Review Article - Interventional Cardiology (2011) Volume 3, Issue 3
Selecting patients for percutaneous mitral valve therapy
- Corresponding Author:
- Samir R Kapadia
Cleveland Clinic, Department of Cardiovascular Medicine, Cleveland, OH, USA
Tel: +1 216 444 6735
Fax: +1 216 445 6176
E-mail: kapadis@ccf.org
Abstract
Keywords
annuloplasty,MitraClip™,mitral regurgitation,mitral valve repair
The current standard of care for patients with severe mitral regurgitation (MR) is cardiac surgery. However, there is a large patient population suffering from MR that is currently not treated with heart surgery because of significant morbidity and mortality risks [1]. In eligible cases, mitral valve (MV) repair should be preferred over MV replacement. Lack of universal expertise for MV repair and moderate long-term success in correcting functional MR are some of the current challenges associated with surgery. Over the last decade we have witnessed important advances in the development of transcatheter techniques for MV repair. Multiple new devices are under different stages of evaluation for percutaneous mitral valve repair. Percutaneous annuloplasty devices are currently in the earlier stages of clinical investigation. In contrast, the edge-to-edge repair using the MitraClip device (Evalve, CA, USA), simulating the surgical Alfieri stitch via percutaneous approach, has been demonstrated to be a safe and feasible technique.
Anatomy & mechanism of regurgitation
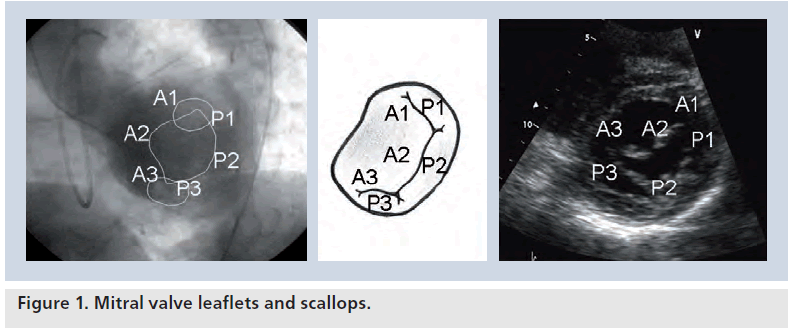
The MV tissue is composed of two leaflets: anterior and posterior. The leaflets are divided into three or more scallops by small indentations. These scallops are identified as P1, P2 and P3 from lateral to medial in the posterior leaflet. The middle scallop (P2) is generally larger than the other two. The corresponding scallops of the anterior leaflet are defined as A1, A2 and A3. Edge-to-edge repair primarily depends on stitching the corresponding leaflets responsible for the MR (Figure 1).
The mitral annulus is a saddle-shaped structure composed of fibrous and muscular fibers. It is a dynamic structure and moves synchronously during the cardiac cycle. It undergoes substantial area changes throughout the cardiac cycle reaching maximum in diastole and minimum in systole. Mitral annular dysfunction generally occurs in the posteromedial region of the valve, which is mostly composed of muscular tissue and hence vulnerable to ischemia. Annuloplasty procedures aim to limit the further dilatation of the annulus, which contributes to malcoaptation of mitral leaflets in dilated LV. Coronary sinus (CS), owing to its close proximity to the posterior mitral annulus, provides a percutaneous access route for percutaneous annuloplasty procedures.
A wide variety of disease conditions, including degenerative (myxomatous) disease, ischemic heart disease, cardiomyopathy, rheumatic disease and infective endocarditis, can lead to MR by causing anatomical abnormalities in any of the components of MV apparatus. MR is basically classified as degenerative and functional. In the Western world, MV prolapse is the most common abnormality associated with degenerative MV disease, resulting from both leaflet redundancy and chordal elongation [2]. In contrast to degenerative MR, MV tissue and attached structures are intact in functional MR cases. Functional MR is caused by malcoaptation of normal leaflets secondary to remodeling of the left ventricle (LV) [3]. Both ischemic (coronary artery disease) and nonischemic heart diseases (e.g., idiopathic dilated cardiomyopathy) may cause ‘functional’ MR via multiple different mechanisms, including impaired LV wall motion, LV dilatation, and papillary muscle displacement and dysfunction. Annular dilatation and papillary muscle displacement are typical characteristics of ischemic LV remodeling and dilatation. Because tendinous chords are not extensible, papillary muscle displacement exerts traction on the leaflet, causing tethering, apical leaflet displacement and impaired coaptation between the two leaflets. Together with annular flattening, enlargement and reduced contraction, MV tenting affects leaflet coaptation and causes functional MR.
Need for percutaneous treatment option
▪ High-risk patients
There is a large patient population suffering from MR that is currently not treated with heart surgery because of significant morbidity and mortality risks [1]. Whether repair or replacement, the surgical approach to MR carries all the risks of open-heart surgery, and many of the patients considered for surgery are limited by comorbidities that are common in this patient group. The Euro Heart Survey revealed that the patients with valvular heart disease are often elderly with a high frequency of cardiovascular risk factors [4]. In this survey, 396 patients had severe symptomatic (New York Heart Association [NYHA] class II or greater) MR as assessed by Doppler echocardiography1 (grade ≥3). A decision not to operate was taken in 193 patients (49%) [5]. In multivariable analysis, decreased left ventricular ejection fraction (odds ratio [OR]: 1.39 per 10% decrease; 95% CI: 1.17–1.66; p = 0.0002), nonischemic etiology (OR: 4.44; 95% CI: 1.96–10.76; p = 0.0006), older age (OR: 1.40 per 10‑year increase; 95% CI: 1.15–1.72; p = 0.001), increased Charlson comorbidity index (OR: 1.38 per 1 point increase; 95% CI: 1.12–1.72; p = 0.004), and grade 3 MR (OR: 2.23; 95% CI: 1.28–3.29; p = 0.005) were associated with the decision not to operate. 1-year survival was 96.0 + 1.4% in patients with a positive decision for intervention versus 89.5 + 2.3% in those with a negative decision (p = 0.02).
▪ Surgical perspective Options
Two different MV operations are currently used for surgical treatment: MV repair and MV replacement. In general, MV repair is preferred to replacement because of improved survival, better preservation of left ventricular function, and increased freedom from thromboembolism and side effects of chronic anticoagulation [6,7]. In a meta-analysis of 29 studies including nearly 10,000 subjects, OR for early mortality, comparing replacement to repair, was 2.24 (1.78–2.80), and total survival hazard ratio was 1.58 (1.41–1.78), indicating worse outcomes among those undergoing MV replacement [8]. The Society of Thoracic Surgeons (STS) database demonstrated an uprising trend for the overall MV repair-rate from 51 to 69% between 2000 and 2007 in the USA [9].
The classic MV repair technique developed by Carpentier primarily involved quadrangular leaflet resection for those with prolapse, transposition of normal chords to other areas of prolapsing leaflet tissue if needed, and a remodeling annuloplasty with a complete ring prosthesis [10].
Another MV repair method in which the leading edges of the mitral leaflets are approximated by use of a suture (Alfieri procedure or ‘bow-tie’ repair), creating a double orifice MV, was introduced by Alfieri and colleagues [11]. The double-orifice repair is technically simple, but careful evaluation of the MV is necessary in selecting the right site for the approximation of the leaflets and the appropriate extension of the suture. The aim of the procedure is to completely abandon the regurgitation while maintaining the largest possible MV orifice area. Inadequate application of the technique may result either in residual MR or in mitral stenosis.
Annuloplasty is the mainstay of the MV repair and is performed in almost all of the surgical repairs regardless of the technique used. Annuloplasty corrects the annular dilatation, improves leaflet coaptation by decreasing anteroposterior dimension of the annulus, improves the durability of repair by reducing the tension on suture lines and prevents future annular dilatation. Because of its supportive role on surgical sutures, annuloplasty is generally included in every kind of surgical repair. Annular remodeling can be achieved by prosthetic annuloplasty devices, suture alone or suture with other supportive materials such as pericardial or dacron strips. Whichever material is used, the purpose is to plicate the annulus and reduce the annular circumference.
In patients with degenerative MV disease, the annuloplasty procedure serves as an adjunct to MV repair and increases the durability of the repair [12]. Functional MR is considered to be principally a ‘ventricular problem’ associated with annular dilatation and papillary muscle displacement and consequent leaflet tethering. In case of functional MR, the annuloplasty itself generally constitutes the whole repair procedure.
▪ Surgical outcomes Degenerative MR
The most common lesion encountered in degenerative MV disease is posterior mitral leaflet (PML) prolapse. In general clinical practice, approximately 40% of MVs are repaired and 60% replaced [13]. However, in experienced centers, repairs exceed 90% [14,15]. Gillinov et al. reported the results of 1072 patients who underwent primary isolated MV repair for valvular regurgitation caused by degenerative disease [12]. At 10 years, freedom from reoperation was 93%. Among 30 patients who required reoperation for late MV dysfunction, the repair failed in 16 (53%) patients as a result of progressive degenerative disease. Repair durability was found to be greatest in patients with isolated PML prolapse who had posterior leaflet resection and annuloplasty.
In a report of 3383 patients undergoing surgery for isolated PML prolapse, repair was performed in 97% [16]. In this series, 15‑year survival was 76%, superior to the age- and sex-matched US population. At 10 years, freedom from mitral reoperation was 97%, and 77% had no or 1+ MR; 11% had ≥3+ MR. It was reported that repair durability was jeopardized by failure to use a prosthetic annuloplasty, left atrial enlargement, and left ventricular remodeling and dysfunction.
Repair of anterior mitral leaflet (AML) prolapse is somewhat more challenging than that of PML prolapse. However, with the utilization of newer surgical techniques such as chordae replacement, chordae transposition, chordae shortening or papillary muscle repositioning, better outcomes are achieved. In a recent report by Seeburger et al., 5-year freedom from reoperation rate was 96.1% (95% CI: 94.3–97.4) for patients with isolated PML prolapse and 92.4% (95% CI: 84–96.6) for patients with isolated AML prolapse (p = 0.5) [17]. In contrast to previous studies that showed poorer outcomes for patients with AML prolapse, this group extensively used neochordae construction with premeasured loops in those patients.
Functional MR
Functional MR is the result of left ventricular remodeling, dilatation and dysfunction leading to geometric reconfiguration of the mitral apparatus, including papillary muscle displacement and annular dilatation. MV leaflets become tethered resulting in failure in proper leaflet coaptation. The most common cause of functional MR is ischemic heart disease. Contrary to degenerative MR, the role of valvular surgery on outcomes in functional MR is controversial. Surgical treatment options include coronary artery bypass grafting (CABG) alone or with concomitant MV annuloplasty or replacement. The most common repair technique in functional MR is placing an undersized annuloplasty ring to reduce mitral annulus size. In a serial transthoracic echocardiographic follow-up study of 51 patients who underwent CABG and restrictive annuloplasty with stringent downsizing of the mitral annulus, residual MR was absent/minimal at 2‑year follow-up, associated with a significant reduction in left atrial dimension and LV reverse remodeling [18]. However, whether or not MV annuloplasty improves outcomes over and above CABG alone is debated. In a study comparing CABG alone (100 patients) to CABG plus annuloplasty (290 patients) in patients with chronic, severe (≥3+) functional ischemic MR due to previous myocardial infarction and low ejection fraction (<45%), it was reported that addition of MV annuloplasty to CABG had no evident survival benefit [19]. In this report, 10-year survival in the CABG alone and CABG plus annuloplasty group was 47 and 39%, respectively (p = 0.6). Patients undergoing CABG alone were more likely to have ≥3+ postoperative MR than those undergoing CABG plus annuloplasty (48 vs 12% at 1 year; p < 0.0001). However, after the procedures, the NYHA functional class substantially improved in both groups (p < 0.001) and remained improved. At 5 years, 23% of patients undergoing CABG plus annuloplasty and 25% undergoing CABG alone were in NYHA functional class III/IV.
Wu et al. analyzed the impact of MV annuloplasty on mortality risk in patients with MR and left ventricular systolic dysfunction [20]. In their report, 126 patients with dilated cardiomyopathy underwent MV annuloplasty and no clear mortality benefit was observed with MV annuloplasty compared with medical therapy. Even after excluding the patients with coronary artery disease, the lack of clinical benefit persisted in the MV annuloplasty group.
In functional MR patients, current literature suggests revascularization in ischemic patients. However, whether ischemic or not, the benefits of MV annuloplasty in dilated cardiomyopathy patients is still controversial.
Percutaneous treatment options
▪ Percutaneous edge-to-edge repair
The simplicity of Alfieri stitch, with its potential percutaneous applicability, quickly drew the attention of the interventional cardiologists. St Goar was the first to demonstrate that an endovascular system can be successfully used to perform the edge-to-edge repair technique in a nondiseased porcine model [21]. After extensive testing in animals revealed considerable efficacy, a US FDA Investigational Device Exemption-approved Phase I safety feasibility trial (Endovascular Valve Edge-to-Edge Repair Study [EVEREST]) was initiated for the percutaneous edge-to-edge repair device, MitraClip™ Mitral Valve Repair System (Evalve Inc., CA, USA).
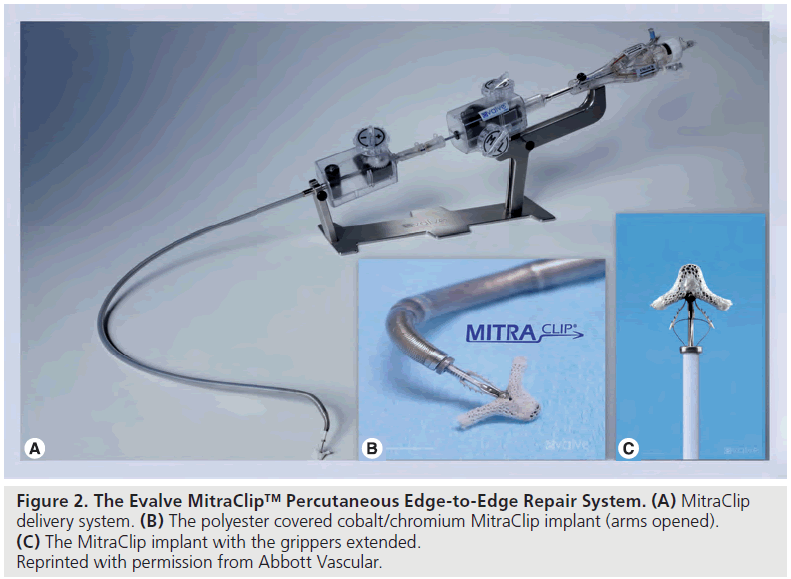
MitraClip Mitral Valve Repair System is a multiaxial catheter system utilizing a clip to grasp and stitch the mitral leaflets percutaneously by a transvenous transseptal route. The system is composed of three main subsystems (Figure 2):
▪▪A steerable guide catheter
▪▪A clip delivery system
▪▪The MitraClip device (implant)
The guide catheter is steerable using a steering knob on the proximal end of the catheter, which allows flexion and lateral movement of the distal tip. The 24 F guide catheter tapers to 22 F at the distal tip. After a transseptal puncture, the distal tip of the guide catheter is advanced into the left atrium over a guidewire with a tapered dilator.
The clip delivery system is advanced through the guide catheter with the clip attached to its distal end. The clip delivery system is steerable using a two knob co-axial system that permits 3D positioning.
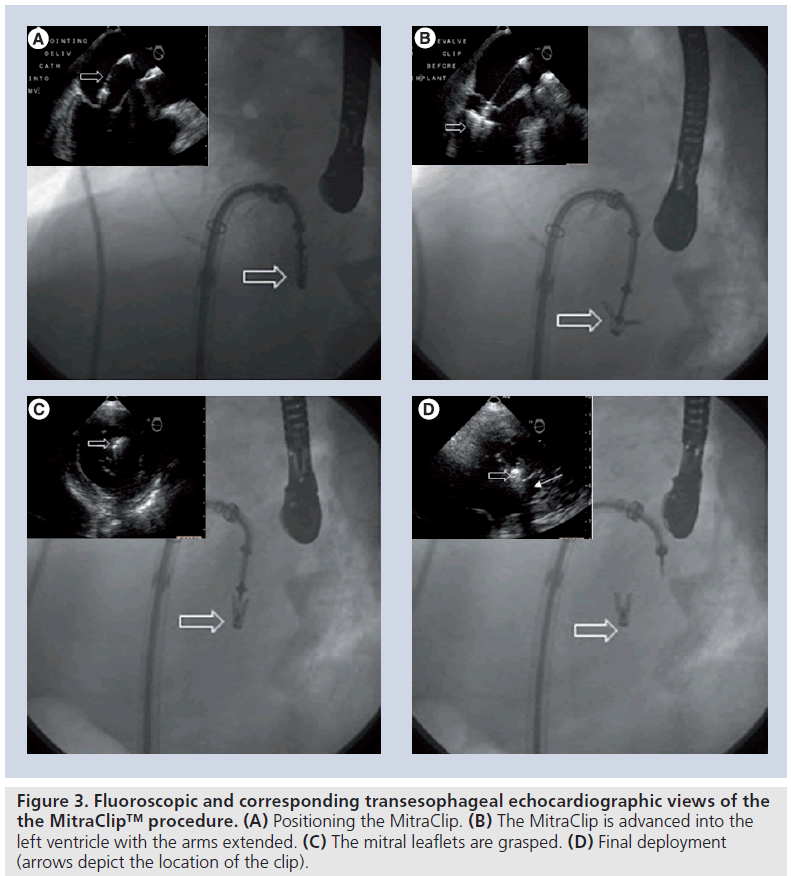
The clip is a polyester-covered cobalt/chromium implant with two arms that are opened and closed by control mechanisms on the clip delivery system. In the closed position, the clip has an outside diameter of 15 F and in the fully opened position the two arms have a span of 20 mm. The clip is designed to grasp a valve tissue of up to 8 mm in height and 4 mm in width in order to replicate the surgical Alfieri stitch. U-shaped gripping elements are placed in the inner portion of the clip. These grippers are small, flexible, multi-prolonged friction elements that appose and stabilize the leaflet tissue against the clip arms. When the clip is closed, leaflet tissue is secured by clip arms on the ventricular side and by the grippers on the atrial side (Figure 3). The clip can be repositioned using echocardiographic guidance to attain the best possible result before final deployment.
Figure 3: Fluoroscopic and corresponding transesophageal echocardiographic views of the the MitraClip™ procedure. (A) Positioning the MitraClip. (B) The MitraClip is advanced into the left ventricle with the arms extended. (C) The mitral leaflets are grasped. (D) Final deployment (arrows depict the location of the clip).
▪ Is percutaneous edge-to-edge repair an option?
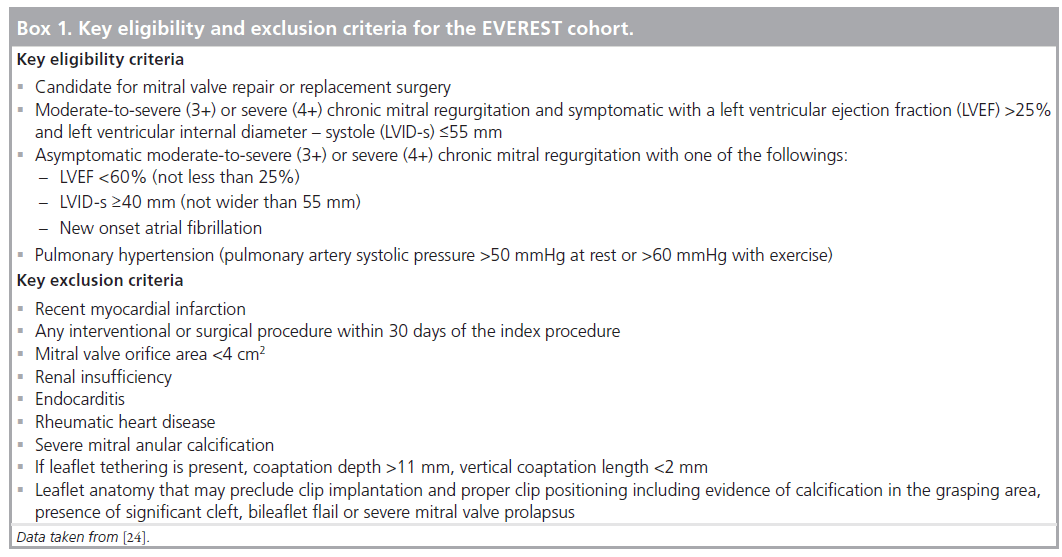
After the encouraging results in animal models a FDA Investigational Device Exemptionapproved Phase I safety feasibility trial (EVEREST) was initiated. The Phase I study aimed to evaluate the safety and the feasibility of the percutaneous edge-to-edge MV repair technique [22]. The key eligibility and exclusion criteria for the EVEREST cohort is listed in Box 1. The primary end point for the EVEREST I trial was safety at 30 days. Safety was defined as freedom from death, myocardial infarction, cardiac tamponade, cardiac surgery for failed clip or device, clip detachment, permanent stroke or septicemia. Secondary safety end points included in-hospital vascular complications, 30-day and 6-month bleeding, endocarditis, clip thrombosis, hemolysis and cardiac surgery for late device failure. Feldman et al. reported the midterm durability and safety of the MitraClip device in the initial EVEREST cohort [23]. This report included the midterm results of the 107 patients of which 23 (21%) had pure functional MR and the rest had either pure degenerative MR or degenerative disease combined with functional MR. One clip was placed in 65 patients (61%) and two clips in 31 patients (29%). The composite primary efficacy end point (freedom from MR >2+, freedom from cardiac surgery for valve dysfunction and freedom from death at 12 months) was 66%. In patients with acute procedural success, freedom from death was 95.9, 94.0 and 90.1% and freedom from surgery was 88.5, 83.2 and 76.3% at 1, 2 and 3 years, respectively. The 23 patients with functional MR showed similar acute results and durability compared with the overall population.
After an initial Phase I feasibility trial demonstrated that percutaneous edge-to-edge MV repair can be performed safely and the degree of MR can be reduced significantly with the percutaneous approach, the trial proceeded to Phase II (EVEREST II) in 2007 to evaluate the performance of endovascular mitral repair in comparison to open MV surgery [24]. The trial is being conducted at 37 sites in the USA and Canada and has enrolled 279 patients. A total of 184 patients were randomized to the MitraClip procedure (device group) and 95 patients were randomized to surgery (control group). The primary efficacy end point of the EVEREST II trial is freedom from the composite end point of death from any cause, surgery for valve dysfunction, and moderate-severe (3+) or severe (4+) MR at 12 months. The composite primary safety end point was major adverse events (MAEs) at 30 days, defined as freedom from death, myocardial infarction, nonelective cardiac surgery for adverse events, renal failure, transfusion of ≥2 U of blood, reoperation for failed surgery, stroke, gastrointestinal complications requiring surgery, ventilation for >48 h, deep wound infection, septicemia and new onset of permanent atrial fibrillation (determined at 12 months).
EVEREST II is designed and powered with a pre-specified superiority safety margin and noninferiority effectiveness margin to show the superiority of the device regarding safety and to show the noninferiority of the device compared with control treatment [24]. It was reported that the primary safety end points were observed in 9.6 and 57% in the device and control group, respectively, which reveals a clear superiority for the MitraClip device regarding safety (pre-specified margin: 6%; observed difference: 47.4%; Psup < 0.0001). However, it must be noted that this clear superiority mostly derived from the difference of need for transfusion ≥two units between the groups. Need for transfusion ≥2 units was 8.8 versus 53.2% in the device and control group, respectively (p < 0.0001). Conventional repair surgery obviously requires more transfusions. Within this context, some commentators argued that radiation exposure due to long f luoroscopy times should also be included in the safety end points. However, even without the transfusion criteria, MitraClip™ still retains its superiority regarding to safety end points (0.7 vs 16.5%; p < 0.0001). In both groups, patients demonstrated significant improvement in left ventricular function, NYHA functional class and quality of lifeIn the EVEREST cohort, 41 patients in the device group had unsuccessful initial procedures. Of those patients, 28 were referred to surgery. Nine patients with an initially successful MitraClip procedure also required surgery for late onset device failure, which makes a total of 37 cases who required surgery after the the MitraClip procedure. In the 12-month followup, the outcomes of this patient subgroup was found to be as successful as the initial control group, which shows surgery is still a safe and effective option after a failed or aborted procedure [25].
▪ Mitraclip in high-risk patients
EVEREST researchers formed an additional cohort of 78 high-risk patients who were not randomized in the EVEREST II trial. This registry, the EVEREST High Risk Registry (EVEREST HRR), included patients who have a prohibitively high risk for surgery. Symptomatic patients with 3+ or 4+ MR (either functional or degenerative) and predicted procedural mortality risk >12% (STS calculated or surgeon estimated based on pre-specified comorbidities) were included in this registry. In September 2009, Whitlow from the Cleveland Clinic (OH, USA) presented the results of this registry at the Transcatheter Cardiovascular Therapeutics meeting [26]. In this registry, the primary end point was 30-day mortality and the secondary end point was MAEs. Major effectiveness end points were MR reduction, freedom from death, NYHA class, left ventricular dimensions and re-hospitalization for congestive heart failure at 12 months. Patients with a left ventricular ejection fraction ≤20% and/or left ventricular end systolic diameter >60 mm, unsuitable mitral leaflet anatomy for the procedure and MV area <4 cm2 were not enrolled into this registry. Patients who were eligible for inclusion in the HRR but for some reason (e.g., institutional review board approval, patient refusal, insurance reasons) could not be treated with MitraClip served as a control population. Whitlow reported that the procedural success was 96% in the HRR. A total of 46 patients required one clip, and 29 patients required two clips for procedural success. In three patients, the procedure was unsuccessful and no clips were implanted. The predicted 30-day mortality – based on the STS scores – of the patient cohort was 18.2%. The observed mortality in those patients was 7.7% (p = 0.006). The total number of patients who met a MAE was 20 (six deaths, one renal failure, one permanent atrial fibrillation, one prolonged [>48 h] ventilation and 11 blood transfusions [≥two Units]). There was no difference in 30-day mortality between the HRR group and HRR control group. However, freedom from death at 12 months was 76.4 and 55.3%, respectively, in the HRR group and HRR control group (p = 0.037). There was a 45% reduction in the rate of re-hospitalizations from congestive heart failure in the HRR group (p = 0.02) compared with their pre-procedural re-hospitalization rates. Both end systolic and end diastolic diameters and ejection fractions were significantly improved in the HRR group compared with their baseline. EVEREST HRR revealed that the MitraClip device can be successfully implanted in high-risk patients who are considered ineligible for surgery and clinical benefits are found to be superior to the conventional medical therapy. The improvement in mortality rate, NYHA class, ventricular functions and re-hospitalization rates were sustained at 12 months.
▪ Percutaneous annuloplasty
As with surgical annuloplasty, the aim of the percutaneous annuloplasty procedures is to decrease the annular dimensions. There are several experimental methods still under investigation for percutaneous mitral annuloplasty. CS annuloplasty is the most intriguing approach. The CS is in close relationship with the posterior mitral annulus and is easily accessible through the venous system and right atrium. CS annuloplasty would address the annular dilatation component of the functional MR. However, in the majority of cases, the CS is located along the wall of the left atrium (i.e., superior to) rather than at the same level as the MV annulus. The distance between the CS and the MV annulus is also variable. Another potential concern with CS device implantation is compression of the coronary arterial system.
Currently, there are three main CS annuloplasty systems under clinical evaluation: The Monarch™ annuloplasty system (Edwards Lifesciences LLC, CA, USA), The Carillon™ Mitral Contour System (Cardiac Dimensions®, Inc., WA, USA) and the Viacor Shape Changing Rods system (Viacor, Inc., MA, USA).
The Monarch device consists of two selfexpandable nitinol stent-like anchors connected by a bridge. One anchor is deployed distally in the posterior interventricular vein and the second anchor is deployed proximally in the CS adjacent to the ostium. After deployment in the CS, tension develops progressively as the spring shortens during the following weeks. The Clinical Evaluation Of the Edwards Lifesciences Percutaneous Mitral Annuloplasty System for the Treatment of Mitral Regurgitation (EVOLUTION-I) feasibility and safety trial of the Monarch annuloplasty system reported successful device implantation in 82% of patients (59 out of 72 patients) and a significant reduction in MR in the majority of patients. However, the 30-day rate of major adverse coronary events (comprising death, myocardial infarction and cardiac tamponade) was 9% and there was evidence of coronary compression in more than 25% (15 out of 59 patients) of all cases [27]. The EVOLUTION-II trial with long-term follow-up and control group was started but has currently been halted.
The Carillon device is a double-anchor nitinol device that is introduced via the jugular vein and advanced to the CS. During deployment, the central segment is progressively shortened and the immediate effect on the posterior mitral annulus is monitored. Results of the prospective multicenter Carillon Mitral Annuloplasty Device European Union Study (AMADEUS) have been published [28]. Successful device implantation was achieved in 30 out of 48 patients (60%). In over 30% of patients, the device had to be recaptured owing to various reasons (e.g., coronary compromise, insufficient reduction of MR and device failure). The overall rate of major adverse coronary events was 14.6% at 30 days and included death, myocardial infarction and CS perforation and/or dissection. A third device, the Percutaneous Transvenous Mitral Annuloplasty system (Viacor Inc.) uses a multilumen polytetrafluoroethylene catheter that is placed in the CS. Subsequently, up to three nitinol rods with variable stiffness can be inserted into the catheter in order to affect CS conformation. The safety of the device was assessed in a preliminary study. The Phase I Percutaneous Transvenous Mitral Annuloplasty (PTOLEMY) trial enrolled 27 patients with heart failure and moderate-to-severe functional MR from five centers in Europe and Canada [29]. Eight patients were excluded before implantation because of unsuitable CS anatomy. Of the 19 patients who underwent implantation, 13 had a reduction in MR severity and in the device was ineffective in six. Four patients subsequently required removal of the device: one patient at day 7 for device fracture and three patients referred to surgery because of device migration and/or diminished efficacy. Five patients (18.5%) had long-term implants with reductions in MR severity. Following the completion of the Phase I studies and the ensuing device iterations, the PTOLEMY II trial is currently underway and will treat up to 60 patients at investigational sites in Europe, Canada and the USA.
There are several other investigational methods for percutaneous mitral annuloplasty. However, because of the lack of clinical data, these procedures are beyond the scope of this article.
Selection of patients
▪ Is intervention indicated?
Mitral regurgitation is considered severe if any of the following are present: angiographic 3–4+ regurgitation; vena contracta width greater than 0.7 cm with large central MR jet (area >40% of left atrium area) or with a wall impinging jet of any size, swirling in LA; regurgitant volume ≥60 ml/beat; regurgitant fraction ≥50%; and regurgitant orifice area ≥0.40 cm2 [30]. According to American College of Cardiology/American Heart Association 2006 guidelines for the management of patients with valvular heart disease, MV surgery is a class I recommendation for patients with acute symptomatic severe MR, for patients with chronic severe MR who have NYHA functional class II, III or IV symptoms, and for asymptomatic patients with chronic severe MR and mild-to-moderate LV dysfunction defined as ejection fraction 0.30–0.60, and/or end-systolic dimension ≥40 mm. MV surgery is a class IIa recommendation for patients with chronic severe MR who have new-onset atrial fibrillation, pulmonary hypertension (pulmonary artery systolic pressure >50 mmHg at rest or >60 mmHg with exercise). If MV repair is highly likely, MV surgery is class IIa indication for patients with chronic severe MR due to a primary abnormality of the mitral apparatus and NYHA functional class III–IV symptoms and severe LV dysfunction. MV repair is reasonable in experienced surgical centers for asymptomatic patients with chronic severe MR and preserved LV function (class IIa). MV repair may be considered for patients with chronic severe secondary MR due to severe LV dysfunction (ejection fraction <0.30) who have persistent NYHA functional class III–IV symptoms despite optimal therapy for heart failure, including biventricular pacing (class IIb).
Mitral valve surgery is not recommended for asymptomatic patients with MR and preserved LV function in whom significant doubt about the feasibility of repair exists. Isolated MV surgery is not indicated for patients with mild or moderate MR.
▪ Is surgery a good option?
Despite the well-known advantages of MV repair, a study conducted on the STS Adult Cardiac Surgery Database revealed a repair rate of 41% among the patients undergoing surgery for MR [31]. Several patient characteristics were independently associated with a decreased odds of mitral repair (versus replacement), including mitral stenosis (OR: 0.09; 95% CI: 0.08–0.11) and active endocarditis (OR: 0.21; 95% CI: 0.17–0.25). While substantial variability in repair rates was observed among low-volume surgeons, increased surgeon-level mitral volume was independently associated with an increased probability of mitral repair. The presence of mitral stenosis had the strongest observed negative association against mitral repair.
Cardiosurgical operative risk can be assessed using the logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE) and the STS score. These scoring systems predict the risk of operative mortality and morbidity after adult cardiac surgery on the basis of patient demographics and clinical variables. Several studies have demonstrated that the EuroSCORE overestimates mortality for various cardiac surgery populations including patients undergoing MV surgery [32]. The STS registry includes data from nearly 90% of cardiac surgery providers in the USA [33]. STS models provide estimates of risk of mortality as well as several nonfatal complications such as stroke, renal failure and prolonged ventilation.
Patients with degenerative MR should be considered for surgical MV repair as long as they have favorable valvular anatomy and acceptable surgical risk. For functional MR there is no clear evidence favoring surgical MV repair.
▪ Selection for percutaneous procedures
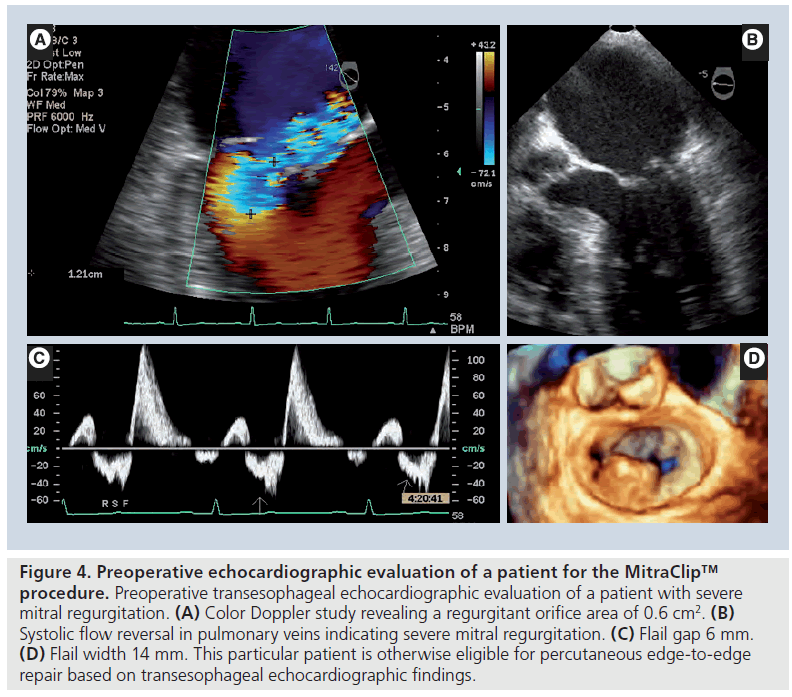
Current data suggest that the MitraClip procedure yields favorable clinical results in patients who are ineligible for MV surgery. In patients who are surgical candidates, it has a better safety profile than the conventional MV repair surgery [25]. The lack of an adjunctive annuloplasty did not have an adverse impact on midterm outcomes and patients with functional MR showed similar benefits compared with the patients with degenerative MR. Pre-procedural echocardiography is vital for patient selection (Figure 4). Owing to technical limitations, the MitraClip device cannot be used in patients with a flail segment width ≥15 mm, or flail gap ≥10 mm. If leaflet tethering is present, coaptation depth >11 mm, or vertical coaptation length <2 mm also renders patient ineligible for the procedure. Evidence of calcification in the grasping area, presence of a significant cleft in the leaflets, bileaflet flail or severe bileaflet prolapse, and lack of both primary and secondary chordal support also preclude clip implantation and the procedure should be avoided in such patients. Patients with concurrent mitral stenosis are also ineligible for the procedure. The aforementioned strict inclusion criteria of the EVEREST studies should be kept in mind during patient selection. With this caveat, it appears that the MitraClip procedure should be considered for selected patients who have degenerative or functional MR. It is an attractive treatment modality for patients with severe symptomatic MR patients who have prohibitively high surgical risk for open MV surgery.
Figure 4: Preoperative echocardiographic evaluation of a patient for the MitraClip™ procedure. Preoperative transesophageal echocardiographic evaluation of a patient with severe mitral regurgitation. (A) Color Doppler study revealing a regurgitant orifice area of 0.6 cm2. (B) Systolic flow reversal in pulmonary veins indicating severe mitral regurgitation. (C) Flail gap 6 mm. (D) Flail width 14 mm. This particular patient is otherwise eligible for percutaneous edge-to-edge repair based on transesophageal echocardiographic findings.
Defining the appropriate patient population is much more difficult for percutaneous annuloplasty devices. The distance between the CS and mitral annulus is quite variable in humans. The success of the CS annuloplasty approach depends on close proximity of the CS to the mitral annulus. Therefore, unusual anatomic separation between the CS and mitral annulus may result in a device being inefficient in modifying the mitral annulus. In 80% of patients, the left circumflex (LCX) coronary artery crosses under the CS [34]. The LCX artery impingement could occur due to a close anatomic relationship between the artery and CS. Appreciation of the relationship between the CS and LCX has been defined as a critical factor for the safety of these devices. Detailed imaging studies are vital for procedural success. Contrast-enhanced multidetector computed tomography (MDCT) can help identify patients in whom the LCX artery will not be a problem when annuloplasty via the CS is attempted [34].
Another issue with the annuloplasty procedures is the tethering pattern of the AML. As a result of the separate sites of chordal insertion, the differential tethering effect on the basal and distal portions of AML varies according to the predominant direction of the tethering forces. Lee et al. described the tethering patterns of AML by measuring the angle between the annular plane and the basal (ALAbase) portion and tip (ALAtip) of AML [35]. Three distinct types of AML tethering pattern can be recognized on echocardiography. In type I, the AML is minimally tethered along its entire length. The rapid visual clues on echocardiography for this type of tethering are a small ALAtip with minimal AML bend. Type II represents basal chordae, posteriorly directed tethering and is characterized by a prominent AML bend on echocardiography. Type III represents severe apical tethering of both basal and distal AML and is recognizable by the large ALAtip with a variable, but usually milder, degree of AML bend. In general, patients with types I and II AML tethering have a good chance of sustained success in mitral annuloplasty, whereas patients with type III are at high risk of MR recurrence. Detailed evaluation of the tethering patterns seems essential for procedural success.
Role of multimodality imaging
Considering the complex 3D nature of the mitral apparatus, no single imaging modality is entirely adequate alone in percutaneous MV procedures. The annulus of the MV can be best assessed by 3D transesophageal echocardiography as well as MDCT. Fluoroscopy is very helpful to see the extent of mitral annular calcification and its circumferential involvement. Annulus size should be measured in different planes to evaluate its contribution to MR and if it would respond to percutaneous treatment. If the annulus is enlarged considerably, edgeto- edge repair may not be sufficient. On the other hand, if the MV annulus size is small, one may create mitral stenosis if edge-to-edge repair is attempted, especially if more than one clip is needed. Coaptation length and depth of the segment close to the prolapsing part is also critical for the success of the procedure. If the closure is too deep to the annular plane (coaptation depth >11 mm) or the leaflets do not have enough apposition (coaptation length <2 mm) it makes the grasp difficult and clip detachment more likely. If there is flail segment, the flail gap should not be more than 10 mm. Most of these measurements are easily made with transesophageal echocardiography.
Assessment of leaflets for functional MR is even more challenging. Functional MR causes leaflet motion restriction along with prolapse, such as motion of the opposing leaflet. It is important to analyze which segment of the leaflet is restricted (A1–3 or P1–3) and also which part of the anterior leaflet in the vertical plane is tethered (apical vs basal segment). The apical segment can be assessed by measuring the angle of closure compared with annulus in specific cuts using transesophageal echocardiography or MDCT. It is unlikely that far lateral (anterior) or far medial (posterior) abnormalities can be treated successfully with either a clip or CS device. Furthermore, annuloplasty devices are unlikely to work if there is considerable apical tethering. If there is enough coaptation length, clip may be a better option for these patients.
Another important point is the systolic displacement of posterior papillary muscle due to inferior myocardial infarction, which may ‘rotate’ the leaflets causing a funnel like defect in the posteromedial commissure and a prolapse- like deformity on the anterolateral side. This can be analyzed by 3D transesophageal echocardiography or MDCT scan by measuring left ventricular circumference at the tips and bases of the papillary muscle. If there is significant ‘twist’ to mitral apparatus in systole, it may be impossible to correct that by annuloplasty. Edge-to-edge repair may normalize this twist, making subvalvular apparatus more aligned.
Future perspective
Limited clinical data have provided promising results for percutaneous edge-to-edge MV repair with MitraClip, regardless of whether the etiology is functional or degenerative, provided sufficient leaflet tissue is available for a successful grasping. Future large-scale randomized trials may help to refine the technology and establish the clinical applicability. Even though current evidence, based on relatively short-term follow-up, has not revealed a clear need for annuloplasty, there are still ongoing concerns about long-term durability of the percutaneous procedure in functional MR patients without an annuloplasty. Percutaneous annuloplasty procedures are still under development and will provide additional durability to the MitraClip procedure. Clinical evidence for percutaneous annuloplasty devices is limited. Detailed anatomic evaluation of CS and tethering patterns of the mitral leaflets may predict the patients who may benefit from the percutaneous annuloplasty procedures. Future studies are required to evaluate the potential benefits of adjunctive catheter-based annuloplasty systems. At present, surgical repair is still the treatment of choice considering durability and low procedural risk in patients with degenerative MV disease without significant comorbidities especially in young patients [9]. The percutaneous treatment should be chosen in selected cases where surgical options are not as durable or risk is high, for example, in high-risk operative candidates, especially with functional MR. Furthermore, patients who are otherwise ineligible for surgical repair may benefit from percutaneous approaches. Evolving technologies and rigorous clinical trials may expand the indications in the future. Transcatheter MV stent implantation is another step forward, which has yielded promising results in animal studies [36]. The atrioventricular passage is not tubuler, therefore, fixation of the valve is demanding [37]. On the other hand, advances in surgical techniques have to be taken into consideration when applicability of percutaneous procedures is being assessed. Minimally invasive MV surgery such as right-sided minithoracotomy or robotic repair are associated with good perioperative results and less hospital stay, even in high-risk patients [38]. There is an increasing trend of surgical MV repair even in octogenarians, who are considered as a high-risk group [39]. With this wide variety of options and moving targets, patient selection is quite challenging and needs to be assessed by a multidisciplinary team of cardiologists and cardiac surgeons while appropriate clinical trial data become available.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
▪▪ Surgical mitral valve repair is the preferred treatment for significant mitral regurgitation.
▪▪ There is a large patient population suffering from mitral regurgitation that is currently not treated with heart surgery owing to significant morbidity and mortality risks.
▪▪ Transcatheter techniques have been developed to overcome the challenges of surgical repair.
▪▪ The Endovascular Valve Edge-to-Edge Repair study (EVEREST) tested the feasibility and safety of the MitraClip™ device.
▪▪ Percutaneous edge-to-edge repair using the MitraClip device, simulating the surgical Alfieri stitch via percutaneous approach, proved to be a safe and feasible technique.
▪▪ Mitraclip is an attractive treatment modality for patients with severe symptomatic mitral regurgitation who have high surgical risk for mitral valve surgery.
References
- Goodney PP, Stukel TA, Lucas FL, Finlayson EV, Birkmeyer JD: Hospital volume, length of stay, and readmission rates in high-risk surgery. Ann. Surg. 238(2), 161–167 (2003).
- Foster E: Clinical practice. Mitral regurgitation due to degenerative mitral-valve disease. N. Engl. J. Med. 363(2), 156–165 (2010).
- Hussaini A, Kar S: Percutaneous mitral valve repair: potential in heart failure management. Curr. Heart Fail. Rep. 7(1), 22–26 (2010).
- Iung B, Baron G, Butchart EG et al.: A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur. Heart J. 24(13), 1231–1243 (2003).
- Mirabel M, Iung B, Baron G et al.: What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur. Heart J. 28(11), 1358–1365 (2007).
- Lawrie GM: Mitral valve repair vs replacement. Current recommendations and long-term results. Cardiol. Clin. 16(3), 437–448 (1998).
- Lee EM, Shapiro LM, Wells FC: Superiority of mitral valve repair in surgery for degenerative mitral regurgitation. Eur. Heart J. 18(4), 655–663 (1997).
- Shuhaiber J, Anderson RJ: Meta-analysis of clinical outcomes following surgical mitral valve repair or replacement. Eur. J Cardiothorac. Surg. 31(2), 267–275 (2007).
- Gammie JS, Sheng S, Griffith BP et al.: Trends in mitral valve surgery in the United States: results from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann. Thorac. Surg. 87(5), 1431–1437; discussion 7–9 (2009).
- Carpentier A, Chauvaud S, Fabiani JN et al.: Reconstructive surgery of mitral valve incompetence: ten-year appraisal. J. Thorac. Cardiovasc. Surg. 79(3), 338–348 (1980).
- Alfieri O, Maisano F, De Bonis M et al.: The double-orifice technique in mitral valve repair: a simple solution for complex problems. J. Thorac. Cardiovasc. Surg. 122(4), 674–681 (2001).
- Gillinov AM, Cosgrove DM, Blackstone EH et al.: Durability of mitral valve repair fordegenerative disease. J. Thorac. Cardiovasc. Surg. 116(5), 734–743 (1998).
- Savage EB, Ferguson TB Jr, DiSesa VJ: Use of mitral valve repair: analysis of contemporary United States experience reported to the Society of Thoracic Surgeons National Cardiac Database. Ann. Thorac. Surg. 75(3), 820–825 (2003).
- David TE, Ivanov J, Armstrong S, Christie D, Rakowski H: A comparison of outcomes of mitral valve repair for degenerative disease with posterior, anterior, and bileaflet prolapse. Thorac. Cardiovasc. Surg. 130(5),1242–1249 (2005).
- Suri RM, Schaff HV, Dearani JA et al.: Survival advantage and improved durability of mitral repair for leaflet prolapse subsets in the current era. Ann. Thorac. Surg. 82(3), 819–826 (2006).
- Johnston DR, Gillinov AM, Blackstone EH et al.: Surgical repair of posterior mitral valveprolapse: implications for guidelines and percutaneous repair. Ann. Thorac. Surg. 89(5), 1385–1394 (2010).
- Seeburger J, Borger MA, Doll N et al.: Comparison of outcomes of minimally invasive mitral valve surgery for posterior, anterior and bileaflet prolapse. Eur.Cardiothorac. Surg. 36(3), 532–538 (2009).
- Bax JJ, Braun J, Somer ST et al.: Restrictive annuloplasty and coronary revascularization in ischemic mitral regurgitation results in reverse left ventricular remodeling. Circulation 110(11 Suppl. 1), II103–II108(2004).
- Mihaljevic T, Lam BK, Rajeswaran J et al.: Impact of mitral valve annuloplasty combined with revascularization in patients with functional ischemic mitral regurgitation. Am. Coll. Cardiol. 49(22), 2191–2201(2007).
- Wu AH, Aaronson KD, Bolling SF et al.: Impact of mitral valve annuloplasty on mortality risk in patients with mitral regurgitation and left ventricular systolic dysfunction. J. Am. Coll. Cardiol. 45(3), 381–387 (2005).
- St Goar FG, Fann JI, Komtebedde J et al.: Endovascular edge-to-edge mitral valve repair: short-term results in a porcine model. Circulation 108(16), 1990–1993 (2003).
- Feldman T, Wasserman HS, Herrmann HC et al.: Percutaneous mitral valve repair usingthe edge-to-edge technique: six-month results of the EVEREST Phase I Clinical Trial. Am. Coll. Cardiol. 46(11), 2134–2140(2005).
- Feldman T, Kar S, Rinaldi M et al.: Percutaneous mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST (endovascular valve edge-to-edge repair study) cohort. J. Am. Coll. Cardiol. 54(8), 686–694 (2009).
- Mauri L, Garg P, Massaro JM et al.: The EVEREST II Trial: design and rationale for a randomized study of the evalve mitraclip system compared with mitral valve surgery for mitral regurgitation. Am. Heart J. 160(1), 23–29 (2010).
- Feldman T, Foster E, Glower DG et al.: Percutaneous repair or surgery for mitral regurgitation. N. Engl. J. Med. 364(15), 1395–1406 (2011).
- Whitlow PL: Percutaneous edge-to-edge evalve mitral valve repair in the US ‘High Risk’ Registry. Presented at: Transcatheter Cardiovascular Therapeutics (TCT) Meeting.San Francisco, CA, USA, 21–25 September (2009).
- Kuck HK, Webb JG, Harnek J et al.: Percutaneous treatment of functional mitral regurgitation: interim EVOLUTION study results with the MONARC system. Am.Cardiol. 100(8 Suppl. 1), 58L (2007).
- Schofer J, Siminiak T, Haude M et al.: Percutaneous mitral annuloplasty for functional mitral regurgitation: results of the CARILLON Mitral Annuloplasty Device European Union Study. Circulation 120(4), 326–333 (2009).
- Sack S, Kahlert P, Bilodeau L et al.: Percutaneous transvenous mitral annuloplasty: initial human experience with a novel coronary sinus implant device. Circ. Cardiovasc. Interv. 2(4), 277–284 (2009).
- Bonow RO, Carabello BA, Chatterjee K et al.: ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 48(3), e1–e148 (2006).
- Bolling SF, Li S, O’Brien SM, Brennan JM, Prager RL, Gammie JS: Predictors of mitral valve repair: clinical and surgeon factors. Ann. Thorac. Surg. 90(6), 1904–1912 (2010).
- Kaartama T, Heikkinen L, Vento A: An evaluation of mitral valve procedures using the European system for cardiac operative risk evaluation. Scand. J. Surg. 97(3), 254–258 (2008).
- Shahian DM, O’Brien SM, Filardo G et al.: The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 1 – coronary artery bypass grafting surgery. Ann. Thorac. Surg. 88(Suppl. 1), S2–S22(2009).
- Choure AJ, Garcia MJ, Hesse B et al.: In vivo analysis of the anatomical relationship of coronary sinus to mitral annulus and left circumflex coronary artery using cardiac multidetector computed tomography: implications for percutaneous coronary sinus mitral annuloplasty. J. Am. Coll. Cardiol. 48(10), 1938–1945 (2006).
- Lee AP, Acker M, Kubo SH et al.: Mechanisms of recurrent functional mitral regurgitation after mitral valve repair in nonischemic dilated cardiomyopathy: importance of distal anterior leaflet tethering. Circulation 119(19), 2606–2614 (2009).
- Lozonschi L, Bombien R, Osaki S et al.: Transapical mitral valved stent implantation: a survival series in swine. J. Thorac. Cardiovasc. Surg. 140(2), 422–426 (2010).
- Goetzenich A, Dohmen G, Hatam N et al.: A new approach to interventional atrioventricular valve therapy. J. Thorac. Cardiovasc. Surg. 140(1), 97–102 (2010).
- Seeburger J, Borger MA, Falk V et al.: Minimal invasive mitral valve repair for mitral regurgitation: results of 1339 consecutive patients. Eur. J. Cardiothorac. Surg. 34(4), 760–765 (2008).
- Nloga J, Henaine R, Vergnat M et al.: Mitral valve surgery in octogenarians: should we fight for repair? A survival and quality-of-life assessment. Eur. J. Cardiothorac. Surg. 39(6), 875–880 (2011).