Research Article - Imaging in Medicine (2019) Volume 11, Issue 1
Saffron Extract Prevents Vancomycin-induced Nephrotoxicity
Maryam Jenabi1*, Ali Asghar Hemmati2, Katayoon Hafezi3 & Esrafil Mansouri41School of Pharmacy, Ahvaz Jundishapur University of Medical Sciences, Iran
2Department of Pharmacology, School of Pharmacy, Medicinal Plant Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
3Diabetes Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
4Department of Anatomical Sciences, Faculty of Medicine, Cellular and Molecular Research Center, Ahvaz Jundishapur University of Medical Sciences, Iran
- Corresponding Author:
- Maryam Jenabi
School of Pharmacy
Ahvaz Jundishapur University of Medical Sciences, Iran
E-mail: jenabi_maryam@yahoo.com
Abstract
Background: Vancomycin, a glycopeptide antibiotic, is commonly used against methicillin-resistant Gram-positive cocci. However, the nephrotoxic side-effects of Vancomycin results in dose restriction and reduces the duration of administration.
Objectives: The present study was designed to evaluate the protective effects of aqueous saffron (Crocus sativus) extract on vancomycin-induced nephrotoxicity in rats.
Materials and methods: In this study 32 adult male Wistar rats were randomly divided into 4 groups of 8 rats; (i) control (ii) saffron (80 mg/kg, I.P.), (iii) vancomycin (200 mg/kg/BD, I.P.) and (iv) vancomycin plus saffron. The saffron treatment began 24 hours before the administration of the vancomycin therapy and lasted 8 days, while the duration of the vancomycin therapy was 7 days. Serum creatinine and blood urea nitrogen (BUN), renal malondialdehyde (MDA) levels, superoxide dismutase (SOD) activity and histopathological changes of renal tissue were analyzed.
Results: Administration of vancomycin caused a significant increase in serum creatinine, BUN and renal MDA levels, whereas, SOD activity was decreased, when compared to the control group. Aqueous saffron extract, on the other hand, decreased serum creatinine, BUN concentration and renal MDA levels but increased the level of renal SOD activity. Substantial histopathological alterations including destruction of kidney tubules, interstitial edema, epithelia vacuolization and epithelial desquamation, were observed with the vancomycin-only treatment group. However, the administration of saffron extract resulted in a significant reduction of damage to the kidneys.
Conclusion: The present study demonstrated that oxidative stress plays an important role in vancomycin induced nephrotoxicity and saffron extract has a potent renoprotective effect against vancomycin induced lipid peroxidation and nephrotoxicity.
Keywords
vancomycin ■ nephrotoxicity ■ saffron extract
Background
Vancomycin is a glycopeptide antibiotic which is commonly used against methicillinresistant gram-positive cocci. In recent years, it has been used more frequently due to the increasing incidences of infections caused by methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis [1,2].
One major concern with vancomycin is its considerable nephrotoxicity. The incidence of vancomycin-induced nephrotoxicity has been reported to be between 5% and 35%, thus, restricting dose and duration of administration [3].
Although the exact mechanism of vancomycin is not fully understood, recent studies suggest that vancomycin has oxidative effects on the proximal renal tubule and production of reactive oxygen species (ROS) which causes changes in the energy dependent renal reabsorption and mitochondrial functions [2-8]. It has also been demonstrated that oxidative stress dysfunction is responsible for the tubular necrosis associated with vancomycin treatments. Therefore the use of antioxidants can be a potential therapeutic strategy to protect against renal damage caused by medications like vancomycin [8,9].
Medicinal herbs with anti-inflammatory constituents have been used to manage a number of inflammatory diseases. Grape seed extract and pomegranate are two examples of the many herbs that have been documented as effective for inflammatory diseases [10-12]. Crocus sativus L., commonly known as saffron, has traditionally been used as an antidepressant, anti-inflammatory, antioxidant, hypnotic agent, antispasmodic, expectorant, hepatoprotective agent and inducer of labor in folk medicine [13,14].
Recent studies have demonstrated that saffron has many pharmacological effects, such as, antioxidant, anti-tumor, antiplatelet, antiatherosclerosis, radical scavenger, anticonvulsant, genoprotective, and chemoprotective properties. In addition, saffron has been linked to improved learning behavior and elevating the oxygen diffusivity in the various tissues. The majority of research published on saffron has reported an acceptable safety profile. However, reports showed adverse reactions such as dry mouth, headaches, nausea [15-22]. Chemical studies have shown that saffron extract has crocin, crocetin and safranal constituents that are responsible for its pharmacological activity [23].
Several investigations have reported that saffron extract protects renal tissue from destruction caused by ischemia/reperfusion and nephrotoxicity induced by gentamicin and cisplatin [13,24-28]. This protective effect is mainly due to the anti-oxidative properties of saffron. However, this present study is the first one regarding the effect of saffron extract on vancomycin nephrotoxicity.
Objectives
This study was designed to evaluate the protective effects of aqueous saffron extract on vancomycin-induced nephrotoxicity in rats. The results of this study were then used to suggest a potential new strategy for preventing the nephrotoxicity of vancomycin.
Materials and Methods
The use of animals in the study and the experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of Ahvaz Jundishapur University of Medical Sciences. All efforts were made to minimize the number of animals used. Particular attention was given to animal cruelty and all measures were taken to ensure humane treatment of all the rats used in this study. Adult male Wistar rats (220-280 g) were purchased from the animal house and research center of the Jundishapur University of Medical Sciences, Ahvaz, Iran. They were all housed in the same room at a controlled temperature of 23 ± 2°C and were exposed to a 12 h light/dark cycle. All the rats were allowed access to food and water, ad libitum, for at least one week before experiments.
■ Preparation of aqueous extract of saffron
The saffron that was used in this experiment was supplied by Saharkhiz Saffron Co. The component of the saffron flower that was used for the formation of the herbal medicine was the stigma. The stigmas were air-dried in the shade and then powdered for 10 min with the use of a mortar and pestle. After grinding, 15 g of ground stigma was extracted with 400 ml of distilled water for 18 h in a Soxhlet extractor. The aqueous extract was concentrated to 100 ml with a rotary evaporator in low pressure, and then was dried at 35-40°C. The extract was kept at 2–8°C.
To prepare the solution for injection, the active ingredients in saffron were quantitatively determined using a high-performance liquid chromatography (HPLC). The powder was first weighed and dissolved in distilled water, then filtered through a 0.2 mm filter, and then immediately administered to the rats. The HPLC analyzed the saffron extract using a UV detector with wavelengths of 308 nm and 443 nm showed the presence of safranal, crocin and picrocrocin with a concentration of 0.520%, 94.98% and 3.01% respectively.
■Experimental design
Adult male Wistar rats were randomly divided into 4 groups of 8 rats: (i) control (ii) saffron (iii) vancomycin (iv) vancomycin plus saffron.
Aqueous saffron extract was prepared and given to both group 2 and 4 at a dose of 80 mg/kg per day, i.p in 24 h intervals for 8 days. This dosage scheme was reported to protect from marked nephrotoxicity caused by gentamicin and oxidative stress following renal ischemia-reperfusion injury in rats [13,28]. Vancomycin (500 mg, Exir Pharmaceutical Co.) was dissolved in water for injection and administrated to Group 3 and 4 at a dose of 200 mg/kg, i.p., twice daily for 7 days. Vancomycin treatment was started 1 day after the first administration of saffron. First, the saffron treatment was given at 8:00 am then an hour later, at 9:00 am, the first dose of vancomycin was administrated. The second dose of vancomycin was then administrated 12 h later at 21:00. An Isotonic saline solution, with an equal volume to vancomycin, was administrated to group 1 and an injection of sterile water, with an equal volume to saffron, was given i.p. to both groups 1 and 3.
After 24 hours from the last injection, the animals were anaesthetized with xylazine (10 mg/kg/d; Chanelle Pharmaceutical Co., Galway, Ireland) and ketamine HCL (50 mg/kg/day; Parke Davis Pharmaceutical Co., Cambridge, UK) and exterminated in a rapid and painless method. Blood samples were collected from the inferior vena cava. Serum samples were separated by centrifugation at 1600 g for 5 min and frozen (-80°C) for measurements of serum creatinine (Cr) and blood urea nitrogen (BUN) levels. Concentration of BUN and Cr were determined using a commercial kit (Pars Azmoon, Tehran, Iran) and Hitachi multianalyser (Hitachinaka, Japan) according to the manufacturer’s instructions.
The abdomen was opened and both kidneys were removed, weighed and de-capsulated. The left kidneys were placed in formaldehyde (10% solution) and then embedded in paraffin wax, sectioned (4 μm) and stained with Hematoxylin and Eosin (H&E) for the standard histopathological examination by light microscopy [28]. Coded slides from each group were examined by a pathologist, whereby, the corresponding treatment regimens remained undisclosed.
The right kidneys were washed with physiological saline and maintained at -80°C and used for analysis of malondialdehyde (MDA) and superoxide dismutase (SOD). On the day of analysis, the kidney tissues were homogenized in a motor driven tissue homogenizer with a cold phosphate buffer solution to give a 10% homogenate suspension. Unbroken cells, cells debris and nuclei were sedimented by centrifugation at 4000rmp for approximately 20 min. The supernatants were collect and used for biochemical assays.
■Malondialdehyde measurement
Malondialdehyde (MDA) levels, an index of lipid peroxidation, were determined in renal tissues. MDA, as a thiobarbituric acid reactive substance (TBARS), reacts with thiobarbituric acid (TBA) to generate a color complex that has a peak absorbance of 532 nm, that can be measured in the visible range using an ELISA plate reader [29]. The MDA levels were evaluated, in accordance to the manufacturers instructions (Zell bio, Germany), using diagnostic Zell Bio kits.
■ Superoxide dismutase activity measurement
One unit of SOD activity was defined as the amount of sample necessary to catalyze 1 micromole of superoxide radicals to hydrogen peroxide and oxygen in the span of one minute. The product transformed the color of the chromogen and SOD activity was determined colorimetrically to be 420 nm by using an ELISA plate reader [30]. Again, superoxide dismutase activity was evaluated using diagnostic Zell Bio kits in accordance to the manufacturer’s instructions (Zell Bio, Germany).
■Ethical issues
The research was both in compliance with the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. Prior to the experiment, the protocols were confirmed to be in accordance with the Guidelines of Animal Ethics Committee of Ahvaz Jundishapur University of Medical Sciences.
■Statistical analysis
Biochemical data were analyzed using the SPPS 16.0 software program (SPSS, Chicago, IL, USA) and presented as means ± SEM. Means of groups were compared by one-way analysis of variance (ANOVA) then Post-Hoc analysis (Tukey test). The level of statistical significance was accepted as p<0.05.
Results
■ Baseline characteristics
All the rats survived the experimental phase despite the considerable variance in kidney weights amongst the different test groups. Kidney weight was significantly higher in the vancomycin and the vancomycin plus saffron study group in contrast to the control group (p<0.05). However, the ratio of kidney to body weight in the vancomycin plus saffron group was significantly lower than the vancomycinonly group (p<0.05), and showed no significant change in comparison with control group (p>0.05) (TABLES 1 and 2).
| Control | Saffron | Vancomycin | Vancomycin + saffron | |
|---|---|---|---|---|
| Body weight (g) | 264.5 ± 4.89 | 249.38 ± 7.82 | 248.75 ± 7.05 | 238.75 ± 4.70 |
| Kidney weight (g) | 0.75 ± 0.05 | 0.87 ± 0.05# | 1.82 ± 0.24* | 1.35 ± 0.12* |
| Kidney weight to body weight ratio in % | 0.28 ± 0.02 | 0.35 ± 0.02# | 0.74 ± 0.09* | 0.57 ± 0.06# |
Table 1. Mean of body weight, kidney weight and the ratio of kidney weight to body weight in each groups.
| Control | Saffron | Vancomycin | Vancomycin + saffron | |
|---|---|---|---|---|
| BUN (mg/dL) | 18.81 ± 1.31 | 15.36 ± 1.23### | 112.29 ± 17.68*** | 49.18 ± 5.92### |
| Creatinine (mg/dL) | 0.47 ± 0.03 | 0.48±0.02### | 2.06 ± 0.49*** | 0.77 ± 0.05## |
| MDA (nmol/g protein) | 62.38 ± 2.73 | 70.57±2.15## | 89.43 ± 3.99** | 60.23 ± 5.13### |
| SOD (U/g protein) | 44.37 ± 3.60 | 49.49±2.86## | 30.38 ± 2.12* | 44.94 ± 3.64# |
Table 2. Blood urea nitrogen (BUN) and creatinine levels in serum and malondialdehyde (MDA) and superoxide dismutase activity (SOD) in kidney homogenates of rats.
■ Effects of vancomycin and saffron extract treatment on levels of renal MDA and activity of renal SOD
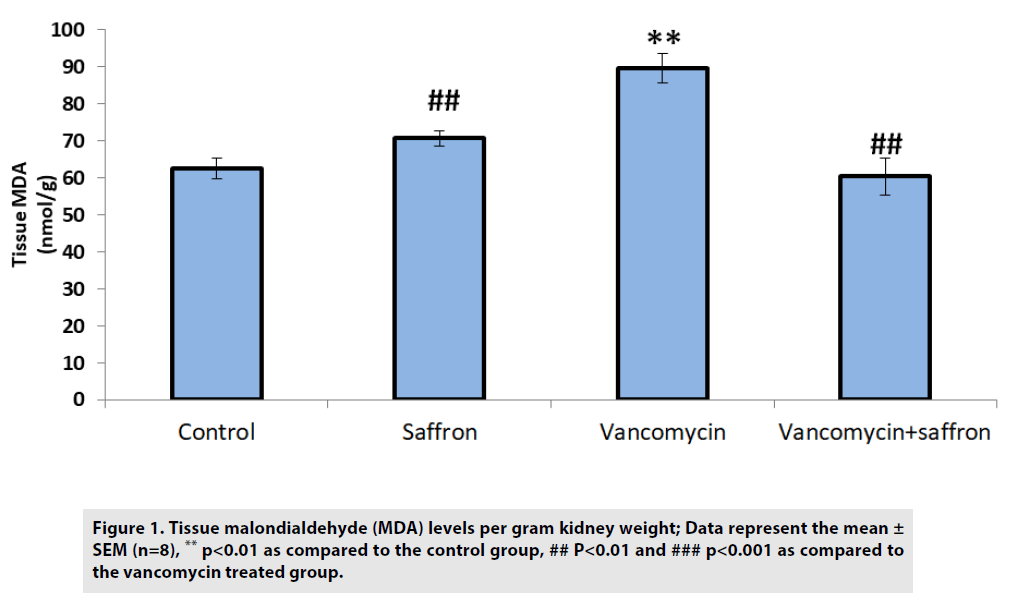
As shown in FIGURE 1, the MDA levels in the vancomycin-only group were elevated compared with the control group (p<0.01). Conversely, the saffron treatment lowered the levels of MDA in the vancomycin plus saffron group compared to the vancomycin-only group (p<0.001).
Figure 1: Tissue malondialdehyde (MDA) levels per gram kidney weight; Data represent the mean ± SEM (n=8), ** p<0.01 as compared to the control group, ## P<0.01 and ### p<0.001 as compared to the vancomycin treated group.
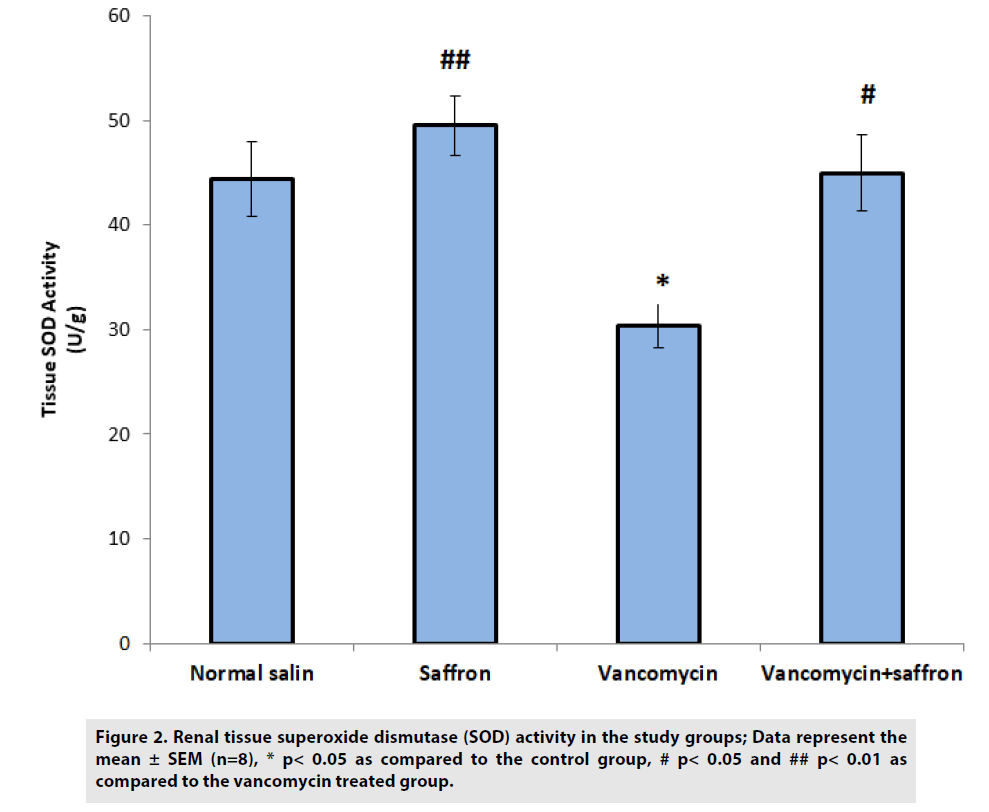
In FIGURE 2, a significant reduction in SOD activity was observed in the vancomycin group when compared to the control group (p<0.05). The saffron treatment caused a significant elevation in SOD activities in the vancomycin plus saffron group when compared with the vancomycin only group (p<0.05).
Figure 2: Renal tissue superoxide dismutase (SOD) activity in the study groups; Data represent the mean ± SEM (n=8), * p< 0.05 as compared to the control group, # p< 0.05 and ## p< 0.01 as compared to the vancomycin treated group.
■Effects of vancomycin and saffron extract treatment on blood urea nitrogen (BUN) and serum creatinine
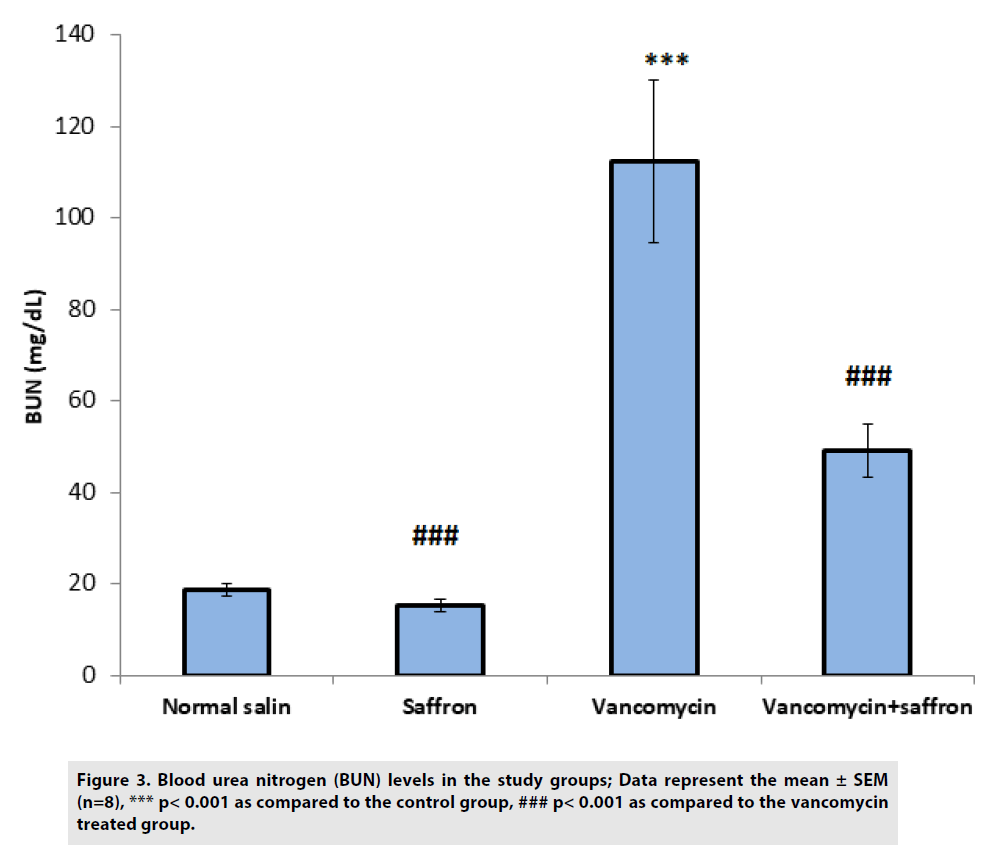
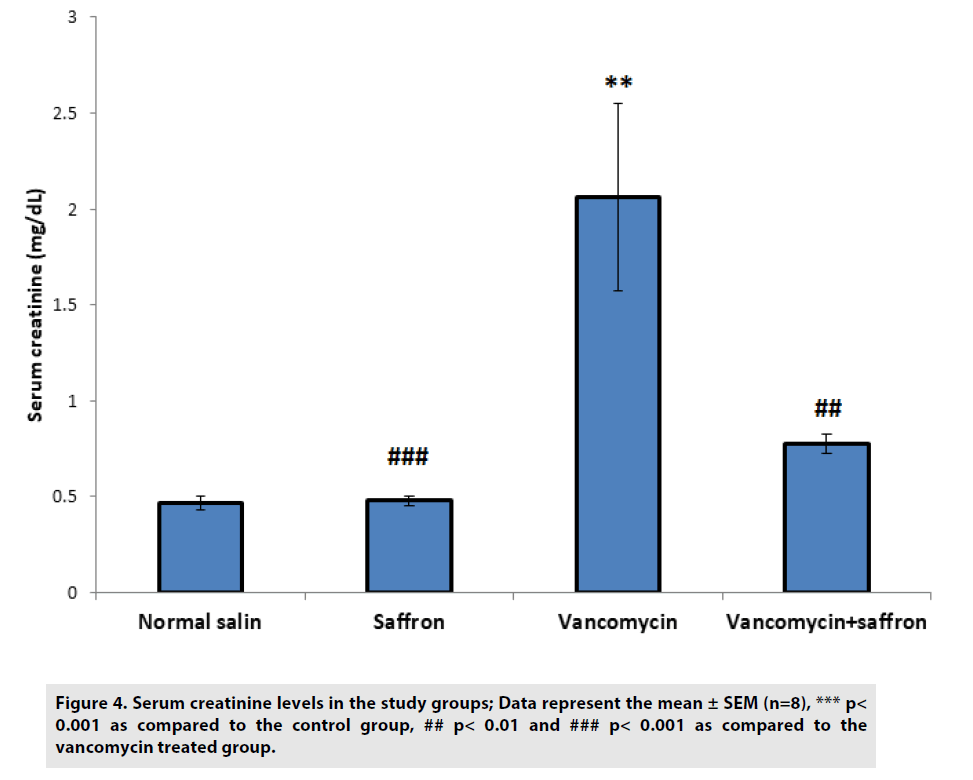
According to FIGURES 3 and 4, BUN and serum creatinine levels were substantially (p<0.001) higher in the vancomycin-treated group than in the control. In the vancomycin plus saffron group, saffron produced a marked reduction in the serum BUN and creatinine concentration, in contrast to the vancomycinonly group (p<0.001 BUN, p<0.01 creatinine).
Figure 3: Blood urea nitrogen (BUN) levels in the study groups; Data represent the mean ± SEM (n=8), *** p< 0.001 as compared to the control group, ### p< 0.001 as compared to the vancomycin treated group.
Figure 4: Serum creatinine levels in the study groups; Data represent the mean ± SEM (n=8), *** p< 0.001 as compared to the control group, ## p< 0.01 and ### p< 0.001 as compared to the vancomycin treated group.
■Effects of vancomycin and saffron extract treatment on kidney histology
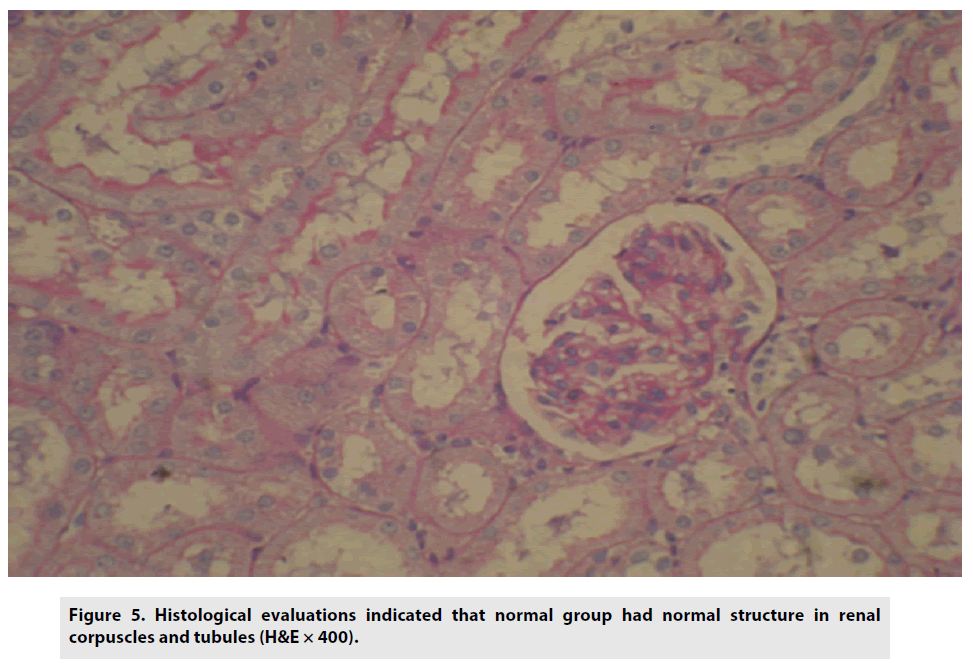
Histological evaluations indicated that the control group had normal structure in renal corpuscles and tubules (FIGURE 5).
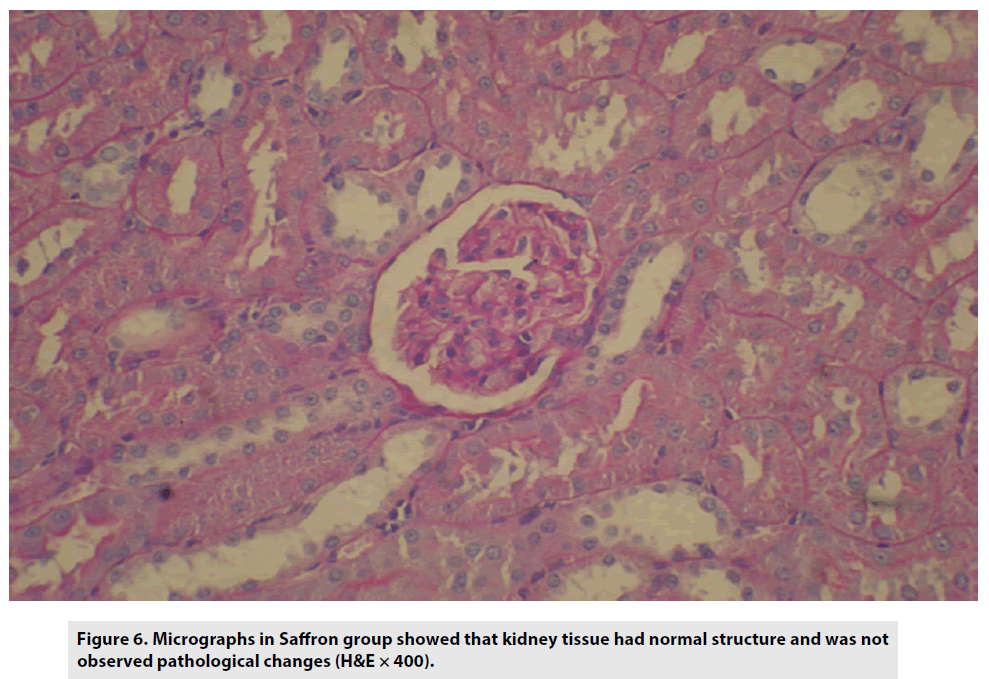
The micrographs in the saffron-treated group also demonstrated no pathological changes (FIGURE 6).
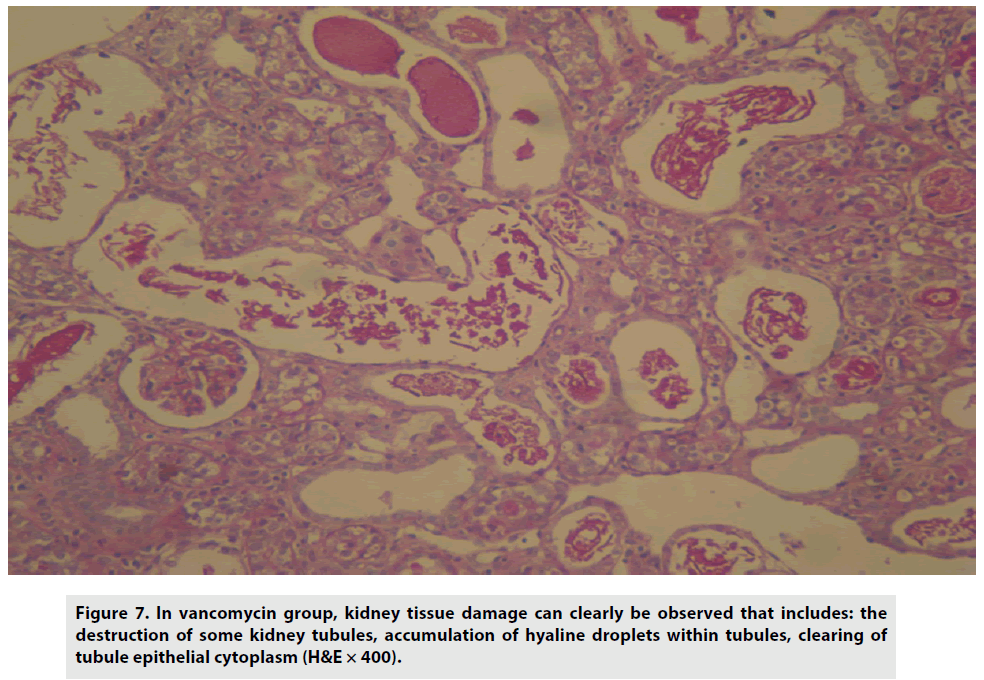
Treatment with vancomycin, on the other hand, caused tissue damage and partial destruction of kidney tubules, with the accumulation of hyaline droplets, and diminishing tubule epithelial cytoplasm (FIGURE 7).
Figure 7: In vancomycin group, kidney tissue damage can clearly be observed that includes: the destruction of some kidney tubules, accumulation of hyaline droplets within tubules, clearing of tubule epithelial cytoplasm (H&E × 400).
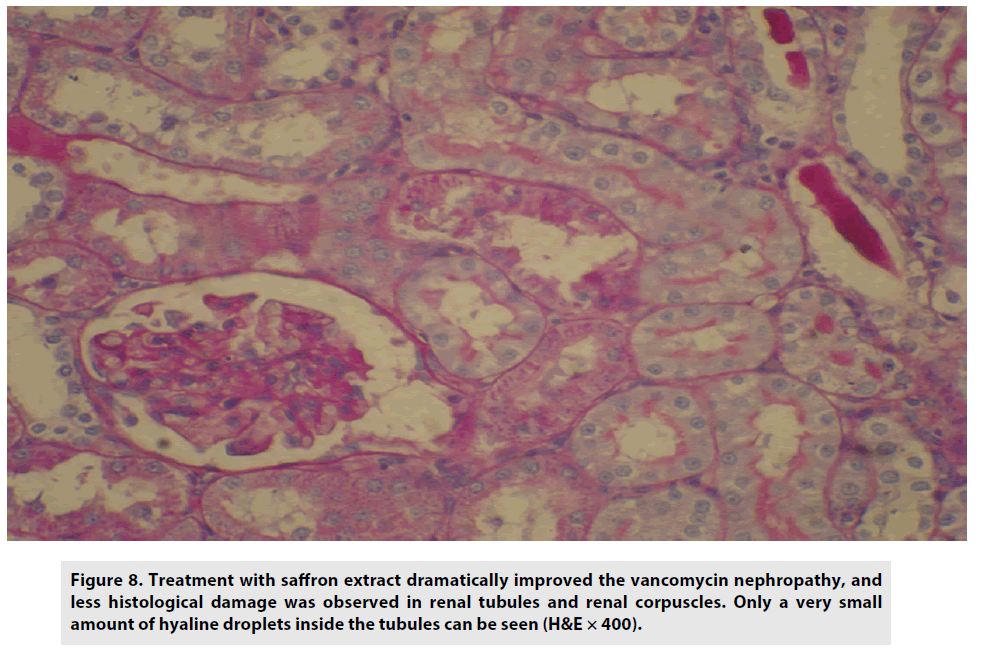
Treatment with saffron dramatically improved vancomycin nephropathy, and less histological damage was observed in renal tubules and renal corpuscles. Only negligible amounts of hyaline droplets inside the tubules were detected (FIGURE 8).
Figure 8: Treatment with saffron extract dramatically improved the vancomycin nephropathy, and less histological damage was observed in renal tubules and renal corpuscles. Only a very small amount of hyaline droplets inside the tubules can be seen (H&E × 400).
Discussion
Vancomycin has considerable nephrotoxic side effects causing serious complications when administered. Therefore amelioration of nephrotoxicity would enhance its clinical use [3]. Despite the fact that the mechanism of vancomycin nephrotoxicity is not fully known, several studies have suggested that the main mechanism of vancomycin nephrotoxicity could be oxidative stress. Some studies have also reported that vancomycin-induced nephrotoxicity was prevented with the use of different antioxidants [2,8,9,31,32]. In the present study, we have explored the possible protective effects of saffron extract, which has a powerful antioxidant effect, on nephrotoxic injury induced by vancomycin. After reviewing the literature, we realized that our study was the first experiment that considered the protective effects of saffron extract on vancomycin-induced nephrotoxicity.
In this study, with the systemic administration of high doses of vancomycin (400 mg/kg BW/day, i.p.), we created marked nephrotoxicity in the vancomycin group. This was corroborated by the significant histological changes that were observed. Then we evaluated the protective effect of saffron using oxidative and biochemical markers such as MDA, SOD, BUN and serum creatinine that are generally used to survey the progress of renal injury. The biological and histological results suggested that saffron effectively protects against vancomycin-induced nephrotoxicity.
Reactive oxygen species (ROS) have been considered to be significant mediators of aminoglycoside and cisplatin induced nephrotoxicity [3,33,34]. Nishino et al., considered that ROS play a very important role in the pathophysiology of nephrotoxicity induced by vancomycin treatment [4]. King and Smith, demonstrated that vancomycin stimulates production of both free radicals and oxidative phosphorylation and increases cellular adenosine triphosphate concentration and oxygen consumption [35]. Cellular and DNA damage, protein denaturation and peroxidation of membrane lipids are caused by free radicals [36]. Lipid peroxidation can cause damage in both membrane structure and function which results in the creation of different end-products, such as, malondialdehyde (MDA) [31]. Therefore there is a direct relation between MDA and lipid peroxidation [37]. In the present study we measured MDA levels, which is a result of free radical damage to membrane structure and is considered as an indicator of lipid peroxidation. There was a significant increase of renal MDA concentration in the vancomycin-treated group when compared to the control group. Nishino et al., Oktem et al., Celik et al. and Basarslan et al., reported significant increases in the levels of renal MDA in vancomycin-induced nephrotoxicity models in rats, and also suggested that oxidative damage and lipid peroxidation play important roles in the pathogenesis of nephrotoxicity induced by vancomycin [4,31,32,38]. In our study, saffron extract significantly attenuated the increase of MDA concentration in renal tissue. The nephroprotective effect of saffron may be due to its capacity to eliminate free radicals. In addition, it’s a recognized powerful antioxidant, therefore, it reduces lipid peroxidation of renal tubular cells in the kidneys of rats [13]. Thus, our study demonstrated that saffron extract could inhibit renal toxicity induced by vancomycin and supports the theory that free radicals are responsible for its pathogenesis.
Studies on the mechanism of vancomycininduced nephrotoxicity have mentioned the role of renal tubules in the excretion of vancomycin [39]. Once administered, vancomycin moves out of the blood and accumulates in the proximal tubular epithelium which is associated with nephrotoxicity [8]. Increased serum BUN and creatinine levels indicate cell injury in the renal proximal tubules [3]. Several studies have reported that vancomycin, as a treatment, significantly increases the levels of serum BUN and creatinine [2,9,32,40-42]. Levels of serum BUN and creatinine are indicators of renal function and marked for the assessment of late and early renal failure respectively [31,43]. Our study found that the levels of serum BUN and creatinine in the vancomycin group were significantly higher than that of the control group. This reveals that the administration of vancomycin causes serious nephrotoxicity. Moreover, a comparison to the control group showed a significant increase in the kidney weight ratio and the histopathological examination of the kidney demonstrated significant renal damage. Treatment with aqueous saffron extract lead to lower levels of serum BUN and creatinine than the vancomycin-treated group, demonstrating improvement in renal function. Antioxidant molecules and enzymes are present in cells to protect against the destructive effects of free radicals. The treatment of vancomycin induced oxidative stress which suppressed and rendered most of the antioxidant enzymes inactive, allowing the function of free radical scavenging [31]. Vancomycin either directly binds to enzyme active sites containing ̶ SH groups, or it displaces metal cofactors from active sites [44,45] SOD is the essential protective enzyme against oxidative stress in renal tubules, and its highest concentration is found in the proximal tubules [46]. In our study, the vancomycin group displayed a significant reduction in SOD activity when compared to the control group. Several of the latest studies have also reported a significant decrease in SOD activity in vancomycin nephrotoxicity rat models [2,3,8,31,32,40,45]. In this study, treatment with saffron extract completely inhibited the decrease of SOD activity in the vancomycin plus saffron group when compared to the vancomycin-only group.
Although the biochemical mechanism of the preventive effect of saffron extract on the inactivation of SOD activity has not yet been proven, we suggested that its effectiveness may be due to its free radical scavenging and antioxidant activity.
Oklem et al., found erdosteine treatment caused an increase in the activity of SOD and a decrease in MDA levels in vancomycininduced nephrotoxicity in rats and rationalized that it was due to the free radical scavenging activity of erdostein [8]. Basarslan et al., have shown that the administration of thymoquinone protects the kidney from damage that is induced by vancomycin. This is observed by the diminishing levels of MDA, BUN and creatinine and the increase in SOD levels. Therefore, the efficacy of thymoquinone is a result of its interaction with ROS, which is a general radical scavenger, during vancomycin toxicity [31]. Sadeeshkumar et al., reported that pre-treatment with Gallic acid increased the activity of SOD in vancomycin treated rats. This may be due to Gallic acid’s ability to act as a chelator and eliminate vancomycin from renal tissue, as well as, reducing the accumulation of free radical generation during lipid peroxidation [45]. Cetin et al., found that the treatment of erythropoietin on vancomycin treated rats caused a decrease in MDA, serum creatinine and BUN levels and an increase in SOD activity. This indicated that the renoprotective effect of erythropoietin was due to the inhibition of apoptosis, as well as, acted as an antioxidant agent against vancomycininduced renal damage [32]. Recent animal studies used various antioxidants, such as, vitamin E, vitamin C, N-acetylcysteine, caffeic acid phenethyl ester, atorvastatin and curcumin and results supported the beneficial effects of antioxidants in the prevention of vancomycin-induced nephrotoxicity [2,9,38,40,47].
Yarijani et al., suggested that crocin has protective effects against gentamycin-induced nephrotoxicity and that might be due to the vasodilatory effect of the kidneys which corrects the glomerular filtration rate and also has antioxidant and anti-inflammatory properties [25]. Recent studies have also reported the renoprotective effects of saffron extract on nephrotoxicities induced by cisplatin, gentamycin and ceftazidime in rats [17,26-28,34].
Hosseinzadeh et al., evaluated the protective effect of aqueous saffron extract and its active constituent, crocin, on renal ischemiareperfusion induced oxidative damage in rats. He reported that although both components might be useful agents for the prevention of renal oxidative injury, saffron extract consist of various constituents which have roles in protection [13].
In the present study, we demonstrated that vancomycin treatment causes kidney damage. It raised the levels of serum creatinine, BUN and renal MDA and decreased SOD activity. In addition, the histological analysis clearly showed partial destruction of kidney tubules, interstitial edema, epithelial vacuolization and epithelial desquamation. Therefore, we concluded that oxidative stress is the main factor in vancomycin-induced nephrotoxicity. Treatment with aqueous saffron extract significantly normalized biochemical and histological changes seen in vancomycin treated rat. Saffron extract has been proved to be a highly efficient scavenger of oxygen radicals and other excited species and it protects against the oxidation of lipid, protein and DNA [17,22]. It can also decrease oxidative stress by increasing the expression of antioxidant enzymes, such as, superoxide dismutase and catalase [48]. Accordingly, it seems that more than one mechanism of protection involved.
Conclusion
In conclusion, the present study demonstrated that saffron extract has a potent renoprotective effect against vancomycininduced nephrotoxicity and it is suggested as a potential new therapeutic strategy.
Conflicts of Interest
The authors have declared that no conflict of interest exists.
References
- Nishino Y, Takemura S, Minamiyama Y et al. Inhibition of vancomycin-induced nephrotoxicity by targeting superoxide dismutase to renal proximal tubule cells in the rat. Redox. Rep. 7: 317-329 (2002).
- Celik I, Cihangiroglu M, Ilhan N et al. Protective effects of different antioxidants and amrinone on vancomycin-induced nephrotoxicity. Basic. Clin. Pharmacol. Toxicol. 97: 325-332 (2005).
- Bamgbola O. Review of vancomycin-induced renal toxicity: an update. Ther. Adv. Endocrinol. Metab. 7: 136-147 (2016).
- Nishino Y, Takemura S, Minamiyama Y et al. Targeting superoxide dismutase to renal proximal tubule cells attenuates vancomycin-induced nephrotoxicity in rats. Free. Radic. Res. 37: 373-379 (2003).
- Toyoguchi T, Takahashi S, Hosoya J et al. Nephrotoxicity of vancomycin and drug interaction study with cilastatin in rabbits. Antimicrob. Agents. Chemother. 41: 1985-1990 (1997).
- Dieterich C, Puey A, Lin S et al. Gene expression analysis reveals new possible mechanisms of vancomycin-induced nephrotoxicity and identifies gene markers candidates. Toxicol. Sci. 107: 258-269 (2009).
- Hodoshima N, Nakano Y, Izumi M et al. Protective effect of inactive ingredients against nephrotoxicity of vancomycin hydrochloride in rats. Drug. Metab. Pharmacokinet. 19: 68-75 (2004).
- Oktem F, Arslan MK, Ozguner F, Candir O, Yilmaz HR, Ciris M, et al. In vivo evidences suggesting the role of oxidative stress in pathogenesis of vancomycin-induced nephrotoxicity: protection by erdosteine. Toxicology. 215: 227-233 (2005).
- Elyasi S, Khalili H, Hatamkhani S et al. Prevention of vancomycin induced nephrotoxicity: a review of preclinical data. Eur. J. Clin. Pharmacol. 69: 747-754 (2013).
- Hemmati AA, Nazari Z, Samei M. A comparative study of grape seed extract and vitamin E effects on silica-induced pulmonary fibrosis in rats. Pulm. Pharmacol. Ther. 21: 668-674 (2008).
- Bhaskaran N, Shukla S, Srivastava JK et al. Chamomile: an anti-inflammatory agent inhibits inducible nitric oxide synthase expression by blocking RelA/p65 activity. Int. J. Mol. Med. 26: 935-940 (2010).
- Hemmati AA, Rezaie A, Darabpour P. Preventive effects of pomegranate seed extract on bleomycin-induced pulmonary fibrosis in rat. Jundishapur. J. Nat. Pharm. Prod. 8: 76-80 (2013).
- Hosseinzadeh H, Sadeghnia HR, Ziaee T et al. Protective effect of aqueous saffron extract (Crocus sativus L.) and crocin, its active constituent, on renal ischemia-reperfusion-induced oxidative damage in rats. J. Pharm. Sci. 8: 387-393 (2005).
- Hosseinzadeh H. Saffron: a herbal medicine of third millennium. Jundishapur. J. Nat. Pharm. Prod. 9: 1-2 (2014).
- Imenshahidi M, Hosseinzadeh H, Javadpour Y. Hypotensive effect of aqueous saffron extract (Crocus sativus L.) and its constituents, safranal and crocin, in normotensive and hypertensive rats. Phytother. Res. 24: 990-994 (2010).
- Hosseinzadeh H, Talebzadeh F. Anticonvulsant evaluation of safranal and crocin from Crocus sativus in mice. Fitoterapia. 76: 722-724 (2005).
- Naghizadeh B, Mansouri SM, Mashhadian NV. Crocin attenuates cisplatin-induced renal oxidative stress in rats. Food. Chem. Toxicol. 48: 2650-2655 (2010).
- Abdullaev FI, Espinosa-Aguirre JJ. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer. Detect. Prev. 28: 426-432 (2004).
- Abdullaev FI, Riveron-Negrete L, Caballero-Ortega H et al. Use of in vitro assays to assess the potential antigenotoxic and cytotoxic effects of saffron (Crocus sativus L.). Toxicol. In. Vitro. 17: 731-736 (2003).
- Akhondzadeh S, Sabet MS, Harirchian MH et al. Saffron in the treatment of patients with mild to moderate Alzheimer's disease: a 16-week, randomized and placebo-controlled trial. J. Clin. Pharm. Ther. 35: 581-588 (2010).
- Akhondzadeh S, Tahmacebi-Pour N, Noorbala AA et al. Crocus sativus L. in the treatment of mild to moderate depression: a double-blind, randomized and placebo-controlled trial. Phytother. Res. 19: 148-151 (2005).
- Schmidt M, Betti G, Hensel A. Saffron in phytotherapy: pharmacology and clinical uses. Wien. Med. Wochenschr. 157: 315-319 (2007).
- Bathaie SZ, Mousavi SZ. New applications and mechanisms of action of saffron and its important ingredients. Crit. Rev. Food. Sci. Nutr. 50: 761-786 (2010).
- Naghizadeh B, Boroushaki MT, Vahdati MN et al. Protective effects of crocin against cisplatin-induced acute renal failure and oxidative stress in rats. Iran. Biomed. J. 12: 93-100 (2008).
- Yarijani ZM, Najafi H, Hamid MS. Protective effect of crocin on gentamicin-induced nephrotoxicity in rats. Iran. J. Basic. Med. Sci. 19: 337-343 (2016).
- Dhar MH, Shah KU, Ghongane BB et al. Nephroprotective activity of crocus sativus extract against gentamicin and/or ceftazidime-induced nephrotoxicity in rats. Int. J. Pharm. Bio. Sci. 4: 864-870 2013.
- Derakhshanfar A, Hashempour SM, Abbasabadi N et al. Histopathologic and biachemical study of the effect of saffron extract on gentamicin-induced nephrotoxicity in rats. Comp. Clin. Pathol. 24: 1347-1351 (2015).
- Ajami M, Eghtesadi S, Pazoki-Toroudi H et al. Effect of crocus sativus on gentamicin induced nephrotoxicity. Biol. Res. 43: 83-90 (2010).
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95: 351-358 (1979).
- Kuthan H, Haussmann HJ, Werringloer J. A spectrophotometric assay for superoxide dismutase activities in crude tissue fractions. Biochem. J. 237: 175-180 (1986).
- Basarslan F, Yilmaz N, Ates S et al. Protective effects of thymoquinone on vancomycin-induced nephrotoxicity in rats. Hum. Exp. Toxicol. 31: 726-733 (2012).
- Cetin H, Olgar S, Oktem F et al. Novel evidence suggesting an anti-oxidant property for erythropoietin on vancomycin-induced nephrotoxicity in a rat model. Clin. Exp. Pharmacol. Physiol. 34: 1181-1185 (2007).
- Ali BH, Al Za'abi M, Blunden G et al. Experimental gentamicin nephrotoxicity and agents that modify it: a mini-review of recent research. Basic Clin. Pharmacol. Toxicol. 109: 225-232 (2011).
- El Daly-ES. Protective effect of cysteine and vitamin E, Crocus sativus and Nigella sativa extracts on cisplatin-induced toxicity in rats. J. Pharm. Belg. 53: 87-93 (1998).
- King DW, Smith MA. Proliferative responses observed following vancomycin treatment in renal proximal tubule epithelial cells. Toxicol. In. Vitro. 18: 797-803 (2004).
- Dean RT, Hunt JV, Grant AJ et al. Free radical damage to proteins: the influence of the relative localization of radical generation, antioxidants, and target proteins. Free. Radic. Biol. Med. 11: 161-168 (1991).
- Nielsen F, Mikkelsen BB, Nielsen JB et al. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin. Chem. 43: 1209-1214 (1997).
- Ocak S, Gorur S, Hakverdi S et al. Protective effects of caffeic acid phenethyl ester, vitamin C, vitamin E and N-acetylcysteine on vancomycin-induced nephrotoxicity in rats. Basic. Clin. Pharmacol. Toxicol. 100: 328-333 (2007).
- Nakamura T, Kokuryo T, Hashimoto Y et al. Effects of fosfomycin and imipenem-cilastatin on the nephrotoxicity of vancomycin and cisplatin in rats. J. Pharm. Pharmacol. 51: 227-232 (1999).
- Ahmida MH. Protective role of curcumin in nephrotoxic oxidative damage induced by vancomycin in rats. Exp. Toxicol. Pathol. 64: 149-153 (2012).
- Dalaklioglu S, Tekcan M, Gungor NE et al. Role of the poly(ADP-ribose)polymerase activity in vancomycin-induced renal injury. Toxicol Lett. 192: 91-96 (2010).
- Elyasi S, Khalili H, Dashti-Khavidaki S et al. Vancomycin-induced nephrotoxicity: mechanism, incidence, risk factors and special populations. A literature review. Eur. J. Clin. Pharmacol. 68: 1243-1255 (2012).
- Erdem A, Gundogan NU, Usubutun A et al. The protective effect of taurine against gentamicin-induced acute tubular necrosis in rats. Nephrol Dial Transplant. 15: 1175-1182 (2000).
- Casalino E, Calzaretti G, Sblano C et al. Cadmium-dependent enzyme activity alteration is not imputable to lipid peroxidation. Arch. Biochem. Biophys. 383: 288-295 (2000).
- Sadeeshkumar V, Arul D, Ravichandran S. Protective effects of gallic acid in the renal markers, histopathology and immunohistochemical studies on vancomycin induced nephrotoxic rats. Int. J. Adv. Life. Sci. 6: 356-365 (2013).
- Inoue M, Nishikawa M, Sato E et al. Synthesis of superoxide dismutase derivative that specifically accumulates in renal proximal tubule cells. Arch. Biochem. Biophys. 368: 354-360 (1999).
- Panonnummal R, Varkey J, Dinoop DR. protective effect of atorvastatin against vancomycin induced nephrotoxicity in albino rats. Pharmacie. Globale. 2: 1-6 (2011).
- El-Beshbishy HA, Hassan MH, Aly HA et al. Crocin "saffron" protects against beryllium chloride toxicity in rats through diminution of oxidative stress and enhancing gene expression of antioxidant enzymes. Ecotoxicol. Environ. Saf. 83: 47-54 (2012).