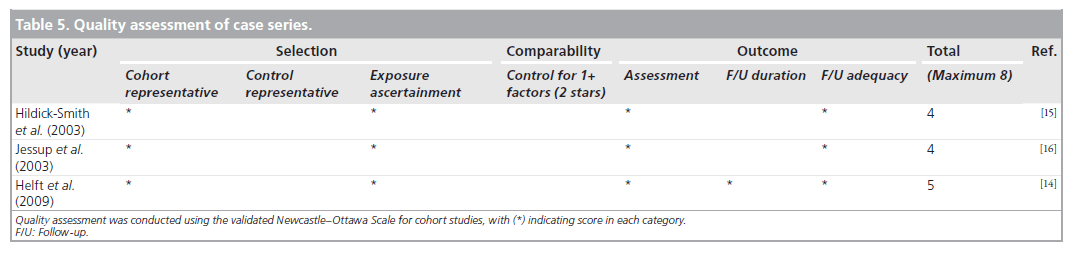
We performed a quality assessment using the validated Newcastle–Ottawa Scale (NOS) for cohort studies [8]. We used the NOS to perform quality assessment of the study by El-Jack et al., as this study was partially randomized and partially cohort [9]. NOS for cohort studies was also used to evaluate the quality of case series. To evaluate the randomized study, we used Cochrane Collaboration’s tool (Oxford, UK) for assessing risk of bias [101]. Disagreements were resolved through consensus.
▪ Data abstraction
Data abstraction was performed independently by two reviewers (Imdad Ahmed and Chad M House) using a standard form, with disagreement being resolved through consensus.
▪ Statistical analyses
Statistical analyses were performed using Review Manager 5.0 (Cochrane Collaboration) and Comprehensive Meta-Analysis Version 2 (Biostat, NJ, USA). Statistical pooling of ORs and proportions were calculated. Statistical heterogeneity beyond chance was evaluated using the I2 value. The more conservative random effects model was used for pooling of the outcomes. Between-study heterogeneity was analyzed by means of I2 = ([Q – df ] / Q) × 100%, where Q is the /2 statistic, and df represents its degrees of freedom. This describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance). A value greater than 50% may be considered substantial heterogeneity.
Results
▪ Literature search
We searched Medline, PubMed, EMBASE, ClinicalTrials.gov and the Cochrane library and yielded a total of 883 results. Of the 883 results, 862 were excluded based on title and abstract review. The remaining 21 articles were retrieved for independent full review. From these, eight were chosen to be included in the final review. Figure 1 shows the Quality of Reporting of Meta-analysis (QUORUM) flow chart, which provides a detailed description of study screening and reasons for exclusions.
▪ Study characteristics & quality assessment
We found three studies with cohort design [10–12], one study with a combination of randomized and cohort design [9], and one study with randomized design [13]. The remaining three studies were case series without any comparative groups [14–16].
Study details
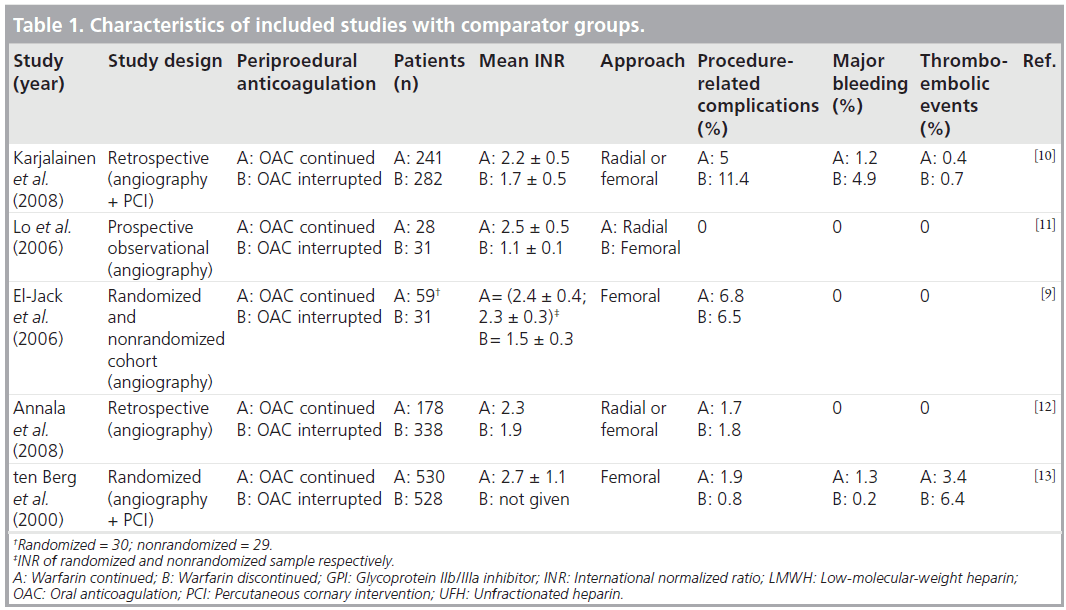
Tables 1 & 2 detail the studies included in the review. El-Jack et al. reported a randomized study along with a concurrent, consecutive cohort of nonrandomized patients who underwent coronary angiography while receiving therapeutic warfarin [9]. In those who were randomized, warfarin was discontinued 48 h before angiography. A total of 61 patients were randomized to include 30 in the continued-warfarin group and 31 in the discontinued-warfarin group. There were a total of 29 patients in the nonrandomized cohort group in which warfarin was continued. A total of 59 patients (randomized and nonrandomized) were included for analysis in continued warfarin group. All patients had a femoral approach. The mean INR was 2.4 ± 0.3 for the 59 patients who underwent coronary angiography while maintaining warfarin therapy. Three patients (10%) in the randomized continued-warfarin group and one patient (3%) in the nonrandomized cohort in the warfarin-continued group developed access site hematoma. Two patients (6%) in the warfarin- discontinued group developed hematoma. No major bleeding or thromboembolic events were reported.
In a retrospective study, Karjalainen et al. evaluated 523 patients on warfarin therapy undergoing coronary angiography and PCI (femoral or radial approach) [10]. Warfarin was continued in 241 patients and discontinued in 282 patients prior to the procedure. The mean INR in the warfarin- continuation group was 2.2 ± 0.5 and in the warfarin-discontinued group was 1.7 ± 0.5. Major bleeding occurred more often in the warfarindiscontinued group compared with the warfarincontinued group (5 vs 1.2%; p = 0.02). Access site complications occurred more frequently in the warfarin-discontinued group than the warfarincontinued group (11.3 vs 5%; p = 0.01). In multivariate analysis, femoral access, closure device and old age were the significant predictors for access site complications.
Lo et al., in a prospective observational study, recruited 59 patients of whom 28 were anticoagulated and 31 were nonanticoagulated patients [11]. Anticoagulated patients had a radial approach and control patients had a femoral approach. In the radial approach, the Seldinger technique was used to puncture the radial artery at the anticubital fossa and a 5-or 6-Fr sheath was inserted. The mean INR was 2.5 ± 0.5 for the warfarin-continued group and 1.1 ± 0.1 in the nonanticoagulated group. No thromboembolic or bleeding complications occurred in any of the cohorts.
Annala et al. retrospectively analyzed all consecutive patients (n = 258) on warfarin therapy who underwent coronary angiography and compared them with age- and gender-matched control groups (n = 258) [12]. The incidence of access site complications was 1.7% in the warfarincontinuation group and 2.5% in the warfarindiscontinued group with or without bridging therapy. There were 2% access site complications in the control group. In multivariate analysis, supratherapeutic INR (INR >3) remained the only significant predictor for access site and bleeding complications in the warfarin group.
ten Berg et al. conducted a prospective study to comparing the effects of aspirin alone and aspirin plus warfarin started before angioplasty with a target INR of 2.1–4.8 [13]. Major bleeding or false aneurysm formation (access site complications) within 30 days was reported in 3.2% of warfarintreated patients compared with only 1% in the aspirin-only group. However, all patients were given a high dose of heparin – 10,000 unit bolus plus infusion – during angioplasties performed via a femoral approach.
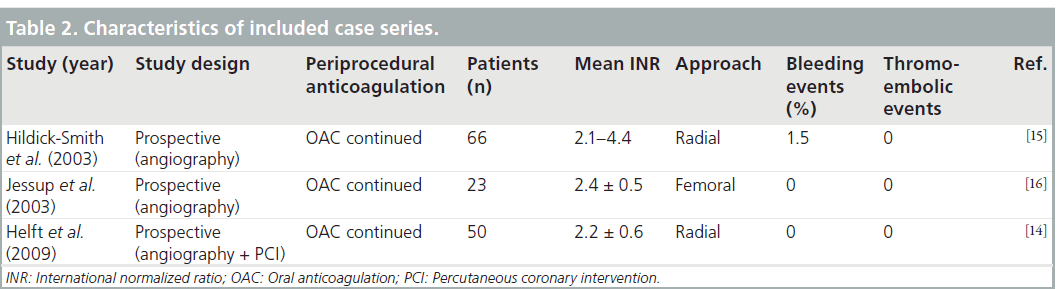
Helft et al. conducted a prospective observational study of 50 patients receiving warfarin who underwent PCI via right radial (94%) and left radial (6%) access [14]. Procedures were performed with an INR range of 1.4–3.4 with a mean of 2.2 ± 0.6. Dual antiplatelet pretreatment was prescribed at the discretion of the physician for 38 patients. In total, 10% of the patients received either LMWH or UFH, 12% received a glycoprotein inhibitor and 3% received both heparin and a GP inhibitor. No hemorrhagic or thrombotic complications were observed during hospitalization.
Hildick-Smith et al. reviewed 66 patients who underwent coronary angiography through radial approach and were anticoagulated with an INR greater than 2 and less than 4.5 [15]. One patient had a mild post-procedural hemorrhage.
Jessup et al. included 23 fully anticoagulated patients who underwent coronary angiography using a femoral approach [16]. Mean INR prior to the procedure was 2.4 ± 0.5. No patient had any occurrences of major or minor bleeding.
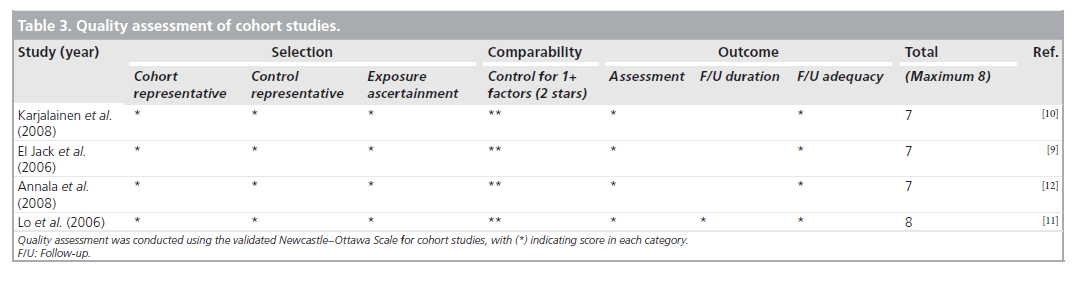
The three cohort studies were good quality, with seven to eight of the NOS criteria being satisfied. The study with the combined randomized and cohort study was also of good quality, with seven of the NOS criteria being satisfied. The case series were of poor quality and satisfied only four to five of the NOS criteria, as they lacked a comparator group. Agreement between reviewers regarding the NOS was moderate. The one randomized trial was of high quality. Tables 3–5 summarize the quality ratings for each study.
▪ Study outcomes
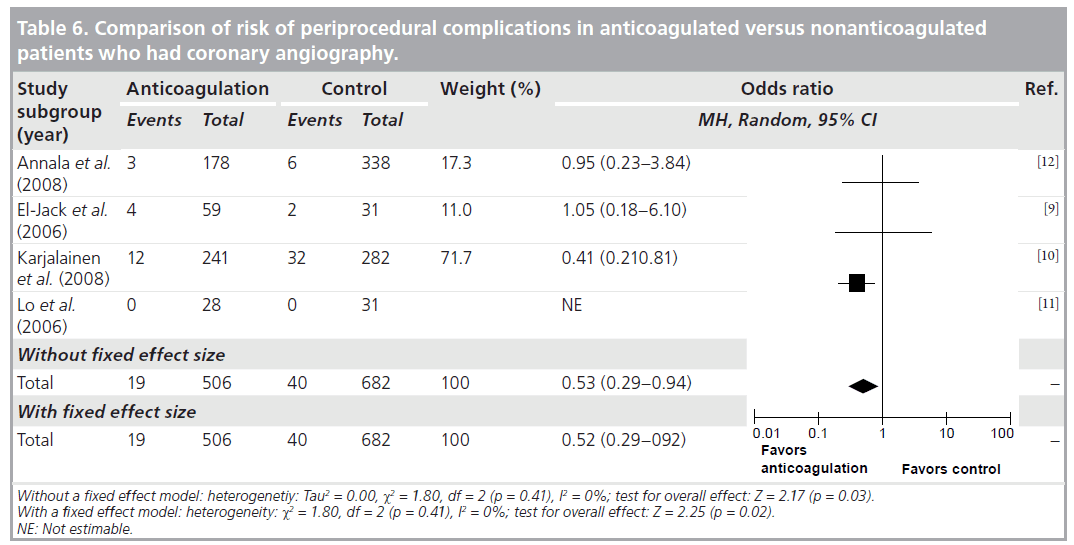
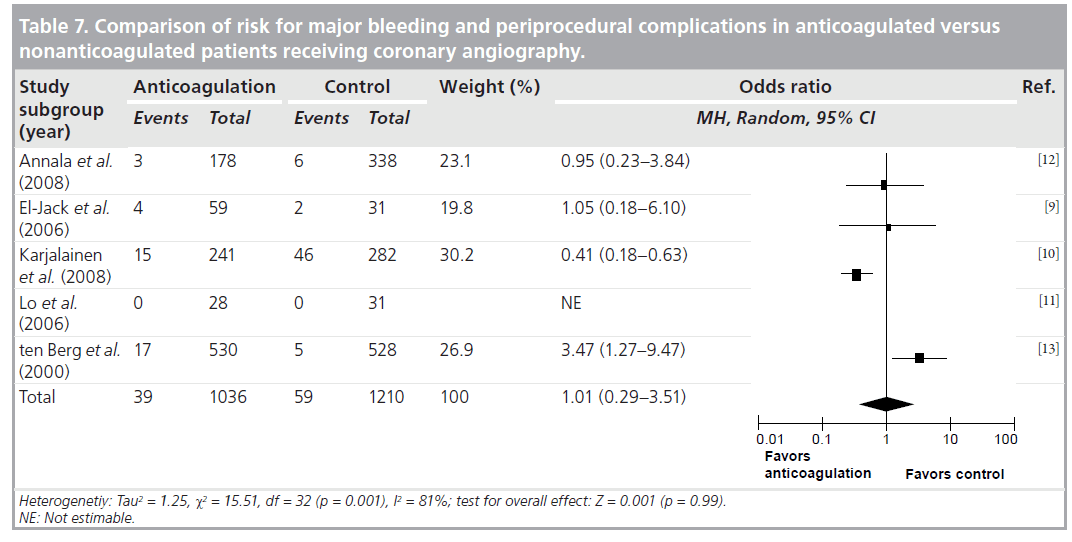
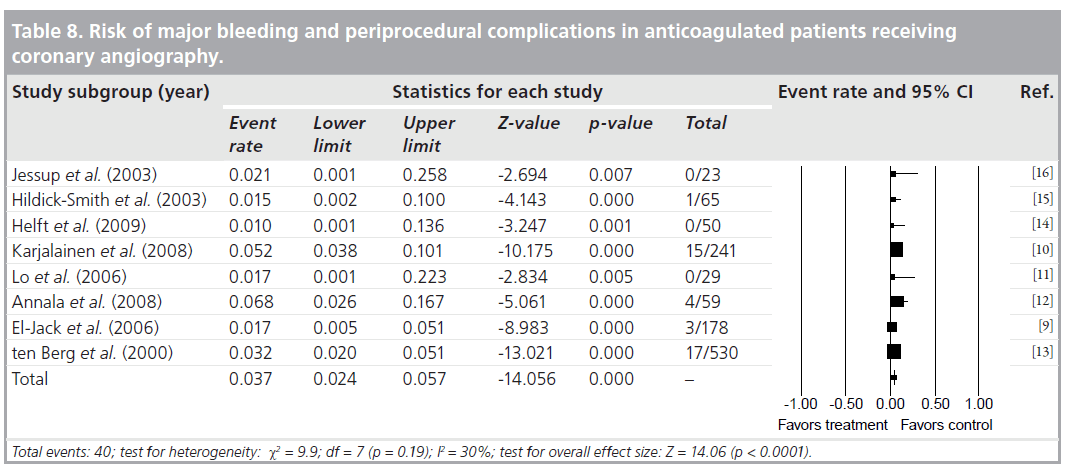
Agreement between the reviewers on the data abstraction forms was excellent. As demonstrated in Table 6, the pooled OR (95% CI) for procedure related complications in continued warfarin versus nonanticoagulated patients, in those studies that had comparison groups, was 0.53 (0.29–0.94). There was no significant heterogeneity for this outcome across this subgroup of included studies (I2 = 0%; p = 0.41). As demonstrated in Table 7, the pooled OR (95% CI) for combined major bleeding and procedure-related complication was 1.01 (0.29–3.51). However, there was significant heterogeneity (I2 = 81%; p = 0.001). To explore the heterogeneity present among the studies, we conducted an analysis between retrospective versus prospective studies, which revealed that all heterogeneity was due to retrospective studies, as prospective I2 = 25%. In Table 8, the pooled incidence (95% CI) of major bleeding and periprocedural complications for all studies was 0.037 (0.024–0.057). There was no significant heterogeneity (I2 = 30%; p = 0.19) for this outcome across all the studies.
Discussion
The main finding of our review is that uninterrupted warfarin therapy in patients undergoing CA, with or without PCI, does not increase the pooled incidence of major bleeding. Although the incidence of major bleeding and periprocedural complications was 1.7–6.8%, the majority of the bleeding events were self-limiting without major clinical consequences. We also analyzed the bleeding risk across all the studies and found no increased risk of bleeding events (OR: 0.037) in patients who were receiving uninterrupted warfarin therapy. Major bleeding events requiring blood transfusion were reported by Annala et al. and ten Berg et al. [12,13]. None of the bleeding events were fatal. A supratherapeutic INR was found to be a significant predictor of access site bleeding complications in the study by Annala et al., whereas Karjalainen et al. did not find any association between access site complications or major bleeding events with INR levels [10,12]. Femoral access, closure device and old age were independent predictors of access site complications in this study. In the subgroup analysis, Karjalainen et al. demonstrated that bridging therapy with LMWH was a significant predictor of access site complications [10]. All studies used either a femoral or radial approach except two case series by Helft et al. and Hildick-Smith et al., where only the radial approach was used [14,15]. Helft et al. and Hildick-Smith et al. showed that transradial coronary angiography is feasible and safe in fully anticoagulated patients [14,15]. In a prospective study of 56 patients by Ziakas et al., a radial approach was found to have fewer access site complications in patients who underwent PCI compared with femoral access [17]. In this study, when performing PCI, patients with an INR of 1.8 or higher were found to have a lower risk of access site complications with a radial approach compared with a femoral approach. However, the authors suggested that this finding should be validated in large-scale trials.
It is estimated that 5% of patients undergoing CA are on long-term warfarin therapy owing to atrial fibrillation or mechanical heart valves [18]. Interruption of warfarin therapy to perform CA may increase the risk of thromboembolic complications. Although bridging therapy is the recommended strategy for these patients [18], studies by MacDonald et al. and Annala et al. determined that bridging therapy was associated with an increased incidence of bleeding complications [12,19]. Morever, reinitiation of warfarin after angiography may result in a transient prothrombic state due to protein C and S suppression leading to more thromboembolic events [20].
We have performed a rigorous systematic review with clear a priori definitions for study eligibility, a validated quality assessment and a standard data abstraction form. These measures should reduce study selection bias and allow the most precise estimate of any effect of periprocedural continuation of oral anticoagulants on major bleeding or procedure-related complications. All the studies included had bleeding as an outcome event and, therefore, reporting bias is unlikely.
We also acknowledge potential limitations of our systematic review and meta-analysis. There were very few relevant studies and the relative sample sizes were small. In addition, study outcomes may have been affected by selection bias and from center-specific surgical techniques. There was no uniform definition of procedure-related complications in all of the studies. Bleeding events may have been influenced by concurrent antithrombotic therapy (UFH or LMWH) and glycoprotein IIb/IIIa inhibitor, and also a compression device. There are no randomized control trials with a large-scale population.
Conclusion
Continuing warfarin therapy during CA and PCI appears to be safe, with no increased incidence for major bleeding and periprocedural complications. However, the quality of evidence is low due to several limitations of the studies included in our review. A multicenter randomized trial is necessary to determine the optimal periprocedral strategy for patients on warfarin therapy undergoing CA and PCI.
Future perspective
Safety of coronary angiography with or without PCI in orally anticoagulated patients needs to be established in prospective randomized studies. Performing angiography in anticoagulated patients will save healthcare costs related to hospitalization and the administration of blood products or reversal agents to correct the INR. This will also prevent the delay in performing coronary angiography in those patients.
Executive summary
▪ Coronary angiography with or without percutaneous coronary intervention in orally anticoagulated patients appears to be safe without increased risk for major bleeding.
▪ Transradial coronary angiography is feasible and safe in anticoagulated patients.
▪ Performing coronary angiography in anticoagulated patients may reduce healthcare costs related to administration of blood products or reversal agents to correct international normalized ratio.
▪ A mutlicenter randomized study is necessary to determine the optimal periprocedural anticoagulation management in patients on chronic warfarin therapy undergoing coronary angiography.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Popma JJ, Bittl JA: Coronary angiographyand intravascular ultrasonography. In: HeartDisease: Textbook of Cardiovascular Medicine.(6th Edition). Braunwald E, Zipes DP,Libby P (Eds). WB Saunders, PA, USAP387–P421 (2001).
- Grossman W: Historical perspective andpresent practice of cardiac catheterization.In: Grossman’s Cardiac Catheterization,Anmgiography and Intervention (6th Edition). Baim D (Ed.). LippincottWilliams & Wilkins, PA, USA 1–8(2000).
- American College of Cardiology/AmericanHeart Association Task Force on PracticeGuidelines; Society of CardiovascularAnesthesiologists, Society for CardiovascularAngiography and Interventions; Society ofThoracic Surgeons; Bonow RO,Carabello BA, Kanu C et al.: ACC/AHA2006 guidelines for the management ofpatients with valvular heart disease: a reportof the American College of Cardiology/ American Heart Association Task Force onPractice Guidelines (writing committee torevise the 1998 Guidelines for theManagement of Patients With Valvular HeartDisease): developed in collaboration with theSociety of Cardiovascular Anesthesiologists:endorsed by the Society for CardiovascularAngiography and Interventions and theSociety of Thoracic Surgeons. Circulation114, E84–E231 (2006).
- Gohlke-Bärwolf C, Acar J, Oakley C et al.:Guidelines for prevention of thromboembolicevents in valvular heart disease. Study Group of the Working Group on Valvular HeartDisease of the European Society ofCardiology. Eur. Heart J. 16(10), 1320–1330(1995).
- Wazni OM, Beheiry S, Fahmy T et al.:Atrial fibrillation ablation in patients withtherapeutic international normalized ratio:comparison of strategies of anticoagulationmanagement in the periprocedural period.Circulation 116(22), 2531–2534 (2007).
- Kearon D, Hirch J: Management ofanticoagulation before and after electivesurgery. N. Engl. J. Med. 84, 478–4809(1997).
- Spandorfer JM, Lynch S, Weitz HH, Fertel S,Merli GJ: Use of enoxaparin for thechronically anticoagulated patient before andafter procedures. Am. J. Cardiol. 84(4),478–480 (1999).
- Wells G, Shea B, O’Connell D et al.: TheNewcastle–Ottawa Scale (NOS) for assessingquality of nonrandomized studies inmeta-analysis. Proceedings of the 3rdSymposium on Systematic Reviews. Beyond theBasic:Improving Quality and Impact. Oxford,UK, July 3–5 2000.
- El-Jack SS, Ruygrok PN, Webster MW et al.:Effectiveness of manual pressure hemostasisfollowing transfemoral coronary angiographyin patients on therapeutic warfarinanticoagulation. Am. J. Cardiol. 97(4),485–488 (2006).
- Karjalainen PP, Vikman S, Niemelä M et al.:Safety of percutaneous coronary interventionduring uninterrupted oral anticoagulanttreatment. Eur. Heart J. 29(8), 1001–1010(2008).
- Lo TS, Buch AN, Hall IR, Hildick-Smith DJ,Nolan J: Percutaneous left and right heartcatheterization in fully anticoagulatedpatients utilizing the radial artery andforearm vein: a two-center experience.J. Interv. Cardiol. 19(3), 258–263 (2006).
- Annala AP, Karjalainen PP, Porela P,Nyman K, Ylitalo A, Airaksinen KE: Safetyof diagnostic coronary angiography duringuninterrupted therapeutic warfarintreatment. Am. J. Cardiol. 102(4), 386–390(2008).
- ten Berg JM, Kelder JC, Suttorp MJ et al.:Effect of coumarins started before coronaryangioplasty on acute complications andlong-term follow-up: a randomized trial.Circulation 102(4), 386–391 (2000).
- Helft G, Dambrin G, Zaman A et al.:Percutaneous coronary intervention inanticoagulated patients via radial arteryaccess. Catheter Cardiovasc. Interv. 73(1),44–47 (2009).
- Hildick-Smith DJ, Walsh JT, Lowe MD,Petch MC: Coronary angiography in the fullyanticoagulated patient: the transradial route issuccessful and safe. Catheter Cardiovasc.Interv. 58(1), 8–10 (2003).
- Jessup DB, Coletti AT, Muhlestein JB,Barry WH, Shean FC, Whisenant BK:Elective coronary angiography andpercutaneous coronary intervention duringuninterrupted warfarin therapy. CatheterCardiovasc. Interv. 60(2), 180–184 (2003).
- Ziakas A, Koskinas KC, Gavrilidis S et al.:Radial versus femoral access for orallyanticoagulated patients. Catheter Cardiovasc.Interv. 76(4), 493–499 (2010).
- Helft G, Gilard M, Le Feuvre C, Zaman AG:Drug insight: antithrombotic therapy afterpercutaneous coronary intervention inpatients with an indication foranticoagulation. Nat. Clin. Pract. Cardiovasc.Med. 3, 673–680 (2006).
- MacDonald LA, Meyers S, Bennett CL et al.:Post-cardiac catheterization access sitecomplications and low molecular-weightheparin following cardiac catherization. J. Invasive Cardiol. 15, 60–62 (2003).
- Hirsh J, Dalen J, Anderson DR et al.: Oralanticoagulation:mechanism of action, clinicaleffectiveness, and optimal therapeutic range.Chest 119(Suppl.), 8S–21S (2001).
▪ Website
101 Higgins JPT, Green S (Eds). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 (2009) www.cochrane-handbook.org (Accessed 10 January 2010)