Review Article - Interventional Cardiology (2011) Volume 3, Issue 3
Selecting patients for transcatheter aortic valve implantation
- Corresponding Author:
- Victoria Delgado
Department of Cardiology, Leiden University Medical Center, Albinusdreef 2, 2333 ZA Leiden, The Netherlands
Tel: +31 715 262 020
Fax: +31 715 266 809
E-mail: v.delgado@lumc.nl
Abstract
Keywords
aortic stenosis,clinical assessment,multimodality imaging,Tavi
Aortic valve replacement (AVR) is the standard treatment for patients with symptomatic severe aortic stenosis (AS) [1,2]. Despite the fact that AVR can improve survival and provide symptomatic relief for these patients, as many as one third of elderly patients with indications for surgery were not operated on [3]. This is at least partly due to the perceived high operative risk associated with a combination of factors including advanced age, multiple comorbidities and/or left ventricular dysfunction. As the survival of unoperated patients with symptomatic severe AS is dismal [4], this has led to the search and development for a less invasive but effective therapeutic alternative for this group of patients, who are deemed unsuitable or have contraindications for surgery.
Since the first successful experience in humans in 2002 [5], transcatheter aortic valve implantation (TAVI) has been rapidly expanding with more than 17,000 procedures performed worldwide to date [6]. Besides the promising procedural success rates of 91–94% observed in recent studies [7–9], a marked improvement in transvalvular hemodynamics [8,10] and functional status have been reported following TAVI [10,11]. More importantly, the results of the first randomized controlled trial (the PARTNER trial) comparing TAVI and standard medical therapy (including balloon valvuloplasty) have demonstrated that TAVI was associated with a superior survival rate at 1 year (69 vs 49%) [12].
Advances in transcatheter technology and its delivery systems over the last few years have helped to improve the clinical results of TAVI resulting in a significant reduction in the 30-day mortality rate (from the initial 14.3 to 6% in the most recent series) [8,9,12–15]. However, there are still several areas of concern, including vascular complications, stroke, atrioventricular conduction block, coronary artery obstruction, prosthesis malpositioning/malfunctioning and paravalvular leakage. Careful patient selection is therefore crucial to minimize procedural complications and optimize the success of TAVI. In this selection process, noninvasive imaging plays a key role in providing information on procedural feasibility and helps to select the most appropriate TAVI approach. This article will describe the steps involved in patient selection (both clinical and anatomic aspects) and TAVI strategy planning.
Clinical risk assessment & evaluation of symptoms
Transcatheter aortic valve implantation is currently restricted to patients with high operative risk for AVR and a life expectancy of ≥1 year, as recommended by a joint position statement from the European Association of Cardio- Thoracic Surgery and the European Society of Cardiology, in collaboration with the European Association of Percutaneous Cardiovascular Interventions [16]. As risk assessment and decision making is a complex process in these elderly patients, a multidisciplinary team approach is essential in making accurate and unbiased clinical assessments on an individual basis [17]. Often, the multidisciplinary team (or so-called ‘Heart Team’) involves the clinical cardiologist, imaging specialist, surgeon, interventionalist, anesthesiologist and geriatrician. To evaluate the risk of surgery, multiple risk scores are available such as the logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE) [18], the Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score [101] or the Ambler score [19]. However, as these risk scores are not designed for isolated AVR, there is a discrepancy between the expected and observed mortality for patients undergoing AVR based on these scoring systems [20–22]. While the widely used logistic EuroSCORE has been shown to overestimate mortality [20,21], the STS-PROM score appears to underestimate but closely approximates the actual observed perioperative mortality for this high-risk group undergoing AVR [20]. As a general guide, several risk scores should be used together to provide a better estimate of the risk involved. The current position statement considers a high-risk patient for surgery when the expected mortality is >20% as calculated with the Logistic EuroSCORE and >10% as calculated with the STS-PROM score [16]. However, owing to the aforementioned limitations, clinical judgment takes precedence over these currently applied scoring systems. In addition, some important factors, which are not reflected in the risk scores, such as chest radiation, porcelain aorta, liver cirrhosis and prior aorto-coronary bypass surgery with patent grafts, may preclude an open heart surgery, and in such cases, TAVI may be the next best therapeutic option [16].
In addition, detailed assessment of comorbidities and physical activity status are an integral part of the pre-procedural screening as these factors have an impact on the life expectancy of the elderly patients [23]. Currently, TAVI should not be performed in patients with a life expectancy <1 year [16]. Moreover, the baseline physical frailty score, as assessed with the Karnofsky index [24], is an independent predictor of in-hospital outcomes following TAVI [7]. Therefore, detailed clinical assessment is critical to ensure that TAVI should be reserved for patients who would derive the maximal benefit from such a procedure.
Next, the evaluation of patients’ symptoms is another important aspect of the pre-procedural patient screening. Often, there is a myriad of reasons that may account for the symptoms experienced in the elderly population. At the present stage, TAVI is only recommended for patients with symptoms that are attributed to AS [16].Thus, a complete clinical assessment may need to involve inputs from other expertise such as those from respiratory physicians and geriatricians in order to be certain that these patients truly have symptoms related to AS before they proceed to TAVI.
Assessment & confirmation of AS severity
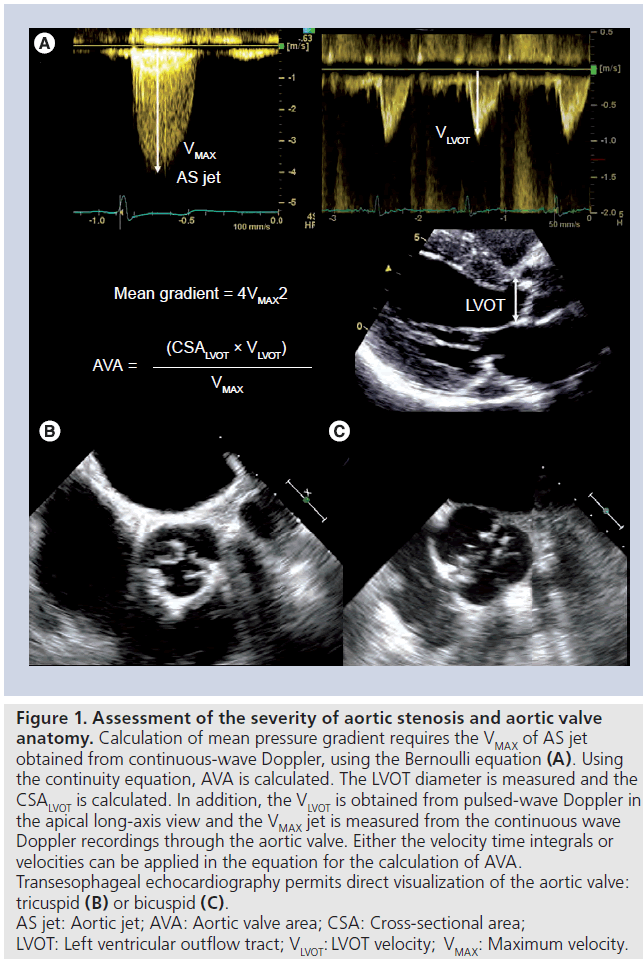
Transthoracic echocardiography (TTE) is the initial modality of choice to assess AS as it readily provides information on valve anatomy, transvalvular hemodynamics and left ventricular response to chronic pressure overload state [25]. Quantification of AS severity relies primarily on the hemodynamic parameters, obtained from echocardiographic Doppler measurements (Figure 1A). Severe AS is defined as an aortic jet velocity >4 m/s and/or a mean pressure gradient >40–50 mmHg and/or an aortic valve area <1 cm2 (or 0.6 cm2/m2 indexed to body surface area) [1,2,25]. In the presence of severe left ventricular systolic dysfunction (ejection fraction ≤40%), patients with true severe AS can present with a relatively low transvalvular pressure gradient (<40 mmHg). The diagnosis of this subgroup of patients with ‘low flow, low gradient AS’ may be challenging and differentiation from other patients with a primary cardiomyopathic disease and a nonstenotic aortic valve (pseudosevere AS) may be difficult [26]. In such cases, dobutamine stress echocardiography should be performed to distinguish between true severe AS from pseudosevere AS [25]. During dobutamine infusion, an increase in transvalvular flow will occur and patients with pseudosevere AS will show an increase in valve area, with little change in transvavular gradient. By contrast, patients with true severe AS will respond by an increase in transvalvular gradient while the aortic valve area remains unchanged [27]. Intervention should be performed in patients with true severe AS who have developed symptoms [1,2].
Figure 1: Assessment of the severity of aortic stenosis and aortic valve anatomy. Calculation of mean pressure gradient requires the VMAX of AS jet obtained from continuous-wave Doppler, using the Bernoulli equation (A). Using the continuity equation, AVA is calculated. The LVOT diameter is measured and the CSALVOT is calculated. In addition, the VLVOT is obtained from pulsed-wave Doppler in the apical long-axis view and the VMAX jet is measured from the continuous wave Doppler recordings through the aortic valve. Either the velocity time integrals or velocities can be applied in the equation for the calculation of AVA. Transesophageal echocardiography permits direct visualization of the aortic valve: tricuspid (B) or bicuspid (C). AS jet: Aortic jet; AVA: Aortic valve area; CSA: Cross-sectional area; LVOT: Left ventricular outflow tract; VLVOT: LVOT velocity; VMAX: Maximum velocity.
Evaluation of TAVI feasibility & selection of procedural approach
After confirmation of severe AS and detailed clinical assessment, a comprehensive and accurate evaluation of the feasibility of TAVI is important to ensure the success of the procedure and minimize procedural related complications. Currently, two types of prosthesis are available: the balloon expandable Sapien XT™ prosthesis (Edwards Lifesciences, Irvine, CA, USA) and the self expandable Medtronic CoreValve Revalving™ (MCR) prosthesis (Medtronic Inc., Luxembourg).
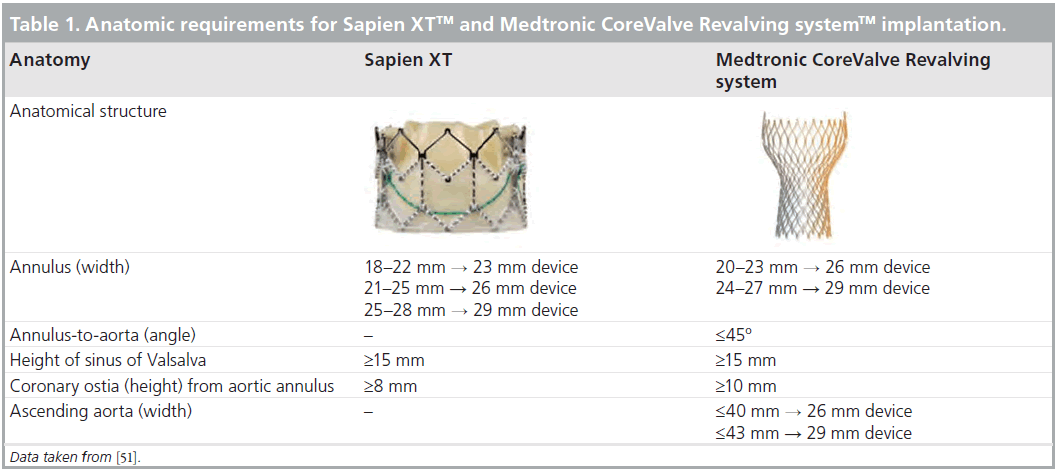
The Sapien XT prosthesis is a trileaflet pericardial bovine valve, mounted within a cobalt-chromium frame that permits thinner struts and lower crimped profile. The available sizes are 23 and 26 mm for an aortic valve annulus of 18–22 mm and 21–25 mm, respectively. The 23 mm prosthesis is mounted onto an 18F transfemoral NovaFlex delivery system (Edwards Lifesciences), whereas the 26-mm valve is crimped onto a 19F NovaFlex delivery system. In addition to a retrograde transfemoral approach, this transcatheter aortic valve can also be implanted antegrade via a transapical approach using the 22F Ascendra 2 delivery system (Edwards Lifesciences). Recently, a 29 mm device has been launched for aortic valve annular dimensions between 25–28 mm. This device can be implanted through a transapical approach. The MCR prosthesis has a different design and is characterized by a 50 mm nitinol frame with three different functional levels: the upper third level that exerts a low radial flow and is placed in the ascending aorta; the middle third level that includes the trifoliate porcine valve and has a constraint design to avoid jailing of the coronary ostia; and the lower third level that exerts a high radial force and anchors the prosthesis within the left ventricular outflow tract. In addition, the lowest 12-mm skirt portion helps to prevent significant paravalvular regurgitation after deployment. This prosthesis is currently available in two sizes (26 and 29 mm for aortic valve annulus of 20–23 and 23–27 mm, respectively) and can be implanted via a transfemoral or transsubclavian approach [28]. These different designs and procedural approaches demand accurate evaluation of the aortic valve annulus and peripheral artery anatomy in order to plan the most appropriate therapeutic strategy. Besides these two key aspects, evaluation of the anatomy of the aortic valve, dimensions of the aortic root and its spatial relationship with coronary ostia and exclusion of contraindications complete the pre-procedural evaluation of patients who are candidates for TAVI (Table 1). The use of multimodality imaging is therefore essential to assess these requirements, and to ensure a successful procedure and prevent complications.
▪ Aortic valve anatomy
Transcatheter aortic valve implantation is indicated in patients with a severely stenotic tricuspid aortic valve. The current position statement considers bicuspid aortic valve anatomy as a contraindication for TAVI due to the risk of incomplete and unfavorable deployment [29]. However, several reports have demonstrated that TAVI is a feasible and safe treatment for bicuspid aortic valve [30,31]. Echocardiography remains the mainstay imaging technique to evaluate the anatomy of the aortic valve. However, poor acoustic windows may challenge the diagnosis of this valvular phenotype. Transesophageal echocardiography (TEE) may be an alternative as it provides superior image quality, permitting better visualization of valve anatomy (Figure 1). In addition, multidetector row computed tomography (MDCT) or MRI may help to differentiate truly bicuspid anatomy from functional bicuspid valves [32].
▪ Aortic valve calcification
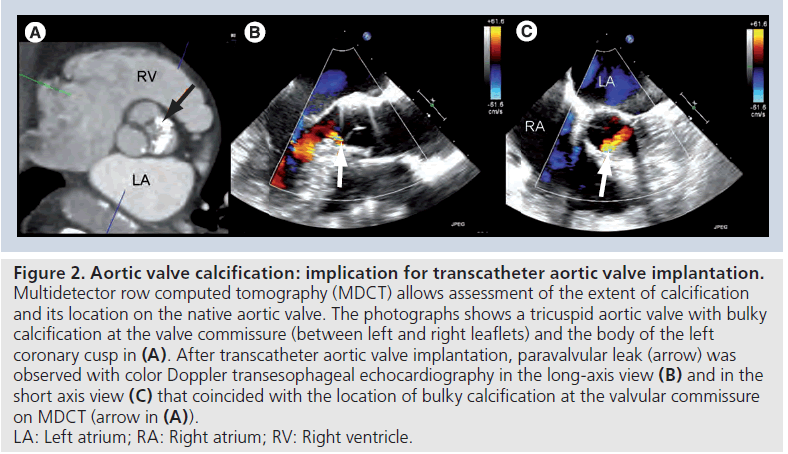
Degenerative aortic valve stenosis is characterized by thickening, retraction and calcification of the aortic valve leaflets. This calcification helps to anchor the transcatheter aortic valve prosthesis. However, extensive and bulky calcifications of the aortic valve may challenge deployment of the prosthesis and has been related to the presence of significant postprocedural aortic valve regurgitation [29,33,34]. Fluoroscopy and echocardiography permit gross evaluation of the extent and location of calcification. However, the spatial resolution of MDCT provides improved image quality to evaluate this aspect (Figure 2). Several studies have demonstrated the role of MDCT to evaluate native aortic valve calcification and have related the extent of valve calcification to the presence of paravalvular aortic regurgitation post-TAVI [33,34]. In a recent study by John and coworkers including 100 patients who underwent TAVI, acute post-procedural valvular regurgitation was significantly correlated with the extent of aortic valve calcification in the landing zone [34]. Furthermore, in a series of 53 patients who underwent TAVI, the extent of valve calcification detected with MDCT was significantly higher in patients with noncircular deployment of prosthesis (3862 vs 1837 Hounsfield unit; p = 0.04) and significant valvular regurgitation (4174 vs 2444 Hounsfield unit; p < 0.001) [33]. Moreover, valve calcification located at the native valve commissures (but not at the valve hinge points or free edge of leaflets) seemed to play a role in determining significant valvular regurgitation following TAVI.
Figure 2: Aortic valve calcification: implication for transcatheter aortic valve implantation. Multidetector row computed tomography (MDCT) allows assessment of the extent of calcification and its location on the native aortic valve. The photographs shows a tricuspid aortic valve with bulky calcification at the valve commissure (between left and right leaflets) and the body of the left coronary cusp in (A). After transcatheter aortic valve implantation, paravalvular leak (arrow) was observed with color Doppler transesophageal echocardiography in the long-axis view (B) and in the short axis view (C) that coincided with the location of bulky calcification at the valvular commissure on MDCT (arrow in (A)). LA: Left atrium; RA: Right atrium; RV: Right ventricle
▪ Aortic valve annular dimensions
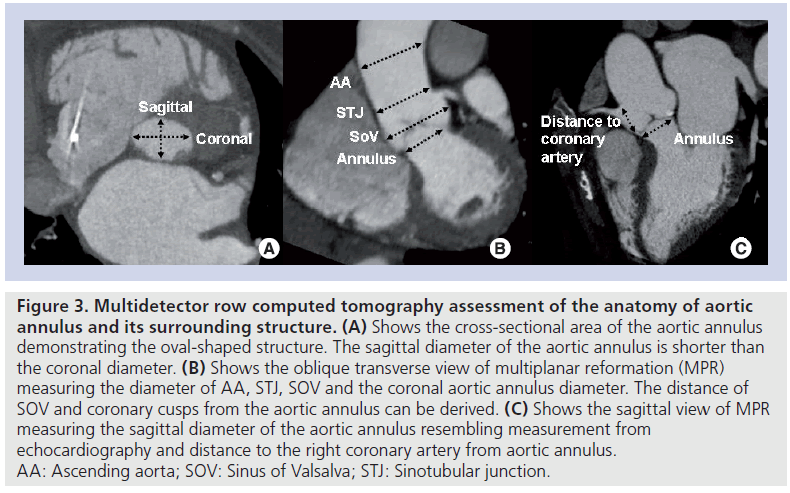
Accurate assessment of the aortic valve annular dimensions is key to select the most appropriate transcatheter prosthesis size. Migration of the prosthesis, when the prosthesis size is too small, or rupture of the aortic valve annulus, when the prosthesis size is too large, are two potential procedural complications that can be avoided by accurate measurement of the aortic valve annulus and appropriate sizing of the prosthesis. 2D echocardiography is the most commonly used technique to measure the aortic valve annulus [35,36]. However, 3D imaging techniques have demonstrated that the aortic valve annulus is not circular, but oval shaped (Figure 3A). Currently, the gold standard imaging technique for the measurement of aortic valve annulus has not been established. However, several studies have demonstrated the superior accuracy of 3D imaging techniques to assess this functional structure [37]. In a recent series of 53 patients undergoing TAVI, the accuracy of 2D and 3D TEE to measure the aortic valve annulus was evaluated, using MDCT as the gold standard [38]. Circular areas calculated with 2D and 3D TEE significantly underestimated the planimetered cross-sectional areas obtained with MDCT (16.4 and 12.9% underestimation, respectively). By contrast, planimetered areas of the aortic valve annulus, measured with 3D TEE, had better agreement with MDCT planimetered cross-sectional areas and the percentage of underestimation was significantly lower (9.6%). Although the clinical implications of these different evaluations have not been fully elucidated, 3D imaging techniques such as 3D TEE or MDCT may be helpful in patients with borderline aortic annular measurement. In a recent study including AS patients who underwent MDCT prior to TAVI with MCR prosthesis, Schultz et al. demonstrated that prosthesis sizing based on the mean annular diameter ([minimal diameter + maximal diameter]/ 2) obtained from MDCT had the best agreement with the operator choice (74%) [39]. By contrast, the agreement with the operator choice was only 44 or 32% if only the minimal diameter or maximal diameter was used, respectively. Similarly, the study by Messika-Zeitoun et al. reported that using the mean annular diameters measured by MDCT would have changed the prosthesis size in 38% of patients who received Edwards Sapien™ prosthesis [35]. These findings suggest the clinical relevance from incorporating detailed anatomy assessment of the aortic annulus with 3D imaging modalities such as that of 3D TEE or MDCT or MRI.
Figure 3: Multidetector row computed tomography assessment of the anatomy of aortic annulus and its surrounding structure. (A) Shows the cross-sectional area of the aortic annulus demonstrating the oval-shaped structure. The sagittal diameter of the aortic annulus is shorter than the coronal diameter. (B) Shows the oblique transverse view of multiplanar reformation (MPR) measuring the diameter of AA, STJ, SOV and the coronal aortic annulus diameter. The distance of SOV and coronary cusps from the aortic annulus can be derived. (C) Shows the sagittal view of MPR measuring the sagittal diameter of the aortic annulus resembling measurement from echocardiography and distance to the right coronary artery from aortic annulus. AA: Ascending aorta; SOV: Sinus of Valsalva; STJ: Sinotubular junction.
▪ Assessment of aortic root anatomy
In addition to the measurement of the aortic valve annular diameter, the dimensions of the sinus of Valsalva, sino-tubular junction and ascending aorta should be assessed (Figure 3B). Particularly, the dimensions of the aortic root and the ascending aorta are of importance when a MCR prosthesis is implanted. The distal part of this prosthesis accommodates these two anatomical structures, exerting a low radial force and orienting the prosthesis in the direction of blood flow. Dilated ascending aorta (>43 mm) are considered contraindications for self-expandable prostheses (Table 1) [16].
Furthermore, measurement of the height of the coronary ostia, relative to the aortic valve annular plane, is important in order to anticipate potential fatal complications such as occlusion of one of the coronary ostia by a bulky calcified cusp [40,41]. The design of the current prostheses, with open struts in the upper two-thirds of the frame, assures normal flow through the coronary ostia. In particular, the MCR device has a constrained middle part, to avoid jailing of the coronary ostia. By contrast, the Sapien XT device seldom reaches the coronary ostia (11% in a recent series) [33]. A coronary ostia height relative to the aortic valve annular plane of at least 10 mm is currently the recommended minimum height to proceed with the procedure (Figure 3C). These characteristics are best evaluated with MDCT, providing a 3D visualization and accurate measurement.
▪ Assessment of the aorta & peripheral arteries
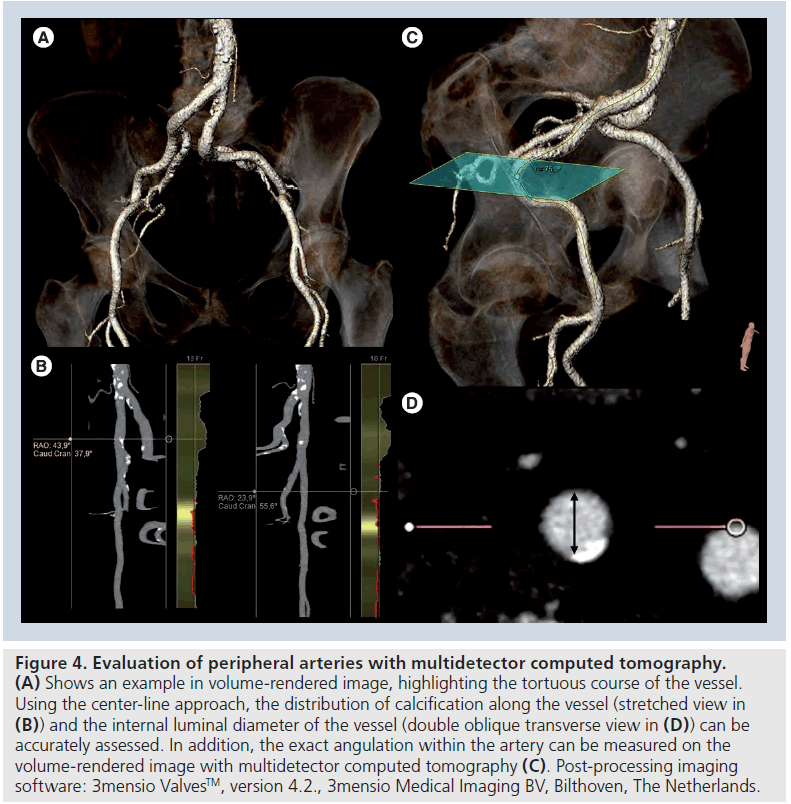
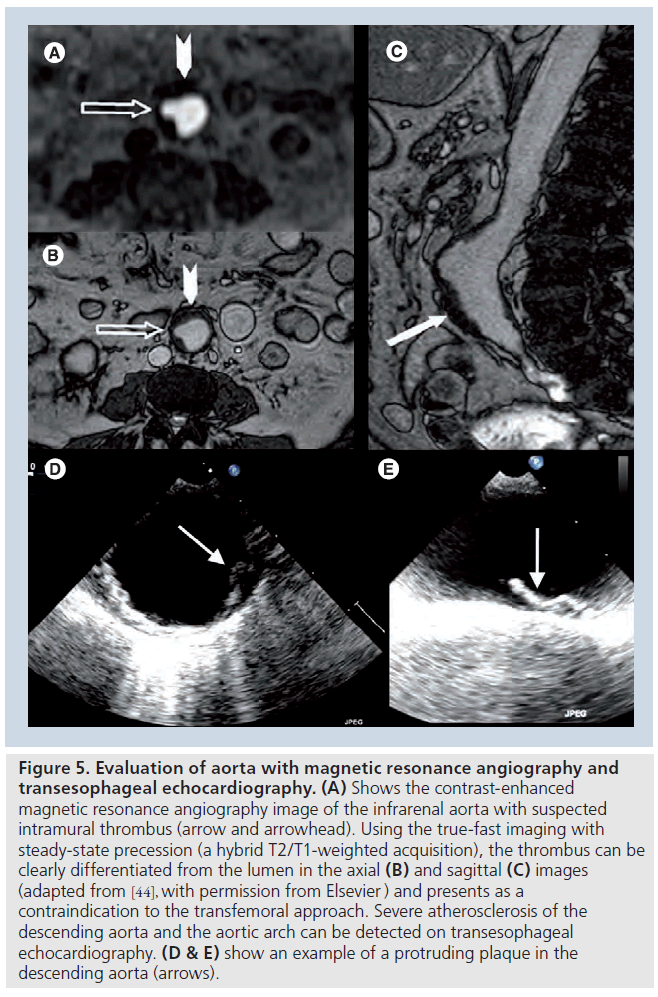
Once the prosthesis size has been selected, the procedural approach (retrograde or antegrade) has to be planned. The retrograde approach via the transfemoral access is usually the approach of first choice. In this aspect, the dimensions of the ilio-femoral arteries will directly determine the feasibility of this procedural approach as the currently available devices require a minimum diameter of at least 6 mm. Traditionally, conventional angiography was considered the gold standard method to assess the dimensions of the ilio-femoral arteries. However, the poor soft-tissue resolution of this imaging technique does not allow accurate assessment of arterial wall disease and calcification. MDCT has demonstrated its superior accuracy to assess the internal diameter of the arterial lumen, the presence of significant atherosclerosis and calcification and the tortuosity of the arteries. These are the crucial determinants of success and feasibility of the transfemoral approach (Figure 4) [42,43]. In a recent series including 37 patients undergoing TAVI, it was demonstrated that MDCT had a high accuracy to evaluate the anatomy of the peripheral arteries, with the advantage of using a lower volume of iodinated contrast compared with invasive angiography [42]. In addition, a new sequence of magnetic resonance angiography, the true-fast imaging with steady-state precession (a hybrid T2/T1-weighted acquisition), provides excellent visualization of vessels without the need for a paramagnetic contrast agent such as gadolinium (Figure 5) [44].
Figure 4: Evaluation of peripheral arteries with multidetector computed tomography. (A) Shows an example in volume-rendered image, highlighting the tortuous course of the vessel. Using the center-line approach, the distribution of calcification along the vessel (stretched view in (B)) and the internal luminal diameter of the vessel (double oblique transverse view in (D)) can be accurately assessed. In addition, the exact angulation within the artery can be measured on the volume-rendered image with multidetector computed tomography (C). Post-processing imaging software: 3mensio ValvesTM, version 4.2., 3mensio Medical Imaging BV, Bilthoven, The Netherlands.
Figure 5: Evaluation of aorta with magnetic resonance angiography and transesophageal echocardiography. (A) Shows the contrast-enhanced magnetic resonance angiography image of the infrarenal aorta with suspected intramural thrombus (arrow and arrowhead). Using the true-fast imaging with steady-state precession (a hybrid T2/T1-weighted acquisition), the thrombus can be clearly differentiated from the lumen in the axial (B) and sagittal (C) images (adapted from [44], with permission from Elsevier ) and presents as a contraindication to the transfemoral approach. Severe atherosclerosis of the descending aorta and the aortic arch can be detected on transesophageal echocardiography. (D & E) show an example of a protruding plaque in the descending aorta (arrows).
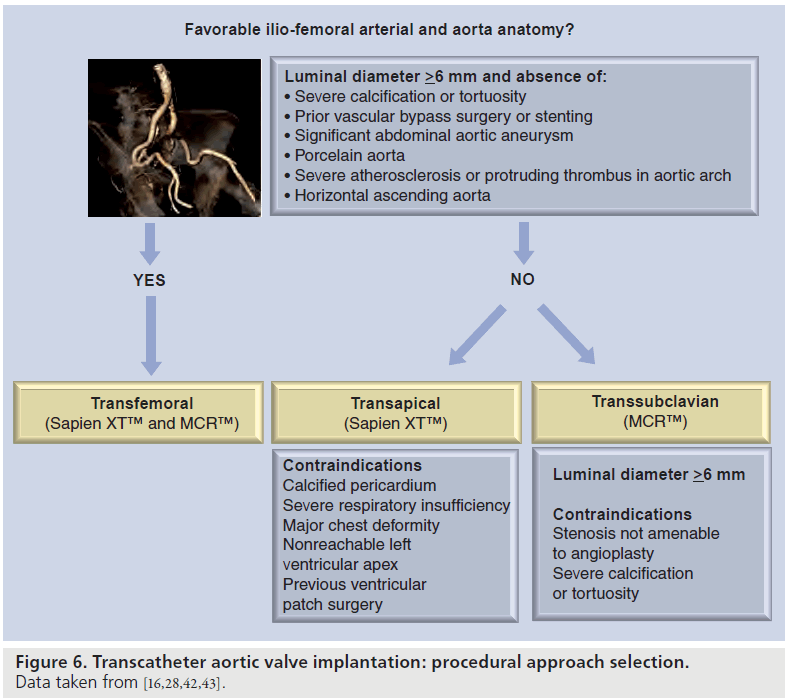
Evaluation of the aortic arch and descending aorta is also of importance when a transfemoral approach is considered. According to current recommendations, severe angulation of the aorta, severe atherosclerosis of the arch, and coarctation and aneurysm of the abdominal aorta with protruding mural thrombosis contraindicate the transfemoral approach. Severe atherosclerosis of the descending aorta and aortic arch can be detected with TEE (Figure 5). In addition, intravascular ultrasound permits characterization of atherosclerotic plaque [45]. MDCT provides 3D visualization of the aorta and is the method of choice to evaluate the presence of extensive calcification (porcelain aorta). Moreover, MRI provides accurate assessment of the aortic wall without need for paramagnetic contrast. Presence of severe aortic atherosclerosis indicates the need for careful manipulation of the catheters during the procedure, in order to avoid thrombo-embolic complications. In such cases, transfemoral approach may not be suitable and an alternative approach (transapical or transsubclavian) should be considered. Figure 6 summarizes the procedural approach selection algorithm. The transapical approach has the advantage of overcoming the problem of aorto–ilio-femoral vascular disease. However, it requires general anesthesia and a small anterior minithoracotomy with direct puncture of the left ventricular apex. This approach is contraindicated in patients with calcified pericardium, severe respiratory insufficiency, major chest deformity and previous left ventricular surgery using a patch [16]. In patient candidates for TAVI with MCR prosthesis who show unsuitable iliofemoral anatomy, transsubclavian approach has been demonstrated to be feasible and safe [28,46].
Exclusion of contraindications
Additional factors concerning the left ventricular function, coronary artery disease and presence of concomitant valvular disease (i.e., severe organic mitral valve regurgitation) must be evaluated prior to TAVI.
A detailed evaluation of left ventricular dimensions and ejection fraction should be performed in patients undergoing TAVI. Impaired left ventricular ejection fraction may increase the risk of hemodynamic instability during TAVI procedures. In addition, severe left ventricular hypertrophy with pronounced sigmoid septum at the level of the left ventricular outflow tract may prove challenging when attempting this procedure using MCR prosthesis. Moreover, the presence of left ventricular thrombus is an established contraindication for TAVI and can be better evaluated by contrast echocardiography [16].
Evaluation of coronary artery anatomy is mandatory before TAVI, as the presence of significant proximal lesions, not amenable to percutaneous coronary intervention, is considered a contraindication to TAVI [16]. Invasive coronary angiography is the gold standard for evaluation of coronary artery anatomy. By contrast, the high prevalence of severe coronary artery calcification in this group of patients may challenge the assessment of coronary artery anatomy using MDCT. In patients with concomitant coronary artery stenoses, the decision of whether to perform coronary revascularization prior to TAVI has to be based on the clinical condition and the anatomy of individual patients. In a recent study of 136 patients with pre-procedural coronary angiogram who underwent TAVI, Masson and coworkers [47] reported that the early mortality and overall 1‑year mortality rates did not differ between patients with no coronary artery stenoses (n = 32), patients with completely revascularized coronary lesions (n = 41) and patients with nonrevascularized or incompletely revascularized coronary lesions of varying ischemic burden (n = 63) as assessed with the Duke Myocardial Jeopardy Score [48]. Of these 136 patients, 15 patients were treated with percutaneous coronary interventions prior to TAVI (with a median time interval of 26 days between procedures; range: 3–100 days). Currently, it is generally advisable to perform revascularization in cases with severe lesions of the left main or the proximal coronary arteries before TAVI as per the current EACTS, ESC and EAPCI position statement [16].
Finally, the presence of significant organic mitral valve regurgitation increases the procedural risk. Particularly, using a MCR prosthesis, the ventricular end of the prosthesis frame may interfere with the motion of the anterior mitral leaflet resulting in or worsening mitral regurgitation [49].
Conclusion
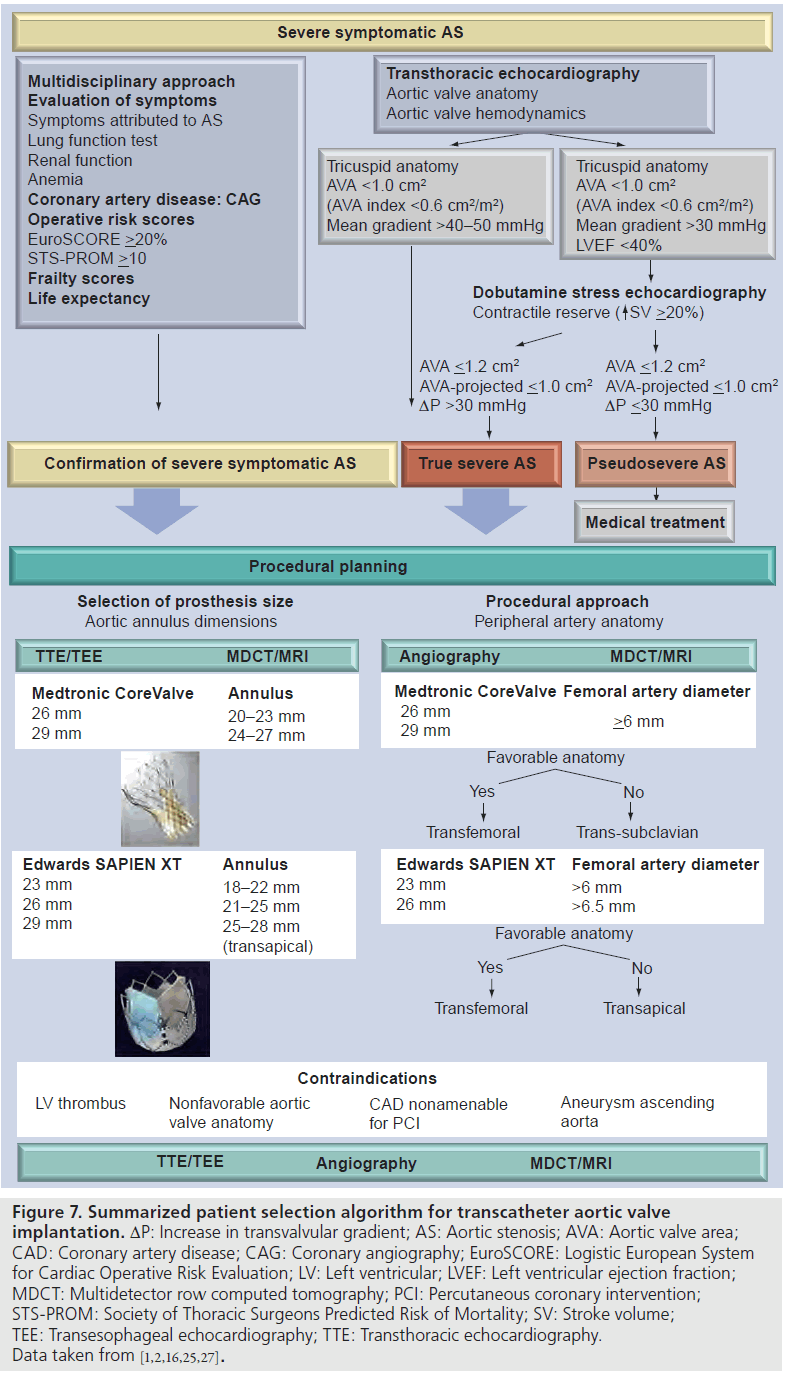
TAVI is a feasible alternative to surgical AVR in patients with severe symptomatic AS and high operative risk. Multidisciplinary evaluation, including accurate clinical evaluation and precise assessment of aortic valve anatomy and function, aortic valve annular dimensions and peripheral artery anatomy, are crucial to optimize the results while reducing the procedural complications rate. Importantly, multimodality imaging plays an important role in the patient selection process. A summarized patient selection algorithm is detailed in Figure 7, highlighting the important factors that need to be considered before TAVI.
Figure 7:Summarized patient selection algorithm for transcatheter aortic valve implantation. DP: Increase in transvalvular gradient; AS: Aortic stenosis; AVA: Aortic valve area; CAD: Coronary artery disease; CAG: Coronary angiography; EuroSCORE: Logistic European System for Cardiac Operative Risk Evaluation; LV: Left ventricular; LVEF: Left ventricular ejection fraction; MDCT: Multidetector row computed tomography; PCI: Percutaneous coronary intervention; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality; SV: Stroke volume; TEE: Transesophageal echocardiography; TTE: Transthoracic echocardiography. Data taken from [1,2,16,25,27].
Future perspective
The number of TAVI procedures is increasing rapidly. Selecting the appropriate patients by detailed clinical assessment and multimodality imaging techniques (the Heart team approach) can improve the results and reduce the rate of complications to a minimum. However, a number of questions still need to be answered. In particular, the reference method and imaging modality to size the aortic valve annulus (TTE/TEE/MDCT/MRI) needs to be defined. In addition, vascular complications and a high rate of atrioventricular conduction block (and the need for a pacemaker, especially with MCR prosthesis) are the main safety issues that remain to be improved. Ongoing research aims to improve the current technology and provide lower profile delivery systems that may help to reduce the number of vascular complications. In the coming years, data on prosthesis durability are eagerly awaited. Degeneration and dysfunction of current transcatheter prosthesis is currently uncommon [10,50]. A transcatheter prosthesis, with durability comparable to that of conventional surgical bioprosthesis, would probably allow this technique to be expanded to younger patients in the future. While awaiting further developments in this technology, patient selection, according to the current recommendation, should be adhered to stringently to increase the success rate of the procedures and minimize the procedural-related complications.
Financial & competing interests disclosure
Kai-Hang Yiu is financially supported by the Hong Kong Heart Foundation. See Hooi Ewe is financially supported by the Ministry of Health Training Scholarship, Singapore. Robert Klautz is a member of one of the advisory boards of Medtronic and receives research support from Edwards Lifesciences, St Jude Medical and Medtronic. Martin J Schalij receives grants from Biotronik, Boston Scientific and Medtronic. Jeroen J Bax receives grants from Biotronik, Medtronic, Boston Scientific Corporation, Lantheus Medical Imaging, St Jude Medical, GE Healthcare and Edwards Lifesciences. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
.No writing assistance was utilized in the production of this manuscript.
Executive summary
.Survival after transcatheter aortic valve implantation in patients with symptomatic aortic stenosis who are not suitable for surgical aortic valve replacement
▪▪ The PARTNER trial demonstrated that transcatheter aortic valve implantation (TAVI) was associated with better survival at 1-year, compared with standard therapy.
▪▪ Requires longer follow-up study to provide data on long-term prosthesis durability and outcomes of patients who underwent TAVI.
Clinical risk assessment before TAVI
▪▪ A multidisciplinary team approach is essential when making accurate and unbiased clinical assessment.
▪▪ TAVI is currently restricted to patients with very high operative risk for surgery and a life expectancy of ≥1year.
▪▪ High operative risk patients are those with a logistic European System for Cardiac Operative Risk Evaluation (EuroScore) >20% and Society of Thoracic Surgeons Predicted Risk of Mortality (STSPROM score) >10%.
Confirmation of aortic stenosis severity
▪▪ Severe aortic stenosis (AS) is primarily assessed by echocardiography and is defined as an aortic jet velocity >4m/s and/or a mean pressure gradient >40–50 mmHg and/or an aortic valve area <1 cm2 (or 0.6 cm2/m2 indexed to body surface area).
▪▪ Dobutamine stress echocardiography should be performed in patients with low flow, low gradient AS to distinguish between true severe AS from pseudosevere AS.
There are two currently available transcatheter prostheses
▪▪ The prostheses are balloon expandable Sapien XTTM (size 23 and 26 mm for aortic valve annulus 18–21 and 22–25 mm, respectively) and self-expandable Medtronic CoreValve Revalving system™ (size 26 and 29 mm for aortic valve annulus 20–23 and 23–27 mm, respectively).
▪▪ Retrograde transarterial (transfemoral or transsubclavian) or antegrade transapical are the procedural approaches.
Evaluation of TAVI feasibility & procedural planning
▪▪ Multimodality imaging is essential to optimize procedural success and to avoid complications.
▪▪ Aortic valve annular dimensions will determine the prosthesis size, whereas peripheral artery anatomy will determine the procedural approach.
▪▪ Exclusion of contraindications: bicuspid aortic valve, severe proximal coronary artery stenosis not amenable to percutaneous intervention and presence of left ventricular thrombus are established contraindications for TAVI.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Vahanian A, Baumgartner H, Bax J et al.: Guidelines on the management of valvular heart disease: the Task Force on the management of valvular heart disease of the European Society of Cardiology. Eur. Heart J. 28, 230–268 (2007).
- Bonow RO, Carabello BA, Chatterjee K et al.: 2008 Focused update incorporated into the ACC/AHA 2006 Guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 52, e1–e142 (2008).
- Iung B, Cachier A, Baron G et al.: Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur. Heart J. 26, 2714–2720 (2005).
- Kojodjojo P, Gohil N, Barker D et al.: Outcomes of elderly patients aged 80 and over with symptomatic, severe aortic stenosis: impact of patient’s choice of refusing aortic valve replacement on survival. Q. J. Med. 101, 567–573 (2008).
- Cribier A, Etchaninoff H, Bash A et al.: Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 106, 3006–3008 (2002).
- Eltchaninoff H. The French TAVI registry. Presented at: EuroPCR. Paris, France, 23 August 2010.
- Buellesfeld L, Wenaweser P, Gerckens U et al.: Transcatheter aortic valve implantation:predictors of procedural success-the Siegburg-Bern experience. Eur. Heart J. 31, 984–991 (2010).
- Rodes-Cabau J, Webb JG, Cheung A et al.: Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J. Am. Coll. Cardiol. 55, 1080–1090 (2010).
- Thomas M, Schymik G, Walther T et al.: Thirty-day results of the SAPIEN aortic bioprosthesis European outcome (SOURCE) registry. a European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation 122, 62–69 (2010).
- Gurvitch R, Wood DA, Tay EL et al.: Transcatheter aortic valve implantation: durability of clinical and hemodynamic outcomes beyond 3 years in a large patient cohort. Circulation 122, 1319–1327 (2010).
- Ussia GP, Mule M, Barbanti M et al.: Quality of life assessment after percutaneous aortic valve implantation. Eur. Heart J. 30, 1790–1796 (2009).
- Leon M, Smith C, Mack M et al.: Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 363, 1597–1607 (2010).
- Grube EM, Buellesfeld LM, Mueller RM et al.: Progress and current status of percutaneous aortic valve replacement: results of three device generations of the CoreValve Revalving System. Circ. Cardiovasc. Interv. 1, 167–175 (2008).
- Webb JG, Altwegg L, Masson JB et al.: A new transcatheter aortic valve and percutaneous valve delivery system. J. Am. Coll. Cardiol. 53, 1855–1858 (2009).
- Zahn R, Gerckens U, Grube E et al.: Transcatheter aortic valve implantation: first results from a multi-centre real-world registry. Eur. Heart J. 32, 198–204 (2011).
- Vahanian A, Alfieri O, Al-Attar N et al.: Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 29, 1463–1470 (2008).
- Fajadet J. Transcatheter aortic valve implantation – patient selection. Presented at: PCR London Valves. London, UK,11 November 2010.
- Roques F, Michel P, Goldstone AR et al.: The logistic EuroSCORE. Eur. Heart J. 24, 882 (2003).
- Ambler G, Omar RZ, Royston P et al.: Generic, simple risk stratification model for heart valve surgery. Circulation 112, 224–231 (2005).
- Brown ML, Schaff HV, Sarano ME et al.: Is the European System for Cardiac Operative Risk Evaluation model valid for estimating the operative risk of patients considered for percutaneous aortic valve replacement?Thorac. Cardiovasc. Surg. 136, 566–571(2008).
- Dewey TM, Brown D, Ryan WH et al.: Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. Thorac. Cardiovasc. Surg. 135, 180–187(2008).
- Wendt D, Osswald BR, Kayser K et al.: Society of Thoracic Surgeons Score is superior to the EuroSCORE determining mortality in high risk patients undergoing isolated aortic valve replacement. Ann. Thorac. Surg. 88, 468–475 (2009).
- Lee SJ, Lindquist K, Segal MR et al.: Development and validation of a prognostic index for 4-year mortality in older adults. JAMA 295, 801–808 (2006).
- Schag CC, Heinrich RL, Ganz PA: Karnofsky performance status revisited: reliability, validity, and guidelines. J. Clin. Oncol. 2, 187–193 (1984).
- Baumgartner H, Hung J, Bermejo J et al.: Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur. J. Echocardiogr. 10, 1–25 (2009).
- Clavel MA, Fuchs C, Burwash IG et al.: Predictors of outcomes in low-flow, low-gradient aortic stenosis: results of the multicenter TOPAS study. Circulation 118, S234–S242 (2008).
- Picano E, Pibarot P, Lancellotti P et al.: The emerging role of exercise testing and stress echocardiography in valvular heart disease. J. Am. Coll. Cardiol. 54, 2251–2260 (2009).
- Petronio AS, De Carlo M, Bedogni F et al.: Safety and efficacy of the subclavian approach for transcatheter aortic valve implantation with the CoreValve Revalving System. Circ. Cardiovasc. Interv. 3, 359–366 (2010).
- Zegdi R, Ciobotaru V, Noghin M et al.: Is it reasonable to treat all calcified stenotic aortic valves with a valved stent?: results from a human anatomic study in adults. J. Am. Coll. Cardiol. 51, 579–584 (2008).
- Chiam PTL, Chao VTT, Tan SY et al.: Percutaneous transcatheter heart valve implantation in a bicuspid aortic valve. JACC Cardiovasc. Interv. 3, 559–561 (2010).
- Delgado V, Tops LF, Schuijf JD et al.: Successful deployment of a transcatheter aortic valve in bicuspid aortic stenosis: role of imaging with multislice computed tomography. Circ. Cardiovasc. Imaging 2, e12–e13 (2009).
- Buchner S, Hulsmann M, Poschenrieder F et al.: Variable phenotypes of bicuspid aorticvalve disease: classification by cardiovascular magnetic resonance. Heart 96, 1233–1240 (2010).
- Delgado V, Ng ACT, Van de Veire NR et al.: Transcatheter aortic valve implantation: role of multi-detector row computed tomography to evaluate prosthesis positioning and deployment in relation to valve function. Eur. Heart J. 31, 1114–1123 (2010).
- John D, Buellesfeld L, Yuecel S et al.: Correlation of device landing zone calcification and acute procedural success in patients undergoing transcatheter aortic valve implantations with the self-expanding CoreValve prosthesis. JACC Cardiovasc. Interv. 3, 233–243 (2010).
- Messika-Zeitoun D, Serfaty JM, Brochet E et al.: Multimodal assessment of the aorticannulus diameter: implications for transcatheter aortic valve implantation. J. Am. Coll. Cardiol. 55, 186–194 (2010).
- Moss RR, Ivens E, Pasupati S et al.: Role of echocardiography in percutaneous aortic valve implantation. JACC Cardiovasc. Imaging 1, 15–24 (2008).
- Smid M, Ferda J, Baxa J et al.: Aortic annulus and ascending aorta: comparison of preoperative and periooperative measurement in patients with aortic stenosis. Eur. J. Radiol. 74, 152–155 (2010).
- Ng ACT, Delgado V, van der Kley F et al.: Comparison of aortic root dimensions and geometries before and after transcatheter aortic valve implantation by 2- and 3-dimensional transesophageal echocardiography and multislice computed tomography. Circ. Cardiovasc. Imaging 3, 94–102 (2010).
- Schultz CJ, Moelker A, Piazza N et al.: Three dimensional evaluation of the aortic annulus using multislice computer tomography: are manufacturer’s guidelines for sizing for percutaneous aortic valve replacement helpful? Eur. Heart J. 31, 849–856 (2010).
- Masson JB, Kovac J, Schuler G et al.: Transcatheter aortic valve implantation: review of the nature, management, and avoidance of procedural complications. JACC Cardiovasc. Interv. 2, 811–820 (2009).
- Webb JG, Chandavimol M, Thompson CR et al.: Percutaneous aortic valve implantationretrograde from the femoral artery. Circulation 113, 842–850 (2006).
- Joshi SB, Mendoza DD, Steinberg DH et al.: Ultra-low-dose intra-arterial contrast injection for iliofemoral computed tomographic angiography. JACC Cardiovasc. Imaging 2, 1404–1411 (2009).
- Kurra V, Schoenhagen P, Roselli EE et al.: Prevalence of significant peripheral artery disease in patients evaluated for percutaneous aortic valve insertion: preprocedural assessment with multidetector computed tomography. J. Thorac. Cardiovasc. Surg. 137, 1258–1264 (2009).
- Iozzelli A, D’Orta G, Aliprandi A et al.: The value of true-FISP sequence added to conventional gadolinium-enhanced MRA of abdominal aorta and its major branches. Eur. J. Radiol. 72, 489–493 (2009).
- Kpodonu J, Ramaiah VG, Diethrich EB. Intravascular ultrasound imaging as applied to the aorta: a new tool for the cardiovascular surgeon. Ann. Thorac. Surg. 86, 1391–1398 (2008).
- Modine T, Obadia JF, Choukroun E et al.: Transcutaneous aortic valve implantation using the axillary/subclavian access: feasibility and early clinical outcomes. J. Thorac. Cardiovasc. Surg. 141, 487–491(2010).
- Masson J, Lee M, Boone R et al.: Impact of coronary artery disease on outcomes after transcatheter aortic valve implantation. Catheter Cardiovasc. Interv. 76, 165–173(2010).
- Dash H, Johnson RA, Dinsmore R et al.: Cardiomyopahtic syndrome due to coronary artery disease. I: Relation to angiographic extent of coronary disease and to remote myocardial infarction. Br. Heart J. 39, 733–739 (1977).
- Tzikas A, Piazza N, van Dalen B et al.: Changes in mitral regurgitation after transcatheter aortic valve implantation. Catheter Cardiovasc. Interv. 75, 43–49 (2010).
- Linke A, Hollrieger R, Walther T et al.: Ingrowths of a percutaneously implanted aortic valve prosthesis (CoreValve) in a patient with severe aortic stenosis. Circ. Cardiovasc. Interv. 1, 155–158 (2008).
- Wilson A, Webb J: Transcatheter treatment approach for aortic valve disease. Int. J. Cardiovasc. Imaging (2011) (In Press).
- STS National Database Risk Calculator www.sts.org/quality-research-patient-safety/ quality/risk-calculator-and-models/ risk-calculator (Accessed 26 May 2011)
▪ Landmark study demonstrating the mortality benefit of transcatheter aortic valve implantation in patients with severe aortic stenosis who are not suitable for surgery.
▪ Illustrates the important role of multidetector row computed tomography in the assessment of patients before transcatheter aortic valve implantation.
▪ Highlights the potential differences in measurement of aortic annulus diameter using various imaging modalities.
▪ Website