Review Article - Interventional Cardiology (2014) Volume 6, Issue 2
Septal reduction therapies in hypertrophic cardiomyopathy: comparison of surgical septal myectomy and alcohol septal ablation
- Corresponding Author:
- Kyle W
Klarich
Cardiovascular Diseases, Mayo Clinic College of Medicine,
Gonda 06, 200 First Street SW, Rochester, MN 55905, USA
Tel: +1 507 284 1226
Fax: +1 507 284 3968
E-mail: klarich.kyle@mayo.edu
Abstract
Hypertrophic cardiomyopathy (HCM) is the most common heritable cardiomyopathy, defined by cardiac hypertrophy in the absence of an identifiable hemodynamic etiology.Keywords
alcohol septal ablation, hypertrophic cardiomyopathy, septal myectomy, septal reduction therapy
Hypertrophic cardiomyopathy (HCM) is the most common heritable cardiomyopathy, defined by cardiac hypertrophy in the absence of an identifiable hemodynamic etiology [1,2]. Within HCM, there is a large spectrum of disease, highlighted by heterogeneous genetic underpinnings as well as varied manifestations of myocardial hypertrophy. Clinical presentation is diverse, ranging from asymptomatic to refractory heart failure symptoms or sudden cardiac death (SCD). Left ventricular outflow tract (LVOT) obstruction, either at rest or with physiologic provocation, occurs in approximately 70% of patients with HCM [3]. Left ventricular outflow obstruction is a major cause of exertional symptoms of dyspnea, angina and syncope, and is associated with a poorer prognosis. The majority of patients with obstruction can be managed with medical therapy consisting of β-blockade, verapamil, or disopyramide alone or in combination, as well as avoidance of gradient provocation (decreases in preload or afterload) [4].
There is a subset of patients with obstructive HCM who continue to have severe limiting symptoms despite optimal medical therapy or who are intolerant to medical therapy; at the Mayo Clinic these comprise approximately one third of patients, reflecting our referral practice, with smaller proportions reported elsewhere [5]. In this subset of patients with medically refractory symptoms and significant LVOT obstruction (defined as a gradient of 50 mmHg at rest or with physiologic provocation, associated with septal hypertrophy and systolic anterior motion [SAM] of the mitral valve), septal reduction therapies should be considered. The 2011 ACCF/AHA Guidelines for Diagnosis and Treatment of Hypertrophic Cardiomyopathy recommend septal myectomy at experienced centers (those with an individual operator >20 procedures or a program with >50 procedures, with mortality rates less than 1%, complication rates less than 3% and documented success at symptom relief) as the first consideration for septal reduction therapy (class IIa, level of evidence B) [4]. Alcohol septal infarction, commonly referred to as alcohol septal ablation (ASA), is an alternative to myectomy in selected patients either with contraindication to myectomy, such as excessive surgical risk, or who have a preference for ablation after informed discussion (class IIb, level of evidence B) [4]. Percutaneous intervention has been rapidly adopted, with rates of alcohol septal ablation presently exceeding myectomy at many institutions. The optimal approach to treatment of obstruction remains controversial [5–8].
Pathophysiology of obstruction
Recognition and understanding of the underlying anatomy, mechanism and hemodynamics of LVOT obstruction in HCM is key to any discussion of septal reduction therapies. In HCM, the mitral leaflets are anteriorly positioned with extension of leaflet tips past the point of coaptation into the outflow tract [9]. The papillary muscles are anteriorly malpositioned and can insert directly onto the mitral leaflet. These geometric papillary muscle changes, in combination with mitral leaflet elongation, lead to less posterior leaflet tethering and thus provide sufficient leaflet mobility to result in SAM [10–13].
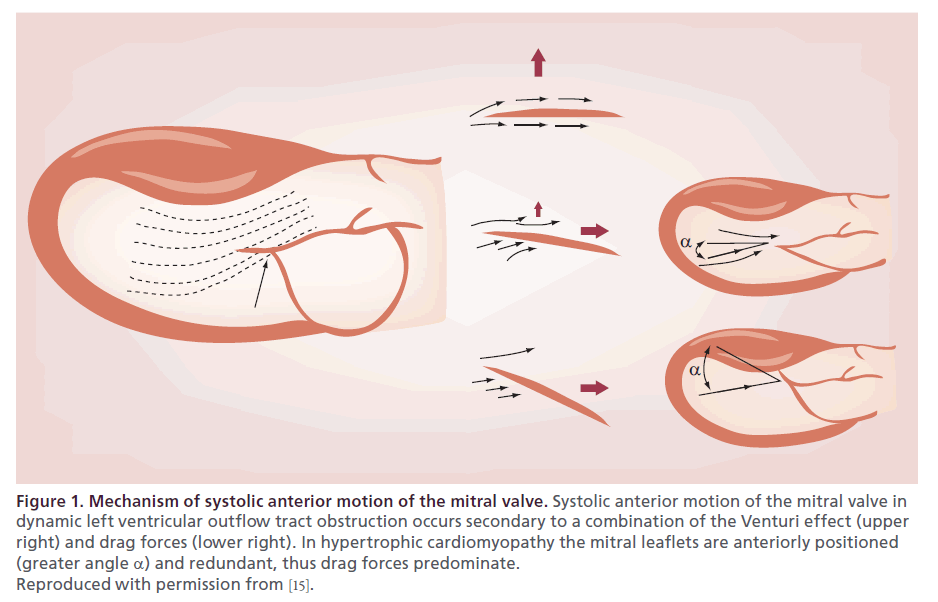
Studies have demonstrated that given the mitral anatomy in HCM, the predominant mechanism of SAM is related to drag on the mitral valve leaflet, generated by blood flow acceleration across the septum during ventricular systole, ‘pushing’ the leaflets into the LVOT (Figure 1) [14–18]. As opposed to a fixed stenosis in aortic stenosis, subvalvular obstruction is dynamic and fluctuates significantly based upon changes in numerous of clinical factors, including fluctuations in volume status, autonomic nervous activity, diurnal variation, pharmacotherapy, exercise and physical position during assessment [18–22]. Despite the fact that the degree of obstruction is highly dynamic (indeed, fluctuating on a minute-to-minute basis) [20,23–24], it is important to document whether severe obstruction does occur and relate its presence to the patient’s symptoms [25–33].
Figure 1. Mechanism of systolic anterior motion of the mitral valve. Systolic anterior motion of the mitral valve in dynamic left ventricular outflow tract obstruction occurs secondary to a combination of the Venturi effect (upper right) and drag forces (lower right). In hypertrophic cardiomyopathy the mitral leaflets are anteriorly positioned (greater angle α) and redundant, thus drag forces predominate. Reproduced with permission from [15].
Nonobstructive hypertrophic cardiomyopathy
Although not the primary focus of this review, it is important to recognize that outflow obstruction is not the only cause of symptoms in patients with HCM. On a cellular basis, HCM is characterized by cardiomyocyte hypertrophy, myofibrillar disarray and interstitial fibrosis [34], with mounting evidence that myocardial ischemia plays a significant role [35]. These cellular changes result in regional and global diastolic dysfunction in essentially all patients with HCM, which in turn is a major contributor to symptoms in both obstructive and nonobstructive HCM [36,37].
Patients with nonobstructive HCM represent approximately one third of all patients with HCM, and prognosis is generally considered favourable [3]. In symptomatic patients with nonobstructive HCM, pharmacotherapy remains the mainstay of treatment. Our practice is use of β-blockade or verapamil in these patients, with selected use of diuretics [38]. Medically refractory patients with nonobstructive HCM remain a therapeutic dilemma and investigations into alternative pharmacotherapies are ongoing [39]. In nonobstructive patients with apical HCM and severe hypertrophy to the point of limiting diastolic left ventricular filling, apical myectomy is a recently emerging option. Apical myectomy improves functional status by decreasing left ventricular end-diastolic pressure, improving operative compliance and increasing stroke volume [40]. The only other option in this highly symptomatic subset of HCM patients is cardiac transplantation. Understanding of when to choose apical myectomy over transplant and which patients receive the most benefit is evolving and few centers offer this intervention at present. The primary indication for myocardial reduction therapy in medically refractory patients remains relief of obstruction.
Septal myectomy
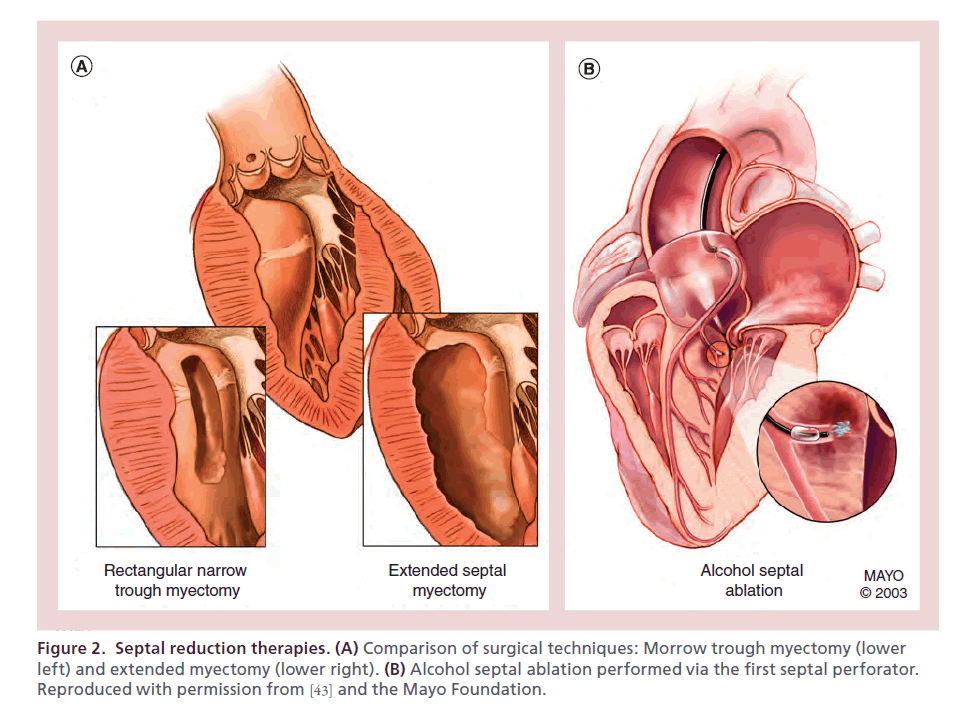
Ever since it was initially described in the 1960s, septal myectomy (muscle excision) has been considered the primary invasive treatment for drug-refractory symptoms in HCM patients with obstructive pathophysiology. Initially performed by Cleland et al. [41], myectomy was subsequently refined to a narrow rectangular trough by Morrow and Brockenbrough (Figure 2A) [42].
Figure 2. Septal reduction therapies. (A) Comparison of surgical techniques: Morrow trough myectomy (lower left) and extended myectomy (lower right). (B) Alcohol septal ablation performed via the first septal perforator. Reproduced with permission from [43] and the Mayo Foundation.
The success of myectomy is dependent upon surgical experience; therefore, guidelines recommend it be performed at high-volume referral centers of excellence [4]. Myectomy must be approached in a detail-oriented, systematic fashion. Operative management should be guided by preoperative comprehensive transthoracic echocardiography, performed by an experienced laboratory. This evaluation is crucial to assess structural and hemodynamic features of the disease; septal morphology, level and severity of flow obstruction(s) (including, when necessary, provocative measures such as Valsalva maneuver, amyl nitrite or exercise), presence of true LVOT obstruction with SAM and an open distal cavity as opposed to cavity obliteration, delineation of mitral valve anatomy, as well as the degree and mechanism of mitral regurgitation [2,44–50].
If obstruction is not identified intraoperatively, administration of isoproterenol should be performed, as anesthetic administration alters loading conditions and may mask dynamic obstruction. Our institution has adopted an extended surgical myectomy, as the most common reason for residual obstruction following myectomy is incomplete resection rather than regrowth of myocardium. Extended myectomy is performed with continuation of the initial incision leftward towards the mitral apparatus and apically towards the papillary muscles (Figure 2A, lower right) [51,52]. Dependent upon the septal morphology and level of obstruction, transapical myectomy or a combination of transaortic and transapical myectomy can be employed to ensure adequate myocardial resection [53,54].
Papillary muscle anomalies in HCM can play a critical role in obstruction [55]. In cases of obstruction due to hypertrophied papillary muscles, additional resection around the base of the muscles is performed. Anomalous chordal structures and fibrous attachments to the ventricular septum are divided or excised, but any attachments to the mitral leaflet free edge are left intact, so as to avoid a flail leaflet [56]. Plication of the anterior mitral leaflet in combination with myectomy has been utilized to restrict mitral leaflet motion [57,58]. In our practice, we do not routinely perform mitral plication, given excellent results with isolated extended septal myectomy in the absence of organic mitral pathology.
Determination of adequate resection is based upon direct visual inspection and digital palpation, with postoperative confirmation of obstruction resolution by direct hemodynamic assessment of left ventricular and aortic pressures with isoproterenol and via transesophageal echocardiography.
Alcohol septal ablation
Alcohol septal ablation was first performed by Sigwart in 1994 [59] and has been rapidly adopted as a percutaneous alternative to myectomy without randomized clinical data. Conceptually, ASA is a chemically induced myocardial infarction targeted at the myocardium that is causing flow obstruction (Figure 2B). Patient appeal from ASA largely stems from avoidance of sternotomy, a fact that may significantly affect informed decision-making [6].
As with myectomy, operator experience is pivotal to ASA success; a case volume >50 patients has been shown to be an independent predictor of survival free of severe symptoms [60]. Greater success rates and lower complication rates are seen at experienced centers for a myriad of reasons: selective identification of proper septal perforators, use of small volumes of injected alcohol, understanding intraprocedural predictors of success, as well as progressive adaptation of procedural technique based upon results and complications.
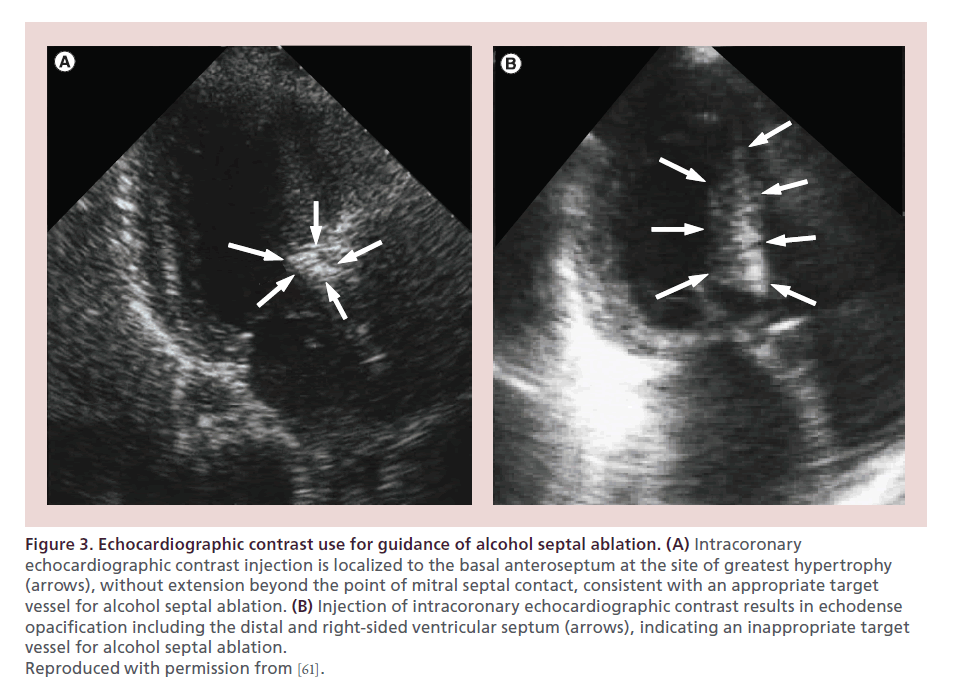
Our approach to ASA includes assessment of septal perforator myocardial distribution via both angiographic contrast (1–2 ml) and echocardiographic contrast injection (Figure 3) [61]. In our laboratory, perflutren protein type A microspheres (OptisonTM, General Electric Company, CT, USA) are utilized for echocardiographic contrast injection, with image acquisition utilizing contrast-optimized presets. Preablation contrast injection allows for determination that the cannulated septal perforator artery supplies the site of SAM septal contact and that infarction will not involve a site distal to septal contact, a papillary muscle, or the right ventricular free wall. Implementation of contrast echocardiography guidance has virtually eliminated apical and lateral wall infarctions in experienced centers [61,62]. Contrast administration assesses the extent of collateral flow, as determined by rapid contrast washout, which may necessitate slower ethanol injection.
Figure 3. Echocardiographic contrast use for guidance of alcohol septal ablation. (A) Intracoronary echocardiographic contrast injection is localized to the basal anteroseptum at the site of greatest hypertrophy (arrows), without extension beyond the point of mitral septal contact, consistent with an appropriate target vessel for alcohol septal ablation. (B) Injection of intracoronary echocardiographic contrast results in echodense opacification including the distal and right-sided ventricular septum (arrows), indicating an inappropriate target vessel for alcohol septal ablation. Reproduced with permission from [61].
Slower alcohol administration and smaller alcohol volume may reduce the incidence of heart block [63], although we routinely place a temporary transvenous pacemaker implantation in all patients undergoing ASA. We typically inject small amounts of alcohol (1–1.5 ml) over a prolonged period of time. Following injection, the catheter is flushed to ensure alcohol is not delivered into the left anterior descending coronary artery.
Comparison of septal reduction therapies
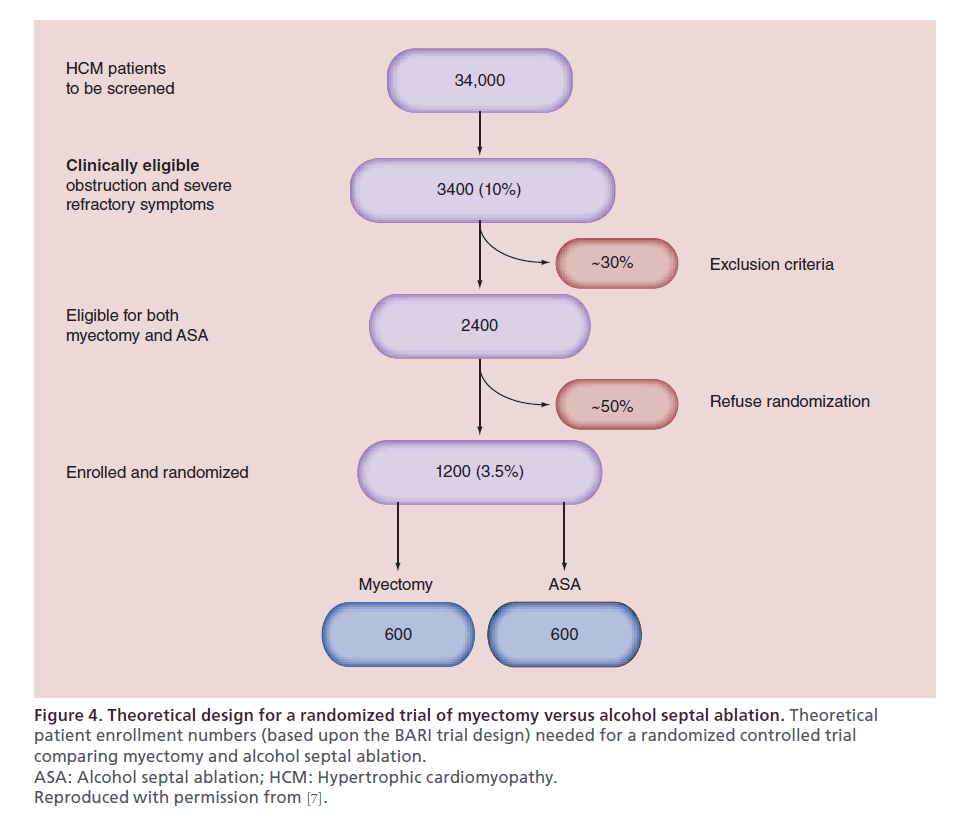
There is a substantial body of literature attempting to compare ASA and myectomy. Olivotto et al. have elegantly outlined the practical issues in designing a randomized controlled trial to compare these interventions (Figure 4) [7]. In order to obtain a trial comparing 600 patients undergoing myectomy to 600 undergoing ASA, 34,000 HCM patients would need be screened, a number nearly ten times our high-volume institution’s cumulative referral practice. Thus, at present the highest level of evidence stems from single-center cohort studies, meta-analyses and registry experiences [64–66].
Figure 4. Theoretical design for a randomized trial of myectomy versus alcohol septal ablation. Theoretical patient enrollment numbers (based upon the BARI trial design) needed for a randomized controlled trial comparing myectomy and alcohol septal ablation. ASA: Alcohol septal ablation; HCM: Hypertrophic cardiomyopathy. Reproduced with permission from [7].
Mortality
As evidenced by early surgical experiences describing complications of valvular injury, heart block, iatrogenic ventricular septal defect, lack of complete obstruction relief and higher operative mortality, myectomy has a steep learning curve [52]. Some institutions have reported higher complication rates [67,68], with multinational data in less experienced centers showing mortality rates of 14% [69]. It is recommended that each center assess their own statistics and compare with published data from centers of excellence. However, in experienced institutions, such as ours, the risk of death from lone myectomy has been reduced to less than 1% (Figure 5A) [32]. Furthermore, a multiinstitutional analysis of consecutive isolated myectomies in experienced institutions revealed zero deaths in >1500 consecutive patients [5]. More complex surgeries, such as those in patients undergoing myectomy after failed ASA, have been shown to have higher operative risk [70]. Given a surgical experience spanning more than 50 years, long-term efficacy of myectomy is well established [32,71,72].
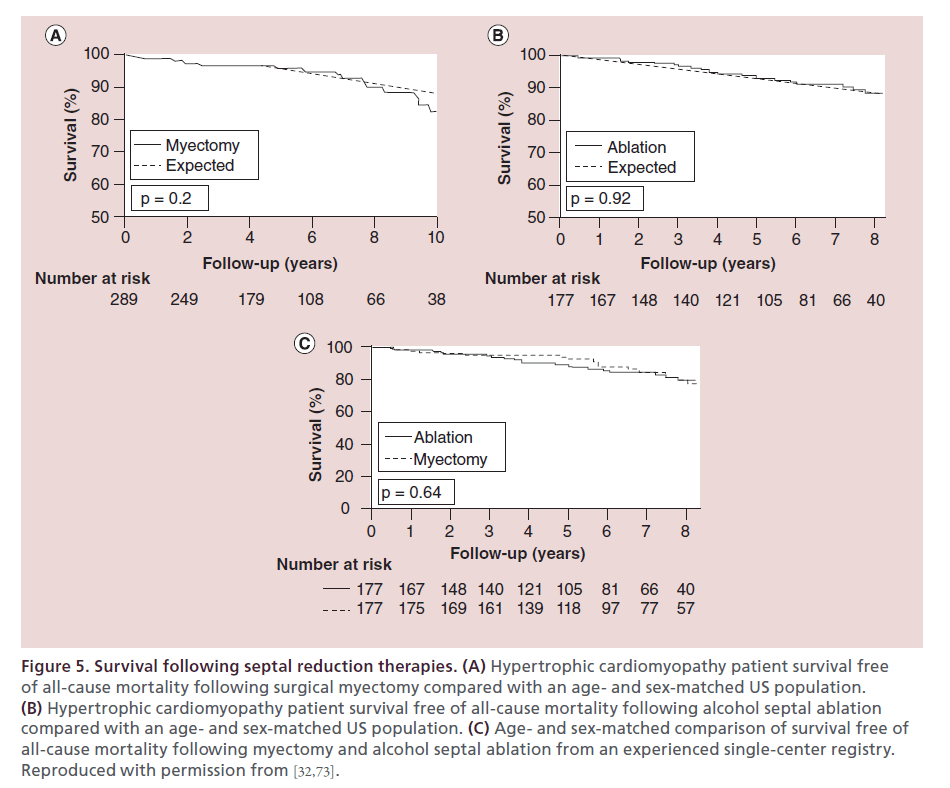
Figure 5. Survival following septal reduction therapies. (A) Hypertrophic cardiomyopathy patient survival free of all-cause mortality following surgical myectomy compared with an age- and sex-matched US population. (B) Hypertrophic cardiomyopathy patient survival free of all-cause mortality following alcohol septal ablation compared with an age- and sex-matched US population. (C) Age- and sex-matched comparison of survival free of all-cause mortality following myectomy and alcohol septal ablation from an experienced single-center registry. Reproduced with permission from [32,73].
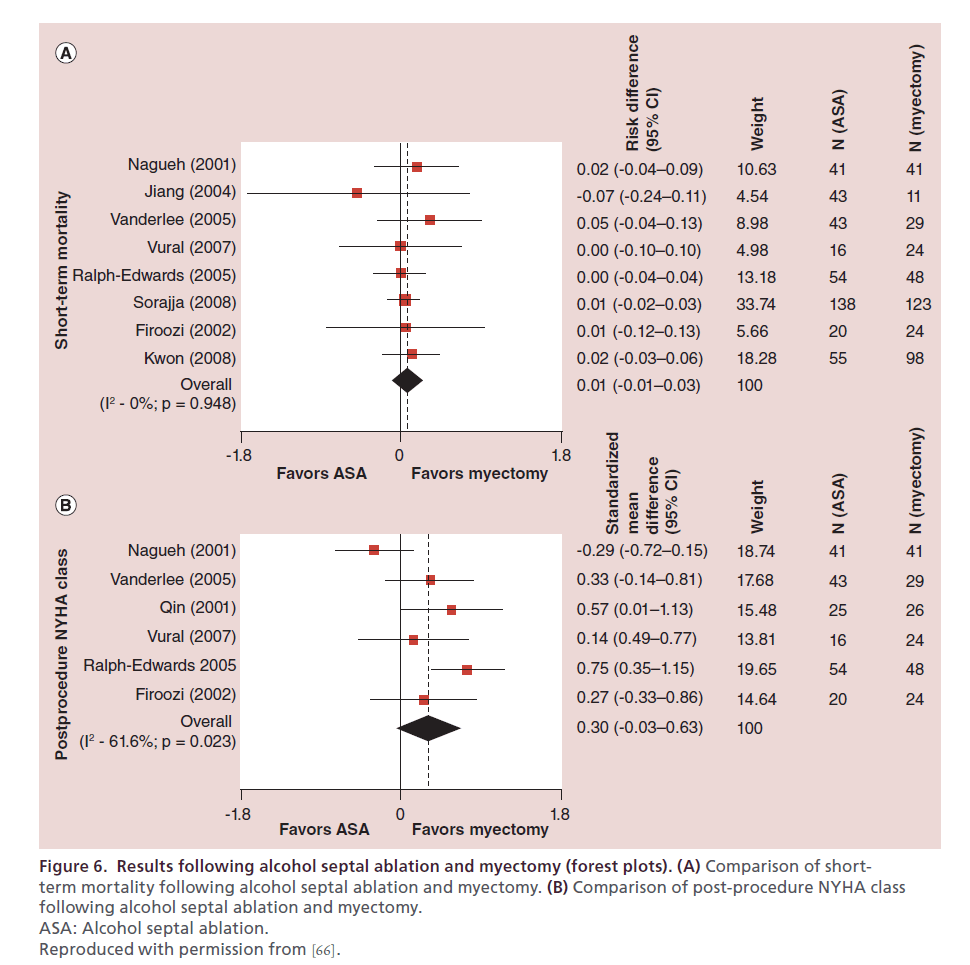
The majority of existing ASA literature has focused on short-term survival, as the procedure is newer than myectomy [28,74,75–78]. There has been concern raised within the literature that ASA is associated with a higher risk of cardiac death or aborted cardiac death [8]. However, pooled data across eight studies assessing short-term post-procedural mortality has shown no significant difference between ASA and myectomy (Figure 6A) [66]. Furthermore, in recent studies of intermediate- term outcome, post-ablation survival has been demonstrated to be similar to that of an age- and sexmatched US population (Figure 5B) and comparable to myectomy (Figure 5C) at our center [60,73]. These data have been mirrored in other studies [79,80], including a recent study with longer follow-up (mean: 8.4 years) [81]. With regards to cardiac mortality, there has been concern raised regarding increased risk of ventricular arrhythmias following ASA [8] and long-term consequences of adverse remodeling, although data on the latter is lacking. Long-term data with follow-up beyond 10 years is required to assess both the overall mortality and arrhythmia risk.
Figure 6. Results following alcohol septal ablation and myectomy (forest plots). (A) Comparison of shortterm mortality following alcohol septal ablation and myectomy. (B) Comparison of post-procedure NYHA class following alcohol septal ablation and myectomy. ASA: Alcohol septal ablation. Reproduced with permission from [66].
Regardless of approach, it is important to recognize that this improvement in natural history does not obviate the need for assessment of SCD risk with appropriate defibrillator implantation when dictated by risk factors. It is beyond the scope of this paper to discuss the assessment and we refer interested readers to the ACCF/ ACC Guidelines for Diagnosis and Management of Hypertrophic Cardiomyopathy [4].
Symptom reduction
Given that the indication for septal reduction therapies is medically refractory symptoms, appraisal of symptom improvement following intervention is paramount. There is substantial literature demonstrating improvement in NYHA functional class following myectomy in patients with resting obstruction [52,72] and more recent data reveal similar improvements are seen in patients with latent obstruction [82]. Similarly, there have been a large number of studies supporting symptom relief in ASA [74,83–85].
While myectomy has previously been reported to result in more symptomatic improvement when compared with ASA [76], meta-analysis data have not shown a statistically significant difference between the techniques (Figure 6B), although there was a trend towards less symptoms with myectomy [66]. Our data clearly shows that myectomy in younger patients (<65 years old) results in significantly more freedom from NYHA class III or IV symptoms when compared with ASA [74]. This may be the result of our referral practice, or the meta-analysis data may reflect heterogeneity of operator experience and patient demographics. Regardless, objective measures of exercise capacity improve more with myectomy than with ASA [76,78].
Relief of obstruction
Despite the dynamic nature of LVOT obstruction in HCM, abolishment of gradient post-procedurally is utilized to determine technical success of septal reduction therapies. Post-myectomy, gradient should be determined intraoperatively by direct pressure measurement and by transesophageal echocardiography, as is routinely performed at our institution. Reduction of gradient post-myectomy is immediate given resection of the offending substrate. In ASA, procedural success is determined via a combination of invasive catheter measures and transthoracic echocardiography. Initial gradient reduction post-ablation likely reflects myocardial stunning (lack of contraction) as opposed to true relief of obstruction from reduction in myocardial mass, as evidenced by immediate gradient reduction, a rise in gradient 1–3 days post-procedurally, and subsequent fall in gradient at 3-month follow-up [86,87]. Because of this stunning phenomenon, prediction of lasting hemodynamic results is difficult. Although not published, it has been our experience that if the gradient is >20 mmHg immediately post ablation, then there will be a poor long-term hemodynamic result. Conversely, if the residual gradient immediately following ASA is <20 mmHg, one cannot predict the degree of gradient reduction, as this decrease may relate entirely to stunning.
Both myectomy and ASA have been shown to alleviate LVOT obstruction. Studies consistently report more complete reduction of the gradient with myectomy [75–77,88–89]. Furthermore, this relationship has remained significant in multiple meta-analyses [65,66].
Improvement in diastolic function
Although not the indication for septal reduction therapy, there is evidence that myectomy results in improvement in diastolic function [90,91], improvement in pulmonary hemodynamics [92], reduction in left ventricular mass disproportionate to the amount of myocardium resected [93] and differential strain improvement [94], all implying positive ventricular remodeling. The reported effects of ASA on diastolic dysfunction are heterogeneous. Some studies have demonstrated improvement in parameters of diastolic function following ASA [95–98], while another study showed no significant difference between diastolic assessment following ASA and myectomy when assessed 5 months post-procedurally [99]. At our institution, we have seen variable change in diastolic function, likely reflecting a balance of obstruction relief and intrinsic effect of the infarct itself [100].
Need for pacemaker implantation
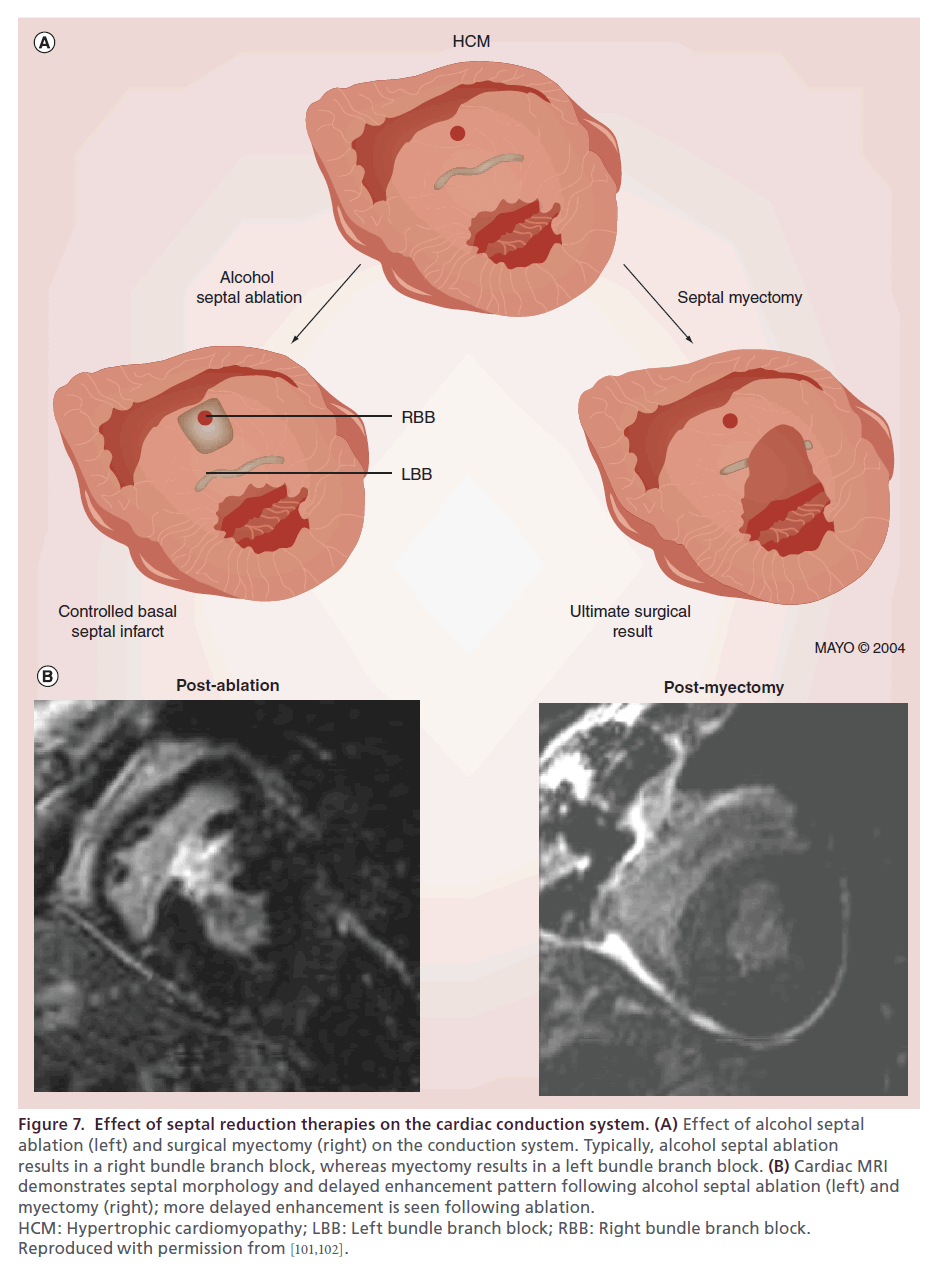
Alcohol septal ablation and myectomy result in differing effects on the conduction system because of the anatomic distribution of the bundle branches within the ventricular septum. The first septal perforator supplies the myocardium containing the right bundle, and thus development of a right bundle branch block is the most common electrical change seen post-ASA. In contrast, myectomy involves resection of tissue containing the left bundle, more typically resulting in a new left bundle branch block (Figure 7A) [101]. The anatomic differences in affected tissues are clearly visualized on post-intervention cardiac MRI (Figure 7B). The presence of an existing left bundle branch block significantly increases the risk of complete heart block in ASA, whereas preexisting right bundle branch block predisposes to complete heart block following myectomy.
Figure 7. Effect of septal reduction therapies on the cardiac conduction system. (A) Effect of alcohol septal ablation (left) and surgical myectomy (right) on the conduction system. Typically, alcohol septal ablation results in a right bundle branch block, whereas myectomy results in a left bundle branch block. (B) Cardiac MRI demonstrates septal morphology and delayed enhancement pattern following alcohol septal ablation (left) and myectomy (right); more delayed enhancement is seen following ablation. HCM: Hypertrophic cardiomyopathy; LBB: Left bundle branch block; RBB: Right bundle branch block. Reproduced with permission from [101,102].
The frequency of complete heart block necessitating pacemaker implantation following ASA varies widely, from a multicenter trial reporting 8% [103] to rates as high as 33% [101]. Higher rates may in part stem from larger volumes of alcohol injected or early operator experience [63]. At our institution, new permanent pacemaker placement occurs in 17% of patients [60]. Post-ablation patients who undergo permanent pacemaker implantation for iatrogenic complete heart block have a comparable intermediate-term prognosis to other post-ASA patients [104]. In the absence of right bundle branch block, risk of complete heart block necessitating permanent pacemaker in patients undergoing myectomy is <1% [45]. Meta-analysis data demonstrate that ASA carries an increased risk of need for permanent pacemaker implantation relative to myectomy [66]. Patients who require alcohol administration in multiple vessels are more likely to require permanent pacing [63]. Furthermore, given the fact that ASA and myectomy affect different aspects of the conduction system, patients undergoing myectomy following unsuccessful ASA have a significantly higher rate of pacemaker implantation [70].
Arrhythmogenic risk
The clinical importance of post-ablation scar formation has been an area of controversy within HCM literature. While some have proposed that myectomy does not result in scar formation [5], postmortem data show that following ASA, segmental infarction occurs, albeit with histopathologic differences from atherosclerotic coronary occlusion [105]. Importantly, Valeti et al. found essentially no myocardial enhancement on cardiac MRI following myectomy compared with following ASA, which resulted in delayed enhancement quantitated at 8.0 ± 3.0% of left ventricular mass.
Whether localized infarction related to septal reduction predisposes to arrhythmogenic risk and incidence of SCD remains a topic of debate. While some literature reports rates of sudden death following ASA are comparable to patients not undergoing ablation [81,106] or similarly low as post-myectomy [107], other literature raises a significant concern of increased risk of potentially lethal arrhythmias. Ten Cate et al. reported a fivefold increase in cardiac death and aborted sudden cardiac death following ASA compared with myectomy, primarily driven by ventricular arrhythmia [8], and Noseworthy et al. reported post-ASA annual rates of ventricular arrhythmia, cardiac arrest or appropriate ICD therapy at similar rates [108]. At our institution, we have cared for patients who have experienced sustained ventricular tachycardia following ASA. Conversely, we have demonstrated that myectomy is associated with lower than expected risk of SCD [109].
Patient selection
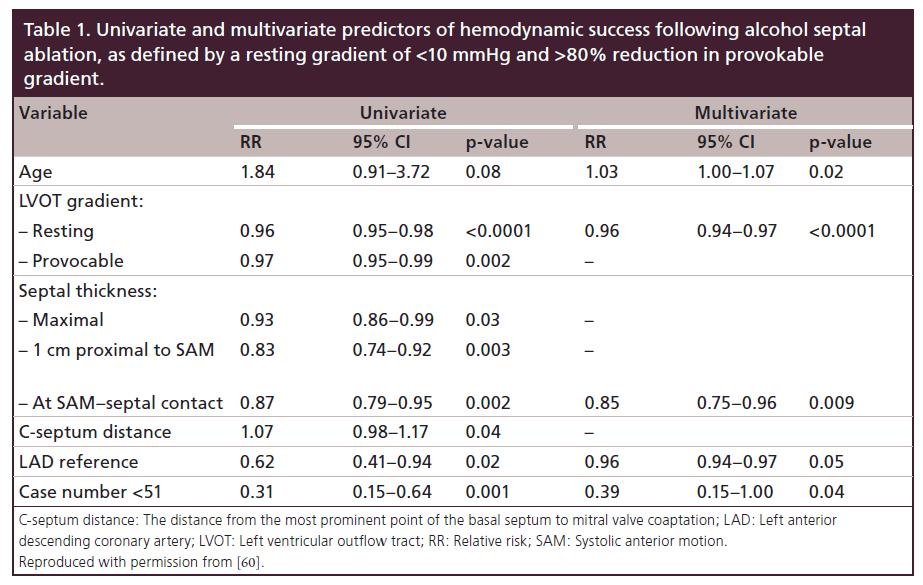
Decision-making and informed consent with regards to choice of septal reduction therapy must include a myriad of factors. In our practice, the “gold standard” of therapy remains myectomy, consistent with guideline recommendations. However, clinical variables, anatomic factors and patient preferences must be considered for each patient as ASA remains a viable and successful therapy in many HCM patients. We have previously published determinants of success for ASA (Table 1) [60]. When pursuing a discussion with patients, we consider each of the following:
Institutional experience
It cannot be overemphasized that regardless of choice of therapy, site experience remains paramount [4,60]. It is clear that operator experience directly affects both morbidity and mortality. In reports from less experienced centers, reported mortality rates can be dramatically higher [110,111], which can skew comparison of septal reduction techniques. In addition, procedural complication rates for both ASA and myectomy from less experienced centers are likely underestimated given lack of published outcomes from these institutions. However, unsuccessful procedure results and complications are apparent in our referral practice [70].
Coexistent valvular or other structural heart disease
The presence of concomitant structural heart disease must be considered when selecting a procedure:
• Patients with primary mitral valve disease (seen in up to 10% of HCM patients), including flail mitral valve necessitating repair, should undergo myectomy;
• Outflow obstruction may have both fixed and dynamic components [112] or be present at multiple levels [113], and these patients should be referred for surgical management;
• Anomalous attachments of papillary muscles may contribute to obstruction and mandate myectomy [55].
Recognition of these anatomic considerations at the time of septal reduction referral is crucial.
Septal perforator anatomy
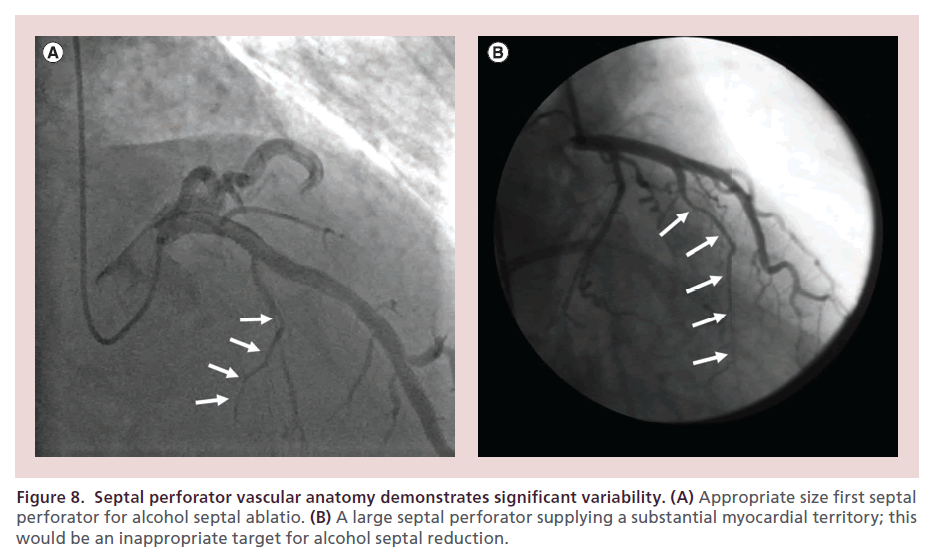
There is significant variability in septal perforator anatomy [114]. ASA procedural success is constrained by identification of an appropriate therapeutic target (Figure 8). Use of angiographic and echocardiographic contrast, as outlined above, allows for evaluation of candidate vessels when considering ASA. Even in patients deemed appropriate for ASA, septal perforators have been shown to supply extra-septal anatomy in >25% [115], which may necessitate superselective approaches to branch vessels [116]. A larger left anterior descending dimension (>4.0 cm) is associated with poorer outcome in ASA [60].
Figure 8. Septal perforator vascular anatomy demonstrates significant variability. (A) Appropriate size first septal perforator for alcohol septal ablatio. (B) A large septal perforator supplying a substantial myocardial territory; this would be an inappropriate target for alcohol septal reduction.
Coronary artery disease
Significant coronary artery disease may preclude ASA. In the presence of hemodynamically significant obstructive coronary disease, concomitant coronary revascularization and septal reduction therapy may be warranted. Simultaneous myectomy and coronary artery bypass grafting have been demonstrated to have excellent results [117] and the presence of significant coronary atherosclerotic disease should prompt surgical consideration.
Patient age
Myectomy provides much greater symptom reduction in younger patients (<65 years old) when compared with ASA [74] and benefits of myectomy have been proven even in pediatric populations [91,118–119]. There is a greater body of evidence supporting longterm improvement in hemodynamics and symptoms in myectomy. Additionally, hemodynamic success with septal ablation is more likely achieved in older patients [60]. Therefore, in younger populations (those <65 years of age) we recommend myectomy, whereas in more elderly patients, ASA is considered.
Underlying conduction disease
Preexistent left bundle branch block increases the risk of permanent pacemaker need in ASA and should prompt consideration of myectomy. Conversely, preexistent right bundle branch block is associated with post-myectomy complete heart block and may alter therapy decision-making. In patients with normal conduction, ASA does pose higher risk of complete heart block, necessitating pacemaker implantation.
Resting gradient
Lower LVOT gradient is a predictor of success in ASA [60], whereas symptomatic success in myectomy appears to be independent of preoperative gradient [45]. In patients with resting gradients >100 mmHg, myectomy should be considered.
Septal thickness & morphology
Myectomy is preferred in patients with more severe septal hypertrophy. Myectomy allows individual tailoring of the degree of myocardium resected. The presence of asymmetric hypertrophy has been associated with better outcome [45]. In comparing a series of patients undergoing ASA, patients with septal thickness <18 mm at the site of SAM–septal contact were more likely to achieve hemodynamic success [60]. In cases of midventricular obstruction or apical HCM, septal vascular anatomy may not allow for isolation of target myocardium.
Sudden death risk
While our data do not show increased risk of SCD following ASA [73], ICD registry data is concerning [108]. Our data does suggest that myectomy may favorably alter risk [109]. As such, we presently do not advise decision- making be altered by consideration of this factor.
We continue to recommend defibrillator implantation based upon established guidelines [4], irrespective of septal reduction therapy.
Frailty & operative risk
Determination of operative risk is critical when ascertaining appropriateness for myectomy. In patients with prohibitive surgical risk, contraindications to circulatory bypass or those who are deemed too frail for surgery, ASA is a reasonable alternative to myectomy.
Patient preference
Although many risks and outcomes are equal in metaanalysis, patients often perceive alcohol septal ablation as favorable or lower risk given a percutaneous approach as opposed to sternotomy. Patient preference should be considered after an informed discussion weighing the risks and benefits of each procedure. At the Mayo Clinic, we still have a 5:1 ratio of myectomy to ablation after discussing all risks and benefits.
Conclusion
Septal reduction therapy in the form of ASA or myectomy remains the treatment of choice in HCM patients with obstructive physiology and medically refractory symptoms. Both myectomy and ASA should be performed at referral centers of excellence. Myectomy is an established technique with decades of experience that remains the ‘gold standard’ for septal reduction therapy, with greater LVOT gradient reduction and reduced need for permanent pacemaker implantation. In experienced centers, ASA has similar short-term mortality when compared with myectomy. Clinical characteristics, anatomic features and patient preference should be considered when determining the optimal technique for septal reduction.
Future perspective
Septal myectomy and ASA will remain effective therapies to reduce LVOT gradient and improve symptoms. Given that a randomized trial comparing these techniques is unlikely, future data will continue to be reliant upon cohort studies and meta-analyses. There is a need for further investigation and recognition of ‘real-world’ complication rates from septal reduction therapies performed at lower volume institutions. These data will further underscore the need for myectomy and ASA to be performed at centers of excellence. Additional data are needed with regards to long-term follow-up and arrhythmogenic risk of ASA.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Excecutive summary
Controversy of myectomy versus alcohol septal ablation
• Guidelines recommend myectomy as the ‘gold standard’ for symptomatic, medically refractory hypertrophic cardiomyopathy (HCM) patients with left ventricular outflow tract (LVOT) obstruction, but there remains significant controversy with regards to choice of myectomy or ablation.
• Both septal myectomy and alcohol septal ablation (ASA) have been shown to reduce the LVOT gradient and improve symptoms.
Septal myectomy
• With more than a half-century of experience, the long-term results of myectomy are well-established.
• Success of myectomy is dependent upon surgical experience; in experienced centers, myectomy carries low operative mortality and results in excellent reduction of LVOT gradient, symptomatic improvement and longterm postoperative survival similar to the general population.
Alcohol septal ablation
• ASA is a newer technique than myectomy, with favorable intermediate-term prognosis but unknown longterm results.
• As with myectomy, the success of ASA is experience dependent and referral centers of excellence are crucial.
Comparison of septal reduction therapies
• Limitations to study enrollment preclude a randomized controlled trial comparing myectomy and ASA.
• In experienced centers, intermediate-term mortality following ASA and myectomy is comparable, but longterm results are uncertain.
• Both ASA and myectomy result in symptom reduction, although myectomy is more efficacious in patients <65 years of age.
• Myectomy consistently results in more gradient reduction compared with ASA.
• There is debate regarding arrhythmogenic risk related to post-ASA scar, with long-term results unknown.
Patient selection
• Institutional experience is critical for both myectomy and ASA.
• Coexistent primary valvular (including mitral valve disease other than SAM) or other structural heart disease mandates surgical myectomy.
• Septal perforator anatomy and coronary artery disease may preclude ASA.
• Younger patients (age <65 years) achieve greater symptom reduction and more hemodynamic improvement with myectomy.
• In patients with higher resting gradients (>100 mmHg) or greater septal thickness (>18 mm), myectomy is more efficacious.
• Operative risk and informed patient preference should be considered when choosing between ASA and myectomy.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- Maron BJ, Epstein SE. Hypertrophic cardiomyopathy: a discussion of nomenclature. Am. J. Cardiol. 43(6), 1242–1244 (1979).
- Sorajja P, Nishimura RA, Ommen SR, Ackerman MJ, Tajik AJ, Gersh BJ. Use of echocardiography in patients with hypertrophic cardiomyopathy: clinical implications of massive hypertrophy. J. Am. Soc. Echocardiogr. 19(6), 788–795 (2006).
- Maron MS, Olivotto I, Zenovich AG et al. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation 114(21), 2232–2239 (2006).
- Gersh BJ, Maron BJ, Bonow RO et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 124(24), e783–831 (2011).
- Maron BJ. Controversies in cardiovascular medicine. Surgical myectomy remains the primary treatment option for severely symptomatic patients with obstructive hypertrophic cardiomyopathy. Circulation 116(2), 196–206; discussion 206 (2007).
- Fifer MA. Controversies in cardiovascular medicine. Most fully informed patients choose septal ablation over septal myectomy. Circulation 116(2), 207–216; discussion 216 (2007).
- Olivotto I, Ommen SR, Maron MS, Cecchi F, Maron BJ. Surgical myectomy versus alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Will there ever be a randomized trial? J. Am. Coll. Cardiol. 50(9), 831–834 (2007).
- Ten Cate FJ, Soliman OI, Michels M et al. Long-term outcome of alcohol septal ablation in patients with obstructive hypertrophic cardiomyopathy: a word of caution. Circ. Heart Fail. 3(3), 362–369 (2010).
- Shah PM, Taylor RD, Wong M. Abnormal mitral valve coaptation in hypertrophic obstructive cardiomyopathy: proposed role in systolic anterior motion of mitral valve. Am. J. Cardiol. 48(2), 258–262 (1981).
- Klues HG, Maron BJ, Dollar AL, Roberts WC. Diversity of structural mitral valve alterations in hypertrophic cardiomyopathy. Circulation 85(5), 1651–1660 (1992).
- Levine RA, Vlahakes GJ, Lefebvre X et al. Papillary muscle displacement causes systolic anterior motion of the mitral valve. Experimental validation and insights into the mechanism of subaortic obstruction. Circulation 91(4), 1189–1195 (1995).
- Schwammenthal E, Levine RA. Dynamic subaortic obstruction: a disease of the mitral valve suitable for surgical repair? J. Am. Coll. Cardiol. 28(1), 203–206 (1996).
- Cape EG, Simons D, Jimoh A, Weyman AE, Yoganathan AP, Levine RA. Chordal geometry determines the shape and extent of systolic anterior mitral motion: in vitro studies. J. Am. Coll. Cardiol. 13(6), 1438–1448 (1989).
- Jiang L, Levine RA, King ME, Weyman AE. An integrated mechanism for systolic anterior motion of the mitral valve in hypertrophic cardiomyopathy based on echocardiographic observations. Am. Heart J. 113(3), 633–644 (1987).
- Sherrid MV, Gunsburg DZ, Moldenhauer S, Pearle G. Systolic anterior motion begins at low left ventricular outflow tract velocity in obstructive hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 36(4), 1344–1354 (2000).
- Wigle ED, Rakowski H, Kimball BP, Williams WG. Hypertrophic cardiomyopathy. Clinical spectrum and treatment. Circulation 92(7), 1680–1692 (1995).
- Wigle ED, Sasson Z, Henderson MA et al. Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review. Prog. Cardiovasc. Dis. 28(1), 1–83 (1985).
- Shah JS, Esteban MT, Thaman R et al. Prevalence of exercise-induced left ventricular outflow tract obstruction in symptomatic patients with non-obstructive hypertrophic cardiomyopathy. Heart 94(10), 1288–1294 (2008).
- Braunwald E, Brockenbrough EC, Morrow AG. Hypertrophic subaortic stenosis – a broadened concept. Circulation 26, 161–165 (1962).
- Wilson WS, Criley JM, Ross RS. Dynamics of left ventricular emptying in hypertrophic subaortic stenosis. A cineangiographic and hemodynamic study. Am. Heart J. 73(1), 4–16 (1967).
- Glancy DL, Shepherd RL, Beiser D, Epstein SE. The dynamic nature of left ventricular outflow obstruction in idiopathic hypertrophic subaortic stenosis. Ann. Intern. Med. 75(4), 589–593 (1971).
- Kizilbash AM, Heinle SK, Grayburn PA. Spontaneous variability of left ventricular outflow tract gradient in hypertrophic obstructive cardiomyopathy. Circulation 97(5), 461–466 (1998).
- Geske JB, Sorajja P, Ommen SR, Nishimura RA. Left ventricular outflow tract gradient variability in hypertrophic cardiomyopathy. Clin. Cardiol. 32(7), 397–402 (2009).
- Geske JB, Sorajja P, Ommen SR, Nishimura RA. Variability of left ventricular outflow tract gradient during cardiac catheterization in patients with hypertrophic cardiomyopathy. JACC Cardiovasc. Interv. 4(6), 704–709 (2011).
- Schoendube FA, Klues HG, Reith S, Flachskampf FA, Hanrath P, Messmer BJ. Long-term clinical and echocardiographic follow-up after surgical correction of hypertrophic obstructive cardiomyopathy with extended myectomy and reconstruction of the subvalvular mitral apparatus. Circulation 92(9 Suppl.), II122–127 (1995).
- Mccully RB, Nishimura RA, Tajik AJ, Schaff HV, Danielson GK. Extent of clinical improvement after surgical treatment of hypertrophic obstructive cardiomyopathy. Circulation 94(3), 467–471 (1996).
- Franke A, Schondube FA, Kuhl HP et al. Quantitative assessment of the operative results after extended myectomy and surgical reconstruction of the subvalvular mitral apparatus in hypertrophic obstructive cardiomyopathy using dynamic three-dimensional transesophageal echocardiography. J. Am. Coll. Cardiol. 31(7), 1641–1649 (1998).
- Nagueh SF, Ommen SR, Lakkis NM et al. Comparison of ethanol septal reduction therapy with surgical myectomy for the treatment of hypertrophic obstructive cardiomyopathy. J. Am. Coll. Cardiol. 38(6), 1701–1706 (2001).
- Kofflard MJ, Ten Cate FJ, Van Der Lee C, Van Domburg RT. Hypertrophic cardiomyopathy in a large communitybased population: clinical outcome and identification of risk factors for sudden cardiac death and clinical deterioration. J. Am. Coll. Cardiol. 41(6), 987–993 (2003).
- Maron MS, Olivotto I, Betocchi S et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N. Engl. J. Med. 348(4), 295–303 (2003).
- Autore C, Bernabo P, Barilla CS, Bruzzi P, Spirito P. The prognostic importance of left ventricular outflow obstruction in hypertrophic cardiomyopathy varies in relation to the severity of symptoms. J. Am. Coll. Cardiol. 45(7), 1076–1080 (2005).
- Ommen SR, Maron BJ, Olivotto I et al. Long-term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 46(3), 470–476 (2005).
- Elliott PM, Gimeno JR, Tome MT et al. Left ventricular outflow tract obstruction and sudden death risk in patients with hypertrophic cardiomyopathy. Eur. Heart J. 27(16), 1933–1941 (2006).
- Marian AJ, Roberts R. The molecular genetic basis for hypertrophic cardiomyopathy. J. Mol. Cell Cardiol. 33(4), 655–670 (2001).
- Maron MS, Olivotto I, Maron BJ et al. The case for myocardial ischemia in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 54(9), 866–875 (2009).
- Nishimura RA, Holmes DR Jr. Clinical practice. Hypertrophic obstructive cardiomyopathy. N. Engl. J. Med. 350(13), 1320–1327 (2004).
- Ommen SR, Nishimura RA. Hypertrophic cardiomyopathy. Curr Probl Cardiol 29(5), 239–291 (2004).
- Spirito P, Seidman CE, Mckenna WJ, Maron BJ. The management of hypertrophic cardiomyopathy. N. Engl. J. Med. 336(11), 775–785 (1997).
- Yamazaki T, Suzuki J, Shimamoto R et al. A new therapeutic strategy for hypertrophic nonobstructive cardiomyopathy in humans. A randomized and prospective study with an angiotensin II receptor blocker. Int. Heart J. 48(6), 715–724 (2007).
- Schaff HV, Brown ML, Dearani JA et al. Apical myectomy: a new surgical technique for management of severely symptomatic patients with apical hypertrophic cardiomyopathy. J. Thorac. Cardiovasc. Surg. 139(3), 634–640 (2010).
- Goodwin JF, Hollman A, Cleland WP, Teare D. Obstructive cardiomyopathy simulating aortic stenosis. Br. Heart J. 22, 403–414 (1960).
- Morrow AG, Brockenbrough EC. Surgical treatment of idiopathic hypertrophic subaortic stenosis: technic and hemodynamic results of subaortic ventriculomyotomy. Ann. Surg. 154, 181–189 (1961).
- Maron BJ (Ed.). Diagnosis and Management of Hypertrophic Cardiomyopathy. Blackwell Futura, MA, USA, 223 (2004).
- Nishimura RA, Ommen SR. Hypertrophic cardiomyopathy: the search for obstruction. Circulation 114(21), 2200–2202 (2006).
- Mccully RB, Nishimura RA, Bailey KR, Schaff HV, Danielson GK, Tajik AJ. Hypertrophic obstructive cardiomyopathy: preoperative echocardiographic predictors of outcome after septal myectomy. J. Am. Coll. Cardiol. 27(6), 1491–1496 (1996).
- Ommen SR. Echocardiographic assessment of diastolic function. Curr. Opin Cardiol. 16(4), 240–245 (2001).
- Vaglio JC Jr, Ommen SR, Nishimura RA, Tajik AJ, Gersh BJ. Clinical characteristics and outcomes of patients with hypertrophic cardiomyopathy with latent obstruction. Am. Heart J. 156(2), 342–347 (2008).
- Ommen SR, Park SH, Click RL, Freeman WK, Schaff HV, Tajik AJ. Impact of intraoperative transesophageal echocardiography in the surgical management of hypertrophic cardiomyopathy. Am. J. Cardiol. 90(9), 1022–1024 (2002).
- Grigg LE, Wigle ED, Williams WG, Daniel LB, Rakowski H. Transesophageal Doppler echocardiography in obstructive hypertrophic cardiomyopathy: clarification of pathophysiology and importance in intraoperative decision making. J. Am. Coll. Cardiol. 20(1), 42–52 (1992).
- Ommen SR. Hypertrophic cardiomyopathy. Curr. Probl. Cardiol. 36(11), 409–453 (2011).
- Dearani JA, Danielson GK. Septal myectomy for obstructive hypertrophic cardiomyopathy. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 86–91 (2005).
- Dearani JA, Ommen SR, Gersh BJ, Schaff HV, Danielson GK. Surgery insight: Septal myectomy for obstructive hypertrophic cardiomyopathy – the Mayo Clinic experience. Nat. Clin. Pract. Cardiovasc. Med. 4(9), 503–512 (2007).
- Kunkala MR, Schaff HV, Nishimura RA et al. Transapical approach to myectomy for midventricular obstruction in hypertrophic cardiomyopathy. Ann. Thorac. Surg. 96(2), 564–570 (2013).
- Said SM, Schaff HV, Abel MD, Dearani JA. Transapical approach for apical myectomy and relief of midventricular obstruction in hypertrophic cardiomyopathy. J. Card. Surg. 27(4), 443–448 (2012).
- Maron BJ, Nishimura RA, Danielson GK. Pitfalls in clinical recognition and a novel operative approach for hypertrophic cardiomyopathy with severe outflow obstruction due to anomalous papillary muscle. Circulation 98(23), 2505–2508 (1998).
- Minakata K, Dearani JA, Nishimura RA, Maron BJ, Danielson GK. Extended septal myectomy for hypertrophic obstructive cardiomyopathy with anomalous mitral papillary muscles or chordae. J. Thorac. Cardiovasc. Surg. 127(2), 481–489 (2004).
- Balaram SK, Sherrid MV, Derose JJ Jr, Hillel Z, Winson G, Swistel DG. Beyond extended myectomy for hypertrophic cardiomyopathy: the resection-plication-release (RPR) repair. Ann. Thorac. Surg. 80(1), 217–223 (2005).
- Mcintosh CL, Maron BJ, Cannon RO 3rd, Klues HG. Initial results of combined anterior mitral leaflet plication and ventricular septal myotomy-myectomy for relief of left ventricular outflow tract obstruction in patients with hypertrophic cardiomyopathy. Circulation 86(5 Suppl.), II60–II67 (1992).
- Sigwart U. Non-surgical myocardial reduction for hypertrophic obstructive cardiomyopathy. Lancet 346(8969), 211–214 (1995).
- Sorajja P, Binder J, Nishimura RA et al. Predictors of an optimal clinical outcome with alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Catheter Cardiovasc. Interv. 81(1), E58–67 (2013).
- Holmes DR Jr, Valeti US, Nishimura RA. Alcohol septal ablation for hypertrophic cardiomyopathy: indications and technique. Catheter Cardiovasc. Interv. 66(3), 375–389 (2005).
- Lakkis NM, Nagueh SF, Kleiman NS et al. Echocardiography-guided ethanol septal reduction for hypertrophic obstructive cardiomyopathy. Circulation 98(17), 1750–1755 (1998).
- Chang SM, Nagueh SF, Spencer WH 3rd, Lakkis NM. Complete heart block: determinants and clinical impact in patients with hypertrophic obstructive cardiomyopathy undergoing nonsurgical septal reduction therapy. J. Am. Coll. Cardiol. 42(2), 296–300 (2003).
- Zeng Z, Wang F, Dou X, Zhang S, Pu J. Comparison of percutaneous transluminal septal myocardial ablation versus septal myectomy for the treatment of patients with hypertrophic obstructive cardiomyopathy – a meta analysis. Int. J. Cardiol. 112(1), 80–84 (2006).
- Alam M, Dokainish H, Lakkis NM. Hypertrophic obstructive cardiomyopathy-alcohol septal ablation vs. myectomy: a meta-analysis. Eur. Heart J. 30(9), 1080–1087 (2009).
- Agarwal S, Tuzcu EM, Desai MY et al. Updated metaanalysis of septal alcohol ablation versus myectomy for hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 55(8), 823–834 (2010).
- Heric B, Lytle BW, Miller DP, Rosenkranz ER, Lever HM, Cosgrove DM. Surgical management of hypertrophic obstructive cardiomyopathy. Early and late results. J. Thorac. Cardiovasc. Surg. 110(1), 195–206; discussion 206–198 (1995).
- Woo A, Williams WG, Choi R et al. Clinical and echocardiographic determinants of long-term survival after surgical myectomy in obstructive hypertrophic cardiomyopathy. Circulation 111(16), 2033–2041 (2005).
- Veselka J, Lawrenz T, Stellbrink C et al. Early outcomes of alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a European multicenter and multinational study. Catheter Cardiovasc. Interv. doi:10.1002/ccd.25236 (2013) (Epub ahead of print).
- Elbardissi AW, Dearani JA, Nishimura RA, Ommen SR, Stulak JM, Schaff HV. Septal myectomy after previous septal artery ablation in hypertrophic cardiomyopathy. Mayo Clin. Proc. 82(12), 1516–1522 (2007).
- Fighali S, Krajcer Z, Leachman RD. Septal myomectomy and mitral valve replacement for idiopathic hypertrophic subaortic stenosis: short- and long-term follow-up. J. Am. Coll. Cardiol. 3(5), 1127–1134 (1984).
- Krajcer Z, Leachman RD, Cooley DA, Coronado R. Septal myotomy–myomectomy versus mitral valve replacement in hypertrophic cardiomyopathy. Ten-year follow-up in 185 patients. Circulation 80(3 Pt 1), I57–64 (1989).
- Sorajja P, Ommen SR, Holmes DR Jr et al. Survival after alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Circulation 126(20), 2374–2380 (2012).
- Sorajja P, Valeti U, Nishimura RA et al. Outcome of alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Circulation 118(2), 131–139 (2008).
- Qin JX, Shiota T, Lever HM et al. Outcome of patients with hypertrophic obstructive cardiomyopathy after percutaneous transluminal septal myocardial ablation and septal myectomy surgery. J. Am. Coll. Cardiol. 38(7), 1994–2000 (2001).
- Firoozi S, Elliott PM, Sharma S et al. Septal myotomymyectomy and transcoronary septal alcohol ablation in hypertrophic obstructive cardiomyopathy. A comparison of clinical, haemodynamic and exercise outcomes. Eur. Heart J. 23(20), 1617–1624 (2002).
- Ralph-Edwards A, Woo A, Mccrindle BW et al. Hypertrophic obstructive cardiomyopathy: comparison of outcomes after myectomy or alcohol ablation adjusted by propensity score. J. Thorac. Cardiovasc. Surg. 129(2), 351–358 (2005).
- Vural AH, Tiryakioglu O, Turk T et al. Treatment modalities in hypertrophic obstructive cardiomyopathy: surgical myectomy versus percutaneous septal ablation. Heart Surg. Forum 10(6), 493–497 (2007).
- Seggewiss H, Rigopoulos A, Welge D, Ziemssen P, Faber L. Long-term follow-up after percutaneous septal ablation in hypertrophic obstructive cardiomyopathy. Clin. Res. Cardiol. 96(12), 856–863 (2007).
- Jensen MK, Almaas VM, Jacobsson L et al. Long-term outcome of percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: a Scandinavian multicenter study. Circ. Cardiovasc. Interv. 4(3), 256–265 (2011).
- Jensen MK, Prinz C, Horstkotte D et al. Alcohol septal ablation in patients with hypertrophic obstructive cardiomyopathy: low incidence of sudden cardiac death and reduced risk profile. Heart 99(14), 1012–1017 (2013).
- Schaff HV, Dearani JA, Ommen SR, Sorajja P, Nishimura RA. Expanding the indications for septal myectomy in patients with hypertrophic cardiomyopathy: results of operation in patients with latent obstruction. J. Thorac. Cardiovasc. Surg. 143(2), 303–309 (2012).
- Nagueh SF, Groves BM, Schwartz L et al. Alcohol septal ablation for the treatment of hypertrophic obstructive cardiomyopathy. A multicenter North American registry. J. Am. Coll. Cardiol. 58(22), 2322–2328 (2011).
- Fernandes VL, Nagueh SF, Wang W, Roberts R, Spencer WH 3rd. A prospective follow-up of alcohol septal ablation for symptomatic hypertrophic obstructive cardiomyopathy – the Baylor experience (1996–2002). Clin. Cardiol. 28(3), 124–130 (2005).
- Faber L, Meissner A, Ziemssen P, Seggewiss H. Percutaneous transluminal septal myocardial ablation for hypertrophic obstructive cardiomyopathy: long term follow up of the first series of 25 patients. Heart 83(3), 326–331 (2000).
- Veselka J, Duchonova R, Prochazkova S et al. The biphasic course of changes of left ventricular outflow gradient after alcohol septal ablation for hypertrophic obstructive cardiomyopathy. Kardiol. Pol. 60(2), 133–136; discussion 137 (2004).
- Yoerger DM, Picard MH, Palacios IF, Vlahakes GJ, Lowry PA, Fifer MA. Time course of pressure gradient response after first alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Am. J. Cardiol. 97(10), 1511–1514 (2006).
- Van Der Lee C, Ten Cate FJ, Geleijnse ML et al. Percutaneous versus surgical treatment for patients with hypertrophic obstructive cardiomyopathy and enlarged anterior mitral valve leaflets. Circulation 112(4), 482–488 (2005).
- Jiang TY, Wu XS, Lu Q, Meng X, Jia CQ, Zhang Y. Transcoronary ablation of septal hypertrophy compared with surgery in the treatment of hypertrophic obstructive cardiomyopathy. Chin. Med. J. (Engl.) 117(2), 296–298 (2004).
- Monteiro PF, Ommen SR, Gersh BJ et al. Effects of surgical septal myectomy on left ventricular wall thickness and diastolic filling. Am. J. Cardiol. 100(12), 1776–1778 (2007).
- Menon SC, Ackerman MJ, Ommen SR et al. Impact of septal myectomy on left atrial volume and left ventricular diastolic filling patterns: an echocardiographic study of young patients with obstructive hypertrophic cardiomyopathy. J. Am. Soc. Echocardiogr. 21(6), 684–688 (2008).
- Geske JB, Konecny T, Ommem SR et al. Surgical myectomy improves pulmonary hypertension in obstructive hypertrophic cardiomyopathy. Eur. Heart J. doi:10.1093/ eurheartj/eht537 (2013) (Epub ahead of print).
- Deb SJ, Schaff HV, Dearani JA, Nishimura RA, Ommen SR. Septal myectomy results in regression of left ventricular hypertrophy in patients with hypertrophic obstructive cardiomyopathy. Ann. Thorac. Surg. 78(6), 2118–2122 (2004).
- Moravsky G, Bruchal-Garbicz B, Jamorski M et al. Myocardial mechanical remodeling after septal myectomy for severe obstructive hypertrophic cardiomyopathy. J. Am. Soc. Echocardiogr. 26(8), 893–900 (2013).
- Rovner A, Smith R, Greenberg NL et al. Improvement in diastolic intraventricular pressure gradients in patients with HOCM after ethanol septal reduction. Am. J. Physiol. Heart Circ. Physiol. 285(6), H2492–2499 (2003).
- Veselka J. Improvement of left ventricular diastolic function after alcohol septal ablation for obstructive hypertrophic cardiomyopathy? Yes, of course, but. Eur. Heart J. 27(23), 2901–2902; author reply 2902 (2006).
- Steendijk P, Meliga E, Valgimigli M, Ten Cate FJ, Serruys PW. Acute effects of alcohol septal ablation on systolic and diastolic left ventricular function in patients with hypertrophic obstructive cardiomyopathy. Heart 94(10), 1318–1322 (2008).
- Chen S, Duan F, Yuan J et al. Effect of septal ablation on regional diastolic dysfunction and diastolic asynchrony in patients with hypertrophic obstructive cardiomyopathy: a follow-up study using speckle tracking echocardiography. Echocardiography 30(5), 564–571 (2013).
- Sitges M, Shiota T, Lever HM et al. Comparison of left ventricular diastolic function in obstructive hypertrophic cardiomyopathy in patients undergoing percutaneous septal alcohol ablation versus surgical myotomy/myectomy. Am. J. Cardiol. 91(7), 817–821 (2003).
- Sorajja P, Nishimura RA, Ommen SR, Rihal CS, Gersh BJ, Holmes DR Jr. Effect of septal ablation on myocardial relaxation and left atrial pressure in hypertrophic cardiomyopathy an invasive hemodynamic study. JACC Cardiovasc. Interv. 1(5), 552–560 (2008).
- Talreja DR, Nishimura RA, Edwards WD et al. Alcohol septal ablation versus surgical septal myectomy: comparison of effects on atrioventricular conduction tissue. J. Am. Coll. Cardiol. 44(12), 2329–2332 (2004).
- Valeti US, Nishimura RA, Holmes DR et al. Comparison of surgical septal myectomy and alcohol septal ablation with cardiac magnetic resonance imaging in patients with hypertrophic obstructive cardiomyopathy. J. Am. Coll. Cardiol. 49(3), 350–357 (2007).
- Veselka J, Lawrenz T, Stellbrink C et al. Low incidence of procedure-related major adverse cardiac events after alcohol septal ablation for symptomatic hypertrophic obstructive cardiomyopathy. Can. J. Cardiol. 29(11), 1415–1421 (2013).
- Veselka J, Krejci J, Tomasov P et al. Outcome of patients after alcohol septal ablation with permanent pacemaker implanted for periprocedural complete heart block. Int. J. Cardiol. 171(2), e37–e38 (2014).
- Raute-Kreinsen U. Morphology of necrosis and repair after transcoronary ethanol ablation of septal hypertrophy. Pathol. Res. Pract. 199(3), 121–127 (2003).
- Cuoco FA, Spencer WH 3rd, Fernandes VL et al. Implantable cardioverter-defibrillator therapy for primary prevention of sudden death after alcohol septal ablation of hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 52(21), 1718–1723 (2008).
- Leonardi RA, Kransdorf EP, Simel DL, Wang A. Metaanalyses of septal reduction therapies for obstructive hypertrophic cardiomyopathy: comparative rates of overall mortality and sudden cardiac death after treatment. Circ. Cardiovasc. Interv. 3(2), 97–104 (2010).
- Noseworthy PA, Rosenberg MA, Fifer MA et al. Ventricular arrhythmia following alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Am. J. Cardiol. 104(1), 128–132 (2009).
- Mcleod CJ, Ommen SR, Ackerman MJ et al. Surgical septal myectomy decreases the risk for appropriate implantable cardioverter defibrillator discharge in obstructive hypertrophic cardiomyopathy. Eur. Heart J. 28(21), 2583–2588 (2007).
- Samardhi H, Walters DL, Raffel C et al. The long-term outcomes of transcoronary ablation of septal hypertrophy compared to surgical myectomy in patients with symptomatic hypertrophic obstructive cardiomyopathy. Catheter Cardiovasc. Interv. 83(2), 270–277 (2013).
- Naidu S, Wang Y. Comparative in-hospital safety and cost of alcohol septal ablation versus isolated surgical myectomy for symptomatic hypertrophic obstructive cardiomyopathy: insights from the multi-center PREMIER database. Abstracts of the Society for Cardiovascular Angiography and Interventions 34th Annual Scientific Sessions. May 4–7, 2011. Baltimore, Maryland, USA. Catheter Cardiovasc. Interv. 77(Suppl. 1), S135 (2011).
- Scantlebury DC, Geske JB, Nishimura RA. Limitations of Doppler echocardiography in the evaluation of serial stenoses. Circ. Cardiovasc. Imaging 6(5), 850–852 (2013).
- Geske JB, Scantlebury DC, Nishimura RA. Doubly obstructive hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 61(20), 2107 (2013).
- Singh M, Edwards WD, Holmes DR Jr, Tajil AJ, Nishimura RA. Anatomy of the first septal perforating artery: a study with implications for ablation therapy for hypertrophic cardiomyopathy. Mayo Clin. Proc. 76(8), 799–802 (2001).
- Wallace EL, Thompson JJ, Faulkner MW, Gurley JC, Smith MD. Septal perforator anatomy and variability of perfusion bed by myocardial contrast echocardiography: a study of hypertrophic cardiomyopathy patients undergoing alcohol septal ablation. J. Interv. Cardiol. 26(6), 604–612 (2013).
- Rigopoulos AG, Seggewiss H. A decade of percutaneous septal ablation in hypertrophic cardiomyopathy. Circ. J. 75(1), 28–37 (2011).
- Minami K, Boethig D, Woltersdorf H, Seifert D, Korfer R. Long term follow-up of surgical treatment of hypertrophic obstructive cardiomyopathy (HOCM): the role of concomitant cardiac procedures. Eur. J. Cardiothorac. Surg 22(2), 206–210 (2002).
- Altarabsheh SE, Dearani JA, Burkhart HM et al. Outcome of septal myectomy for obstructive hypertrophic cardiomyopathy in children and young adults. Ann. Thorac. Surg. 95(2), 663–669; discussion 669 (2013).
- Minakata K, Dearani JA, O’Leary PW, Danielson GK. Septal myectomy for obstructive hypertrophic cardiomyopathy in pediatric patients: early and late results. Ann. Thorac. Surg. 80(4), 1424–1429; discussion 1429–1430 (2005).
•• Guidelines for diagnosis and treatment of hypertrophic cardiomyopathy; emphasizes the need for experienced centers and outlines recommendations and level of evidence for septal myectomy and alcohol septal ablation (ASA).
• A well-written paper that emphasizes the enrollment limitations of a hypothetical randomized controlled trial of myectomy versus ASA.
• In this cohort comparison of ASA and myectomy, rates of cardiac death and aborted sudden cardiac death were five times higher post-ASA, raising concern for scar-mediated arrhythmias.
•• A large cohort study of long-term survival following myectomy in a referral center of excellence demonstrating operative mortality of less than 1% and postoperative longterm survival equivalent to that of the general population.
•• The most recent meta-analysis (2010) comparing myectomy and ASA.
• Moderate-sized cohort study of intermediate-term survival following ASA in a referral center of excellence demonstrating survival similar to that of the general population.