Research Article - International Journal of Clinical Rheumatology (2018) Volume 13, Issue 5
Serum vitamin D in Egyptian patients with systemic lupus erythematous and its association with lupus nephritis
- *Corresponding Author:
- Tamer O. Elsaid
Department of Rheumatology and Rehabilitation
Mansoura University, Egypt
E-mail: tamer-rheum@hotmail.com
Abstract
Aim: To assess VD levels in serum of patients with LN in comparison with patients with extra-renal lupus and healthy controls, and to assess the relation between VD levels and the various clinical and laboratory disease parameters.
Subjects and Methods: Serum levels of 25(OH)D in 30 SLE without LN (SLE/noLN), 30 SLE with LN (SLE/LN), and 30 matched controls were assessed and estimated for deficiency and insufficiency at 10 and 30 ng/mL, respectively. SLE-related clinical and biochemical data were collected and the disease activity score for all patients was calculated. The association of serum VD with SLE-related features was evaluated Results - VD deficiency and insufficiency are more prevalent in SLE/LN patients (26.7% and 66.7%, respectively) than in SLE/noLN (20% and 63.3% respectively) and then in controls (13.3% and 43.3% respectively) (p=0.013). Serum VD levels were inversely correlated with the SLE-DAI score in SLE/LN patients (p=0.012) and in SLE/noLN (p=0.037). Low serum VD level is significantly associated with fatigue and photosensitivity in SLE/LN patients (p=0.002 and p=0.014 respectively) and in SLE/noLN patients (p=0.021 and p=0.044 respectively). In SLE/LN patients, low VD is associated with proteinuria and anti-dsDNA.
Conclusion: VD deficiency and insufficiency are prevalent in patients with SLE and is more prevalent in SLE/LN patients. Low serum VD is significantly correlated with higher disease activity and ESR. Low serum VD is significantly associated with presence of fatigue and photosensitivity. The strongest factors determining the serum VD level among the SLE patients was presence of LN and photosensitivity.
Keywords
systemic lupus erythematous, lupus nephritis, vitamin D, disease activity score
Introduction
Vitamin D (VD) is a lipo-soluble vitamin that plays a key role in calcium and phosphorus metabolism and bone mineralization. The main source of VD in our body comes from the conversion of 7-dehydrocholesterol into pre- VD3 in the skin, while smaller amounts of VD come from dietary sources [1]. However, it had been reported that several immune cells express VD receptors (VDRs) on their surfaces [2], and many immune cells synthesize the 1α-hydroxylase enzyme responsible for synthesis of the active form of VD in the microenvironment of lymph tissues [3]. These findings indicate that VD is involved in the immune modulation [4,5].
Systemic Lupus Erythematous (SLE) is a chronic inflammatory multisystem disease predominantly affecting females of childbearing period. SLE is characterized by a wide spectrum of clinical manifestations in addition to prototypic abnormalities of the immune response [6]. Low serum VD is prevalent in SLE patients, ranging from 16% [7] to 95% [8] and can be attributed to several factors such as photosensitivity, sunscreen application, renal damage, chronic glucocorticoids or anti-malarial therapy [9].
Accordingly, many studies had been performed to identify potential link between low VD and lupus. Musculoskeletal pain, fatigue and depression are common in SLE, and low VD is implicated in these conditions [10]. Disease activity in SLE was found to be inversely correlated with serum VD [11,12]. However, Orbach et al. found no relationship between SLE activity and serum VD level [13].
Lupus Nephritis (LN) is one of the most serious consequences of SLE and is one of the major factors predicting poor outcome [14]. Active LN can be the initial presentation in ~30% of the patients with SLE [15] and 10%-30% of SLE patients may develop End-Stage Renal Disease (ESRD) 10 years after onset of LN [16]. Renal involvement can interfere with 1-hydroxylation that is essential to make active form of VD. Renal involvement is strongest predictor for the VD levels <10 ng/ml [17].
This study aims to assess VD levels in serum of patients with LN in comparison with patients with extra-renal lupus and healthy controls, and to assess the relation between VD levels and the various clinical and laboratory disease parameters.
Subjects and methods
Participants
The study included 30 consecutive SLE without LN (SLE/noLN), and 30 consecutive SLE with LN (SLE/LN), collected from the Outpatient Clinics of the Rheumatology and Rehabilitation and Internal Medicine departments (Mansoura University Hospitals) during the period from March 2015 to October 2017. All patients with SLE fulfilled at least 4 of 11 American College of Rheumatology (ACR) criteria from 1997 for the diagnosis of SLE [18]. This study adopted the criteria for LN described by the ACR [18]:
• Persistent proteinuria >0.5 grams/day or >3+ by dipstick, or
• Cellular casts (red cells, hemoglobin, granular, tubular, or mixed). For inclusion of patients with SLE/LN, only patients who underwent a recent renal biopsy were included in this study. Pathologic lesions were evaluated according to the International Society of Nephrology and the Renal Pathology Society (ISN/RPS) systems [19].
The study included also 30 healthy matched controls. Prior to inclusion, the procedures of the study was explained to all participants and an informed written consent was obtained from all the participants in the study. The study was approved by the local Ethical Committee.
Exclusion criteria
Patients with kidney disease due to causes other than SLE, ESRD with or without dialysis, granulomatous disorders (sarcoidosis, tuberculosis), malignancy or suspected malignancy and those on VD therapy were excluded from the study.
Clinical assessment
Patients were clinically assessed through full history taking in addition to both general and local examinations as well as review of their medical records. The following variables were recorded: age, sex, duration of SLE, photosensitivity, skin changes, active arthritis, and activity of LN and drug intake. All patients were taking hydroxychloroquine and glucocorticoids while none of the patients were taking VD and calcium supplements.
Laboratory assessment
All patients had provided a blood sample and urine sample for complete urine analysis in the same day of clinical evaluation. All patients underwent following laboratory analysis: concentration of parathyroid hormone, urinalysis, erythrocyte sedimentation rate (ESR), C-Reactive Protein (CRP), serum creatinine, C3 and C4 concentration, presence of Anti-Nuclear Auto-antibodies (ANA) and the anti-ds-DNA. Urine samples were obtained for evaluation of 24-hours protein in urine and presence of urine casts.
Assessment of SLE disease activity
The disease activity in the SLE patient was assessed by the modified SLE Disease Activity Index 2000 (SLE-DAI). The SLE-DAI involves both clinical and laboratory parameters and defines disease activity within past 10 days. The total score falls between 0 and 109, with higher scores representing increased activity [20].
Determination of vitamin D status
VD status was determined by measuring serum 25-hydroxy vitamin (25(OH)D) concentration, which is the main circulating form of VD. VD insufficiency was defined as a serum 25(OH)VD level of <30 ng/ml and higher than 15 ng/ml, whereas VD deficiency was defined as a serum 25(OH)VD level of <15 ng/ml. The 25(OH)D concentration was measured using a commercial ELISA kit according to the manufacturer’s instructions (Immunodiagnostic Systems Inc, Fountain Hills, AZ, USA).
Statistical analysis
All statistical analyses were performed using SPSS for windows version 20.0 (SPSS, Chicago, IL). Continuous data were expressed as mean ± Standard Deviation (SD), while categorical data were expressed in number and percentage. All continuous data were tested for normality of distribution prior to any calculations. The differences among the groups were determined using independent sample Student`s t-test for continuous data that attained normal distribution or using the Mann-Whitney test for data with abnormal distribution. Chi-square test was used for comparison of categorical data. The correlations between serum 25(OH)VD level and continuous data was evaluated using the correlation co-efficient test. In the present study we played the linear regression analysis to explore the clinical or laboratory manifestations with strongest association with the serum 25(OH)VD levels among the patients with SLE. Statistical significance was set at p<0.05.
Results
The study included 30 SLE/noLN, 30 SLE/ LN patients and 30 healthy controls. The SLE/ noLN group included 28 females (93.3%) and two males (6.7%); their ages ranged from 26 to 53 years with a mean ± SD of 38.3 ±9.2 years and their disease duration ranged from 2 to 16 years with a mean ± SD of 9.0 ± 4.3 years. The SLE/LN group included 27 females (90.0%) and three males (10.0%); their ages ranged from 26 to 53 years with a mean ± SD of 39.7 ± 8.8 years and their disease duration ranged from 1 to 14 years with a mean ± SD of 7.7 ± 3.7 years. The control group included 27 females (90.0%) and three males (10.0%); their ages ranged from 26 to 52 years with a mean ± SD of 41.3 ± 7.5 years. There was no significant difference among the studied groups as regards the age, sex distribution. However, the disease duration was significantly longer in the SLE/LN group than SLE/noLN group (p=0.031).
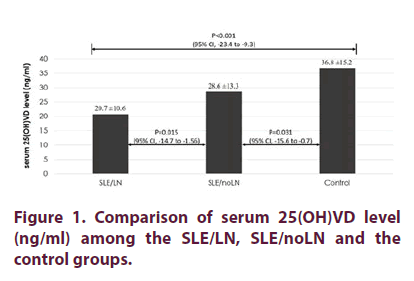
Comparison of the serum 25(OH)VD level among the groups
The average serum 25(OH)VD in the SLE/LN group was 20.7 ± 10.6 ng/ml compared to the 28.6 ± 13.3 ng/ml in the SLE/noLN group. This difference was significant (p=0.015). The average serum 25(OH)VD level was also significantly lower in the SLE/LN group as compared to the control group (20.7 ± 10.6 vs 36.8 ±15.2 ng/ ml, p<0.001). Also, the SLE/noLN group had significantly lower average serum 25(OH)VD level than the control group (28.6 ± 13.3 vs 36.8 ± 15.2 ng/ml, p=0.031) (Figure 1).
Regarding the VD status among the groups, the highest frequency of VD deficiency was found in the SLE/LN group followed by the SLE/noLN group while it is least in the control group. Similarly, VD insufficiency frequency is highest in the SLE/LN group and is least in the control group. These differences were significant (p=0.013) (Table 1).
| SLE/LN group | SLE/noLN group | Control group | Chi square test | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | χ2 | p | |
| VD status | ||||||||
| Deficiency | 8 | 26.7 | 6 | 20 | 4 | 13.3 | ||
| Insufficiency | 20 | 66.7 | 19 | 63.3 | 13 | 43.3 | ||
| Normal | 2 | 6.7 | 5 | 16.7 | 13 | 43.3 | 12.687 | 0.013 |
Table 1. Comparison of serum 25(OH)VD status among the groups.
Comparison between SLE/LN and SLE/noLN groups
Table 2 compares the clinical and laboratory findings between the SLE/LN and SLE/noLN groups. The frequency of the presence of the clinical manifestations and drug intake did not differ significantly between the SLE/LN and SLE/noLN groups. None of the patients in the SLE/noLN group had proteinuria, hematuria or urinary casts, meanwhile 56.7%, 36.7% and 23.3% of the patients in the SLE/LN group had proteinuria, hematuria and urinary casts respectively (p<0.001). SLE/LN group more frequently had low C3 (p=0.037), low C4 (p=0.035) and anti-dsDNA positivity (p=0.018) than SLE/LN group.
| SLE/LN | SLE/noLN | P | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Fatigue | 24 | 80 | 22 | 73.3 | 0.541 |
| Mucosal ulcer | 11 | 36.7 | 9 | 30 | 0.584 |
| Malar rash | 27 | 90 | 25 | 83.3 | 0.447 |
| Photosensitivity | 27 | 90 | 25 | 83.3 | 0.447 |
| Alopecia | 14 | 46.7 | 9 | 30.3 | 0.184 |
| Arthritis | 24 | 80 | 19 | 63.3 | 0.152 |
| Pleurisy | 9 | 30 | 5 | 16.7 | 0.222 |
| Pericarditis | 4 | 13.3 | 3 | 10 | 0.687 |
| Proteinuria | 17 | 56.7 | 0 | 0% | <0.001 |
| Hematuria | 11 | 36.7 | 0 | 0% | <0.001 |
| Urinary casts | 7 | 23.3 | 0 | 0% | 0.005 |
| Low C3 | 26 | 86.7 | 19 | 61.30% | 0.037 |
| Low C4 | 16 | 53.3 | 8 | 26.70% | 0.035 |
| Anti-dsDNA | 22 | 73.3 | 14 | 46.70% | 0.018 |
Table 2. Comparison of the frequency of the clinical and laboratory markers between the SLE/LN patients SLE/noLN patients.
Correlation of the serum 25(OH)VD with clinical and laboratory findings
In SLE/LN group and in SLE/noLN group, the serum 25(OH)VD is inversely correlated with SLE-DAI score (p=0.012 and p=0.037, respectively), with ESR (p=0.016 and 0.049, respectively), and with serum creatinine level (p=0.015) only in SLE/LN group while serum 25(OH)VD show no significant correlation with age of the patients, duration of SLE (Table 3).
| SLE/LN | SLE/noLN | |||
|---|---|---|---|---|
| r | p | r | p | |
| Age | 0.136 | 0.473 | 0.308 | 0.098 |
| Duration of SLE | -0.116 | 0.543 | -0.146 | 0.442 |
| SLE-DAI score | -0.453 | 0.012 | -0.382 | 0.037 |
| ESR | -0.436 | 0.016 | -0.363 | 0.049 |
| CRP | 0.103 | 0.584 | 0.106 | 0.574 |
| Serum creatinine | -0.441 | 0.015 | -0.187 | 0.322 |
| Renal biopsy chronicity index | 0.156 | 0.409 | ||
| Renal biopsy activity index | 0.282 | 0.132 | ||
Table 3. Correlation of the serum 25(OH)VD with clinical and laboratory findings in SLE/LN patients and SLE/ noLN patients.
The associations between serum VD level with SLE-related features
The median value of the serum 25(OH)VD is significantly lower in patients with fatigue than those without fatigue and also is lower in patients with photosensitivity than those without in the SLE/LN and in the SLE/noLN groups. On the other hand, no association had been found between serum 25(OH)VD and other clinical features (Table 4).
| SLE/LN group | SLE/noLN group | ||||
|---|---|---|---|---|---|
| Serum 25(OH)VD (ng/ml) Median [IQR] | P | Serum 25(OH)VD (ng/ml) Median [IQR] | P | ||
| Fatigue | Absent | 40.05 [24.5 – 40.7] | 46.9 [21.8 – 48.4] | ||
| Present | 13.7 [8.4 – 28.3] | 0.002 | 27.1 [13.7 – 36.5] | 0.021 | |
| Mucosal ulcer | Absent | 23.3 [12.6 – 30.7] | 28.7 [17.3 – 38.5] | ||
| Present | 14.6 [7.9 – 29.7] | 0.286 | 30.9 [16.6 – 45] | 0.697 | |
| Malar rash | Absent | 20.3 [8.7 – 29.9] | 20.9 [19.1 – 28.1] | ||
| Present | 7.5 [7 – 13.4] | 0.061 | 31.7 [14.5 – 42] | 0.275 | |
| Photosensitivity | Absent | 22.9 [13.7 – 34.2] | 36.2 [20.4 – 46.1] | ||
| Present | 8.4 [7.3 – 28.2] | 0.014 | 16.5 [14.8 – 32.9] | 0.044 | |
| Alopecia | Absent | 29.5 [10.4 – 38.8] | 27.3 [20.4 – 44.6] | ||
| Present | 14.2 [8.2 – 27.3] | 0.129 | 30.6 [11.3 – 36.7] | 0.497 | |
| Arthritis | Absent | 26.2 [12.3 – 32.3] | 31.7 [19.1 – 41.1] | ||
| Present | 13.9 [7.6 – 29.5] | 0.145 | 13.9 [9.4 – 38.5] | 0.432 | |
| Pleurisy | Absent | 21.6 [12.5 – 31.8] | 31.7 [20.9 – 43.7] | ||
| Present | 12.9 [7.3 – 27.9] | 0.079 | 17.8 [8.8 – 35] | 0.069 | |
| Pericarditis | Absent | 17.9 [8.7 – 29.2] | 30.6 [20.4 – 39.1] | ||
| Present | 11.1 [7.6 – 37.2] | 0.746 | 26.7 [10 – 47.5] | 0.848 | |
| Proteinuria | Absent | 26.7 [13.4 – 35.3] | - | ||
| Present | 8.7 [7.7 – 15.8] | 0.012 | - | ||
| Hematuria | Absent | 21.6 [8.5 – 33.7] | - | ||
| Present | 14.4 [8.6 – 17.9] | 0.335 | - | ||
| Urinary casts | Absent | 20.3 [8.7 – 31.8] | - | ||
| Present | 15.8 [7.7 – 23.5] | 0.416 | - | ||
| Low C3 | Absent | 19.1 [8.6 – 29.4] | 30.6 [12.8 – 39.1] | ||
| Present | 12.8 [7.8 – 28.9] | 0.498 | 26.8 [20.4 – 40.2] | 0.933 | |
| Low C4 | Absent | 19.8 [13.0 – 29.7] | 31.2 [15.4 – 40.3] | ||
| Present | 12.8 [8.2 – 30.2] | 0.377 | 23.9 [20.5 – 39.5] | 0.836 | |
| Anti-dsDNA | Absent | 24.2 [11.8 – 34.1] | 26.8 [11.3 – 43.7] | ||
| Present | 9.9 [7.2 – 15.3] | 0.021 | 35.0 [20.4 – 39.1] | 0.582 | |
Table 4. The association between serum 25(OH)VD level with clinical findings and drug intake in SLE/LN and SLE/noLN groups.
In the SLE/LN group, patients with proteinuria had significantly lower median serum VD than patients without proteinuria (p=0.012). Also, patients with positive anti-dsDNA had significantly lower median serum VD than patients with negative anti-dsDNA (p=0.021), while no significant association was found between the serum VD and hematuria, urinary casts, low C3, low C4 (Table 4). In the SLE/ noLN group, no significant association was found between the serum VD and low C3, low C4 nor presence of anti-dsDNA. No significant correlation had been found between the degree of glomerular disease severity as diagnosed by renal biopsy and the VD status or serum level (Table 3).
Factors determining the serum VD in SLE
The linear regression analysis test had shown that the strongest factors determining the serum 25(OH)VD level among the patients with SLE was presence of LN (OR=6.521, p=0.032), proteinuria (OR=3.860, p=0.039) and photosensitivity (OR=2.506, p=0.047) (Table 5).
| 95% C.I. for EXP(B) | |||||||
|---|---|---|---|---|---|---|---|
| B | S.E. | Wald | Sig. | Exp(B) | Lower | Upper | |
| Fatigue | -0.129 | 0.093 | 1.86 | 0.175 | 1.141 | 0.943 | 1.369 |
| Photosensitivity | -1.198 | 0.66 | 3.341 | 0.048 | 0.31 | 0.079 | 3.102 |
| SLEDAI | -0.059 | 0.046 | 2.078 | 0.152 | 0.942 | 0.859 | 1.025 |
| ESR | -0.629 | 0.627 | 1.021 | 0.309 | 1.877 | 0.549 | 6.399 |
| CRP | -0.118 | 0.109 | 1.161 | 0.279 | 1.131 | 0.907 | 1.408 |
| Proteinuria | -1.067 | 0.547 | 3.981 | 0.041 | 1.422 | 1.17 | 4.509 |
| Anti-dsDNA | -0.119 | 0.108 | 1.167 | 0.279 | 1.131 | 0.907 | 1.408 |
| LN | -0.933 | 0.432 | 4.619 | 0.032 | 2.54 | 1.083 | 7.928 |
| Constant | -30.18 | 11.269 | 7.167 | 0.01 | 0.002 | ||
Table 5. The linear logistic regression analysis for the factors predicting the serum 25(OH)VD level in patients with SLE.
Discussion
The main findings of the current study is that (a) VD deficiency is more prevalent in SLE/LN patients than in SLE/noLN and VD deficiency is more prevalent in the both groups than in controls, (b) serum VD levels were inversely correlated with the SLE-DAI score, (c) low serum VD level is significantly associated with fatigue and photosensitivity in patients with SLE and (d) in SLE/LN patients, low VD is associated with proteinuria and anti-dsDNA.
Among the SLE patients participated in the current study, 88.3% of the patients had low serum VD, with 23.3% had VD deficiency and 65% of the patients had insufficiency. Abaza et al. reported the prevalence of VD deficiency and insufficiency of 73% and 23% among the Egyptian SLE patients respectively [21]. Another study reported that overall prevalence VD insufficiency and VD deficiency among Egyptian SLE patients was 69% and 39% respectively [22]. Also, Korah et al. found lower serum VD level in Egyptian patients with SLE as compared with matched controls [23]. These findings altogether confirm that low serum VD is prevalent among the Egyptian SLE patients, despite the fact that Egypt had a plenty of sunny days around the year.
Moreover, our results agree with the results obtained from studies performed in other countries and across various geographic locations and latitudes. The prevalence of VD insufficiency in SLE patients was 55% in Brazilian [24], 66.7% in Canadian [25], 81.9% in Hungarian [26] and 98.8% in Saudi Arabian SLE patients [27]. In Saudi Arabia 55% of the controls had VD deficiency which can be explained by their traditional clothing that considerably reduces their chance for exposure to sun [27].
Also, in agreement with our findings, previous studies observed that the average serum VD in SLE patients was significantly lower than in the healthy matched controls [2,28]. One study reported that 75% of SLE patients had VD deficiency and 15.2% VD insufficiency [29].
Interestingly, in a recently diagnosed SLE patients, the prevalence of VD insufficiency and deficiency in 67.4% and 17.9% respectively suggesting that VD deficiency is a risk factor for the development of SLE rather than being a consequent of it [17].
The results of our study revealed that, the SLE/ LN group of patients had the highest frequency of VD deficiency and insufficiency followed by the SLE/noLN group while it is least in the control group. Likewise, when serum VD was expressed in ng/ml, our results showed that the SLE/LN group of patients had significantly lower average serum VD than the patients in the SLE/noLN group and also than healthy controls. Also, patients in the SLE/noLN group had significantly lower average serum VD than the healthy controls. In agreement with our results, a strong association between VD deficiency and LN was reported [23] and SLE patients with LN showed higher prevalence of VD deficiency [17]. Another study found that SLE patients with LN exhibited a higher prevalence of VD deficiency in comparison to patients without LN albeit that it is not statistically significant [2].
Our results had shown that VD serum levels were inversely correlated with the SLE-DAI score in SLE/LN group of patients and also in SLE/noLN group of patients. In accordance with our finding, several previous studies reported a strong negative correlation between serum VD and the SLE disease activity measured by SLE-DAI [21,30,31]. It had been reported that SLE patients with severe VD deficiency had significantly higher mean SLE activity [32,33]. Moreover, Amital et al. reported a significant negative correlation between the serum VD level and the SLE disease activity scores as measured by two scales, the SLE-DAI and the European Consensus Lupus Activity Measurement (ECLAM) [34].
Also, consistent to our findings, Attar and Siddiqui found that the mean serum VD level was significantly lower in patients with active SLE than in patients with inactive disease [35]. The association between VD deficiency and SLE activity is expected as the underlying inflammatory process in SLE patients potentially enhances VD catabolism [36]. In this context, our results had revealed that VD is inversely correlated with ESR in SLE patients with LN and those without LN.
In contrast, many other studies did not find this association [2,7,29]. The discrepancy between the findings of these studies and the present study can be attributed to the characteristics of the enrolled population since patients with VD supplements were not excluded from these studies.
In the present study, no significant association between the serum VD level and SLE duration had been found, finding that is supported by many other studies [25,29] indicating that VD status is affected more by the way that disease is clinically manifested rather than disease duration.
The results of the current study revealed that the low serum VD level is significantly associated with fatigue and photosensitivity. In agreement with our findings, Abaza et al. observed a significant negative correlation between VD and fatigue score [21]. Likewise, Ruiz-Irastorza et al. stated that SLE patients with VD deficiency had a greater intensity of fatigue [29]. In support to this concept, it was reported that VD supplementation had decreased the intensity of fatigue in patients with SLE suggesting that elevating serum VD levels had a beneficial effect on fatigue [37]. Photosensitivity was found to be strongly associated with the low VD among the patients with SLE [17] and the resultant avoidance of sun exposure or use of sunscreen contribute to such deficiency [29].
Our results showed that VD is inversely associated with proteinuria and anti-dsDNA in the SLE/LN group of patients. Previous studies reported the association between low VD and renal involvement [38] and the association between low VD and occurrence of proteinuria [39]. The results of the present study had found significant association between low serum VD and anti-dsDNA antibodies. In support to our results, other studies reported a strong inverse association between VD and anti-dsDNA antibodies [26,40].
In the present study we played the linear regression analysis to explore the clinical or laboratory manifestations with strongest association with the serum VD levels among the SLE patients. At the linear regression model, LN was the strongest predictor for lower serum 25(OH)VD levels. On the other hand, presence of proteinuria and photosensitivity are also associated with lower serum VD level in the regression analysis test. In agreement with these findings, it had been found that renal involvement (OR=13.3; p<0.001) followed by photosensitivity (OR=12.9; p<0.001) are the strongest predictors of low VD level [17]. Also, in agreement with our study, serum creatinine level was found to be the strongest predictor for low serum VD in the regression analysis test [23].
Limitations of this study include the small number of patients. Examination of the association between VD and LN in larger number of patients is recommended to confirm the findings of the current study. Also, another area of future study is to investigate the supplementation of VD on the prognosis of SLE and LN.
Conclusion
VD deficiency and insufficiency are prevalent in patients with SLE and is more prevalent in SLE-LN patients. Low serum VD is significantly correlated with higher disease activity and ESR. Low serum VD is significantly associated with presence of fatigue and photosensitivity. The strongest factors determining the serum VD level among the patients with SLE was presence of LN and photosensitivity.
References
- Holick MF. Vitamin D deficiency. N. Engl. J. Med. 357(3), 266–281 (2007).
- Miskovic R, Plavsic A, Raskovic S et al. Vitamin D Status in Patients with Systemic Lupus Erythematosus in Serbia: Correlation with Disease Activity and Clinical Manifestations. Open. Access. Maced. J. Med. Sci. 3(2), 256–261 (2015).
- Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat. Rev. Immunol. 8(9), 685–698 (2008).
- Kamen DL, Tangpricha V. Review Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J. Mol. Med. 88, 441–450 (2010).
- White JH. Review Vitamin D metabolism and signaling in the immune system. Rev. Endocr. Metab. Disord. 13, 21–29 (2012).
- Chizzolini C, Cohen CD, Eisenberger U et al. Towards the Swiss systemic lupus erythematosus cohort study (SSCS). Rev. Med. Suisse. 5, 808–811 (2009)
- Kim HA, Sung JM, Jeon JY et al. Vitamin D may not be a good marker of disease activity in Korean patients with systemic lupus erythematosus. Rheumatol. Int. 31(9), 1189-1894 (2011).
- Cutillas-Marco E, Morales-Suárez-Varela M, Marquina-Vila A et al. Serum 25-hydroxyvitamin D levels in patients with cutaneous lupus erythematosus in a Mediterranean region. Lupus. 19(7), 810-814 (2010).
- Barnes TC, Bucknall RC. Vitamin D deficiency in a patient with systemic lupus erythematosus. Rheumatol. 43, 393–394 (2004).
- Reynolds JA, Bruce IN. Vitamin D in systemic lupus erythematosus: potential beyond bone health. Int. J. Clin. Rheumatol. 4(3), 297–309 (2009).
- Ritterhouse LL, Crowe SR, Niewold TB. Vitamin D deficiency is associated with an increased autoimmune response in healthy individuals and in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 70, 1569–1574 (2011).
- Terrier B, Derian N, Schoindre Y. Restoration of regulatory and effector T cell balance and B cell homeostasis in systemic lupus erythematosus patients through vitamin D supplementation. Arthritis. Res. Ther. 14, R221 (2012).
- Orbach H, Zandman-Goddard G, Amital H et al. Novel biomarkers in autoimmune diseases: prolactin, ferritin, vitamin D, and TPA levels in autoimmune diseases. Ann. NY. Acad. Sci. 1109, 385–400 (2007).
- Feldman CH, Hiraki LT, Liu J et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000-2004. Arthritis. Rheum. 65(3), 753–763 (2013).
- Mok CC, Tang SS. Incidence and predictors of renal disease in Chinese patients with systemic lupus erythematosus. Am. J. Med. 15; 117(10), 791–795 (2004).
- Lateef A, Petri M. Unmet medical needs in systemic lupus erythematosus. Arthritis. Res. Ther. 14(S4) (2012).
- Kamen DL, Cooper GS, Bouali H et al. Vitamin D deficiency in systemic lupus erythematosus. Autoimmun. Rev. 5(2), 114–117 (2006).
- Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis. Rheum. 40(9), 1725 (1997).
- Weening JJ, D'Agati VD, Schwartz MM et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J. Am. Soc. Nephrol. 15, 241–250 (2004).
- Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J. Rheumatol. 29(2), 288–291 (2002).
- Abaza NM, El-Mallah RM, Shaaban A et al. Vitamin D Deficiency in Egyptian Systemic Lupus Erythematosus Patients: How Prevalent and Does It Impact Disease Activity? Integr. Med. Insights. 26(11), 27–33 (2016).
- Abou-Raya A, Abou-Raya S, Helmii M. The effect of vitamin D supplementation on inflammatory and hemostatic markers and disease activity in patients with systemic lupus erythematosus: a randomized placebo-controlled trial. J. Rheumatol. 40(3), 265–272 (2013).
- Korah TE, Soliman SG, Al-Sharaki DR et al. Vitamin D in systemic lupus erythematosus patients with and without nephropathy. Egypt. Rheumatol. Rehabil. 40(3), 165–175 (2013).
- de Souza VA, Bastos MG, Fernandes NM et al. Association of hypovitaminosis D with Systemic Lupus Erythematosus and inflammation. J. Bras. Nefrol. 36(4), 430–436 (2014).
- Toloza SMA, Cole DEC, Gladman DD et al. Vitamin D insufficiency in a large female SLE cohort. Lupus. 19, 13–19 (2010).
- Szodoray P, Tarr T, Bazso A et al. The immunopathological role of vitamin D in patients with SLE: data from a single centre registry in Hungary. Scand. J. Rheumatol. 40(2), 122–126 (2011).
- Damanhouri LH. Vitamin D deficiency in Saudi patients with systemic lupus erythematosus. Saudi. Med. J. 30(10), 1291–1295 (2009).
- Chen S, Sims GP, Chen XX et al. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 179(3), 1634–1647 (2007).
- Ruiz-Irastorza G, Egurbide MV, Olivares N et al. Vitamin D deficiency in systemic lupus erythematosus: prevalence, predictors and clinical consequences. Rheumatology (Oxford). 47(6), 920–923 (2008)
- Borba VZ, Vieira JG, Kasamatsu T et al. Vitamin D deficiency in patients with active systemic lupus erythematosus. Osteoporos. Int. 20, 427–433 (2009).
- Yeap SS, Othman AZ, Zain AA et al. Vitamin D levels: its relationship to bone mineral density response and disease activity in premenopausal Malaysian systemic lupus erythematosus patients on corticosteroids. Int. J. Rheum. Dis. 15, 17–24 (2012).
- Cutolo M, Otsa K, Paolino S et al. Vitamin D involvement in rheumatoid arthritis and systemic lupus erythematosus. Ann. Rheum. Dis. 11, 446–447 (2009).
- Martins DC, Kang J, Kamen DL et al. The threshold of vitamin D required for optimal immune regulation: implications for patients with lupus (abstract). Arthritis. Rheum. 54, S431 (2006).
- Amital H, Szekanecz Z, Szücs G et al. Serum concentrations of 25-OH vitamin D in patients with systemic lupus erythematosus (SLE) are inversely related to disease activity: is it time to routinely supplement patients with SLE with vitamin D? Ann. Rheum. Dis. 69(6),1155–1157.
- Attar SM, Siddiqui AM. Vitamin D deficiency in patients with systemic lupus erythematosus. Oman. Med. J. 28(1), 42–47 (2013).
- Ben-Zvi I, Aranow C, Mackay M et al. The impact of vitamin D on dendritic cell function in patients with systemic lupus erythematosus. PLoS One. 5(2), e9193 (2010).
- Ruiz-Irastorza G, Gordo S, Olivares N et al. Changes in vitamin D levels in patients with systemic lupus erythematosus: Effects on fatigue, disease activity, and damage. Arthritis Care Res (Hoboken). 62(8), 1160–1165 (2010).
- Bogaczewicz J, Sysa-Jedrzejowska A, Arkuszewska C et al. Vitamin D status in systemic lupus erythematosus patients and its association with selected clinical and laboratory parameters. Lupus. 21(5), 477–484 (2012).
- Robinson AB, Thierry-Palmer M, Gibson KL et al. Disease activity, proteinuria, and vitamin D status in children with systemic lupus erythematosus and juvenile dermatomyositis. J. Pediatr. 160, 297–302 (2012).
- Carvalho JF, Blank M, Kiss E et al. Anti-vitamin D, vitamin D in SLE: preliminary results. Ann. NY. Acad. Sci. 1109, 550–557 (2007).