Research Article - International Journal of Clinical Rheumatology (2021) Volume 16, Issue 9
Serum Renalase Level as A Marker of Activity and Severity in Lupus Nephritis Cases
- *Corresponding Author:
- Yasser A Elmotaleb Elsayed
Department of Rheumatology and Rehabilitation, Faculty of Medicine, Al-Azhar University, Cairo, Egypt
E-mail: dr.yasser_rh@yahoo.com
Abstract
Aim: Introduction: Lupus nephritis (LN) is a substantial risk factor for death and morbidity in patients with Systemic Lupus Erythematosus (SLE) (SLE). Despite excellent immunosuppressive therapy, it nevertheless leads to a disproportionate percentage of persons developing chronic kidney disease (CKD) or end-stage renal disease (ESRD). Renalase is a distinctive cytokine-like protein generated by the kidneys that promote cell survival. It has been recently linked to the etiology of LN and may be an ideal candidate as a sensitive biomarker for flare-ups and LN remission.
Aim of the work: The purpose of this study was to evaluate the utility of human serum renalase as a biomarker for assessing disease activity and severity in SLE, as well as to evaluate if it can be used as a sensitive biomarker in this capacity.
Methods: This study consists of around 23 healthy controls and 46 individuals with LN. These participants were separated into two equal groups according to disease activity as determined by the SLEDAI (SLE Disease Activity Index): 23 cases with LN who had disease activity and 23 cases who did not. The concentration of human serum Renalase (RNLS) was evaluated using a very sensitive commercial enzyme immunoassay that captures renalase from serum using (RNLS) antibody.
Results: Renalase concentrations were significantly greater in LN cases than in healthy controls (P-value <0.001). Additionally, cases with active LN exhibited significantly greater serum renalase concentrations than those with inactive LN (P-value <0.005). Serum renalase concentrations were positively connected with 24-h urine protein excretion, SLEDAI, ESR, CRP, and ds-DNA but were negatively related to serum C3 and the class (particularly in the proliferative type) (Class III, IV, more than class V).
Conclusion: Serum renalase levels were associated with disease symptoms in LN and may serve as a biomarker for disease activity in LN.
Keywords
systemic lupus erythematosus • renal disease • lupus nephritis • serum renalase
Introduction
Renal disease is a severe manifestation of systemic lupus erythematosus (SLE), accounting for a significant share of death and morbidity [1]. Up to 90% of persons with SLE will have pathologic indications of kidney damage at the time of biopsy, even though only 50% will develop clinically serious nephritis [2]. Lupus nephritis manifests clinically in a number of different ways, from asymptomatic hematuria and/or proteinuria to a full-blown nephrotic syndrome to a rapidly increasing glomerulonephritis with accelerating renal dysfunction. Although there are exceptions, lupus nephritis normally improves within the first 36 months of the disorder. Thus, screening for nephritis on a monthly basis is crucial for the continuous evaluation and therapy of cases with SLE [3].
Current laboratory markers for lupus nephritis, such as urine protein-to-creatinine ratio, proteinuria, anti-dsDNA, creatinine levels, and complement levels, are insufficient. Owing to a lack of sensitivity and specificity, they are unable to distinguish between renal activity and impairment in lupus nephritis [4].
Serum Renalase is a monoamine oxidase that can be secreted into the bloodstream by the kidneys [5]. Renalase has previously been shown to assist in regulating blood pressure by degrading catecholamines in the bloodstream [6]. Renalase treatment is associated with renal protection and decreased macrophage infiltration in a mouse model of acute kidney injury (AKI), showing that renalase has an anti-inflammatory role in renal dysfunction [7, 8].
The association between renalase and inflammation has been demonstrated in investigations of organ transplantation and serum renalase concentrations in kidney and heart cases [9, 10]. The purpose of this study was to examine whether serum renalase levels were associated with renal pathology and disease activity in lupus nephritis, to hypothesize a function for renalase in this autoimmune and inflammatory disorder, and to assess whether it is an excellent biomarker for lupus nephritis.
Method
This study included 48 patients with SLE who were evaluated through using Systemic Lupus International Collaborating Clinics (SLICC) (2015) [11] or the New EULAR/ACR SLE Classification Criteria 2017 [12] and 30 healthy controls. They were gathered from the rheumatology and rehabilitation departments of the AL-Azhar University hospitals' inpatient and outpatient clinics. The ethical council of Al-Azhar Medical School approved this study, and all subjects were given written informed permission before participation.
All individuals with life-threatening conditions other than LN were eliminated from this investigation (e.g., heart failure, central nervous system lupus, malignant tumor, infectious disease), as well as those with an eGFR of less than 30ml/min/1.73 m2 or who was pregnant at the age of 18 or 50.
On the basis of SLEDAI (SLE Disease Activity Index) ratings, the recruited cases were split into two equal groups [13]:
Group II included 23 patients of lupus with disease activity as determined by the SLEDAI.
Group III had 23 lupus patients with no signs of disease activity, as determined by the SLEDAI.
The control group (Group I) consists of 23 persons who appear to be in good health and are of comparable age and sex.
The following items were covered in a detailed examination of all participants: Complete history taking, investigations of the general and local areas; Upon that, during the study visit, venous blood samples were obtained by all participants in the prescribed sequence: C-reactive protein (CRP), complete blood counting, C-reactive protein (CRP), sedimentation rate of erythrocytes (ESR), liver-function testing, creatinine serum, uric blood urea, and serum, and urine proteins 24- hour. To assess urinary protein excretion, 24-hour urine and spot urine samples were obtained. Autoimmune Profile: C3 and DNA-Anti-double-strand (anti-ds- DNA-Ab) complements were evaluated by the use of an enzyme-related immune-sorbent assay (ELISA). Renal biopsies were percutaneously collected from LN cases involved with this study using an ultrasonographically guided biology or computed tomography needle, and paraformaldehyde-fixed air-dry slices of the frozen LN kidney sample were delivered to the histopathologist. Renal samples were categorized in accordance with the International Society of Nephrology/Renal Pathology (ISN/RPS) [14].
At the study visit, serum renalase was taken, and serum was separated within three hours of collection. To avoid repeated freeze cycles, serum was separated using Rotofix32 (Hettich-zentrifugen) at 2000x for 20 minutes and then gathered into at least four aliquots and kept at -20°C until required for analysis. Serum renalase concentrations were determined as per the manufacturer's procedure by an ELISA kit specific to renalase. The concentration of human serum Renalase (RNLS) was established by a highly sensitive, commercial sandwich enzyme immunoassay employing an (RNLS) serum renalase. This assay is very specific and sensitive for detecting (RNLS). There was no evidence of considerable cross-reactivity or interaction between (RNLS) and analogs [15].
The 2000 SLE Illness Activity Index (SLEDAI-2K) and Renal SLEDAI (rSLEDAI) have been used for evaluating the activity of the disease and renal disease consecutively [13]. RSLEDAI includes haematuria, pyuria, proteinuria, and urine casts (SLEDAI-2K renal scores). Cases with LN were divided into two categories according to their SLEDAI scores, the active LN (SLEDAI <8) and the quiescent LN (SLEDAI <8).
Statistical analysis
The Social Science Statistics Program (SPSS) version 20.0 was used to examine the data. The standard difference and average of quantitative data were calculated (SD). Frequency and percentage of qualitative data were used. The following tests have been performed: The independent samples t-test of significance was used when comparing two means. A one-way variance analysis (ANOVA) is utilized when comparing more than two means. The Chi-square (X2) meaning test was used to examine the proportions between two qualitative parameters. Relationships were created through Pearson's coefficient of relationship (r) test. The trust interval was set at 95%, while the acceptable error margin was set at 5%. Therefore, the following p-value was judged significant: The likelihood (P-value) P-value less than 0.05 was considered important. A P-value less than 0.001 was considered to be very important. P-value >0.05 was considered negligible.
Results
Around 46 LN patients and 23 healthy controls were part of the current study. The LN group comprised five male and forty-one female cases with a mean age of 30.1±9.196. Table 1 outlines the participants' demographic information. Significant changes have been seen between healthy controls and LN cases in the systolic blood pressure (P=0,017).
| Demographic Data and Anthropometric Measurements | Group I: Control | Group II: Active SLE |
|---|---|---|
| Sex | ||
| Male | 3 | |
| (13.0%) | 2 | |
| (8.7%) | 2 | |
| (8.7%) | 0.318# | 0.853 |
| Female | 20 (87.0%) | 21 (91.3%) |
| Age (year) | ||
| Mean±SD | 29.30±8.19 | 29.96±9.42 |
| Range | 18-45 | 18-48 |
| SBP (mmHg) | ||
| Mean±SD | 117.83±11.36 | 129.13±17.03 |
| Range | 95-140 | 100-160 |
| DBP (mmHg) | ||
| Mean±SD | 74.13±8.21 | 77.39±12.14 |
| Range | 60-90 | 60-100 |
Table 1. Comparison between groups as per demographic information.
Also, Table 2 presented the comparison between cases' groups as per Renal biopsy Class where Class III was predominant in both active lupus nephritis group 13 cases (56.5%) and inactive LN group 12 cases (52.2%)
| Class | Group II: Active SLE | Group III: Inactive SLE | x2 | p-value |
|---|---|---|---|---|
| Class II | 1 (4.3%) | 2 (8.7%) | 0.373 | 0.946 |
| Class III | 13 (56.5%) | 12 (52.2%) | ||
| Class IV | 7 (30.4%) | 7 (30.4%) | ||
| Class V | 2 (8.7%) | 2 (8.7%) |
Table 2. Comparison between cases' groups as per Renal biopsy Class.
In LN, the serum renalase level was substantially higher in our research objective (132,35±92,59 vs. 30,26±17,67 μg/ml, P-value less than0,001) (Table 3).
| Renalase (µmg/ml) | Group I: Control | Group II: Active SLE | Group III: Inactive SLE | x2 | p-value |
|---|---|---|---|---|---|
| Mean±SD | 30.26±17.67 | 158.88±65.70 | 105.83±53.78 | 38.325 | <0.001 |
| Range | Oct-65 | 0.2-240 | 10-225 |
Table 3. Comparison between groups as per renalase (μmg/ml).
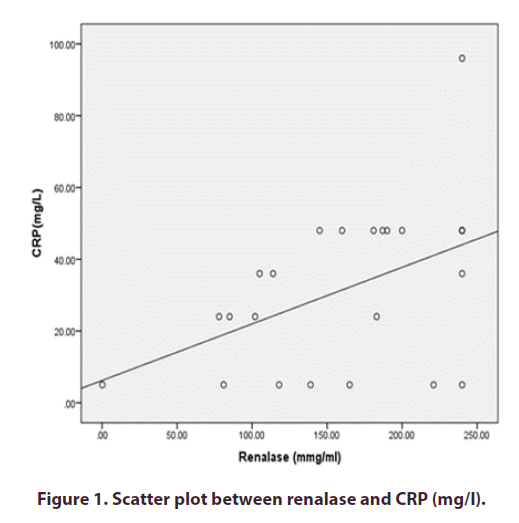
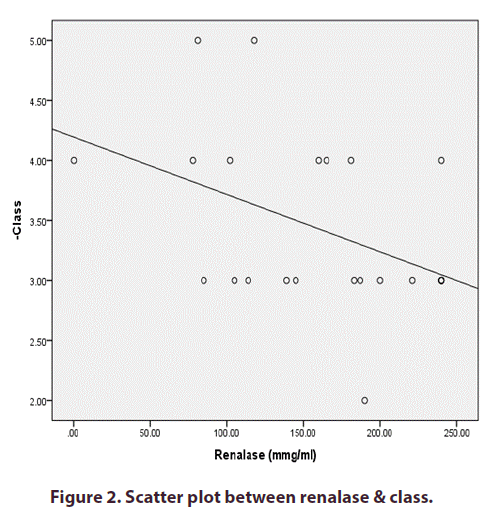
The blood renalase levels of patients with Active LN (group II) were strongly linked with CRP but not with the nephritis class. Figures 1 and 2) However, instances with SLE with inactive LN (group III) did not show a statistically significant correlation between their serum renalase and any of the clinical or laboratory markers. However, they did show a negative correlation with the nephritis class, as shown in (Table 4).
| Parameters | Renalase (µmg/ml) | |
|---|---|---|
| r | p-value | |
| Age (year) | -0.282 | 0.193 |
| SBP (mmHg) | -0.41 | 0.052 |
| DBP (mmHg) | -0.161 | 0.464 |
| WBC (×103) | -0.085 | 0.699 |
| Lymphocyte(×103) | -0.127 | 0.564 |
| Hemoglobin (g/L) | -0.127 | 0.563 |
| Platelet (×103) | 0.066 | 0.764 |
| 24hr urine protein (g/d) | -0.109 | 0.62 |
| Serum creatinine (mmol/L) | -0.01 | 0.963 |
| Serum uric acid(mmol/L) | -0.034 | 0.877 |
| B. urea(mg/dl) | -0.31 | 0.15 |
| ESR(mm/h) | -0.073 | 0.74 |
| CRP(mg/L) | -0.016 | 0.942 |
| TG (mmol/L) | -0.001 | 0.996 |
| TC (mmol/L) | -0.199 | 0.362 |
| HDL (mmol/L) | 0.122 | 0.581 |
| LDL (mmol/L) | -0.197 | 0.367 |
| ds-DNA (IU/L) | 0.215 | 0.325 |
| C3 (mg/dl) | 0.199 | 0.364 |
| C4 (mg/dl) | 0.049 | 0.823 |
| SLEDAI | 0.144 | 0.523 |
| -Class | -.579** | 0.004 |
Table 4. Relation between renalase (μmg/ml) and other factors in inactive LN group III.
Additionally, there was no significant association between markers of renal activity and serum renalase concentrations in subjects with active LN, such as proteinuria, ESR, anti-dsDNA, C4, and C3 (Table 5).
| Parameters | Renalase (µmg/ml) | |
|---|---|---|
| R | p-value | |
| Age (year) | -0.374 | 0.079 |
| SBP (mmHg) | -0.386 | 0.069 |
| DBP (mmHg) | -0.246 | 0.257 |
| WBC (×103) | 0.222 | 0.308 |
| Lymphocyte(×103) | 0.355 | 0.096 |
| Hemoglobin (g/L) | -0.038 | 0.862 |
| Platelet (×103) | 0.102 | 0.645 |
| 24hr urine protein (g/d) | 0.123 | 0.578 |
| Serum creatinine (mmol/L) | -0.01 | 0.964 |
| Serum uric acid(mmol/L) | 0.06 | 0.784 |
| B. urea(mg/dl) | 0.094 | 0.668 |
| ESR(mm/h) | 0.208 | 0.341 |
| TG (mmol/L) | -0.171 | 0.435 |
| TC (mmol/L) | -0.144 | 0.511 |
| HDL (mmol/L) | -0.173 | 0.431 |
| LDL (mmol/L) | 0.174 | 0.426 |
| ds-DNA (IU/L) | 0.082 | 0.709 |
| C3 (mg/dl) | 0.002 | 0.992 |
| C4 (mg/dl) | -0.057 | 0.795 |
| SLEDAI | 0.365 | 0.087 |
| PGA | 0.329 | 0.125 |
Table 5. Relation between renalase (μmg/ml) and other parameters in Active LN patient group (II).
Discussion
Without question, early diagnosis of LN is crucial for persons with SLE to be treated effectively [16]. LN is a well-established risk factor for mortality and morbidity in SLE, and even when immunosuppressive therapies are employed, many patients improve from end-stage renal disease (ESRD) or chronic kidney disease (CKD) [17].
Biomarkers predictive of the development of active LN would be tremendously beneficial, as early diagnosis and treatment could improve renal outcomes. Antibodies against dsDNA complement C3 and C4 were previously used to monitor disease activity in LN. Nonetheless, these serological indicators of autoimmunity are insufficiently highly sensitive and specific to be used as a biomarker for LN activity [18].
Renalase (monoamine oxidase) has been implicated in the pathogenesis of LN and its flare; consequently, we sought to investigate the serum level of renalase in SLE patients with active or inactive LN.
This cross-sectional study included approximately 48 cases of SLE and nephritis, 23 active cases, 23 inactive cases, and 30 healthy controls.
In our current investigation, we discovered that LN cases had considerably greater concentrations of renalase than healthy controls (P-value less than0.001).
Additionally, those with active LN had significantly higher serum renalase levels than those with inactive LN (P-value less than 0.005), indicating that renalase levels may be related to disease severity in LN, especially the proliferating type.
We approved the link between clinical activity and serum renalase in LN cases using relation analysis.
These findings corroborate those of another study published in 2015 by Qi C, Wang L, et al. (2015) (Serum Renalase Concentrations Correlate with Disease Activity in Lupus Nephritis Activity), which discovered that serum renalase concentration levels were greater in patients with active LN than in patients with inactive LN (95.4033.84 vs. 52.6922.37 g/ml, P0.001). Univariate findings show positive correlations between serum renalase levels and ESR (r2 = 0.15, P = 0.003), SLEDAI (r2 = 0.32, P = 0.001), and anti-dsDNA (r2 = 0.10, P = 0.013). Serum renalase levels were shown to be negatively correlated with serum albumin (r2 = 0.25, P0.001) and C3 levels (r2 = 0.17, P = 0.001). Serum renalase concentrations had no correlation with either systolic or diastolic blood pressure.
Additionally, serum renalase levels were found to be significantly linked with rSLEDAI (r2 = 0.37, P-value less than 0.001) and 24-hour urine protein excretion (r2 = 0.417, P = 0.001). Serum Renalase expression was not detected in the glomeruli under normal circumstances. Simultaneously, patients with proliferative LN had significantly greater serum renalase concentrations than those with Class V LN [19].
As revealed in a 2005 study by Xu J, Li G, et al., renalase is involved in the control of cardiovascular function and blood pressure. (Renalase is a novel soluble monoamine oxidase that affects heart function and blood pressure) [5], and Desir GV et al. 2012 .'s study. (Renalase is responsible for ambulatory blood pressure decrease by metabolizing circulating adrenaline [20]. Other several research, on the other hand, revealed no correlation between blood pressure and serum renalase in patients receiving hemodialysis or peritoneal dialysis [21, 22]. Blood pressure may continue to have an effect on serum renalase. Nevertheless, because blood pressure varied little between groups, it is possible that blood pressure had a slight impact on our investigation.
Recent investigations demonstrated that renalase effectively ameliorated renal injury caused by cisplatin and hydrogen peroxide attacks on human proximal tubular (HK–2) cells. The renalase's administration is related to decreased macrophage infiltration and renal protection in the acute kidney injury (AKI) mice model. In contrast, macrophage infiltration and renal injury are more severe in the renalase knockout mice model, implying that renalase plays an anti-inflammatory role in kidney injury [7, 8].
The investigations on organ transplantation and blood renalase concentrations discovered increased renalase concentrations in the heart and kidney recipients, implying a link between inflammation and renalase [9, 10].
Several intriguing studies established a link between renalase and type 1 diabetes, implying that it may also have a role in developing autoimmune pancreatic damage [23-25].
The association of renalase with the development of organ transplant recipients and autoimmune diabetes shows that it has a role in the pathophysiology of immune-mediated illnesses. Even though data from the research outlined above are available, renalase's expression and clinical importance in individuals with LN remain unknown.
The study was cross-sectional and single-center, and a prospective, multi-center experiment will be done to explain further and validate the findings. The future study will evaluate the time point at which renalase levels increase in response to LN, the differences in serum renalase levels across different types of kidney disease, and if renalase is involved in the mechanism or etiology of LN.
Conclusion
Serum renalase was related to disease activity in LN, especially the proliferative form, implying that it may be employed as a biomarker in prospective clinical studies. Further research is necessary to determine the efficiency of serum renalase as a biomarker of disease severity in lupus nephritis.
Acknowledgements
The study was presented at the 2018 EULAR Annual Conference in Madrid as a poster presentation.
Conflict of Interest
The researchers of the presented paper have no conflict to declare.
Funding
The current research has not been supported by a grant from a commercial, municipal, or not-for-profit funding body. The researchers got no funding or assistance from the industry.
Ethics approval and written informed consents statements
All processes involving the recruitment of human subjects are conducted in accordance with the institutional and/or national research committee's ethical standards and the 1964 Helsinki statement and its subsequent revisions, or comparable ethical standards.
The study of Al-Azhar University Registration was authorized by the local ethics committee. (NO. (0000027)).
Before enrolment in the present study, all subjects were instructed about the study's objectives, besides obtaining their informed written consent.
References
- CC Mok, SSK Tang, CH To et al. Incidence and risk factors of thromboembolism in systemic lupus erythematosus: a comparison of three ethnic groups. Arthritis Rheum. 52(9), 2774–2782 (2005).
- Danila MI, Pons-Estel GJ, Zhang J et al. Renal damage is the most important predictor of mortality within the damage index: data from LUMINA LXIV, a multiethnic US cohort. Rheumatology. 48, 542–545 (2009).
- Mok CC, Kwok RC, Yip PS. Effect of renal disease on the standardized mortality ratio and life expectancy of patients with systemic lupus erythematosus. Arthritis Rheum. 65(8), 2154–2160 (2013).
- CC Mok. Update on emerging drug therapies for systemic lupus erythematosus. Expert Opin. Emerg. Drugs. 15, 53–70 (2010).
- Xu J, Li G, Wang P et al. Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J. Clin. Invest. 115, 1275–1280 (2005).
- Desir GV, Wang L, Peixoto AJ. Human renalase: a review of its biology, function, and implications for hypertension. J. Am. Soc. Hypertens. 6, 417–426 (2012).
- Wang L, Velazquez H, Moeckel G, et al. Renalase Prevents AKI Independent of Amine Oxidase Activity. J. Am. Soc. Nephrol. 25, 1226–1235 (2014).
- Lee HT, Kim JY, Kim M et al. Renalase protects against ischemic AKI. J. Am. Soc. Nephrol. 24, 445–455 (2013).
- Przybylowski P, Koc-Zorawska E, Malyszko JS, et al. Renalase and endothelial dysfunction in heart transplant recipients. Transplant. Proc. 45, 394–396 (2013).
- Przybylowski P, Malyszko J, Kozlowska S et al. Serum renalase depends on kidney function but not on blood pressure in heart transplant recipients. Transplant. Proc. 43, 3888–3891 (2011).
- Petri M, Orbai AM, Alarcón GS et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 64, 2677–2686 (2012).
- Johnson S. European League Against Rheumatism and American College of Rheumatology present new SLE classification criteria at the 2017 ACR/ARHP annual meeting. Presentation at: ACR/ARHP 2017 Annual Meeting; November 3-8, San Diego, CA (2017).
- Urowitz MB, Gladman DD. Measures of disease activity and damage in SLE. Baillieres. Clin. Rheumatol. 12, 405–413 (1998).
- Bombardier C, Gladman DD, Urowitz MB et al. Derivation of the SLEDAI: A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. J. Arthritis. Rheum. 35, 630–640 (1992).
- Weening JJ, D'Agati VD, Schwartz MM et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J. Am. Soc. Nephro. 15, 241–250 (2004).
- Xue J, Yang J, Yang L et al. Dickkopf-1 is a biomarker for systemic lupus erythematosus and active lupus nephritis. J. Immunol. Res. 3, 1-13 (2017).
- Almaani S, Meara A, Rovin BH. Update on lupus nephritis. Clin. J. Am. Soc. Nephrol. 12, 825–835 (2017).
- Arto N, Bertolaccini ML, Calabuig E et al. AntiC1q antibodies in nephritis: correlation between titres and renal disease activity and positive predictive value in systemic lupus erythematosus. Ann. Rheum. Dis. 64, 444–448 (2005).
- Qi C, Wang L, Zhang M et al. Serum Renalase Levels Correlate with Disease Activity in Lupus Nephritis. PLoS ONE 10(10), e0139627 (2015).
- Desir GV, Tang L, Wang P et al. Renalase lowers ambulatory blood pressure by metabolizing circulating adrenaline. J. Am. Heart Assoc. 1: e2634 (2012).
- Zbroch E, Malyszko J, Malyszko J et al. Renalase in peritoneal dialysis patients is not related to blood pressure, but to dialysis vintage. Perit. Dial. Int. 32, 348–351 (2012).
- Zbroch E, Malyszko J, Malyszko JS et al. A novel enzyme involved in blood pressure regulation, is related to kidney function but not to blood pressure in hemodialysis patients. Kidney Blood Press. Res. 35: 395–399 (2012).
- Howson JM, Cooper JD, Smyth DJ et al. Evidence of gene-gene interaction and age-at-diagnosis effects in type 1 diabetes. Diabetes. 61, 3012–3017 (2012).
- Barrett JC, Clayton DG, Concannon P et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat. Genet. 41, 703–707 (2009).
- Wallace C, Rotival M, Cooper JD et al. Statistical colocalization of monocyte gene expression and genetic risk variants for type 1 diabetes. Hum. Mol. Genet. 21, 2815–2824 (2012).