Review Article - Interventional Cardiology (2014) Volume 6, Issue 2
Selecting patients likely to benefit from renal artery stenting
- Corresponding Author:
- Jose D Tafur-Soto
John Ochsner Heart & Vascular Institute, Ochsner Medical Center
1514 Jefferson Highway, New Orleans, LA 70121, USA
Tel: +1 504 842 4135
Fax: +1 504 842 4465
E-mail: jtafursoto@ochsner.org
Abstract
Atherosclerotic renal artery stenosis (RAS) has been identified as an independent predictor of death in patients with coronary artery disease. However, the cause-and-effect relation between RAS and mortality remains unproven. It is possible that the presence of RAS is a marker for more diffuse or extensive atherosclerosis, which would result in more vascular-related deaths.Keywords
chronic kidney disease, flash pulmonary edema, ischemic nephropathy, renal artery stenting, renal atherosclerosis, renal FFR, renovascular hypertension
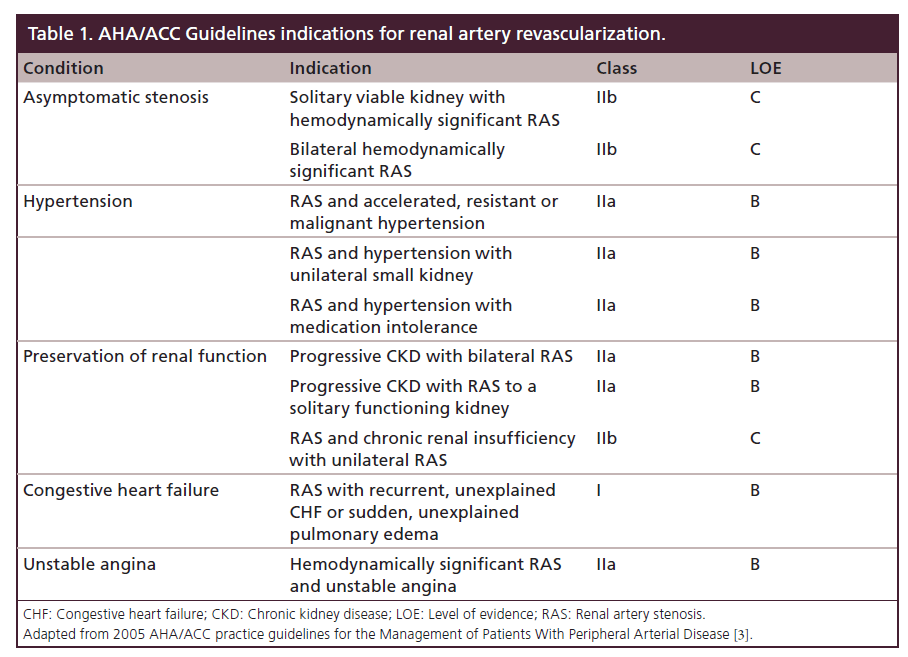
Atherosclerotic renal artery stenosis (RAS) has been identified as an independent predictor of death in patients with coronary artery disease [1]. However, the cause-and-effect relation between RAS and mortality remains unproven. It is possible that the presence of RAS is a marker for more diffuse or extensive atherosclerosis, which would result in more vascular-related deaths. Patients who improve their renal function after renal stent placement have significantly better survival rates compared with those whose renal function does not improve [2]. When selecting patients for renal artery stenting, clinical, anatomical and physiologic data should be considered to optimize the benefit of revascularization. The AHA/ACC Guideline indications for renal artery revascularization are shown in Table 1 [3].
Renal hypoperfusion is a strong stimulus for renal neuro-hormonal activation resulting in renin and subsequent angiotensin II release. Renin–angiotensin–aldosterone system activation occurs with both unilateral and bilateral (or solitary) renal hypoperfusion [4]. This leads to sodium retention, secondary hyperaldosteronism, vasoconstriction and adverse left ventricular remodeling. When renal artery stenosis is unilateral, the ischemic kidney secretes renin, which leads to increased angiotensin formation and hence elevation of blood pressure. As blood pressure rises, sodium excretion by the contralateral kidney increases; therefore, there is no sodium retention or subsequent volume overload. This is the mechanism for the hypertensive hyponatremia syndrome seen in unilateral RAS [5]. With bilateral (or solitary) renal artery stenosis, the lack of compensation from a normal kidney in terms of natriuresis leads to fluid retention, loss of kidney function and congestive heart failure [6]. Improving renal perfusion should have a normalizing effect on these physiologic processes.
Several deleterious metabolic pathways in the poststenotic kidney have been implicated in the progression of this disease including microvascular damage, oxidative stress, inflammation and development of fibrosis [7]. The extent and severity of the damage of the post-stenotic kidney may play an important role in renal recovery and outcomes of renal angioplasty. The reversibility of these processes has not yet been fully established. In a series of 17 revascularized kidneys, despite reversal of renal hypoxia and partial restoration of renal blood flow after revascularization, inflammatory cytokines and injury biomarkers remained elevated and GFR failed to recover in atherosclerotic RAS [8].
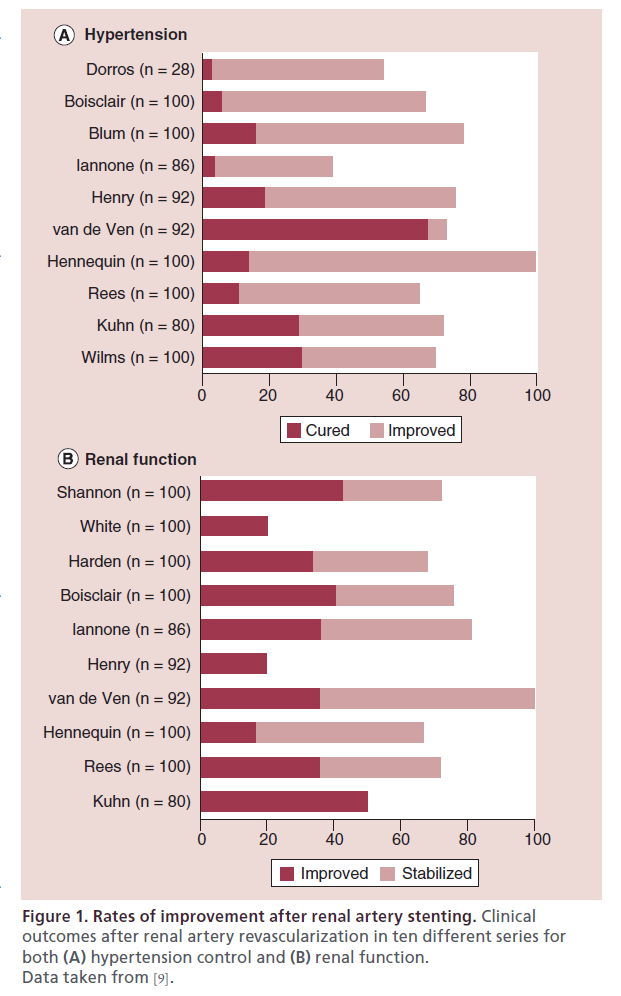
Despite excellent angiographic outcomes achieved with renal stenting, there is a mismatch between angiographic (>97%) and clinical (∼70%) success. Renal arterial stent placement has proved to be highly successful. In a meta-analysis including 14 studies (678 patients) dealing with renal artery stenting for either hypertension or chronic kidney disease (CKD), the initial angiographic success rate was 98% (95% CI: 95–100%) [9]. However, the beneficial clinical response for hypertension was 69%, with a cure rate of 20% and improvement in blood pressure in 49% (Figure 1A). Renal function improved in 30% and stabilized in 38% of patients with an overall favorable response rate of 68% (Figure 1B) [9]. This mismatch between 98% angiographic success and approximately 70% clinical response can be explained by the fact that some of these RAS lesions were not hemodynamically significant, and/or the symptoms (hypertension or CKD) are not caused by renal artery stenosis. The key to successful clinical outcomes is to try to identify which of those patients are likely to benefit from intervention and avoid procedures in those patients unlikely to benefit.
Figure 1: Rates of improvement after renal artery stenting. Clinical outcomes after renal artery revascularization in ten different series for both (A) hypertension control and (B) renal function. Data taken from [9].
A matched cohort study of patients with atherosclerotic RAS collected over 5 years in two large centers compared medical therapy only (n = 182) with primary renal artery stenting plus medical therapy (n = 348). Patients with CKD stage 3, 4 and 5 had improved clinical outcome of 20% or more increase in GFR as opposed to no benefit in CKD stage 1 and 2. It suggested that specific subgroups of patients with atherosclerotic RAS, notably those with a more impaired renal function, might have greater benefit in terms of survival and renal function following revascularization [10].
Several recent randomized clinical trials have attempted to determine the clinical benefit of renal artery stenting. However, these trials have been flawed by poor design and the inability to objectively assess the severity of the RAS. They have failed to select patients with hemodynamically significant RAS lesions that cause renal hypoperfusion. The STAR trial attempted to determine the efficacy and safety of renal artery stenting in patients with RAS and creatinine clearance <80 ml/min per 1.73m2. The primary outcome of 20% decrease in creatinine clearance was similar with renal artery stenting and medical therapy alone. However, 18 out of 64 (28%) patients randomized to stent placement did not actually have a stent placed and the analysis was made on an intention-to treat basis. Twelve (19%) patients were found to have non-obstructive stenosis (less than 50%) at the time of the procedure [11].
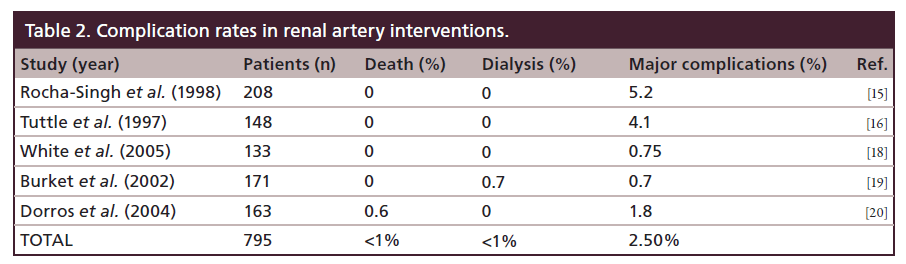
The ASTRAL trial was also seriously flawed [12]. It included patients with clinical suspicion of RAS with imaging-confirmed stenosis in at least one renal artery, in whom the physician was uncertain if that the patient would benefit from revascularization. This excluded patients most likely to benefit from revascularization, creating a selection bias against severe RAS. Additionally, there was no objective measurement of RAS, only visual estimation was used, which has been shown to be inaccurate [13]. Overestimation of RAS creates a significant bias against revascularization because these mild lesions are not likely to be causing renal hypoperfusion that would benefit from intervention. In fact, 40% of patients had RAS <70%. Only 83% of patients randomized to revascularization actually underwent renal artery stenting. Also, due to the inexperience of the operators, the procedural success was 78.6%, which is far below the >97% success rate expected in clinical practice [9]. Moreover, there was a serious complication rate of 9% in the percutaneous revascularization group, which is far higher than the 2–4% reported in prior clinical trials [14–20] (Table 2). Sixty five percent of the centers enrolled <1 patient per year, indicating a very low procedural volume per site and per operator. ASTRAL inappropriately concluded that the risks of renal stenting were not justified by the benefits. A better conclusion would have been that patients with mild RAS do not benefit from renal stent placement.
The recently published CORAL trial was open to patients with RAS of at least 60% with uncontrolled hypertension (systolic blood pressure [SBP] >155 with two or more antihypertensive medications) or chronic kidney disease (eGFR <60 ml/min/1.73m2). Patients were screened with DUS, MRA or CTA. No hemodynamic measurements were reported (see below). The CORAL trial’s original design had to be altered along the way because of slower than expected patient enrollment. A major factor limiting enrollment was a lack of equipoise between medical therapy and stent placement. Patients with severe renal artery lesions, in whom clinicians recommended revascularization, were not likely to be enrolled in CORAL, as indicated by modest average renal artery stenosis of 67% found by the core laboratory. For those patients enrolled, there was no statistical difference in the primary outcome between medical therapy alone and medical therapy and renal artery stenting [21]. CORAL reinforced the need for better strategies to select patients likely to benefit from renal artery revascularization.
Clinical syndromes resulting from renal hypoperfusion
The first step in selecting patients who may benefit from renal artery revascularization is to identify the clinical impact of renal artery hypoperfusion. Renal stent placement may be indicated for asymptomatic patients with hemodynamically significant RAS based upon preserving renal mass, on a case-by-case basis (class IIb, level of evidence [LOE]: C). The main clinical syndromes associated with RAS include renovascular hypertension, ischemic nephropathy and cardiac destabilization syndromes.
Renovascular hypertension
(Percutaneous revascularization is reasonable for patients with hemodynamically significant RAS and accelerated hypertension, resistant hypertension, malignant hypertension, hypertension with an unexplained unilateral small kidney, and hypertension with intolerance to medication [class IIa, LOE: B] [3]) (Table 1). Resistant hypertension is defined as blood pressure above goal on three different classes of anti-hypertensive medications, ideally including a diuretic drug [22,23]. Secondary causes of hypertension should be evaluated in such patients.
Ischemic nephropathy
(Percutaneous revascularization is reasonable for patients with RAS and progressive chronic kidney disease with bilateral RAS or a RAS to a solitary functioning kidney [class IIa level of evidence: B]. Percutaneous revascularization may be considered for patients with RAS and chronic renal insufficiency with unilateral RAS [class IIb, LOE: C]) [3] (Table 1). Ischemic nephropathy is potentially a reversible form of kidney failure. If unrecognized it can lead to end-stage renal disease (ESRD). Some studies suggest that as much as 11–14% of ESRD is attributable to chronic ischemic nephropathy from RAS [26]. Renal salvage criteria include recent increase in serum creatinine concentration, decrease in GFR during ACEI or ARB treatment, absence of glomerular or interstitial fibrosis on kidney biopsy, and kidney longitudinal diameter >8.0 cm [27]. In a study with a mean follow-up of 2 years, renal function improved in 34 out of 59 patients (57.6%). The slope of the reciprocal serum creatinine plot before revascularization was significantly associated with a favorable clinical response, suggesting that rapidly progressive renal failure is associated with a favorable response on renal failure progression after revascularization in patients with vascular nephropathy and renal artery stenosis [28].
Cardiac destabilization syndromes
(Percutaneous revascularization is indicated for patients with hemodynamically significant RAS and recurrent, unexplained congestive heart failure or sudden, unexplained pulmonary edema [class I, LOE: B]. Percutaneous revascularization is reasonable for patients with hemodynamically significant RAS and unstable angina [class IIa, LOE: B]) [3] (Table 1). Exacerbations of coronary ischemia (class IIa, LOE: B) and congestive heart failure (class I, LOE: B) caused by peripheral arterial vasoconstriction and/or volume overload can be attributed to RAS. The most widely recognized example of a cardiac destabilization syndrome is ‘flash’ pulmonary edema (class I, LOE B) or Pickering syndrome [29,30]. Renovascular disease may also complicate the treatment of patients with heart failure by preventing the administration of angiotensin antagonist therapy.
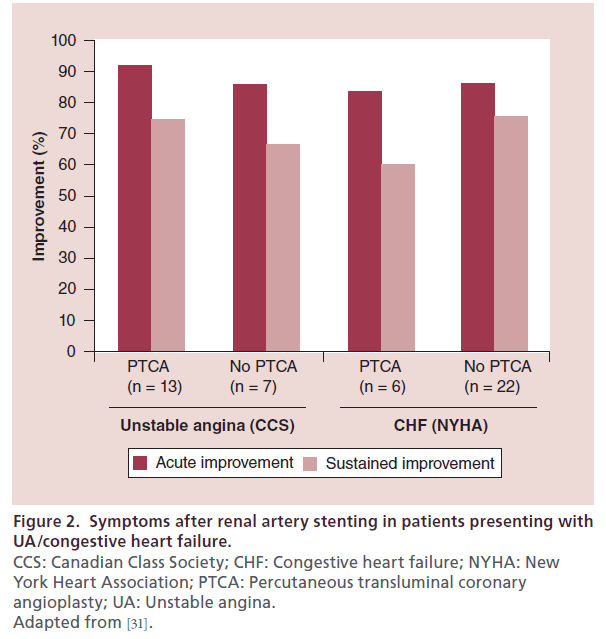
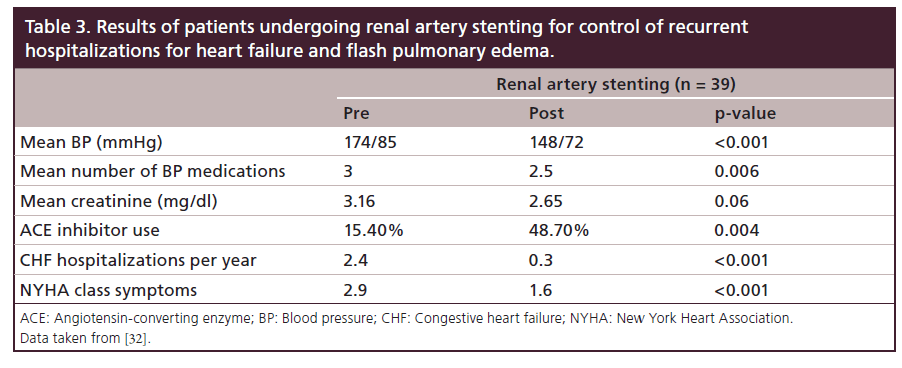
The importance of renal artery stent placement in the treatment of cardiac disturbance syndromes has been described in a series of patients presenting with either congestive heart failure or an acute coronary syndrome [31]. Successful renal stent placement resulted in a significant decrease in blood pressure and symptom improvement in 88% (42 out of 48) of patients. For those patients who presented with unstable angina, renal artery stenting improved the Canadian Class Society (CCS) symptoms at least by one regardless of concomitant coronary intervention. In patients presenting with heart failure, the New York Heart Association Class of symptoms improved by at least one also independent of coronary revascularization (Figure 2). In a study performed in 207 patients with decompensated heart failure, 19% had severe RAS and underwent renal artery stenting with decreased frequency of congestive heart failure admissions, flash pulmonary edema NYHA class symptoms and tolerance to ACE inhibitors (Table 3) [32].
Figure 2: Symptoms after renal artery stenting in patients presenting with
UA/congestive heart failure.
CCS: Canadian Class Society; CHF: Congestive heart failure; NYHA: New
York Heart Association; PTCA: Percutaneous transluminal coronary
angioplasty; UA: Unstable angina.
Adapted from [31].
Table 3: Results of patients undergoing renal artery stenting for control of recurrent hospitalizations for heart failure and flash pulmonary edema.
Noninvasive lesion stratification
Doppler ultrasound evaluation
Renal artery Doppler ultrasound, or duplex ultrasound (DUS), is a noninvasive examination useful for screening for RAS. It carries a sensitivity of 84%, specificity of 97% and positive-predictive value of 94% for the detection of significant RAS [33]. The success of this technology is highly dependent on technical skill in performing the examination.
A peak systolic velocity (PSV) >180 cm/s has a 95% sensitivity and 90% specificity for significant RAS. When the ratio of the PSV of the stenosed renal artery to the PSV in the aorta is >3.5, DUS predicts >60% RAS with a 92% sensitivity [34,35]. Duplex also allows follow-up of stent patency in patients that have undergone renal artery stenting; however, criteria for native renal artery stenosis overestimates the degree of angiographic in-stent restenosis (ISR). Surveillance monitoring for renal stent patency should take into account that PSV and renal to aortic ratio (RAR) obtained by DUS are higher for any given degree of arterial narrowing within the stent. PSV >395 cm/s or RAR >5.1 were the most predictive of angiographically significant ISR >70% [36].
DUS can be performed without risk to the patient, and there is no iodinated contrast or ionizing radiation required. The main limitations for DUS include unsatisfactory exams due to overlying bowel gas or large body habitus. There is a requirement for a capable sonographer who is allowed enough time to perform the examination.
The intra-renal resistive index (RI) is the ratio of the peak systolic to end diastolic velocity within the renal parenchyma at the level of the cortical blood vessels [37]. The RI is a representation of small-vessel glomerulosclerosis. There have been conflicting reports regarding the usefulness of RI to predict individual patient response to revascularization. One retrospective study demonstrated that an elevated RI greater than 0.80 predicted a lack of improvement in blood pressure and renal function after revascularization; however, it included balloon angioplasty without stent procedures, a strategy that is now recognized as less optimal compared with stenting (see below) [38]. A prospective study of renal stenting in 241 patients with an elevated RI (>0.70) showed improvement in blood pressure and renal function after intervention [39]. Patients with a higher RI (>0.8) actually benefitted more from revascularization than did those with milder elevations.
Computed tomographic angiography
Computed tomographic angiography (CTA) can provide high-resolution noninvasive detection of RAS while supplying 3D angiographic images of the aorta, renal and visceral arteries allowing localization and enumeration of the renal arteries, including accessory branches [40]. It carries good sensitivity (59–96%) and specificity (82–99%) for detecting significant RAS compared with invasive angiography [41]. CTA requires the administration of 100–150 ml of iodinated contrast and therefore carries the potential risk of contrastinduced nephropathy (CIN), especially in patients with an estimated glomerular filtration rate (eGFR) <60 ml/1.73m2, diabetes mellitus or anemia [42,43]. However, as CTA scanner technology advances, spatial resolution will improve, scanning time will decrease and the administered contrast load may be reduced [44]. Additionally, iso-osmolar contrast media are now available with decreased potential for nephrotoxicity [45]. CTA allows following patients with prior stents to detect ISR, an advantage over magnetic resonance angiography (MRA) in which metallic stents generate artifact [41].
Contrast-enhanced MRA
This imaging modality allows localization and enumeration of the renal arteries and characterization of the stenosis. When compared with invasive angiography it has a sensitivity of 97% and specificity of 93% for detection of RAS [46]. MRA does not require the use of ionizing radiation. Limitations for contrast-enhanced MRA (CE-MRA) include the association of gadolinium with nephrogenic systemic fibrosis when administered to patients with eGFR <30 ml/1.73m2 and metal causes artifacts on MRA; therefore, it is not a useful test for patients with prior renal stents. Other patients who are not good candidates for MRA include those with claustrophobia or those with implanted medical devices (e.g., artificial joints, permanent pacemakers) [47]. MRA-based flow measurements have been postulated to predict clinical outcomes after revascularization [48]; however, there is still no clinical evidence to support the use of this technique to select patients for treatment.
Captopril renal scans
This test is based on the principle that patients with flow-limiting stenosis to the afferent arteriolar vascular bed are dependent on the tone of the efferent arteriole to maintain their glomerular filtration rate. Therefore, patients in whom angiotensin-converting enzyme (ACE) inhibitors such as captopril (efferent arteriole vasodilators) decrease the GFR are more likely to have hemodynamically significant RAS [49,50]. GFR is measured with renal scintigraphic imaging with technetium-99m mercaptoacetyltriglycine (MAG3) or technetium-99m diethylenetriaminepentaacetic acid (DTPA) [51]. When captopril renal scans (CRS) were compared with invasive angiography in a clinical practice setting, the sensitivity and specificity were 74 and 59%, respectively. The test requires close monitoring of volume status and holding of medications that interact with the renin–angiotensin–aldosterone system, which in the clinical practice setting becomes unreliable. Another limitation of the test includes the relative contraindication to the use of ACE inhibitors in patients with significant azotemia, bilateral RAS or RAS to a single functioning kidney. CRS is not a very useful test for screening patients for RAS [52] and is not recommended by the AHA/ACC guidelines [53]. CRS may have a more important role in providing additional physiologic information in those patients with known RAS and in whom the decision to revascularize is being evaluated.
Invasive angiography
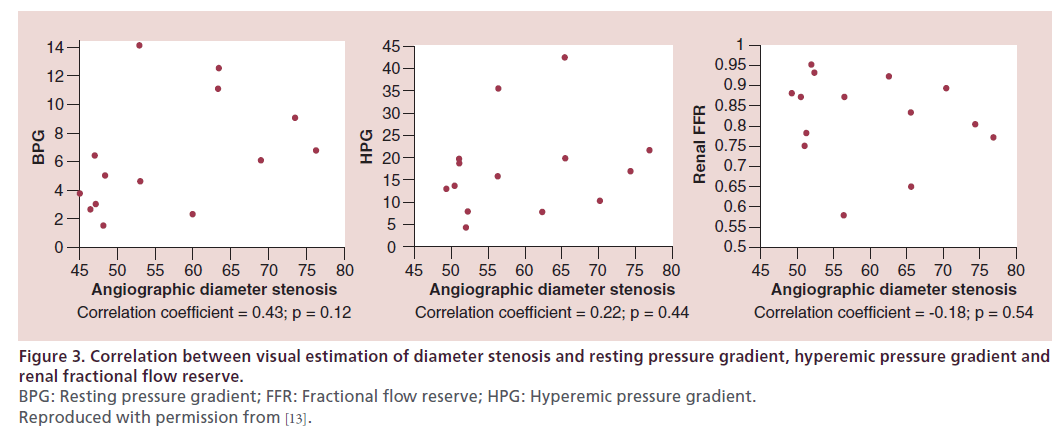
Invasive angiography remains the ‘gold standard’ for the diagnosis of renal artery disease. However, the visual estimate of the severity of stenosis is inaccurate. When a single operator performed visual estimation of angiographic diameter stenosis in patients with moderate RAS (50–90% diameter stenosis), the correlation was poor between the angiographic diameter stenosis and resting mean translesional pressure gradient (r = 0.43; p = 0.12), hyperemic mean translesional pressure gradient (r = 0.22; p = 0.44) and renal FFR (r = 0.18; p = 0.54) (Figure 3) [13]. The 3D anatomy of the renal arteries and the inability to obtain extreme cranio–caudal angulation during invasive angiography contribute to the inaccuracy of determination of angiographic severity of RAS. Therefore, physiologic assessment should always be performed with invasive angiography in moderate lesions.
Pressure gradients
Resting and hyperemic translesional pressure gradients >20 mmHg, as well as a trans-lesional resting pressure ratio of 0.90 (Pd/Pa <0.90) correlate with a significant rise in renin concentration in the ipsilateral renal vein [54,55]. Renin secretion from a hypoperfused kidney is a key element in the development of renovascular hypertension and ischemic nephropathy. Several series have shown improved blood pressure response when treating lesions with resting or hyperemic pressure gradients >20 mmHg [56,57]. Based on these observations, an expert consensus panel of the AHA recommended that a peak systolic gradient of at least 20 mmHg, or a mean pressure gradient of 10 mmHg, be used to identify candidate lesions for revascularization in symptomatic patients with RAS [58]. It is important, however, to understand the limitations of pressure gradients. They can be unreliable indicators of borderline lesions because the gradient is dependent upon the diameter of the catheter placed across the lesions, the aortic pressure, the degree of stenosis, the distal vascular bed and the renal venous pressure. The catheter itself can introduce an artificial gradient [59]. Measurements can be done with either a 4-Fr catheter or a 0.014” pressure wire. Some authors have suggested that the later is a more reliable measure; however, they have compared their measurements to angiographic minimal luminal diameter [60].
Hyperemia has been proposed as a way to increase the accuracy of the pressure measurements, as is the case in the coronary arteries. Renal hyperemia can be achieved with papaverine [13,55], dopamine [57] or acethylcholine [61] (see Table 4). Nevertheless, renal hyperemia is lower when compared with the myocardium: renal artery flow reserve is 1.8 compared with 5 or 6 in the coronary vasculature [62]. The limited renal hyperemia explains why the predictive value of mean resting gradient is similar to that of mean vasodilator-induced gradient. However, vasodilators increased the gradient, allowing identification of hemodynamically significant lesions in the case of resting gradients <20 mmHg [57]. Hyperemic measures should be performed when resting gradients are not significant and there is persistent suspicion of renal hypoperfusion.
Renal artery FFR
Another method to determine the severity of angiographic RAS is to quantify the FFR. This measure of pressure, which is widely used in the coronary circulation, is based on the principle that flow across a conduit artery is proportional to pressure across the vascular bed and inversely proportional to the resistance of the vascular bed. Under conditions of maximum hyperemia (i.e., maximum vasodilation), the flow through the conduit artery is maximal, while the resistance of the vascular bed is at a minimum and constant. Any reduction in flow under these conditions is caused by the stenosis and is proportional to the ratio of pressure distal to the stenosis (Pd) and the pressure proximal to the stenosis (Pa).
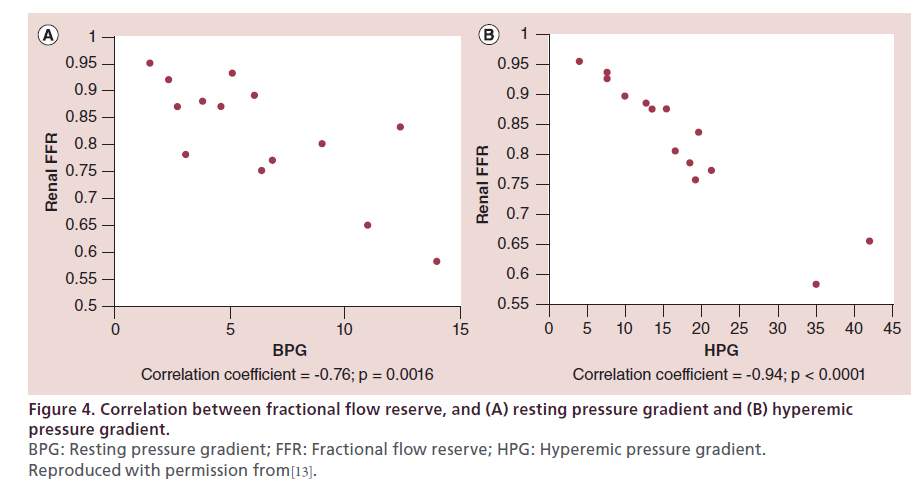
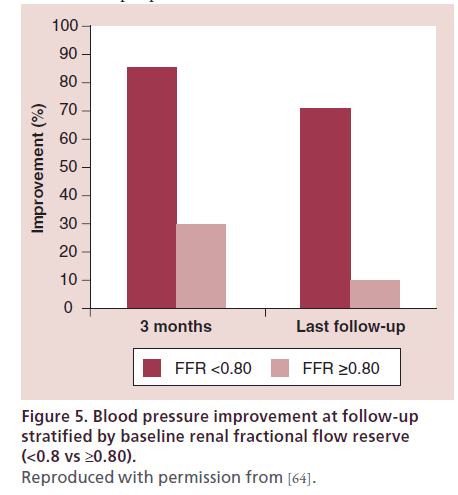
FFR is measured after induction of maximum hyperemia. Renal hyperemia can be obtained with papaverine, dopamine and acethylcoline. Translesional pressure gradients are measured and FFR (Pd/Pa) is calculated using a 0.014’’ pressure guidewire. Renal artery FFR correlates well with other hemodynamic parameters of lesion severity [55,63] (Figure 4) and in some series has been proven to be a better predictor of clinical response. In one study, renal FFR was measured after renal stent placement in 17 patients with refractory hypertension and moderate-to-severe (50–90% stenosis), unilateral RAS. Ten patients had normal baseline renal FFR (defined as FFR ≥0.80), whereas an abnormal baseline renal FFR (<0.80) was recorded in seven patients. At 3 months after intervention, 86% of patients with an abnormal renal FFR experienced improvement in their BP, compared with only 30% of those with normal renal FFR (p = 0.04) (Figure 5). Baseline systolic, mean or hyperemic translesional pressure gradients were not different between patients whose BP improved and those in whom it did not [64].
Figure 4: Correlation between fractional flow reserve, and (A) resting pressure gradient and (B) hyperemic
pressure gradient.
BPG: Resting pressure gradient; FFR: Fractional flow reserve; HPG: Hyperemic pressure gradient.
Reproduced with permission from[13].
Figure 5: Blood pressure improvement at follow-up stratified by baseline renal fractional flow reserve (<0.8 vs ≥0.80). Reproduced with permission from [64].
Intravascular ultrasonography
Intravascular ultrasonography (IVUS) can complement angiography by providing additional information on lumen dimension and stent expansion, aiding in optimizing outcomes after intervention. Primary stent patency is dependent upon maximal size of stents and IVUS allows safe selection of the larger stent that fits the patient [65] (see below in the ‘Optimizing outcomes’ section).
For diagnostic purposes, IVUS with virtual histology (VH) has enabled tissue characterization of atherosclerotic plaque [66] and the plaque composition in coronary plaques detected by VH-IVUS has been associated with impairment of the coronary microcirculation after coronary intervention [67]. Plaque composition of RAS has been postulated to be responsible for some of the observed variability in clinical response in renal function after intervention. VH-IVUS allows characterization of percentage of necrotic core in atherosclerotic plaque, which has been correlated with worsening renal function post intervention, likely due to microembolizaion of necrotic debris. Also, dense calcium was negatively associated with percent change in systolic blood pressure [68,69].
Renal frame counts
In the coronary vasculature, the Thrombolysis in Myocardial Infarction (TIMI) frame count is a quantitative angiographic assessment of coronary blood flow that correlates with clinical outcomes [70–73]. The renal frame count (RFC) has been proposed as an alternative angiographic method to assess renal perfusion. RFC is the number of cine frames required for the contrast to reach the smallest visible distal branch in the renal parenchyma. As in TIMI frame counting, the first frame used for the RFC is the frame in which the contrast first fills the main renal artery. The last frame is when contrast enters the smallest visible branch of the distal renal parenchyma along the axis of the main renal artery. The measurements are done with 30 frames/s angiography. RFC was initially described in patients with fibromuscular dysplasia (FMD) of the renal arteries (15 kidneys), who were compared with subjects with normal renal arteries (50 kidneys) and had a significantly higher (prolonged) mean RFC (26.9 vs 20.4; p = 0.0001) [74]. In a prospective series of 24 patients with uncontrolled hypertension who underwent renal artery stenting, reduction in RFC after stenting was associated with BP reduction and >4 RFC reduction after stenting predicted BP reduction in 78% of subjects [75].
Serum biomarkers
Brain natriuretic peptide (BNP) is a neurohormone released from the ventricular myocardium in conditions that cause myocardial cell stretching such as congestive heart failure and pulmonary embolism [76]. BNP has been shown to directly correlate with pulmonary capillary wedge pressure [77]. In vitro studies have also shown that angiotensin II induces the synthesis and release of BNP, and in rats it has been found that BNP mRNA is upregulated 6 h after clipping of the renal artery [78].
In a series of 27 patients with significant RAS (>70% diameter stenosis) and uncontrolled hypertension, excluding patients with congestive heart failure, recent myocardial infarction and chronic renal insufficiency (Cr ≥2), it was found that a baseline BNP >80 and BNP decreased by at least 30% had a significant correlation with clinical improvement in blood pressure. However, in the safety and efficacy of the HERCULES trial, a single-arm multicenter trial that included 202 patients with RAS and uncontrolled hypertension, BNP levels did not correlate with clinical improvement in blood pressure[79]. The usefulness of BNP as a predictor of good clinical outcome needs to be confirmed in a larger cohort of patients.
Technical aspects of intervention
There are several important technical and procedural considerations to prevent renal artery injury, kidney injury and atheroembolization. Selective renal angiography should be guided by nonselective abdominal aortography; the catheter-in-catheter or no-touch techniques should be used to minimize contact with the aortic wall and injury to the renal ostium during guiding catheter engagement (see below). Adequate hydration and limiting contrast volume are helpful to prevent the development of contrast-induced nephropathy (CIN).
Optimizing outcomes
Radial access
Radial access is increasingly used to perform percutaneous diagnostic and interventional coronary procedures given the lower access site bleeding complications and improved post-procedural patient comfort when compared with femoral access. The access study (n = 900) showed access site-related bleeding complications of 2.3% for the brachial group, 2.3% for the femoral group and 0% for the radial group (p = 0.035) [80]. The radial approach for renal stenting represents a valuable tool to reduce access-site complications and improve the patient’s comfort. However, the operator needs specific technical skills as well as knowledge of device compatibility. Both radial arteries are suitable for renal intervention. Depending on the configuration of the aortic arch, the left radial access allows a shorter distance to renal arteries. The right radial approach is more comfortable for the operator and radiation exposure is less compared with the left. The 125 cm long catheters are appropriate for almost all patients, whereas the 110 cm length catheters may not reach the renal arteries when coming from the right radial artery in taller patients or in patients with excessive aortic arch tortuosity [81]. The feasibility of transradial renal intervention has been clearly demonstrated. A series reported success and safety parameters in 11 patients with the transradial approach comparable to 44 matched transfemoral interventions [82]. Transradial access virtually eliminates access-related bleeding complications.
Catheter-in-catheter technique
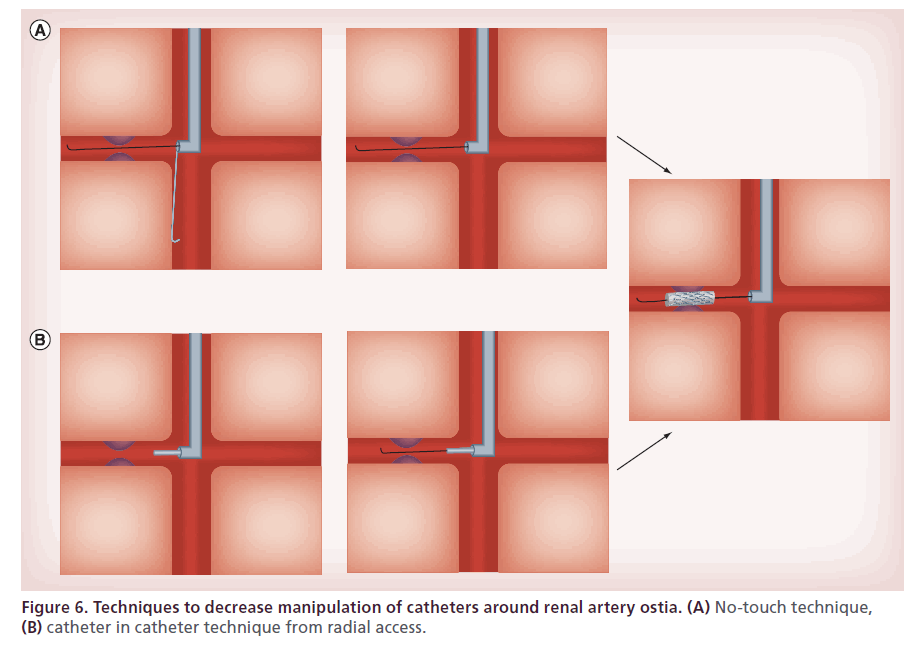
Different catheter techniques involve various degrees of catheter manipulation within the aorta. Interventional cardiologists tend to favor the technique involving direct insertion of the guide catheter as it is most similar to that used during coronary interventions. Nevertheless, there are several differences between abdominal aortic interventions and coronary interventions: more manipulation is required to engage the renal ostium than the coronary, and the descending aorta contains more extensive atherosclerotic disease than the ascending aorta [83]. Increased manipulation in a vessel with higher burden of atherosclerotic disease can be hazardous [84,85]. In order to minimize manipulation, the catheter-in-catheter technique employs a 4 or 5 French soft-tipped diagnostic catheter loaded inside guiding catheter 2 French sizes larger. After the renal artery is engaged with the diagnostic catheter, a 0.014’ wire is advanced across the stenosis and positioned distal to the lesion. The guiding catheter is advanced over the diagnostic catheter, and once positioned the diagnostic catheter is removed [86] (Figure 6B).
No touch technique
This approach involves keeping a 0.035’ J-wire advanced beyond the tip of the guide catheter during initial cannulation of the renal artery. The guide wire prevents the tip of guide catheter from coming into direct contact with the wall of the abdominal aorta during manipulation. When cannulation of the target artery is achieved, an angioplasty guide wire is inserted alongside the 0.035’ J-wire and advanced into the target vessel. After the angioplasty, the guide wire is in position across the target lesion, and the 0.035’ guide wire is removed [87] (Figure 6A).
Embolic protection devices
Atheroembolism has been associated with an increase in surgical morbidity and a dramatic reduction in 5-year survival compared with patients who had no evidence on biopsy of renal atheroembolization (54 vs 85%; p = 0.011) [88]. Since atheroembolism is a potential complication of renal artery stenting, investigators have looked for the role of embolic protection devices (EPDs) in optimizing outcomes after renal intervention. However, distal protection is technically difficult given the early bifurcation of renal arteries. One hundred patients undergoing renal artery stenting were randomly assigned to an open-label EPD or use of abciximab in a 2×2 factorial design. A positive interaction was observed between treatment with abciximab and embolic protection: renal artery stenting alone, stenting with EPD and stenting with abciximab were associated with similar and modest declines in eGFR at 1 month follow-up (-10, -12, -10 ml/min/1.73m2 eGFR change, respectively); however, the group treated with both EPD and abciximab was protected from a decline in eGFR and was superior to the other three groups (+9 ml/min/1.73 m2 eGFR change; p < 0.01) [89]. In an uncontrolled retrospective trial, RFC were measured in 66 patients undergoing renal artery intervention with and without EPD. EPD was associated with improved renal blood flow measured by RFC compared with the control group following RAS (mean reduction in RFC: 14.2 vs 6.7; p = 0.03) [90]. EPDs may be effective in preventing renal atheroembolic injury and a controlled trial measuring the impact of EPDs on renal blood flow following RAS should be performed.
Stent sizing with IVUS
As described above, IVUS can provide anatomical characterization of the atherosclerotic plaque. Twentytwo patients with atherosclerotic renal artery stenosis were studied with IVUS after pre-dilation and after angiographically successful stent deployment (diameter stenosis <10%). Modification based on IVUS including selection of a larger balloon, additional dilation, and placement of a second stent occurred in five patients after predilatation and in one patient after stent deployment. Mean blood pressure and the amount of antihypertensive drugs decreased (p < 0.05). Therefore, IVUS monitoring during renal artery stent placement resulted in additional lumen enlargement not considered necessary at angiography [65].
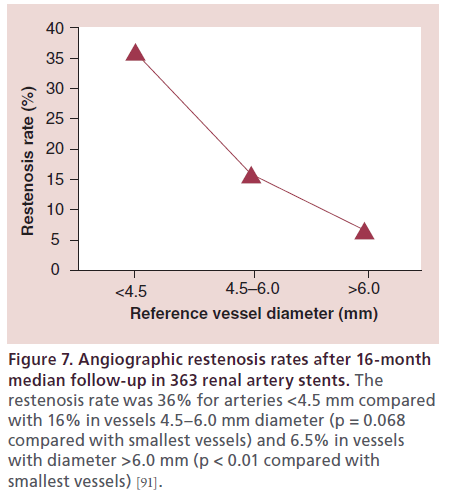
In a series of 363 renal artery interventions, followup angiography was available in 102 patients (34%) at an average of 303 days. Larger diameter arteries were associated with a significantly lower incidence of angiographic restenosis. The restenosis rate was 36% for vessels with a reference diameter <4.5 mm compared with 16% in vessels with a reference diameter 4.5–6 mm (p = 0.068) and 6.5% in vessels with a reference diameter >6 mm (p < 0.01) [91]. IVUS allows a more accurate way to measure vessel diameter than 2D angiography, allowing the operator to safely maximize the stent size. Visual estimate tends to underestimate the size of the vessel, which can translate into higher rates of in-stent restenosis.
Drug-eluting stent vs bare-metal stent
Restenosis after stent-angioplasty of atherosclerotic RAS is a limitation, especially in small-diameter renal arteries (Figure 7). The GREAT study [92] was a prospective, multicenter study of angiographic patency of renal artery stents placed in patients with atherosclerotic RAS. The restenosis rate was determined as binary restenosis with a cutoff point at 50% diameter stenosis. Clinical patency at 6-month follow-up was 92% in the bare-metal stent (BMS) and 98% in the drug-eluting stent (DES) group. Target lesion revascularization was performed in 8% of the patients in the BMS group, and 2% in the DES group. At 1 year follow-up, the clinical patency was 88.5% in the BMS and 98.1% in the DES group. In one study of renal artery stenting, vessels with a diameter smaller than 4.5 mm had a restenosis rate of 36%, compared with a 6.5% rate of restenosis in vessels with diameters greater than 6 mm [91]. Based on these observations, DES should be preferred over BMS, especially in smaller arteries.
Figure 7: Angiographic restenosis rates after 16-month median follow-up in 363 renal artery stents. The restenosis rate was 36% for arteries <4.5 mm compared with 16% in vessels 4.5–6.0 mm diameter (p = 0.068 compared with smallest vessels) and 6.5% in vessels with diameter >6.0 mm (p < 0.01 compared with smallest vessels) [91].
The use of covered stents in the renal arteries has been reported in the management of complications including perforation [93]. Polytetrafluoroethylene (PTFE)-covered stents may offer a way to treat recurrent renal artery stenosis. In case reports, the covered stents remained free of any significant neointimal tissue or obstruction after used for management of recurrent ISR [94]. Covered stents may also have a role in preventing distal embolization. Their safety is currently being evaluated in clinical trials.
Restenosis lesions
The durability of renal artery interventions is limited by the development of in-stent restenosis and the need for secondary or tertiary renal interventions. Two meta-analyses of renal artery intervention have demonstrated mean restenosis rates after stent placement of 16 and 17% [9,95]. A more recent series reported an 11% prevalence of in-stent restenosis over a mean follow-up of 3 years [96]. The optimal treatment of renal artery in-stent restenosis is uncertain. An initial report of treatment of in-stent restenosis included 20 renal arteries in 15 patients. At a mean follow-up of 11 months, the restenosis rate for percutaneous transluminal angioplasty (PTA) treatment of in-stent restenosis was 25% (Figure 8). Renal function remained stable or improved in 80%, and hypertension was classified as improved or cured in 47% [97]. There are no reports of efficacy in treating in stent restenosis with cutting balloons [94,98] and brachytherapy [99].
Follow-up post-revascularization
Patients should be followed clinically in terms of blood pressure control with laboratory results to monitor renal function and surveillance imaging is recommended to evaluate stent patency. DUS is the recommended imaging technique to screen for in-stent restenosis. DUS surveillance monitoring for renal stent patency should take into account that a stented artery is less compliant than a native artery and that PSV and RAR obtained by DUS are higher for any given degree of arterial narrowing within the stent [36]. Therefore, obtaining a postprocedure DUS near to the intervention is a reasonable option to establish a new baseline PSV.
Summary
Hemodynamically significant RAS, causing renal hypoperfusion, may result in uncontrolled renovascular hypertension, ischemic nephropathy and cardiac destabilization syndromes. Screening for RAS can be done with DUS, CTA and MRA. Controlled renovascular hypertension should be treated medically as there is no proven added benefit of percutaneous revascularization. Patients with uncontrolled renovascular hypertension having failed three maximally tolerated antihypertensive medications (one of which is a diuretic), ischemic nephropathy and cardiac destabilization syndromes with severe RAS are likely to benefit from renal artery stenting. In order to select which patients are likely to benefit from intervention several physiologic measurements including FFR, hyperemic/resting gradients and renal frame counts may be performed. Those individuals in whom physiologic testing demonstrates renal hypoperfusion should undergo renal artery revascularization. Experienced operators should perform renal interventions in order to minimize complications. Adequate patient hydration, the use of radial access, and minimal catheter manipulation in the abdominal aorta with or without the use of embolic protection devices can improve procedural outcomes and translate into better clinical response. IVUS can be used to guide stent size selection as well as to evaluate plaque composition and stent apposition. Patients should be followed up after intervention to evaluate recurrence of symptoms and surveillance of in-stent restenosis with DUS.
Future perspective
Physiologic confirmation of the severity of renal artery stenosis severity will likely improve the response rate for reperfusion from 70 to >90%, as has been demonstrated in preliminary trials. A better demonstration of the renal hemodynamic obstruction of renal artery stenosis is paramount in selecting those patients whose symptoms (hypertension, renal insufficiency or heart failure) are caused by renal hypoperfusion. The inaccurate visual estimation of angiographic severity of renal artery stenosis is the primary guide to revascularization versus medical management of renal artery stenosis. However, physiologic severity defined by renal fractional flow reserve (FFR), resting and hyperemic translesional gradients can fundamentally change our practice from anatomically to physiologically guided management. Using physiologic confirmation of renal artery stenosis severity before intervention, intravascular ultrasound to maximize safe deployment diameters and imaging modalities to identify adequate renal parenchymal reserve are some of the strategies that may improve the response rate from the 70 to >90% for patients undergoing renal stenting. Similar to the experience of coronary intervention, physiologicguided revascularization should lead to better outcomes. Randomized trials for revascularization procedures in patients selected by physiologic testing are needed to confirm this strategy.
Executive summary
Introduction
• Hemodynamically significant renal artery stenosis results in the activation of the renin–angiotensin system, which may result in uncontrolled hypertension, ischemic nephropathy or cardiac destabilization syndromes.
• Screening can be done with Doppler ultrasonography, computed tomographic angiography and magnetic resonance angiography.
Selecting patients likely to benefit from stenting
• Renovascular hypertension that is controlled medically should not undergo renal stenting, as there is no added benefit of revascularization.
• Patients with uncontrolled renovascular hypertension having failed three maximally tolerated antihypertensive medications (one of which is a diuretic), ischemic nephropathy and cardiac destabilization syndromes with hemodynamically severe renal artery stenosis are likely to benefit from renal artery stenting.
• Physiologic measurements including fractional flow reserve and hyperemic/resting translesional gradients may be performed to select patients who should undergo renal artery revascularization.
Optimizing procedural outcomes
• Experienced operators should perform renal interventions to minimize complications and optimize late patency.
• Adequate patient hydration, the use of radial access, and minimal catheter manipulation in the abdominal aorta with or without the use of embolic protection devices can improve procedural outcomes and translate into a better clinical response.
• Intravascular ultrasound should be used to guide stent size selection and maximize stent apposition.
Follow-up
• Patients should have routine 30-day, 3-month, 6-month, 12-month and annual clinical, laboratory and imaging follow-up for surveillance of in-stent restenosis.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- Conlon PJ, Little MA, Pieper K, Mark DB. Severity of renal vascular disease predicts mortality in patients undergoing coronary angiography. Kidney Int. 60(4), 1490–1497 (2001).
- Kennedy DJ, Colyer WR, Brewster PS et al. Renal insufficiency as a predictor of adverse events and mortality after renal artery stent placement. Am. J. Kidney Dis. 42(5), 926–935 (2003).
- Hirsch AT, Haskal ZJ, Hertzer NR et al.; American Association for Vascular Surgery; Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines; American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J. Am. Coll. Cardiol. 47(6), 1239–1312 (2006).
- Agarwal M, Lynn KL, Richards AM, Nicholls MG. Hyponatremic–hypertensive syndrome with renal ischemia: an underrecognized disorder. Hypertension 33(4), 1020–1024 (1999).
- Pickering TG. Renovascular hypertension: etiology and pathophysiology. Semin. Nucl. Med. 19(2), 79–88 (1989).
- Textor SC, Lerman L. Renovascular hypertension and ischemic nephropathy. Am. J. Hypertens. 23(11), 1159–1169 (2010).
- Eirin A, Lerman LO. Darkness at the end of the tunnel: poststenotic kidney injury. Physiology 28(4), 245–253 (2013).
- Saad A, Herrmann SM, Crane J et al. Stent revascularization restores cortical blood flow and reverses tissue hypoxia in atherosclerotic renal artery stenosis but fails to reverse inflammatory pathways or glomerular filtration rate. Circ. Cardiovasc. Interv. 6(4), 428–435 (2013).
- Leertouwer TC, Gussenhoven EJ, Bosch JL et al. Stent placement for renal arterial stenosis: where do we stand? A meta-analysis. Radiology 216(1), 78–85 (2000).
- Kalra PA. Medical therapy versus primary renal artery stenting – a twin centre study of outcome in atherosclerotic renovascular disease. Nephrol. Dialysis Transplant. 6(FO014), vi7–vi8 (2007).
- Bax L, Woittiez AJ, Kouwenberg HJ et al. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann. Intern. Med. 150(12), 840–848, W150–W841 (2009).
- Investigators A, Wheatley K, Ives N et al. Revascularization versus medical therapy for renal-artery stenosis. N. Engl. J. Med. 361(20), 1953–1962 (2009).
- Subramanian R, White CJ, Rosenfield K et al. Renal fractional flow reserve: a hemodynamic evaluation of moderate renal artery stenoses. Catheter Cardiovasc. Interv. 64(4), 480–486 (2005).
- Dorros G, Jaff MR, Mathiak L et al. Stent revascularization for atherosclerotic renal artery stenosis. 1-year clinical followup.Tex. Heart Inst. J. 25(1), 40–43 (1998).
- Tuttle KR, Chouinard RF, Webber JT et al. Treatment of atherosclerotic ostial renal artery stenosis with the intravascular stent. Am. J. Kidney Dis. 32(4), 611–622 (1998).
- White CJ, Ramee SR, Collins TJ, Jenkins JS, Escobar A, Shaw D. Renal artery stent placement: utility in lesions difficult to treat with balloon angioplasty. J. Am. Coll. Cardiol. 30(6), 1445–1450 (1997).
- Zeller T, Frank U, Muller C et al. Predictors of improved renal function after percutaneous stent-supported angioplasty of severe atherosclerotic ostial renal artery stenosis. Circulation 108(18), 2244–2249 (2003).
- Rocha-Singh K, Jaff MR, Rosenfield K, Investigators A-T. Evaluation of the safety and effectiveness of renal artery stenting after unsuccessful balloon angioplasty: the ASPIRE-2 study. J. Am. Coll. Cardiol. 46(5), 776–783 (2005).
- Burket MW, Cooper CJ, Kennedy DJ et al. Renal artery angioplasty and stent placement: predictors of a favorable outcome. Am. Heart J. 139(1 Pt 1), 64–71 (2000).
- Dorros G, Jaff M, Mathiak L, He T, Multicenter Registry P. Multicenter Palmaz stent renal artery stenosis revascularization registry report: four-year follow-up of 1,058 successful patients. Catheter Cardiovasc. Interv. 55(2), 182–188 (2002).
- Cooper CJ, Murphy TP, Cutlip DE et al. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N. Engl. J. Med. 370(1), 13-22 (2013).
- Pimenta E, Calhoun DA. Resistant hypertension: incidence, prevalence, and prognosis. Circulation 125(13), 1594–1596 (2012).
- Calhoun DA, Jones D, Textor S et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 117(25), e510–526 (2008).
- Aqel RA, Zoghbi GJ, Baldwin SA et al. Prevalence of renal artery stenosis in high-risk veterans referred to cardiac catheterization. J. Hypertens. 21(6), 1157–1162 (2003).
- Anderson GH Jr, Blakeman N, Streeten DH. The effect of age on prevalence of secondary forms of hypertension in 4429 consecutively referred patients. J. Hypertens. 12(5), 609–615 (1994).
- Preston RA, Epstein M. Ischemic renal disease: an emerging cause of chronic renal failure and end-stage renal disease. J. Hypertens. 15(12 Pt 1), 1365–1377 (1997).
- Garovic VD, Textor SC. Renovascular hypertension and ischemic nephropathy. Circulation 112(9), 1362–1374 (2005).
- Muray S, Martin M, Amoedo ML et al. Rapid decline in renal function reflects reversibility and predicts the outcome after angioplasty in renal artery stenosis. Am. J. Kidney Dis. 39(1), 60–66 (2002).
- Messerli FH, Bangalore S, Makani H et al. Flash pulmonary oedema and bilateral renal artery stenosis: the Pickering syndrome. Eur. Heart J. 32(18), 2231–2235 (2011).
- Messerli FH, Bangalore S. The Pickering syndrome – a pebble in the mosaic of the cardiorenal syndrome. Blood Press. 20(1), 1–2 (2011).
- Khosla S, White CJ, Collins TJ, Jenkins JS, Shaw D, Ramee SR. Effects of renal artery stent implantation in patients with renovascular hypertension presenting with unstable angina or congestive heart failure. Am. J. Cardiol. 80(3), 363–366 (1997).
- Gray BH, Olin JW, Childs MB, Sullivan TM, Bacharach JM. Clinical benefit of renal artery angioplasty with stenting for the control of recurrent and refractory congestive heart failure. Vasc. Med. 7(4), 275–279 (2002).
- Taylor DC, Kettler MD, Moneta GL et al. Duplex ultrasound scanning in the diagnosis of renal artery stenosis: a prospective evaluation. J. Vasc. Surg. 7(2), 363–369 (1988).
- Strandness DE Jr. Duplex imaging for the detection of renal artery stenosis. Am. J. Kidney Dis. 24(4), 674–678 (1994).
- Olin JW, Piedmonte MR, Young JR, Deanna S, Grubb M, Childs MB. The utility of duplex ultrasound scanning of the renal arteries for diagnosing significant renal artery stenosis. Ann. Intern. Med. 122(11), 833–838 (1995).
- Chi YW, White CJ, Thornton S, Milani RV. Ultrasound velocity criteria for renal in-stent restenosis. J. Vasc. Surg. 50(1), 119–123 (2009).
- Radermacher J. Ultrasonography of the kidney and renal vessels. I. Normal findings, inherited and parenchymal diseases. Der Urologe. Ausg. A 44(11), 1351–1363; quiz 1364 (2005).
- Radermacher J, Chavan A, Bleck J et al. Use of Doppler ultrasonography to predict the outcome of therapy for renalartery stenosis. N. Engl. J. Med. 344(6), 410–417 (2001).
- Zeller T, Muller C, Frank U et al. Stent angioplasty of severe atherosclerotic ostial renal artery stenosis in patients with diabetes mellitus and nephrosclerosis. Catheter Cardiovasc. Interv. 58(4), 510–515 (2003).
- Kawashima A, Sandler CM, Ernst RD, Tamm EP, Goldman SM, Fishman EK. CT evaluation of renovascular disease. Radiographics 20(5), 1321–1340 (2000).
- Kim TS, Chung JW, Park JH, Kim SH, Yeon KM, Han MC. Renal artery evaluation: comparison of spiral CT angiography to intra-arterial DSA. J. Vasc. Interv. Radiol. 9(4), 553–559 (1998).
- Mccullough PA, Adam A, Becker CR. Risk prediction of contrast-induced nephropathy. Am. J. Cardiol. 98(6A), 27K–36K (2006).
- Mccullough PA, Adam A, Becker CR et al. Epidemiology and prognostic implications of contrastinduced nephropathy. Am. J. Cardiol. 98(6A), 5K–13K (2006).
- Lufft V, Hoogestraat-Lufft L, Fels LM et al. Contrast media nephropathy: intravenous CT angiography versus intraarterial digital subtraction angiography in renal artery stenosis: a prospective randomized trial. Am. J. Kidney Dis. 40(2), 236–242 (2002).
- Davenport MS, Khalatbari S, Cohan RH, Dillman JR, Myles JD, Ellis JH. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: risk stratification by using estimated glomerular filtration rate. Radiology 268(3), 719–728 (2013).
- Tan KT, Van Beek EJ, Brown PW, Van Delden OM, Tijssen J, Ramsay LE. Magnetic resonance angiography for the diagnosis of renal artery stenosis: a meta-analysis. Clin. Radiol. 57(7), 617–624 (2002).
- Dellegrottaglie S, Sanz J, Rajagopalan S. Technology insight: clinical role of magnetic resonance angiography in the diagnosis and management of renal artery stenosis. Nat. Clin. Pract. Cardiovasc. Med. 3(6), 329–338 (2006).
- Lim SW, Chrysochou C, Buckley DL, Kalra PA, Sourbron SP. Prediction and assessment of responses to renal artery revascularization with dynamic contrast-enhanced magnetic resonance imaging: a pilot study. Am. J. Physiol. Renal Physiol. 305(5), F672–F678 (2013).
- Meier GH, Sumpio B, Setaro JF, Black HR, Gusberg RJ. Captopril renal scintigraphy: a new standard for predicting outcome after renal revascularization. J. Vasc. Surg. 17(2), 280–285; discussion 285–287 (1993).
- Setaro JF, Saddler MC, Chen CC et al. Simplified captopril renography in diagnosis and treatment of renal artery stenosis. Hypertension 18(3), 289–298 (1991).
- Prigent A, Cosgriff P, Gates GF et al. Consensus report on quality control of quantitative measurements of renal function obtained from the renogram: International Consensus Committee from the Scientific Committee of Radionuclides in Nephrourology. Semin. Nucl. Med. 29(2), 146–159 (1999).
- Huot SJ, Hansson JH, Dey H, Concato J. Utility of captopril renal scans for detecting renal artery stenosis. Arch. Intern. Med. 162(17), 1981–1984 (2002).
- Hirsch AT, Haskal ZJ, Hertzer NR et al. ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)–summary of recommendations. J. Vasc. Interv. Radiol. 17(9), 1383–1397; quiz 1398 (2006).
- De Bruyne B, Manoharan G, Pijls NH et al. Assessment of renal artery stenosis severity by pressure gradient measurements. J. Am. Coll. Cardiol. 48(9), 1851–1855 (2006).
- Kapoor N, Fahsah I, Karim R, Jevans AJ, Leesar MA. Physiological assessment of renal artery stenosis: comparisons of resting with hyperemic renal pressure measurements. Catheter Cardiovasc. Interv. 76(5), 726–732 (2010).
- Leesar MA, Varma J, Shapira A et al. Prediction of hypertension improvement after stenting of renal artery stenosis: comparative accuracy of translesional pressure gradients, intravascular ultrasound, and angiography. J. Am. Coll. Cardiol. 53(25), 2363–2371 (2009).
- Mangiacapra F, Trana C, Sarno G et al. Translesional pressure gradients to predict blood pressure response after renal artery stenting in patients with renovascular hypertension. Circ. Cardiovasc. Interv. 3(6), 537–542 (2010).
- Rundback JH, Sacks D, Kent KC et al. Guidelines for the reporting of renal artery revascularization in clinical trials. American Heart Association. Circulation 106(12), 1572– 1585 (2002).
- Nahman NS Jr, Maniam P, Hernandez RA Jr et al. Renal artery pressure gradients in patients with angiographic evidence of atherosclerotic renal artery stenosis. Am. J. Kidney Dis. 24(4), 695–699 (1994).
- Colyer WR Jr, Cooper CJ, Burket MW, Thomas WJ. Utility of a 0.014” pressure-sensing guidewire to assess renal artery translesional systolic pressure gradients. Catheter Cardiovasc. Interv. 59(3), 372–377 (2003).
- Jones NJ, Bates ER, Chetcuti SJ, Lederman RJ, Grossman PM. Usefulness of translesional pressure gradient and pharmacological provocation for the assessment of intermediate renal artery disease. Catheter Cardiovasc. Interv. 68(3), 429–434 (2006).
- Loutzenhiser R, Griffin K, Williamson G, Bidani A. Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290(5), R1153–R1167 (2006).
- White CJ, Olin JW. Diagnosis and management of atherosclerotic renal artery stenosis: improving patient selection and outcomes. Nat. Clin. Pract. Cardiovasc. Med. 6(3), 176–190 (2009).
- Mitchell JA, Subramanian R, White CJ et al. Predicting blood pressure improvement in hypertensive patients after renal artery stent placement: renal fractional flow reserve. Catheter Cardiovasc. Interv. 69(5), 685–689 (2007).
- Leertouwer TC, Gussenhoven EJ, Van Overhagen H, Man in ‘T Veld AJ, Van Jaarsveld BC. Stent placement for treatment of renal artery stenosis guided by intravascular ultrasound. J. Vasc. Interv. Radiol. 9(6), 945–952 (1998).
- Nair A, Kuban BD, Tuzcu EM, Schoenhagen P, Nissen SE, Vince DG. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation 106(17), 2200–2206 (2002).
- Kawamoto T, Okura H, Koyama Y et al. The relationship between coronary plaque characteristics and small embolic particles during coronary stent implantation. J. Am. Coll. Cardiol. 50(17), 1635–1640 (2007).
- Takumi T, Mathew V, Barsness GW et al. The association between renal atherosclerotic plaque characteristics and renal function before and after renal artery intervention. Mayo Clin. Proc. 86(12), 1165–1172 (2011).
- Prasad A, Ilapakurti M, Hu P et al. Renal artery plaque composition is associated with changes in renal frame count following renal artery stenting. J. Invasive Cardiol. 23(6), 227–231 (2011).
- Kunadian V, Harrigan C, Zorkun C et al. Use of the TIMI frame count in the assessment of coronary artery blood flow and microvascular function over the past 15 years. J. Thromb. Thrombolysis 27(3), 316–328 (2009).
- Gibson CM, Cannon CP, Murphy SA et al. Relationship of the TIMI myocardial perfusion grades, flow grades, frame count, and percutaneous coronary intervention to longterm outcomes after thrombolytic administration in acute myocardial infarction. Circulation 105(16), 1909–1913 (2002).
- Gibson CM, Murphy SA, Rizzo MJ et al. Relationship between TIMI frame count and clinical outcomes after thrombolytic administration. Thrombolysis In Myocardial Infarction (TIMI) Study Group. Circulation 99(15), 1945–1950 (1999).
- Gibson CM, Cannon CP, Daley WL et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation 93(5), 879–888 (1996).
- Mulumudi MS, White CJ. Renal frame count: a quantitative angiographic assessment of renal perfusion. Catheter Cardiovasc. Interv. 65(2), 183–186 (2005).
- Mahmud E, Smith TW, Palakodeti V et al. Renal frame count and renal blush grade: quantitative measures that predict the success of renal stenting in hypertensive patients with renal artery stenosis. JACC Cardiovasc. Interv. 1(3), 286–292 (2008).
- Morrison LK, Harrison A, Krishnaswamy P, Kazanegra R, Clopton P, Maisel A. Utility of a rapid B-natriuretic peptide assay in differentiating congestive heart failure from lung disease in patients presenting with dyspnea. J. Am. Coll. Cardiol. 39(2), 202–209 (2002).
- Troughton RW, Prior DL, Pereira JJ et al. Plasma B-type natriuretic peptide levels in systolic heart failure: importance of left ventricular diastolic function and right ventricular systolic function. J. Am. Coll. Cardiol. 43(3), 416–422 (2004).
- Wolf K, Kurtz A, Pfeifer M, Hocherl K, Riegger GA, Kramer BK. Different regulation of left ventricular ANP, BNP and adrenomedullin mRNA in the two-kidney, one-clip model of renovascular hypertension. Pflugers Arch. 442(2), 212–217 (2001).
- Jaff MR, Bates M, Sullivan T et al. Significant reduction in systolic blood pressure following renal artery stenting in patients with uncontrolled hypertension: results from the HERCULES trial. Catheter Cardiovasc. Interv. 80(3), 343–350 (2012).
- Kiemeneij F, Laarman GJ, Odekerken D, Slagboom T, Van Der Wieken R. A randomized comparison of percutaneous transluminal coronary angioplasty by the radial, brachial and femoral approaches: the access study. J. Am. Coll. Cardiol. 29(6), 1269–1275 (1997).
- Trani C, Tommasino A, Burzotta F. Transradial renal stenting: why and how. Catheter Cardiovasc. Interv. 74(6), 951–956 (2009).
- Alli O, Mathew V, From AM, Barsness G, Misra S, Gulati R. Transradial access for renal artery intervention is feasible and safe. Vasc. Endovascular Surg. 45(8), 738–742 (2011).
- Glagov S, Rowley DA, Kohut RI. Atherosclerosis of human aorta and its coronary and renal arteries. A consideration of some hemodynamic factors which may be related to the marked differences in atherosclerotic involvement of the coronary and renal arteries. Arch. Pathol. 72, 558–571 (1961).
- Van De Ven PJ, Beutler JJ, Kaatee R et al. Transluminal vascular stent for ostial atherosclerotic renal artery stenosis. Lancet 346(8976), 672–674 (1995).
- Palmer FJ, Warren BA. Multiple cholesterol emboli syndrome complicating angiographic techniques. Clin. Radiol. 39(5), 519–522 (1988).
- Safian RD, Madder RD. Refining the approach to renal artery revascularization. JACC Cardiovasc. Interv. 2(3), 161–174 (2009).
- Feldman RL, Wargovich TJ, Bittl JA. No-touch technique for reducing aortic wall trauma during renal artery stenting. Catheter Cardiovasc. Interv. 46(2), 245–248 (1999).
- Olin JW. Atheroembolic renal disease: underdiagnosed and misunderstood. Catheter Cardiovasc. Interv. 70(6), 789–790 (2007).
- Cooper CJ, Haller ST, Colyer W et al. Embolic protection and platelet inhibition during renal artery stenting. Circulation 117(21), 2752–2760 (2008).
- Paul TK, Lee JH, White CJ. Renal embolic protection devices improve blood flow after stenting for atherosclerotic renal artery stenosis. Catheter Cardiovasc. Interv. 80(6), 1019–1022 (2012).
- Lederman RJ, Mendelsohn FO, Santos R, Phillips HR, Stack RS, Crowley JJ. Primary renal artery stenting: characteristics and outcomes after 363 procedures. Am. Heart J. 142(2), 314–323 (2001).
- Zahringer M, Sapoval M, Pattynama PM et al. Sirolimuseluting versus bare-metal low-profile stent for renal artery treatment (GREAT Trial): angiographic follow-up after 6 months and clinical outcome up to 2 years. J. Endovasc. Ther. 14(4), 460–468 (2007).
- Rasmus M, Huegli R, Jacob AL, Aschwanden M, Bilecen D. Extensive iatrogenic aortic dissection during renal angioplasty: successful treatment with a covered stent-graft. Cardiovasc. Intervent. Radiol. 30(3), 497–500 (2007).
- Patel PM, Eisenberg J, Islam MA, Maree AO, Rosenfield KA. Percutaneous revascularization of persistent renal artery in-stent restenosis. Vasc. Med. 14(3), 259–264 (2009).
- Isles CG, Robertson S, Hill D. Management of renovascular disease: a review of renal artery stenting in ten studies. QJM 92(3), 159–167 (1999).
- Simone TA, Brooke BS, Goodney PP et al. Clinical effectiveness of secondary interventions for restenosis after renal artery stenting. J. Vasc. Surg. 58(3), 687–694 (2013).
- Bax L, Mali WP, Van De Ven PJ, Beek FJ, Vos JA, Beutler JJ. Repeated intervention for in-stent restenosis of the renal arteries. J. Vasc. Interv. Radiol. 13(12), 1219–1224 (2002).
- Munneke GJ, Engelke C, Morgan RA, Belli AM. Cutting balloon angioplasty for resistant renal artery in-stent restenosis. J. Vasc. Interv. Radiol. 13(3), 327–331 (2002).
- Spratt JC, Leslie SJ, Verin V. A case of renal artery brachytherapy for in-stent restenosis: four-year follow-up. J. Invasive Cardiol. 16(5), 287–288 (2004).
• This article compares translesional pressure gradients both at rest and in hyperemic states, and compares them to renin production in the ipsilateral renal vein.
• This article discusses the use of translesional pressure gradients for further evaluating the severity of moderate renal artery stenosis.
•• This article shows fractional flow reserve as a predictor of blood pressure response after renal artery intervention.
•• This article highlights the importance of stent size as a predictor for in-stent restenosis.