Review Article - Interventional Cardiology (2010) Volume 2, Issue 2
Second-generation atrial tachycardias: adverse repercussions of ablation or a step in the right direction?
- Corresponding Author:
- Tushar V Salukhe
University Hospital
Eppendorf University Heart Center
Department of Cardiology/Electrophysiology Martinistr
52, D-20246, Hamburg, Germany
Tel: +49 40 428 034 120
Fax: +49 40 428 034 125
E-mail: salukhe@aol.com
Abstract
Keywords
ablation, chronic atrial fibrillation, mapping, recurrent atrial tachycardia
Clinical electrophysiology has experienced period of renaissance over the last decade, particularly in the treatment of atrial fibrillation (AF). Catheter ablation of AF has rapidly come to the fore as a treatment of choice with an excellent expectation of cure. Since the landmark paper identifying the pulmonary veins (PVs) as instigators and perpetuators of AF in its paroxysmal form [1], PV isolation has become fundamental to the process of AF ablation [2–5]. As longer lasting forms of AF were tackled, it became clear that more substrates needed to be targeted for ablation as PV isolation alone is rarely successful in treating patients with longer lasting AF [6–10]. This implied that processes within structures outside the PVs are responsible for AF perpetuation in its chronic form and thus, the focus of ablation shifted to the atria and their annexed structures (coronary sinus, atrial appendages and thoracic veins). To this end, sites of complex fractionated atrial electrograms (CFAE), and the use of linear lesions at the left atrial roof, mitral isthmus and cavotricuspid isthmus have emerged as viable targets for successful outcomes [11,12].
The stepwise process of PV isolation, targeting CFAEs and linear ablation leads to the favorable end point of AF termination during ablation in up to 85% of patients with chronic AF [12]. Whether a stepwise approach or other approaches involving extensive left atrium (LA) ablation are used [11–16], AF rarely terminates directly to sinus rhythm. AF termination usually occurs by conversion to one or a series of intermediate atrial tachycardias (ATs). These subsequent ATs are frequently encountered during follow-up and are critically important for several reasons. First, they usually generate more symptoms than the initial presenting AF. Second, they are very resistant to medical therapy and very often require further ablation. Finally, as we begin to understand the pathogenesis of these subsequent ATs, it may eventually change our approach to index AF ablation. This article will focuses on ATs encountered in the context of AF ablation with particular attention to mechanisms, pathogenesis and conventional mapping and ablation.
Termination of AF & the diagnosis of AT
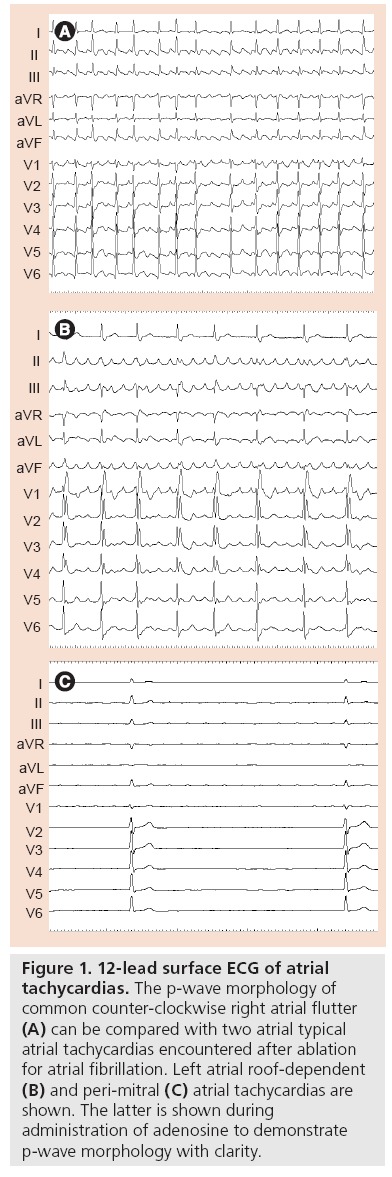
The surface ECG alone is usually sufficient to discern AF from AT. ATs typically display regular monomorphic p‑waves with a regular cycle length (Figure 1). By contrast, p-waves in AF vary in morphology and cycle length. Difficulties can sometimes arise when partial or intermittent regularity in p‑wave morphology and cycle length is observed in AF. When does ‘organized AF’ become an AT? This is resolved by careful examination of intracardiac electrograms and usually with three catheters (in the right atrium, LA and coronary sinus) because consistency in relative timing is equally important to define AT. AT cycle lengths are usually consistent. Organized AF is loosely, and rather arbitrarily, defined as cycle length consistency for over 75% of the time in a 10-min window [17]. In practice, and more important than definition boundaries, the point when AF becomes more organized represents a shift in the mechanism of the perpetuating arrhythmia to one more amenable to mapping and targeted ablation and a step closer to sinus rhythm.
Figure 1: 12-lead surface ECG of atrial tachycardias. The p‑wave morphology of common counter-clockwise right atrial flutter (A) can be compared with two atrial typical atrial tachycardias encountered after ablation for atrial fibrillation. Left atrial roof-dependent (B) and peri-mitral (C) atrial tachycardias are shown. The latter is shown during administration of adenosine to demonstrate p‑wave morphology with clarity.
Mechanisms of atrial tachycardia
Understanding the arrhythmia mechanism is fundamental to electrophysiological mapping and ablation. This is true for any arrhythmia, but perhaps more so for ATs as they are often the most challenging to interrogate and ablate. Thus, an overview of common arrhythmia mechanisms is outlined here.
Re-entry is by far the most common mechanism and is usually considered first during electrophysiological study, particularly because it lends itself well to traditional mapping techniques. Re-entry is a perpetuating wave front of activation rotating in circus fashion around an obstacle. The obstacle can be anatomical (a fixed structure) or functional (myocardium with dynamic conduction properties). In the context of AT surrounding AF ablation, common obstacles include the PVs and the mitral annulus in the LA and the tricuspid annulus in the right atrium (RA). Less frequently after AF ablation, such obstacles are created by ablation lesions, particularly if linear ablation is incomplete. The recipe for re-entry is the same whether the circuit diameter is several centimetres (macro-re-entry) or a few millimetres (localized re-entry). This includes two electrical conduits separated by the obstacle and connected proximally and distally, thus forming a circuit. The conduits have different refractory periods and conduction velocities (one fast with a long refractory period, the other slow with a short refractory period). At first, this complex arrangement may seem implausible, yet myocardium altered by fibrosis, ischemia, surgical incision or indeed ablation often harbors components required for re-entry.
Focal ATs originate from a single point and spread centrifugally. The underlying mechanisms include increased automaticity and triggered activity. As the approach to mapping and ablation of focal AT is the same regardless of mechanism, these are only outlined here. All areas of myocardium have some degree of automaticity, most obviously in the sinoatrial node. This is due to (normal) acceleration of phase 4 of the action potential until threshold potential is reached producing another action potential. Abnormally accelerated phase 4 depolarization results in abnormal automaticity and can occur wherever there is excitable tissue. This mechanism accounts for 10% of ATs. Focal ATs typically exhibit a warm-up and warm-down phenomenon at onset and offset, often due to physiological or metabolic stress such as ischemia, hypoxia, electrolyte or acid–base imbalance [18,19].
Late in phase 3 or early in phase 4 of the action potential, continued leakage of positive ions can cause smaller peaks of depolarization, termed afterdepolarizations. These can be of sufficient magnitude to reach threshold potential and generate another action potential, so-called triggered activity. In this way, triggered activity is similar to automaticity; however, it is not always spontaneous and can be provoked by an extrastimulus, much like re-entry. Thus, triggered activity displays characteristics of both automaticity and re-entry.
Natural history of subsequent atrial tachycardias
Reports of AT incidence after AF ablation vary widely from 4 to 50% [7,20–22]. The spread of rates is largely a reflection of several variables between studies including the chronicity of AF, the approach to ablation, the lesion set applied and the procedural end point accepted. For example, in two studies reporting AT incidence after paroxysmal AF ablation (when PV isolation alone was the accepted end point), incidence rates of subsequent ATs of 3% [20] and 21% [21] were described. Although both studies attributed the majority of subsequent AT to PV reconnection, the large discrepancy in the incidence rates probably reflects different techniques for PV isolation and the extent of ablation applied to achieve the same end point. A simple explanation may be that segmental lesions are more reliable at achieving long-lasting pulmonary vein isolation compared with long continuous lesions, which may be more likely to have gaps. An alternative explanation is that the greater lesion burden encumbered by wide area circumferential ablation itself provides a substrate for subsequent ATs. This is supported by the observation that empirical wide area circumferential, without the intent to isolate the PVs, is associated with a 10–30% risk of developing subsequent ATs [22], the majority of which are not related to PV conduction but rather large macro-re-entrant, LA roof‑dependant or perimitral circuits [23,24].
The critical observations that arose from these and other studies are, first, that AT incidence is directly related to the extent of ablation (forming the basis of the ‘proarrhythmic’ hypothesis), and second, that gaps in attempted linear ablation increase this incidence further [24,25]. This risk can be dramatically reduced by routinely checking integrity and contiguity of linear ablation for bidirectional block [26] as current guidelines would dictate [27]. If the proarrhythmic hypothesis were true, then the risk of extensive and noncontiguous linear ablation may be avoided altogether by focusing ablation to specific electrogram- based targets. The stepwise approach of segmental ablation of PV sleeves for PV isolation, followed by ablation of CFAE, is such a strategy. Although this approach yields high immediate AF termination rates (~85%), the incidence of subsequent ATs are comparable to those after wide area ablation (26–36%) [12,28]. This may be partly explained by the fact that the overall lesion burden in targeted electrogram-based ablation and wide area circumferential ablation are often comparable. This is particularly true for longer-lasting AF, which inevitably requires more extensive ablation.
The range of incidence of subsequent AT after chronic AF ablation is also wide (4–50%), and higher than that after paroxymal AF ablation [7,29]. In addition to a higher incidence rate, a shift in subsequent AT mechanism is observed after chronic AF ablation. This is particularly true after an electrogram-guided approach. As the AF of longer durations are ablated and lesion sets become more extensive and complex, the mechanisms of subsequent ATs shift from predominantly macro-re-entry to focal ATs and localized (small-circuit) re-entrant tachycardias [7,29–31].
While the proarrhythmic hypothesis for subsequent ATs may appeal to first intuitions, Ockham’s razor may actually support a different explanation; atria of patients with longer lasting AF with more diseased myocardial substrates may already harbor properties to support AT, and ablation simply removes their capacity to fibrillate and ‘unmasks’ the underlying AT.
It is noteworthy that up to half of all ATs presenting after AF ablation do so within the 8–12 weeks after ablation, and not all of these were actually observed during the index AF ablation [32–34]. If these early arrhythmia recurrences are treated with cardioversion (particularly after chronic AF ablation), they may not manifest again. This initial time of arrhythmia convalescence is commonly referred to as the blanking period. Investigators who reported high numbers of repeat ablation procedures for subsequent AT often performed them during the initial blanking period. Current practice is to offer ablation for AT persisting beyond this period (2–3 months). Ablation of these ATs yields excellent success rates and is an important step towards stable sinus rhythm [35].
Conventional mapping & ablation
A methodical approach to AT mapping and ablation is critical for success. Following a stepwise routine armed with (but not biased by) prior knowledge of the previous lesion set invariably leads to a satisfying outcome for both the patient and the electrophysiologist.
▪ PV reisolation
Maintained PV isolation is fundamental for freedom from AF. Furthermore, subsequent ATs commonly result from PV reconnection [20,23]. It is therefore reasonable to first ensure that all PVs are still isolated. Mapping of the pulmonary veins is performed with a circular mapping catheter positioned just within the ostia of the PV. In an AT truly related to a reconnected PV, very early potentials are seen on the mapping catheter occurring during the diastolic interval of the AT. Depending on the approach at the index procedure, PV reisolation is performed either by closing the gap in the previous wide area encirclement or by targeted segmental ablation of the reconnected sleeve as suggested by the activation sequence on the circular mapping catheter. Either strategy should terminate the AT to sinus rhythm or another intermediate AT.
▪ Fundamentals of mapping
Enormous technological advances over the last two decades have assisted the electrophysiologist in identifying tachycardia mechanisms and guiding ablation therapy. Of late, these advances, however sophisticated, have done little more than convert data from conventional mapping maneuvers into a more aesthetic 3D picture (a picture that most traditional electrophysiologists construct mentally). These are not described here as they have been extensively reviewed elsewhere [36,37]. Accurate analysis and interpretation of the surface ECG and intracardiac signals remain fundamental to conventional mapping. Two maneuvers most commonly employed, usually in combination, are activation sequence mapping and entrainment. These techniques constitute the essentials of arrhythmia interrogation in any standard electrophysiological study and are applicable to both focal and re-entrant forms of AT.
Continuous monitoring of the AT cycle length (ATCL) is essential for diagnosis and for monitoring the progress of mapping and ablation. Obvious variability in ATCL may suggest a focal mechanism, but this is far from conclusive and differentiation from focal and reentry mechanisms is confirmed with traditional mapping and pacing manoeuvres.
▪ Activation sequence mapping
Activation sequence mapping is performed during tachycardia in an effort to describe a temporal sequence of atrial activation. The intracardiac signal is recorded at the tip of a roving mapping catheter and timed to the chamber in which the arrhythmia mechanism is confined (LA, RA or coronary sinus [CS]). Critically, the timing of the electrogram recorded at the tip of a roving catheter is compared with that of a constant and reliable reference, either a surface ECG or stable intracardiac signal, for example, a CS catheter electrogram. In re-entry tachycardias, this temporal relationship can be used to define the activation sequence during tachycardia around a well-defined circuit. Alternatively, the earliest endocardial signal can aide mapping focal or micro-re-entrant tachycardias. This is performed under fluoroscopic guidance so that the temporal activation can be given spatial connotation.
The origin of a focal ATs is typically characterized by activation occurring less than 50 ms prior to systole timed from the surface p-wave. Conversely, macro-re-entrant arrhythmias demonstrate diastolic activation more than 50 ms prior to systole. However, in reentrant ATs, such signals cannot justifiably be described as ‘early’ because intracardiac activity should be present at some location throughout the tachycardia cycle. In re-entry AT, middiastolic potentials are often observed during tachycardia as discrete or complex signals that precede the p‑wave by more than 50 ms and are thought to represent areas of slow conduction. Once recognized, it is important to establish their relevance to the tachycardia mechanism. If relevant, mid-diastolic potentials must be associated with every beat of tachycardia. This should be particularly evident at the onset of tachycardia. If these criteria are satisfied, these potentials would be in-keeping with a catheter position within the diastolic limb or critical isthmus of the re-entrant AT. Before ablation, confirmation of proximity to the tachycardia circuit should be confirmed with entrainment.
Entrainment
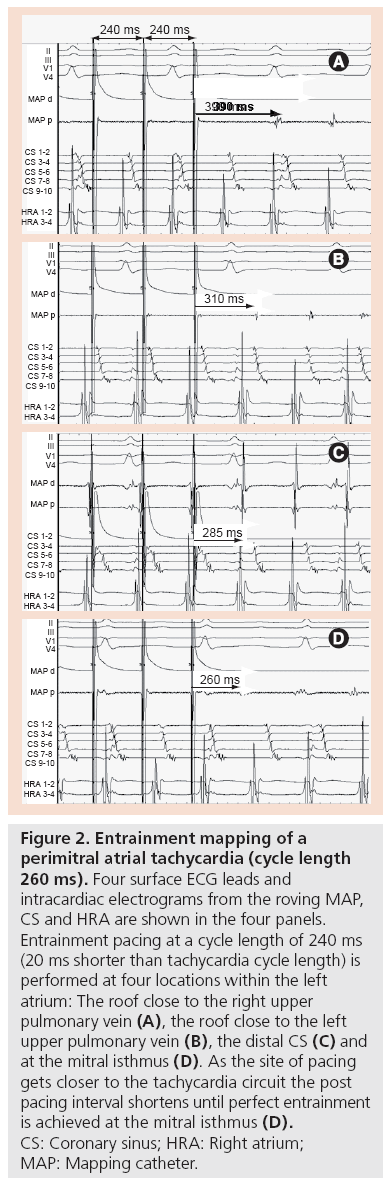
Entrainment (overdrive pacing) provides evidence of an excitable gap, supporting re-entry as the mechanism. In re-entry, the circus wavefront is bound in front by the leading depolarized myocardium and behind by the trailing refractory myocardium. The rest of the circuit remains excitable and therefore vulnerable to intrusion by other activation fronts. By pacing close to the re-entry circuit at a rate slightly faster than the tachycardia cycle length, it is possible to invade the excitable gap, breach the re-entry circuit and thus entrain the tachycardia [38,39]. On the surface ECG, entrainment manifests as progressive fusion of p‑waves. Such fusion occurs as the activation front spreading centrifugally from the pacing site, invades the excitable gap and is physiologically forced around the re-entry circuit. During entrainment, it does not complete the circuit as it collides with the next paced beat, which arrives at the next excitable gap as entrainment continues. When pacing stops, the last paced beat to invade the excitable gap continues uninterrupted around the re-entrant circuit before tachycardia continues. This last paced beat is therefore entrained but not fused. The time taken for the last entrained beat to circumvent the AT circuit and return to the pacing site represents the post-pacing interval (PPI). As the pacing site approaches the re-entry circuit, the PPI shortens. Thus, the PPI minus ATCL is a quantitative measure of the distance of the pacing site to the tachycardia circuit (Figure 2).
Figure 2: Entrainment mapping of a perimitral atrial tachycardia (cycle length 260 ms). Four surface ECG leads and intracardiac electrograms from the roving MAP, CS and HRA are shown in the four panels. Entrainment pacing at a cycle length of 240 ms (20 ms shorter than tachycardia cycle length) is performed at four locations within the left atrium: The roof close to the right upper pulmonary vein (A), the roof close to the left upper pulmonary vein (B), the distal CS (C) and at the mitral isthmus (D). As the site of pacing gets closer to the tachycardia circuit the post pacing interval shortens until perfect entrainment is achieved at the mitral isthmus (D). CS: Coronary sinus; HRA: Right atrium; MAP: Mapping catheter.
Macro-re-entrant mechanisms are likely when consistently short PPI minus ATCL times (<20 ms) are recorded at two or more distant sites, suggesting a wide circuit. Localized re-entry is also supported by consistent PPI minus ATCL from various locations, but with the shortest measurements localized to a small area; whereas, inconsistent or varying PPI minus ATCL recorded repeatedly from any single location is highly suggestive of a focal mechanism [40].
Common ATs mapped after AF ablation
▪ Macro-re-entrant ATs
Perimitral, peritricuspid and LA roof-dependent AT constitute the vast majority of re-entrant ATs observed after AF ablation. They are rapidly diagnosed with activation mapping and entrainment but are often difficult to ablate because linear ablation required to break the circuit can be technically challenging.
Activation mapping of perimitral AT typically demonstrates a septal-to-lateral sequence along the CS with lateral-to-septal sequence along the endocardial surface of the anterior mitral valve annulus (or vice versa if the wave front propagates in the opposite direction). Importantly, the anterior and posterior LA walls both demonstrate inferior-to-superior activation sequence.
If the anterior and posterior LA walls demonstrate opposite activation directions, this is highly suggestive of LA roof-dependent re-entry. LA roof-dependent AT circuits rotating around the left-sided PVs are suggested to be caused by CS activation that is lateral-toseptal while those around right-sided PVs are thought to be caused by CS activation that is septal-to-lateral.
In peritricuspid AT, CS activation is invariably septal-to-distal and the entire cycle length can be mapped around the tricuspid annulus. All three AT can be confirmed by entrainment within the presumed circuit. Once identified, perimitral, LA roof-dependent and peritricuspid AT are ablated with complete linear ablation of the mitral isthmus, LA roof or cavotricuspid isthmus, respectively.
▪ Localized (small circuit) re-entrant atrial tachycardia
Occasionally, a stable AT cycle length and consistently short PPI minus ATCL values appear to propagate centrifugally from a discrete location. These are hallmarks of a localized re-entry mechanism [41]. Such circuits can occur anywhere in the atria, but are invariably located at sites of previous ablation. At these locations, the signal on the roving ablation catheter is often complex, highly fractionated and seen to traverse at least 50% of the entire AT cycle length within the small area of the catheter bipoles (Figure 3). Although often time consuming to map, they are highly sensitive to ablation at a single spot.
Figure 3: Localized re-entry mechanism of an atrial tachycardia. Four surface ECG leads and intracardiac electrograms from the roving MAP, CS and HRA are shown. The long, fractionated electrograms bridging the small distance between the bipoles of the MAP are seen to span the entire tachycardia cycle length. CS: Coronary sinus; HRA: Right atrium; MAP: Mapping catheter.
▪ Focal ATs
The first indication of a focal mechanism may be subtle variation or irregularity in ATCL. Evidence suggests that ATCL variation of more than 15% is highly suggestive of a focal mechanism. Such quantification is difficult without bespoke software for analysis and specificity is not impressive. The response to entrainment and the systematic exclusion of common macro-reentrant mechanisms, followed by careful activation mapping, is a more reliable route to diagnosis. Focal AT is probable if, first, repeated PPI minus ATCL measurements from a single location produce inconsistent measurements, second, PPI minus ATCL values are greater than 30 ms at various distant sites as this is not compatible with a large re-entrant circuit; and, third, PPI minus ATCL measurements diminish towards a centrifugal focus. At the origin of a truly focal tachycardia, the earliest activation is observed approximately 50 ms prior to the p-wave and the signal is usually high frequency and high voltage unlike that of localized reentry. A unipolar mapping configuration is also useful to refine mapping focal AT. As the roving catheter approaches the exact point of origin, the first deflection on the unipolar signal is sharply negative as the activation wave-front moves away from the catheter.
In the vast majority of cases, a careful stepwise approach to AT mapping and ablation [42] will achieve the desired result of termination to SR, either directly or through further ATs. Detailed methods for performing and verifying linear ablation at the mitral isthmus and LA roof are beyond the scope of this review and have been the focus of several previous publications [42–44]. It is important that the operator is open to all possibilities and is not biased by prior knowledge of the previous ablation lesions.
The chronic AF ablation end point conundrum
An end point of AF termination for chronic AF ablation is associated with fewer AF recurrences and repeat procedures. AF may terminate directly to sinus rhythm or, more frequently, to an AT. It is common practice to ablate these ATs further to achieve SR, although the value of this additional ablation beyond AF termination is open to question. This is primarily because the AT(s) that manifest when AF terminates may be clinically irrelevant. Indeed, many subsequent ATs do not manifest during the index AF ablation [35,45]. If the proarrhythmic hypothesis is true, the significant increase in lesion burden encumbered by ablation that is continued beyond AF termination in an effort to achieve sinus rhythm may actually be counterproductive. The question of whether continued ablation to sinus rhythm confers a distinct advantage over ablation to AF termination alone is as yet unanswered.
Conclusion
As AF ablation is more widely performed, new subsequent ATs will become more common. While this may appear to be an undesired side effect of ablation, it is clearly a consequence of continued ablation to the critical end point of AF termination. The presence of subsequent AT over recurrences of AF probably represents a step closer to sustained sinus rhythm and an atrium incapable of maintaining a fibrillatory process. It is important for the physician to recognise the blanking period after the index AF ablation and allow for this period of arrhythmia convalescence with a cardiovert and watch policy. Patients with AT persisting beyond this period should be offered ablation with excellent expectation of success and maintained sinus rhythm.
Future perspective
While it is true that mapping and ablation of recurrent ATs will improve as our exposure to these arrhythmias increases, their very occurrence questions our approach to chronic AF ablation. It is generally observed that the frequency of subsequent ATs is dependant on the amount of ablation delivered during chronic AF ablation and, therefore, also on the end point sought. Simply reducing the lesion sets and compromising on the ablation end point (e.g., accepting no AF termination over termination to AT over ablation to sinus rhythm) may well reduce the incidence of subsequent ATs but perhaps at the cost of more recurrent AF. The elucidation of these critical end points to chronic AF ablation can only be addressed through randomized controlled trials.
Financial & competing interests disclosure
Tushar Salukhe is supported by the British Heart Foundation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
▪ Atrial tachycardia (AT) following atrial fibrillation (AF) ablation is an emergent problem.
▪ The incidence of these recurrent ATs is dependent on the lesion set and amount of ablation applied during chronic AF ablation and the end point sought.
▪ These recurrent ATs are poorly tolerated clinically and are very amenable to conventional mapping and ablation.
▪ Common ATs occurring after AF ablation include macro-re-entry (typically perimitral, left atrium roof-dependent and peritricuspid) and localized re-entry.
References
Papers of special note have been highlighted as:
▪ of interest
- Haïssaguerre M, Jaïs P, Shah DC et al.: Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 339(10), 659–666 (1998).
- Haissaguerre M, Jais P, Shah DC et al.: Catheter ablation of chronic atrial fibrillation targeting the reinitiating triggers. J. Cardiovasc. Electrophysiol. 11(1), 2–10 (2000).
- Haissaguerre M, Jais P, Shah DC et al.: Electrophysiological end point for catheter ablation of atrial fibrillation initiated from multiple pulmonary venous foci. Circulation 101(12), 1409–1417 (2000).
- Jais P HM, Macle L, Choi KJ et al.: Distinctive electrophysiological properties of pulmonary veins in patients with atrial fibrillation. Circulation 106(19), 2479–2485 (2002).
- Hocini M, Ho SY, Kawara T et al.: Electrical conduction in canine pulmonary veins: electrophysiological and anatomic correlation. Circulation 105(20), 2442–2448 (2002).
- Haissaguerre M, Sanders P, Hocini M et al.: Catheter ablation of long-lasting persistent atrial fibrillation: critical structures for termination. J. Cardiovasc. Electrophysiol. 16(11), 1125–1137 (2005).
- Haissaguerre M, Hocini M, Sanders P et al.: Catheter ablation of long-lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias. J. Cardiovasc. Electrophysiol. 16(11), 1138–1147 (2005).
- Takahashi Y, O’Neill MD, Hocini M et al.: Effects of stepwise ablation of chronic atrial fibrillation on atrial electrical and mechanical properties. J. Am. Coll. Cardiol. 49(12), 1306–1314 (2007).
- O’Neill MD, Wright M, Knecht S et al.: Long-term follow-up of persistent atrial fibrillation ablation using termination as a procedural endpoint. Eur. Heart J. 30(9), 1105–1112 (2009).
- Takahashi Y, O’Neill MD, Hocini M et al.: Effects of stepwise ablation of chronic atrial fibrillation on atrial electrical and mechanical properties. J. Am. Coll. Cardiol. 49(12), 1306–1314 (2007).
- Oral H, Chugh A, Yoshida K et al.: A randomised assessment of the incremental role of ablation of complex fractionated electrograms for long-lasting persistent atrial fibrillation. J. Am. Coll. Cardiol. 53(9), 782–789 (2009).
- Elayi CS, Verma A, Di Biase L et al.: Ablation for long-standing permanent atrial fibrillation: results from a randomised study comparing three different strategies. Heart Rhythm 5(12), 1658–1667 (2008).
- Earley MJ, Abrams DJ, Staniforth AD, Sporton SC, Schilling RJ: Catheter ablation of permanent atrial fibrillation: medium term results. Heart 92(2), 233–238 (2006).
- Seow SC, Lim TW, Koay CH, Ross DL, Thomas SP: Efficacy and late recurrences with wide electrical pulmonary vein isolation for persistent and permanent atrialfibrillation. Europace 9(12), 1129–1133 (2007).
- Oral H, Pappone C, Chugh A et al.: Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N. Engl. J. Med. 354(9), 934–941 (2006).
- Seidl K, Schwacke H, Zahn R, Rameken M, Drogemuller A, Senges J: Catheter ablation of chronic atrial fibrillation with noncontact mapping: are continuous linear lesions associated with ablation success? Pacing Clin. Electrophysiol. 26(2 Pt1), 534–543 (2003).
- Haïssaguerre M, Hocini M, Sanders P et al.: Localized sources maintaining atrial fibrillation organized by prior ablation. Circulation 113(5), 616–625 (2006).
- Saoudi N, Cosio F, Waldo A et al.: Classification of atrial flutter and regular atrial tachycardia according to electrophysiologic mechanism and anatomic bases: A statement from a Joint Expert Group from the Working Group of Arrhythmias of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. J. Cardiovasc. Electrophysiol. 12, 852–866 (2001).
- Patel A, Markowitz SM: Atrial tachycardia: mechanisms and management. Expert Rev. Cardiovasc. Ther. 6, 811–822 (2008).
- Gerstenfeld EP, Callans DJ, Dixit S et al.: Mechanisms of organized left atrial tachycardias occurring after pulmonary vein isolation. Circulation 110(11), 1351–1357 (2004).
- Ouyang F, Antz M, Ernst S et al.: Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double Lasso technique. Circulation 111(2), 127–135 (2005).
- Karch MR, Zrenner B, Deisenhofer I et al.: Freedom from atrial tachyarrhythmias after catheter ablation of atrial fibrillation: a randomized comparison between 2 current ablation strategies. Circulation 111(22), 2875–2880 (2005).
- Mesas CE, Pappone C, Lang CC et al.: Left atrial tachycardia after circumferential pulmonary vein ablation for atrial fibrillation: electroanatomic characterization and treatment. J. Am. Coll. Cardiol. 44(5), 1071–1079 (2004).
- Chae S, Oral H, Good E et al.: Atrial tachycardia after circumferential pulmonary vein ablation of atrial fibrillation: mechanistic insights, results of catheter ablation, and risk factors for recurrence. J. Am. Coll. Cardiol. 50(18), 1788–1790 (2007).
- Wazni OM, Saliba W, Fahmy T et al.: Atrial arrhythmias after surgical maze: findings during catheter ablation. J. Am. Coll. Cardiol. 48(7), 1405–1409 (2006).
- Knecht S, Hocini M, Wright M et al.: Left atrial linear lesions are required for successful treatment of persistent atrial fibrillation. Eur. Heart J. 29(19), 2359–2366 (2008).
- Calkins H, Brugada J, Packer DL et al.: HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation developed in partnership with the European Heart Rhythm Association (EHRA) and the European Cardiac Arrhythmia Society (ECAS); in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Endorsed and approved by the governing bodies of the American College of Cardiology, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons and the Heart Rhythm Society. Europace 9(6), 335–379 (2007).
- Oral H, Chugh A, Good E et al.: Radiofrequency catheter ablation of chronic atrial fibrillation guided by complex electrograms. Circulation 115(20), 2606–2612 (2007).
- Rostock T, Steven D, Lutomsky B et al.: Chronic atrial fibrillation is a biatrial arrhythmia. Data from catheter ablation of chronic atrial fibrillation aiming arrhythmia termination using a sequential ablation approach. Circ. Arrhythmia Electrophysiol. 1, 344–353 (2008).
- Takahashi Y, Takahashi A, Miyazaki S et al.: Electrophysiological characteristics of localized reentrant atrial tachycardia occurring after catheter ablation of longlasting persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 20, 623–629 (2009).
- Jais P, Matsuo S, Knecht S, Weerasooriya R et al.: A deductive mapping strategy for atrial tachycardia following atrial fibrillation ablation: importance of localized reentry. J. Cardiovasc. Electrophysiol. 20, 480–491 (2009).
- Chugh A, Oral H, Lemola K, Hall B et al.: Prevalence, mechanisms, and clinical significance of macroreentrant atrial tachycardia during and following left atrial ablation for atrial fibrillation. Heart Rhythm 2(5), 464–471 (2005).
- Themistoclakis S, Schweikert RA, Saliba WI et al.: Clinical predictors and relationship between early and late atrial tachyarrhythmias after pulmonary vein antrum isolation. Heart Rhythm 5(5), 679–685 (2008).
- Bertaglia E, Stabile G, Senatore G et al.: Predictive value of early atrial tachyarrhythmias recurrence after circumferential anatomical pulmonary vein ablation. Pacing Clin. Electrophysiol. 28(5), 366–371 (2005).
- Rostock T, Drewitz I, Steven D: Characterisation, mapping and catheter ablation of recurrent atrial tachycardias following stepwise ablation of long lasting persistent atrial fibrillation. Circ. Arrhythmia Electrophysiol. DOI: 10.1161/ CIRCEP.109.899021 (2010) (In Press).
- Gerstenfeld EP, Marchlinski FE: Mapping and ablation of left atrial tachycardias occurring after atrial fibrillation ablation. Heart Rhythm 4(3 Suppl.), S65–S72 (2007).
- Lickfett L, Schwab JO, Lewalter T: Advanced mapping techniques in atrial fibrillation. J. Interv. Card. Electrophysiol. 22(2), 155–159 (2008).
- Waldo AL: Atrial flutter: entrainment characteristics. J. Cardiovasc. Electrophysiol. 8(3), 337–352 (1997).
- Waldo AL: From bedside to bench: entrainment and other stories. Heart Rhythm 1(1), 94–106 (2004).
- Mohamed U, Skanes AC, Gula LJ et al.: A novel pacing maneuver to localize focal atrial tachycardia. J. Cardiovasc. Electrophysiol. 18(1), 1–6 (2007).
- Sanders P, Hocini M, Jais P et al.: Characterisation of focal atrial tachycardia using high density mapping. J. Am. Coll. Cardiol. 46(11), 2088–2099 (2005).
- Weerasooriya R, Jais P, Wright M et al.: Catheter ablation of atrial tachycardia following atrial fibrillation ablation. J. Cardiovasc. Electrophysiol. 20, 833–838 (2009).
- Jais P, Hocini M, Hsu LF et al.: Technique and results of linear ablation at the mitral isthmus. Circulation 10, 2996–3002 (2004).
- Hocini M, Jais P, Sanders P et al.: Techniques, evaluation and consequences linear block at the left atrial roof in paroxysmal atrial fibrillation: a prospective randomised study. Circulation 112, 3688–3696 (2005).
- Daoud EG, Weiss R, Augostini R et al.: Proarrhythmia of circumferential left atrial lesions for management of atrial fibrillation. J. Cardiovasc. Electrophysiol. 17(2), 157–165 (2006).
▪ Provides excellent illustration of the proper procedure for mitral isthmus line ablation and validation.
▪ Provides excellent illustration of the proper procedure for left atrial roof line ablation and validation.