Research Article - Neuropsychiatry (2017) Volume 7, Issue 3
Serum Brain-derived neurotrophic factor level and Depressive severity in patients with Chronic Temporal lobe Epilepsy: A Case-control study
- *Corresponding Author:
- Chiung-Chih Chang, M.D., Ph.D
Department of Neurology
Cognition and Aging Center
Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine
Kaohsiung, Taiwan
Tel: +886-7-731-7123 ext-3389;
Fax: +886-7-731-7123 ext-3390
E-mail: neur099@adm.cgmh.org.tw
Abstract
Most cases of temporal lobe epilepsy (TLE) had epileptic foci originating from the hippocampal networks. As Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin growth factor and mainly expressed in the hippocampus, it is not known the circulating level of BDNF may correlate with cognitive performance and depression severities in patients with chronic TLE. Forty-eight patients with TLE and 48 age- and gender-matched controls were enrolled for standardized cognitive tests, geriatric depression scale (GDS) and serum BDNF measurement. The study results showed significantly lower BDNF levels in the TLE patients compared with the controls, and the significance was found in the age ranges of 30 to 65 yearold. There was no gender effect of BDNF whether in the patients or in controls. In the patients, the BDNF levels were related to the antiepileptic drug (AED) numbers (σ= -0.287, p =0.048), GDS scores (σ= -0.306, p=0.044) and all cognitive test scores (p<0.05). None of the test scores showed significance in the controls. Using regression model, independent role of BDNF level on predicting verbal memory score and visual memory was found in the patients. Our study suggested that serum BDNF levels were of diagnostic repertoire in reflecting poor cognitive functions, higher depressive severity and greater numbers of AEDs in TLE patients.
Keywords
Cognitive function, Brain-Derived Neurotrophic Factor, Temporal lobe epilepsy, Depression severity
Introduction
With an estimated prevalence of 40-60% in adult epilepsy, temporal lobe epilepsy (TLE) remains a common epilepsy syndrome that is often refractory to antiepileptic drugs [1,2]. In most cases of TLE, the epileptic focus originates from the temporal lobe, particularly the hippocampus [3]. TLE can be a progressive disorder, potentially resulting in structural and neurobehavior deficits over time [4]. Impairment in cognitive performance beyond the memory domain has been frequently reported [5,6] that caused poor life quality. Development of reliable biological biomarker for predicting cognitive performances in TLE patients are of great clinical significance.
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family of growth factors mainly expressed in the hippocampus-connected network. BDNF has protective roles in the maintenance of neurons, synaptic transmission and synaptic plasticity [7,8]. It has been demonstrated that BDNF can cross the blood-brain barrier in two directions, from the brain to the peripheral blood and from the blood to the brain via the high capacity saturable transporter system [9]. The high correlation between cortical and serum BDNF [10,11] has led to a number of human studies on BDNF levels in various neurological diseases. Previous studies have shown decreased serum levels of BDNF in the patients with major depressive disorder [12,13]. Whether the BDNF serum level also predicted the depressive severity and cognitive performances in patients with chronic TLE patients required more evidence.
Compared with age- and gender-matched controls, the present study aimed to investigate the diagnostic value of serum BDNF level. We explored the relationships between serum BDNF levels and the cognitive function and depression severities in patients with chronic TLE and in age-matched controls. In addition to the direct comparisons between patients and controls, we also tested the interactions of age, gender and seizure risk demographics on the BDNF levels.
Methods
▪ Study design
This was a single center, age-and sex-matched case-control study which was approved by the Institutional Review Board of Chang Gung Memorial Hospital and complied with the ethical standards established in the Declaration of Helsinki. The experiments were undertaken with the written, informed consent of each subject and their caregiver (when appropriate).
▪ Patient enrolment
This study was initiated at the epilepsy outpatient clinic of the Kaohsiung Chang Gung Memorial Hospital in 2009. Patients followed up at the epilepsy cohort [14] all underwent an extensive investigation including clinical history, comprehensive neurologic examination, interictal electroencephalography (EEG) and routine visual MRI analysis.
The clinical diagnosis of TLE was based on the International League Against Epilepsy criteria (1997) as follows: (1) seizure semiology consistent with TLE, with abdominal, epigastric, psychic, or autonomic auras, followed by behavioral arrest, progressive alteration of consciousness, oroalimentary, and manual automatisms; (2) mesial and/or anterior temporal interictal spikes from video-electroencephalography (EEG) or bilateral sphenoidal EEG; and (3) no lesions other than increased T2 signal and/or atrophy in hippocampal formation identified by MRI.
Because it was not possible to combine all the influential factors in the TLE group to produce a uniform population, we only included nonsurgical patients. By family history and past medical history review, none of the patients selected in this study had family traits or a childhood febrile seizure history. Additional exclusion criteria in this study included a known history of mental retardation and a psychiatric comorbidity that prevented either a neuropsychiatric interview or neuroimaging. We also excluded patients with any of the following: (1) medication history of psychoactive or central nervous system depressant drugs; and (2) abnormal liver or renal functions. These exclusion criteria were added to avoid the confounding effects of medication and physical disorders on the cognitive test results.
After screening our TLE cohort [14], 48 patients fulfilled the inclusion and exclusion criteria, agreed to participate in the study, and completed it. Data for the age at onset, duration of epilepsy, average seizure frequency per month during the previous year, and numbers of AED were analyzed. Forty-eight age-and sex- paired healthy controls from the normative database were used for BDNF level and neuropsychological testing [15] comparisons.
Neurobehavior testing and depressive severities: All the tests were performed within two hours of EEG showing interictal state. General intellectual function was assessed using the minimental state examination (MMSE) [16]. Verbal and non-verbal episodic memory was assessed by Chinese versions Verbal Learning Test (CVVLT) [15] and the Rey-Osterrieth Complex figure [17] after a 10-minute delay. Language screening included the 15-item Boston Naming Test [18] and 3-step comprehension and semantic verbal fluency using fruit and transportation.
Visuo-spatial abilities were assessed by a modified Rey-Osterrieth Complex Figure and by the number location test from the Visual Object and Space Perception Battery [19]. The frontal lobe function was assessed by digit forward and backward span, design fluency, Stroop Interference test [20], and the Modified Trails B test [21]. The 15 items geriatric depression scale (GDS) was used to access the emotional states related to physical abilities [22].
BDNF analysis: From the literature, a numbers of determinants may influence the BDNF levels. There is a diurnal variation for plasma BDNF levels, but not in serum level [23,24]. Therefore, we measured serum level in this study. In addition, a non-fasting state was related to an attenuated BDNF level [25]. As we hypothesized that patients with more severe cognitive deficits may show lower serum BDNF levels, we decided to unify the time line to a fasting state in the study population to avoid the possible floor effect of BDNF level in the patients. Meanwhile, to explore the diagnostic repertoire of BDNF, we matched the gender and age in the patients and controls, as well as time of sampling in the coolbox (less than 30 minutes) and duration of sample storage (< one week).
The time intervals between blood sampling with last seizure or last secondary generalized tonic-clonic seizure was 2 to 470 days. Blood samples were taken between 8 and 10 am after overnight fasting for BDNF analysis. Blood for serum BDNF was collected in anticoagulant-free tubes with the clotting activator and kept for one hour one ice with a temperature of about 4℃. Serum BDNF levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit (BDNF Emax® ImmunoAssay System Technical Bulletin). The degrees of enzymatic turnover of the substrate were determined by dual wavelength absorbance measurements at 450 nm using a multiscan spectrum reader (Thermo Scientific, Miami, FL, USA). The antigen standards were used to plot a standard curve of absorbance versus antigen concentration from which the antigen concentrations in the unknowns were calculated. The intra- and inter-assay variations were less than 9 and 15%.
▪ Statistical analysis
All values are expressed as mean ± standard deviation. The chi-square test was used to compare the significance between categorical variables. We used paired t test to compare continuous variables between the patients and controls. Pearson correlation analysis or Spearman rank analysis, as appropriate, was used to explore the relationship between BDNF and cognitive domains in all subjects or individual group. Multiple regression analysis was carried out to determine the best predictor of visual or verbal memory scores in the patients. The strategy for the regression analysis was to assess the association between cognitive outcomes and BDNF levels, using age, education, and seizure risk demographics as independent variables.
Previous translational model suggested long term treatment with Phenobarbital (40mg/ kg), valproate (100mg/kg), topiramate (40mg/ kg) or lamotrigine (80mg/kg) may reduce BDNF mRNA level that can interfere with the measured BDNF levels [26]. To assess the influence of AED on serum BDNF levels, the aforementioned AED was separately coded, and the levels of BDNF were compared. A p value < 0.05 was considered to be statistically significant. Statistical analysis was done using the Statistical Package for Social Sciences (SPSS) software package (version 13 for Windows®, SPSS Inc, Chicago, IL).
Results
▪ Clinical data
A comparison of clinical data between patients with TLE with controls is listed in Table 1. There were no significant differences in age and gender between the controls and patients with TLE. However, the patients had significantly lower cognitive scores in verbal and visual memory, speech and language ability and executive test scores as compared with the controls. Meanwhile, the patients scored higher in GDS.
| Group | epilepsy | controls | P value |
|---|---|---|---|
| Age | 40.3 ± 12.6 | 38.7 ± 10.5 | 0.492 |
| Education (year) | 12.7 ± 3.6 | 13.8 ± 2.7 | 0.104 |
| Sex (male/female) | 22 / 26 | 22 / 26 | 0.581 |
| Seizure onset (age, year-old) | 26.8 ± 13 | NA | |
| Seizure duration (years) | 15.4 ± 9.2 | NA | |
| Seizure frequency (per month) | 3.77 ± 7.9 | ||
| Antiepileptic drug number | 2.4 ± 1.0 | NA | |
| Mini-mental state examination | 27.7 ± 2.9 | 28.8 ± 1.4 | 0.023* |
| Verbal memory CVVLT (9) | |||
| T1 | 5.4 ± 1.5 | 6.0 ± 1.2 | 0.021* |
| T2 | 7.5 ± 1.4 | 7.7 ± 1.1 | 0.563 |
| T3 | 8.1 ± 1.1 | 8.2 ± 0.9 | 0.883 |
| T4 | 8.3 ± 1.0 | 8.5 ± 0.8 | 0.271 |
| 30- sec free recall | 7.9 ± 1.8 | 8.5 ± 0.8 | 0.045* |
| 10-min free recall | 7.5 ± 2.0 | 8.1 ± 1.3 | 0.122 |
| Visual memory | |||
| Modified Rey-Osterrieth recall (17) | 11.9 ± 4.4 | 14.4 ± 2.4 | 0.004* |
| Visual-spatial Functions | |||
| Modified Rey-Osterrieth copy (17) | 16.8 ± 0.6 | 17.0 ± 0.0 | 0.067 |
| Visual object and Space Perception (10) | 8.5 ± 1.7 | 8.6 ± 1.8 | 0.697 |
| Speech and Language Ability | |||
| Semantic fluency (1 minute) | |||
| Fruit | 12.1 ± 3.6 | 15.3 ±2.9 | < 0.001* |
| Transportation | 9.1 ± 2.1 | 12.6 ± 3.4 | < 0.001* |
| Boston Naming Test (15) | 15.5 ± 0.8 | 14.3 ± 2.1 | 0.001* |
| Comprehension (4) | 3.5 ± 0.8 | 3.8 ± 0.4 | 0.088 |
| Executive function | |||
| Digit forward | 7.3 ± 1.4 | 8.2± 1.0 | 0.001* |
| Digit backward | 4.7 ± 1.4 | 5.6 ± 1.4 | 0.004* |
| Stroop Interference Correct (1 minute) | 43.5 ± 14.5 | 51.4 ± 11.0 | 0.009* |
| Design fluency | 8.2 ± 5.0 | 10.6 ± 3.1 | 0.003* |
| Trail making test time (<120 seconds) | 26.5 ± 12.0 | 45.8 ± 34.0 | 0.012* |
| Correct line in Trail making (14) | 12.5 ± 3.2 | 13.1 ± 2.5 | 0.294 |
| Geriatric depression scale | 4.4 ± 4.0 | 1.6 ± 2.2 | < 0.001* |
Data present as mean (standard deviation); number in parenthesis following task name = maximal scores; CVVLT: Chinese version verbal learning test; *p<0.05
Table 1: Demographic data of 48 epilepsy patients and 48 age- and sex-matched controls.
Demographic Factors related to serum BDNF level (Table 2): A direct comparison between patients and controls in BDNF level showed a lower levels in the patients, whether in man or woman. The age effect was significantly demonstrated in patients aged 30-65 years. Within the patient or control group, we found no gender or age effects on the serum BDNF levels. In the patients group, the BDNF levels correlated significantly with the numbers of AED used (σ= -0.287, p =0.048) but not with seizure duration (σ= - 0.058, p =0.693), age of onset (σ= -0.191, p=0.193) or seizure frequency (σ= -0.196, p=0.193).
| BDNF values (ng/ml) | Patients | Controls | P value |
|---|---|---|---|
| All (48/48) | 22.04 (9.1) | 27.61 (10.54) | 0.007* |
| Gender | |||
| Male (22/22) | 21.00 (8.86) | 26.84 (10.77) | 0.042* |
| Female (26/26) | 22.93 (9.37) | 28.28 (10.52) | 0.046* |
| Age range (year-old) | |||
| 17~29 (11/11) | 26.09 (7.12) | 27.23 (8.87) | 0.6 |
| 30~39 (16/16) | 20.48 (10.02) | 27.98 (8.43) | 0.011* |
| 40-65 (21/21) | 21.12 (9.04) | 27.54 (12.98) | 0.02* |
Data present as mean (standard deviation); number in parenthesis =casenumbers of patients or controls; Paired t test; * p<0.05
No gender effects in BDNF levels whether in the patients (t=0.728, p=0.47) or controls (t=0.42, p=0.675)
No age effects in the BDNF levels in the controls among the 3 age ranges (f=0.017, P=0.983)
Table 2: Paired Comparisons of Brain-Derived Neurotrophic Factor (BDNF) levels between patients and controls.
Correlations between serum BDNF levels and cognitive performance: For all the participants, the cognitive scores that correlated with BDNF levels were explored (Table 3). It revealed that BDNF levels were significantly related to episodic verbal and visual memory, speech and language ability and executive functions.
| Brain-Derived Neurotrophic Factor | ||
|---|---|---|
| Variables | Pearson Correlation coefficient | p-value |
| Mini-Mental State Examination scores | 0.242 | 0.015* |
| Chinese Version Verbal Learning Test | ||
| T1 | 0.298 | 0.004* |
| T2 | 0.192 | 0.067 |
| T3 | 0.178 | 0.090 |
| T4 | 0.217 | 0.038* |
| 30 seconds free recall | 0.319 | 0.002* |
| 10 minutes free recall | 0.293 | 0.005* |
| Visual memory | ||
| Modified Rey-Osterrieth recall (17) | 0.224 | 0.032* |
| Speech and Language Ability | ||
| Semantic fluency (Fruit) | 0.284 | 0.006* |
| Semantic fluency (transportation) | 0.264 | 0.011* |
| Boston naming test (16) | 0.340 | 0.001* |
| Executive function | ||
| Digit Forward | 0.207 | 0.048* |
| Digit backward | 0.242 | 0.020* |
| Stroop test (1 minute) | 0.247 | 0.0018* |
| Design fluency | 0.278 | 0.007* |
| Modified Trails B test time | -0.351 | 0.001* |
| Geriatric depression scale | -0.216 | 0.039* |
*P<0.05,
Table 3: Correlation analysis of Brain-Derived Neurotrophic Factor and cognitive function in all participants.
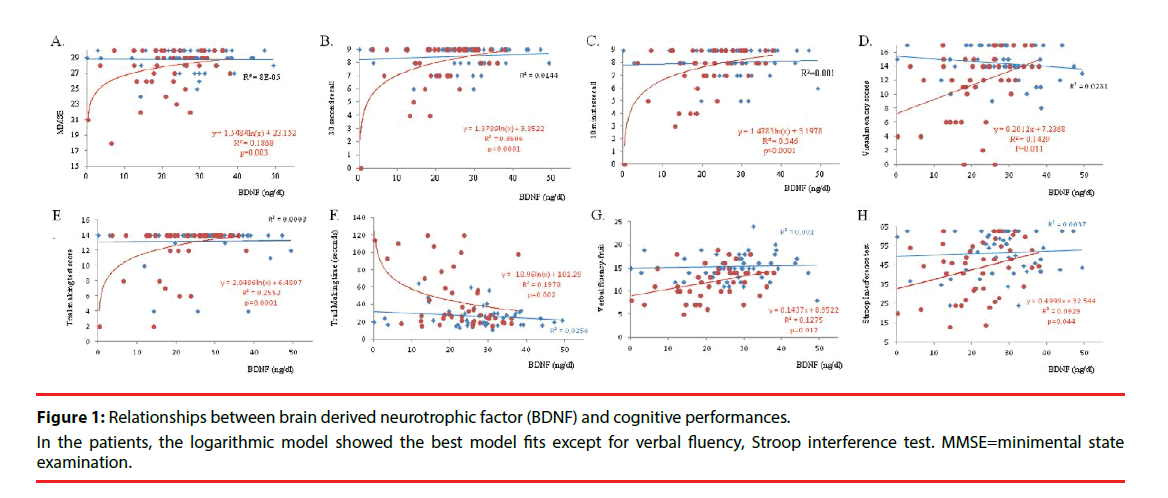
We further explore the interactions between BDNF levels and cognitive performance between two groups using 4 different models (supplementary Figure 1). For the analysis between BDNF and cognition, the best model of BDNF levels for predicting cognitive scores in each group or between-group interactions are shown in Figure 1.
None of the test scores in the control group showed significant relationships with BDNF level. In the patients, the logarithmic model showed the best model fits (R square=0.187, F=9.654, p=0.003), compared with linear model (R square=0.131, F=6.353, p=0.016), quadratic (R square=0.156, F=3.776, p=0.031) or logistic model (R square=0.140, F=6.816, p=0.012) for predicting MMSE scores (Figure 1A). For visual memory, both short delay (30 second recall, Figure 1B) or long delay (10 minutes recalls, Figure 1C) are dependent on the serum level of BDNF in the patients, while the relationship were not shown in the controls. The same interactions were also shown in visual memory recall scores (Figure 1D). For executive function test, only the patients showed relationships between serum BDNF levels with regards to the trail making score (Figure 1E) or time (Figure 1F). Serum BDNF levels also significantly reflected verbal fluency using fruit (Figure 1G) or Stroop interference test score (Figure 1H) in the patients.
GDS and BDNF or cognitive scores: In the control or the patients, the correlation between GDS and cognitive scores were not significant (Supplementary Table 1). However, the BDNF levels showed significant correlation with GDS scores in the patients (σ= -0.306, p=0.044) while the significance was not found in the controls (σ= 0.075, p=0.611).
BDNF showing independent role to verbal and visual memory scores in TLE patient controlling for risk confounders: In the patients, the relationships between verbal memory scores (30 seconds) and age (σ=- 0.438, p=0.003) or educational levels (σ=- 0.505, p<0.001) were significant. After entering these 2 with BDNF levels in multiple regression model (R square=0.61, p<0.0001), only the educational (standardized beta=0.404, p=0.0009) and BDNF (standardized beta=0.2693, p=0.01) significantly predicted the short delay verbal memory scores. For visual memory scores, the regression model showed significance in educational level (standardized beta=4.483, p<0.0001), age (standardized beta=-2.497, p=0.017) and BDNF levels (standardized beta=2.432, p=0.02).
Relationships between BDNF levels with AED and seizure risk demographics: The influence of Phenobarbital, valproate, topiramate or lamotrigine was separately analyzed (Table 4). The result suggested possible interference of Topamax or valproate on the serum BDNF levels. However, while the numbers of AED adjusted, the effect of Topamax (standardized beta=- 0.288, p=0.086) or Valproate (standardized beta=0.271, p=0.057) disappeared.
| BDNF values (ng/ml) | |||
|---|---|---|---|
| Medication name | With medication | Without medication | P value |
| Phenobarbital | N=3, 26.4 (10.1) | N=48, 21.75 (9.07) | 0.396 |
| Valproic acid | N=12, 26.5 (7.9) | N=36, 20.55 (9.06) | 0.027* |
| Topamax | N=10, 16.01 (9.1) | N=38, 23.63 (8.49) | 0.021* |
| Lamictal | N=23, 23.33 (8.6) | N=25. 20.85 (9.5) | 0.35 |
Data present as case numbers (N), mean (standard deviation); * p<0.05
Table 4: Types of anti-epileptic medication and serum Brain-Derived Neurotrophic Factor (BDNF) levels.
Discussion
▪ Major findings
In this study, TLE patients with neurobehavioral deficits were related to lower BDNF levels compared with age- and gender-matched controls especially in those aged more than 30 year-old. While the serum level of BDNF also correlated with the test scores, our results suggest that BDNF may be useful as a prognostic biomarker for predicting global cognitive performance and depressive severity in patients with TLE. In contrast, there was no gender or age effect on BDNF levels in the controls. The only risk demographic factor found to be critical to lower serum BDNF levels in TLE patients was greater AED numbers while the age of onset, seizure frequency or seizure duration did not significantly related to the BDNF levels.
Serum BDNF levels are predictive of Cognitive dysfunction and depression in TLE: Cognitive deficits in TLE are well known. In TLE, cognitive dysfunctions were reported not related to seizure frequency [14]. There is a strong distribution of temporal lobe abnormalities in TLE with respect to epileptogenesis, particularly in the hippocampus and also to a lesser degree on the parahippocampal and entorhinal cortex [27]. As BDNF level is highly associated with hippocampal volume or function [28], decreased BDNF level can be consider as a serum biomarker for hippocampal network integrity.
For aging processes, BDNF decline was reported to relate to the degrees of hippocampal atrophy [28], or exhibit no changes with age [29]. Lower levels of BDNF were associated with decline in hippocampus volume and poorer memory in normal elderly study, controlled for the variations of age [28]. In this study, a significantly lower BDNF level was found in the patients especially in those aged more than 30 year-old. An insignificant effect of age on BDNF levels in normal population with age ranges of 17-65 year-old is purposed based on our controls.
The positive correlations between cognitive performance and BDNF levels in our study validated the protective role of BDNF in TLE. In literature reviews, hippocampus network activity stimulates transcription of the Bdnf gene and translation of Bdnf mRNA, while exogenous BDNF injected into the murine hippocampus was reported to increase proliferation [30- 32]. There was a significant compensatory increase in BDNF mRNA in TLE patients with hippocampus sclerosis, compared with those without [32]. As serum BDNF levels were related to short- and long-delay verbal memory, visual memory, speech and language ability and executive functions in this study, we suggested the measure of BDNF level may extend the prognostic repertoire in relating cognitive severities in TLE patients.
BDNF and depressive severity in TLE: In this study, patients with TLE showed significantly higher GDS scores indicating depressive state and the BDNF levels are highly reflective of the severities. While the associations did not exist in the controls, our observations were consistent with one meta-analysis report showing lower serum BDNF levels in depressed individuals [29]. Patients with TLE had decrease frontotemporal fiber tract integrity [33] that may be linked with greater severity of depression. Symmetric GM atrophy was also found in the extratemporal regions in TLE suggestive of common pathways of epileptogenesis through the thalamus, supplementary motor area and caudate nucleus [14]. There were also several studies that indicated diffusely white matter damages in TLE [34,35]. The importance of kindling effects from the epileptic foci that either deactivate the inhibitory or stimulate the excitatory pathways [36] may augmented the epilepsy-related damages to the interconnected hippocampal neuronal networks that could contributed to the decrement of BDNF levels in the patients. As there is a lack of relationship between GDS scores or any of the cognitive test scores (supplementary Table 1), our study suggested the relationships between BDNF levels and depressive severity in TLE may not be mediated by the identical neuronal networks as in cognition.
Decreased BDNF levels in TLE patients and factors that may interfere with the BDNF levels: Recently, Hong, et al. [37] suggested that the serum concentration of BDNF is associated with disease severity in people with epilepsy. In the report [37], patients with epilepsy did not have significant differences in the serum BDNF levels, as compared with controls. Meanwhile, they pointed out a significant higher level of BDNF in the male gender, whether in the controls or patients. The aforementioned observations were not found in our study report and a few methodology differences may be related to the differences.
First, the epilepsy diagnosis and disease severity between two patient groups were not identical. While our TLE patients demonstrated a significant cognitive dysfunction, patients in the study by Hong, et al. [37] enrolled patients with different types of epilepsy while they also excluded those with cognitive deficits. The differences in study population can demonstrate great differences in epilepsy syndrome. For example, serum BDNF might increase in children with epilepsy [38] but decreased in normal elderly, conversion disorder, psychogenic nonepileptic seizures or epileptic seizure in adult [38,39]. Second, while a lack of gender effects in BDNF levels was previously reported [28,29], the differences in epilepsy types or peripheral origins of BDNF [40] can potentially related to the gender effects. Hong, et al. [37] suggested the gender effect may also be related to the experimental differences in detecting BDNF. As such, the abnormality of the BDNF level needed to be interpreted with great care in different age ranges, disease or cognitive severity, methodological issues, brain neurogenesis ability and other biological sources. In this study, we carefully matched the case numbers, age and gender in the patient and controls group to reduce the potential variations arising from the sample size inhomogeneity.
Relationships with AEDs and possible limitations: We tested the differences of serum BDNF levels in relation to individual AED type for understanding possible confounding. The results should be interpretated with care as these TLE patients used different combinations of AEDs and the numbers of AEDs were also significantly related to lower BDNF levels in this study. Similar to previous translational study [26], those within the Topiramate group showed significantly lower BDNF levels while the observation in the our valproic acid group paradoxically showed higher level in this study. Based on the findings, we speculated complex interactions among AED types did exist in TLE patients. Long term AED use has been shown to contribute to atherosclerosis and oxidative stress [41] and that BDNF levels can be related to other potential peripheral sources such as the vascular endothelial cells [42], monocytes during activation [43] or myocytes [44]. The fluctuation of BDNF levels in the patients with AED uses can be related to effects of AED acting on the peripheral system.
There were several limitations to this study. As this study enrolled only a small number of patients and as the numbers of AEDs, the daily frequencies and dosages of AED used cannot be uniform, the explore of AED types in relation to BDNF levels may require larger samples. Second, the study design was mainly focused on the neurobehavioral parameters and the serum BDNF levels during the interictal state. The observations here cannot represent the ictal pathogenesis in TLE. In surgically-resected hippocampus, increase levels of BDNF mRNA and protein was found, indicating epileptic activity may up-regulate the protein level via BDNF gene expression [32,45]. Finally, there were other factors that can interfere with the serum levels of BDNF that included the oxidative stress, blood brain barrier damage and deregulated neuroinflammation [25,46,47] all coexisted in TLE. Further evidence is required to understand the clinical weightings of these factors.
Conclusion
In conclusion, the present study showed that TLE patients with more AED numbers, aged more than 30 year-old, worse cognitive performances and depressive state might have lower serum BDNF level. While the relationships were not found in the controls, measurement of serum BDNF level may extend the diagnostic repertoire in relating to neurobehavioral status in chronic TLE patients.
Acknowledgement
The authors wish to thank the patients and their caregivers for their time and commitment to this research. This study was supported in part by research grants NSC99-2314-B-182A-054-MY3 and CMRPG 8B1001 to C.C.C.
Disclosure statement
None of the authors have any financial disclosures or conflicts of interest related to this study. The study protocol was approved by the hospital’s Institutional Review Committee on Human Research.
References
- Tellez-Zenteno JF, Hernandez-Ronquillo L. A review of the epidemiology of temporal lobe epilepsy. Epilepsy. Res. Treat 2012(2012), 630853 (2011).
- Picot MC, Baldy-Moulinier M, Daures JP, et al. The prevalence of epilepsy and pharmacoresistant epilepsy in adults: a population-based study in a Western European country. Epilepsia 49(7), 1230-1238 (2008).
- Engel J Jr. Report of the ILAE classification core group. Epilepsia 47(9), 1558-1568 (2006).
- Pitkanen A, Sutula TP. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet. Neurol 1(3), 173-181 (2002).
- Andersson-Roswall L, Engman E, Samuelsson H, et al. Cognitive outcome 10 years after temporal lobe epilepsy surgery: a prospective controlled study. Neurol 74(24), 1977-1985 (2010).
- Baxendale S, Heaney D, Thompson PJ, et al. Cognitive consequences of childhood-onset temporal lobe epilepsy across the adult lifespan. Neurol 75(8), 705-711 (2010).
- Schinder AF, Poo M. The neurotrophin hypothesis for synaptic plasticity. Trends. Neurosci 23(12), 639-465 (2000).
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Ann. Rev. Neurosci 24(1), 677-736 (2001).
- Pan W, Banks WA, Fasold MB, et al. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology 37(12), 1553-1561 (1998).
- Blugeot A, Rivat C, Bouvier E, et al. Vulnerability to depression: from brain neuroplasticity to identification of biomarkers. J. Neurosci 31(36), 12889-12899 (2011).
- Sartorius A, Hellweg R, Litzke J, et al. Correlations and discrepancies between serum and brain tissue levels of neurotrophins after electroconvulsive treatment in rats. Pharmacopsychiatry 42(6), 270-276 (2009).
- Hashimoto K. Brain-derived neurotrophic factor as a biomarker for mood disorders: an historical overview and future directions. Psych. Clin. Neurosci 64(4), 341-357 (2010).
- Hashimoto K, Shimizu E, Iyo M. Critical role of brain-derived neurotrophic factor in mood disorders. Brain research. Brain Res. Rev 45(2), 104-114 (2004).
- Chang CC, Lui CC, Lee CC, et al. Clinical significance of serological biomarkers and neuropsychological performances in patients with temporal lobe epilepsy. BMC. Neurol 12(1), 15 (2012).
- Chang CC, Kramer JH, Lin KN, et al. Validating the Chinese version of the Verbal Learning Test for screening Alzheimer's disease. J. Int. Neuropsychol. Soc 16(2), 244-251 (2010).
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res 12(3), 189-198 (1975).
- Boone KB. The Boston Qualitative Scoring System for the Rey-Osterrieth Complex Figure. J. Clin. Exp. Neuropsychol 22(5), 430-434 (2000).
- Kaplan EF, Goodglass H, Weintraub S. The Boston naming test, Lea & Febiger, Philadelphia, USA (1983).
- Warrington EK, James M, Visual Object and Space Perception Battery., Thames Valley Test Co., Bury St. Edmunds, Suffolk, UK (1991).
- Amieva H, Lafont S, Rouch-Leroyer I, et al. Evidencing inhibitory deficits in Alzheimer's disease through interference effects and shifting disabilities in the Stroop test. Arch. Clin. Neuropsychol 19(), 791-803 (2004).
- Reitan RM. The relation of the trail making test to organic brain damage. J. Consult. Psychol 19(6), 393-394 (1955).
- Pocklington C, Gilbody S, Manea L, et al. The diagnostic accuracy of brief versions of the Geriatric Depression Scale: a systematic review and meta-analysis. Int. J. Geriat. Psych 31(8), 837-857 (2016).
- Choi SW, Bhang S, Ahn JH. Diurnal variation and gender differences of plasma brain-derived neurotrophic factor in healthy human subjects. Psych. Res 186(2-3), 427-430 (2011).
- Piccinni A, Marazziti D, Del Debbio A, et al. Diurnal variation of plasma brain-derived neurotrophic factor (BDNF) in humans: an analysis of sex differences. Chronobiol. Int 25(5), 819-826 (2008).
- Bus BA, Molendijk ML, Penninx BJ, et al. Determinants of serum brain-derived neurotrophic factor. Psychoneuroendocrinology 36(2), 228-239 (2011).
- Shi XY, Wang JW, Cui H, et al. Effects of antiepileptic drugs on mRNA levels of BDNF and NT-3 and cell neogenesis in the developing rat brain. Brain. Dev 32(1), 229-235 (2010).
- Keller SS, Roberts N. Voxel-based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia 49(5), 741-757 (2008).
- Erickson KI, Prakash RS, Voss MW, et al. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J. Neurosci 30(15), 5368-5375 (2010).
- Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol. Psych 64(6), 527-532 (2008).
- Lau AG, Irier HA, Gu J, et al. Distinct 3'UTRs differentially regulate activity-dependent translation of brain-derived neurotrophic factor (BDNF). Proc. Nat. Acad. Sci. USA 107(36), 15945-15950 (2010).
- Scharfman H, Goodman J, Macleod A, et al. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp. Neurol 192(2), 348-356 (2005).
- Wang FJ, Li CM, Hou XH, et al. Selective upregulation of brain-derived neurotrophic factor (BDNF) transcripts and BDNF direct induction of activity independent N-methyl-D-aspartate currents in temporal lobe epilepsy patients with hippocampal sclerosis. J. Int. Med. Res 39(4), 1358-1368 (2011).
- Kucukboyaci NE, Girard HM, Hagler DJ Jr, et al. Role of frontotemporal fiber tract integrity in task-switching performance of healthy controls and patients with temporal lobe epilepsy. J. Int. Neuropsychol. Soc 18(1), 57-67 (2011).
- Keller SS, Ahrens T, Mohammadi S, et al. Voxel-based statistical analysis of fractional anisotropy and mean diffusivity in patients with unilateral temporal lobe epilepsy of unknown cause. J. Neuroimag 23(1), 352-359 (2013).
- Shon YM, Kim YI, Koo BB, et al. Group-specific regional white matter abnormality revealed in diffusion tensor imaging of medial temporal lobe epilepsy without hippocampal sclerosis. Epilepsia 51(4), 529-535 (2009).
- Lanneau D, Wettstein G, Bonniaud P, et al. Heat shock proteins: cell protection through protein triage. Sci. Wor. J 10(1), 1543-1552 (2009).
- Hong Z, Li W, Qu B, et al. Serum brain-derived neurotrophic factor levels in epilepsy. Eur. J. Neurol 21(1), 57-64 (2014).
- Connolly AM, Chez M, Streif EM, et al. Brain-derived neurotrophic factor and autoantibodies to neural antigens in sera of children with autistic spectrum disorders, Landau-Kleffner syndrome, and epilepsy. Biol. Psych 59(4), 354-363 (2006).
- LaFrance WC Jr, Leaver K, Stopa EG, et al. Decreased serum BDNF levels in patients with epileptic and psychogenic nonepileptic seizures. Neurol 75(14), 1285-1291 (2010).
- Lommatzsch M, Zingler D, Schuhbaeck K, et al. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol. Aging 26(1), 115-123 (2005).
- Tan TY, Lu CH, Chuang HY, et al. Long-term antiepileptic drug therapy contributes to the acceleration of atherosclerosis. Epilepsia 50(6), 1579-1586 (2009).
- Nakahashi T, Fujimura H, Altar CA, et al. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS. Lett 470(2), 113-117 (2000).
- Schulte-Herbruggen O, Nassenstein C, Lommatzsch M, et al. Tumor necrosis factor-alpha and interleukin-6 regulate secretion of brain-derived neurotrophic factor in human monocytes. J. Neuroimmunol 160(1-2), 204-209 (2005).
- Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nature reviews. Endocrinology 8(1), 457-465 (2012).
- Murray KD, Isackson PJ, Eskin TA, et al. Altered mRNA expression for brain-derived neurotrophic factor and type II calcium/calmodulin-dependent protein kinase in the hippocampus of patients with intractable temporal lobe epilepsy. J. Compar. Neurol 418(4), 411-422 (2000).
- Yang T, Zhou D, Stefan H. Why mesial temporal lobe epilepsy with hippocampal sclerosis is progressive: uncontrolled inflammation drives disease progression? J. Neurol. Sci 296(1-2), 1-6 (2010).
- Zhao HF, Li Q, Li Y. Long-term ginsenoside administration prevents memory loss in aged female C57BL/6J mice by modulating the redox status and up-regulating the plasticity-related proteins in hippocampus. Neurosci 183(1), 189-202 (2011).