Research Article - Interventional Cardiology (2022)
Short-term outcomes of complete coronary revascularization compared to staged revascularization during primary percutaneous coronary intervention in patients with multivessel coronary artery disease: Presenting with ST segment elevation myocardial infarction
- Corresponding Author:
- Mohamed Zahran
Department of Cardiology,
Ain Shams University,
Cairo,
Egypt,
E-mail: zahrancardiology@yahoo.com
Received date: 01-Jul-2022, Manuscript No. FMIC-22-67852; Editor assigned: 04-Jul-2022, PreQC No. FMIC-22-67852 (PQ); Reviewed date: 25-Jul-2022, QC No. FMIC-22-67852;Revised date: 01-Aug-2022, Manuscript No. FMIC-22-67852 (R);Published date: 08-Aug-2022, DOI: 10.37532/1755-5310.2022.14(S11).274
Abstract
Background: Complete revascularization has been recently popularized for management of ST-Segment–Elevation Myocardial Infarction (STEMI) patients with multivessel disease scheduled for Primary Percutaneous Coronary Intervention (PPCI). We assessed the three months outcomes of Compete Revascularization (CR) compared to staged revascularization in patients with multivessel disease undergoing PPCI.
Materials and methods: We conducted a randomized, open-label, comparative trial on STEMI patients with multivessel disease indicated for PPCI in the setting of STEMI. Patients were randomly assigned to undergo PCI revascularization of the non-culprit lesions during the index procedure, Complete Revascularization (CR) or within 30 days later after discharge, Staged Revascularization (SR). The primary endpoint was the composite of all-cause mortality, re-infarction, Heart Failure (HF), recurrence of angina symptoms, cerebrovascular stroke, and need for revascularization.
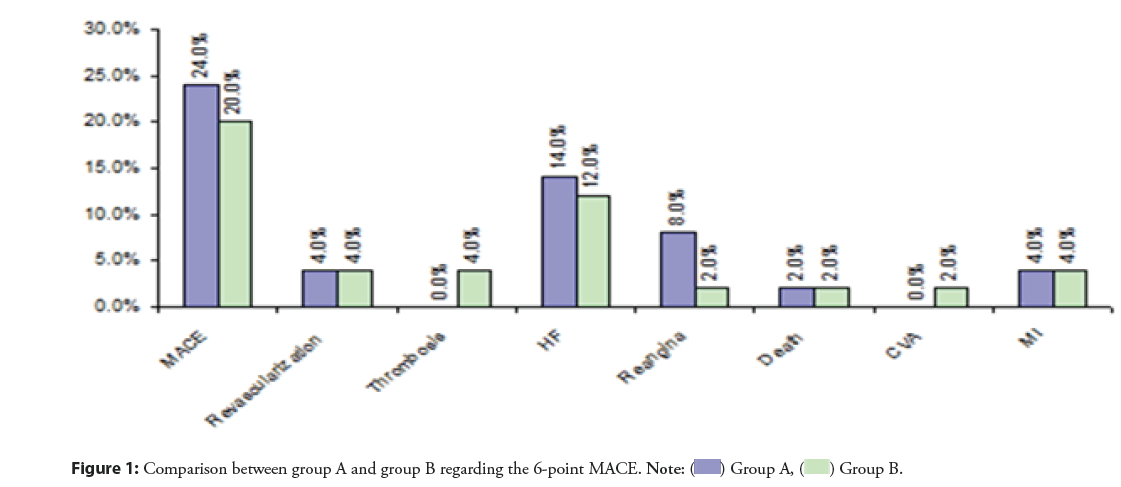
Results: A total of 100 patients were randomized in 1:1 ratio. The primary end point occurred in 24% of the patients in CR and 20% in SR group (p=0.62). The incidence of HF (14% vs. 12%; p=0.76), repeated revascularization (4% in each group), persistent angina (8% vs. 2%, p=0.16), all-cause mortality (2% in each group), MI (4% in each group), stent thrombosis (0% vs. 4%; p=0.15), and cerebrovascular accident (0% vs. 2%; p=0.32).
Conclusion: Staged revascularization provided comparable short-term benefits to complete revascularization in STEMI patients with multivessel disease undergoing PPCI. The present trial demonstrated that complete revascularization was associated with a trend towards higher incidence of stent thrombosis and CVA than staged
Keywords
ST-segment–elevation myocardial infarction • Primary percutaneous coronary intervention • Revascularization • Multivessel disease
Abbreviations
CAD: Coronary Artery Disease; FFR: Fractional Flow Reserve; PCI: Percutaneous Coronary Intervention; PPCI: Primary Percutaneous Coronary Intervention; STEMI: ST-segment–Elevation Myocardial Infarction; CVA: Cerebrovascular Accidents
Introduction
Cardiovascular disorders are a leading cause of mortality and morbidity, affecting over 18 million patients worldwide. These disorders result in complications, disabilities, and diminished productivity making them a major challenge to the healthcare system [1]. Acute Myocardial Infarction (AMI) is the most frequent cause of death among cardiovascular disorders, accounting for over 15% of global deaths [1,2]. For the sake of urgent intervention: patients with AMI are either designated as having ST-Elevation Myocardial Infarction (STEMI) or Non-ST Elevation Myocardial Infarction (NSTEMI) depending on their ECG findings [1]. Primary Percutaneous Coronary Intervention (PPCI) is the standard of care now offered to ST Elevation Myocardial Infarction (STEMI) patients, with lower mortality and lower rates of re-infarction when compared to the fibrinolytic therapy [3].
A considerable proportion of STEMI patients present with multivessel disease (nearly 50%), which negatively impacts the short- and long-term clinical outcomes [4]. The current clinical guidelines show controversy concerning the management approaches of the non-culprit lesions in STEMI patients with multivessel disease. Nonetheless, the current evidence demonstrates that multi-vessel PCI is a safe alternative to culprit-only PCI and provides superior clinical outcomes [5]. Large clinical trials -such as PRAMI and DANAMI-3-PRIMULTI trials- established the benefits of complete revascularization over culprit-only PCI in patients with multivessel diseases in terms of short- and long-term clinical outcomes, including mortality and repeat revascularization [6,7]. More recently, staged revascularization emerged as a clinical-effective and safe approach for revascularization of non-culprit artery, which is based on revascularization of non-culprit lesions through a separate procedure during hospitalization or up to 45 days after discharge. In the COMPLETE trial, staged revascularization resulted in a significantly lower annual incidence of CVD or repeated revascularization compared to culprit-only PCI [8].
Nonetheless, only few reports have compared the clinical outcomes of complete revascularization (Ad-hoc PCI of the non-culprit lesions during the index procedure) vs. staged revascularization of STEMI patients with multivessel disease. The current clinical trial assessed the three months outcomes of total coronary revascularization compared to staged revascularization in patients with multivessel disease undergoing PPCI.
Patients and Methods
The present study was initiated after obtaining the protocol approval from the local ethics committees at Ain shams University Hospital. All patients or their legal representatives signed the written informed consent before enrollment. We confirm that the present study did not violate any of the ethics principles declared by the latest version of the Declaration of Helsinki [9].
Study design and population
We conducted a randomized, open-label, comparative trial on STEMI patients with multivessel disease scheduled to undergo PPCI in the setting of STEMI. All patients were recruited from the Cardiology department of Ain Shams University hospitals and National Heart Institute through the period from the October 2018 to October 2020. Patients were recruited if they had a confirmed STEMI diagnosis with an onset of less than 24 hours until hospital admission. The diagnosis of STEMI was done according to the fourth universal definition of myocardial infarction. Only patients with angiographically-confirmed multivessel disease were included. The multivessel disease was identified as the presence of one or more non-culprit epicardial vessel, or one of its branches, with ≥ 70% diameter stenosis. We excluded patients with left main coronary artery disease, cardiogenic shock, pulmonary edema, creatinine clearance <30 ml/min, contraindication to anti-platelet therapy, thrombolysis therapy, and/or patients with a Chronic Total Occlusion (CTO) of a non-culprit vessel.
Following initial screening and informed consent, eligible patients were randomly allocated, using a computer-generated sequence, to undergo PCI revascularization of the non-culprit lesions during the index procedure (Ad-hoc complete revascularization) or 30 days later after discharge (staged revascularization).
Data collection and revascularization procedures
All patients were subjected to history taking, full clinical examination, routine laboratory investigations, baseline 12-lead Electrocardiogram (ECG), echocardiography, and diagnostic coronary angiography. Before the procedure, all patients received a loading dose of 300 mg aspirin and 180 mg ticagrelor or 600 mg clopidogrel. Unfractionated Heparin (UFH) was administrated during the procedure in the standard doses adjusted to body weight. The PPCI was performed according to local institutional guidelines and PCI with Drug Eluting Stents (DES) was performed. In complete revascularization group, patients underwent Ad-hoc PCI in non-culprit arteries with >70% stenosis. While in staged revascularization group, patients underwent PCI within 30 days from the hospital discharge. All angiographic complications during PCI were noted and recorded. Following the procedure, the patients received the standard regimen for STEMI including Dual Antiplatelet Therapy (DAPT), B-blockers, high dose statins, Angiotensinogen Converting Enzyme (ACE) inhibitors.
Follow-up and study endpoints
All patients were followed-up for three months after the operation. The primary endpoint was the composite of the Major Adverse Cardiovascular Events (MACE), composed of death, stent thrombosis, re-infarction, Heart Failure (HF), recurrence of angina symptoms, cerebrovascular stroke, and need for revascularization. Secondary outcomes involved death, stent thrombosis, re-infarction, Heart Failure (HF), recurrence of angina symptoms, cerebrovascular stroke, and need for revascularization.
Statistics
The conduction of data analysis was performed via SPSS software (SPSS Inc., Chicago, IL, USA) version 22 for Microsoft Windows. Appropriate descriptive measures were used to describe numerical and categorical variables according to the normality of the data. The hypothesize of a significant association between type of revascularization and the 6-point MACE or its individual components was tested by Chi-square test, while association between quantitative data was done using unpaired t-test or Mann-Whitney Rank Sum test per data normality. The statistical associations were considered significant at a p-value of <5%.
Results
A total of 100 patients were randomized in 1:1 ratio to the study’s groups. In group A, 50 patients underwent staged PCI to non-culprit vessels within one month from discharge (staged PCI), while in group B, a similar number of patients had complete revascularization in the same setting. Overall, the mean age of the included patients was 55.67 ± 8.31 years old and the majority of participants (85%) were males. Hypertension, diabetes mellitus, and dyslipidemia history were reported by 45% of the patients, each. Besides, nearly half of the patients (48%) were smokers and 28% had a positive family history of MI. Regarding Killip classification, the majority of participants were type I (94%). The mean systolic blood pressure was 134.10 ± 24.92 mmHg. The serum creatine kinase-MB (CK-MB) level ranged between 15-245 IU/L, with a mean of 77 ± 35.04 IU/L. There were no statistically significant differences between both groups in terms of demographic characteristics, comorbidities, MI-related characteristics, or laboratory findings (p>0.05), (Table 1).
| Group A | Group B | Test value | p-value | ||
|---|---|---|---|---|---|
| No.=50 | No.=50 | ||||
| Age (years) | 54.78 ± 8.70 | 56.56 ± 7.88 | 1.072 | 0.286 | |
| Male gender | 46 (92.0%) | 39 (78.0%) | 3.843* | 0.050 | |
| Hypertension | 21 (42.0%) | 24 (48.0%) | 0.364 | 0.546 | |
| Smoking | 25 (50.0%) | 23 (46.0%) | 0.160 | 0.689 | |
| Diabetes mellitus | 23 (46.0%) | 22 (44.0%) | 0.040 | 0.841 | |
| history of dyslipidemia | 23 (46.0%) | 22 (44.0%) | 0.040 | 0.841 | |
| Positive family history | 16 (32.0%) | 12 (24.0%) | 0.794 | 0.373 | |
| Anterior MI | 25 (50.0% | 27 (54.0%) | 0.160 | 0.689 | |
| Inferior MI | 25 (50.0% | 23 (46.0% | 0.160 | 0.689 | |
| Killip classification | I | 46 92.0% | 48 96.0% | 0.709 | 0.400 |
| II | 4 8.0% | 2 4.0% | |||
| Urea | Median (IQR) | 28 (19-38) | 25 (18-55) | 0.003 | 0.997 |
| Range | 10-85 | 10-85 | |||
| S.creatinine | Mean ± SD | 0.94 ± 0.24 | 0.93 ± 0.22 | 0.285 | 0.777 |
| Range | 0.6-1.6 | 0.6-1.55 | |||
| GFR | Mean ± SD | 88.34 ± 25.47 | 84.24 ± 20.84 | 0.881 | 0.381 |
| Range | 38-148 | 38-125 | |||
| CKMB | Mean ± SD | 75.10 ± 26.99 | 78.90 ± 41.77 | 0.540 | 0.590 |
| Range | 15-165 | 25-245 | |||
Note: *Chi-square test
Table 1: Comparison between group A and group B regarding pre-procedure characteristics.
| Variables | Group A | Group B | Test value* | p-value | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | ||||
| No. of vessels | 2 vessels | 34 | 68.00% | 46 | 92.00% | 9 | 0.003 |
| 3 vessels | 16 | 32.00% | 4 | 8.00% | 9 | 0.003 | |
| Coronary angiography | LAD | 45 | 90.00% | 43 | 86.00% | 0.379 | 0.538 |
| LCX | 33 | 66.00% | 31 | 62.00% | 0.396 | 0.529 | |
| RCA | 37 | 74.00% | 30 | 60.00% | 1.604 | 0.205 | |
| 1st PCI | LAD | 25 | 50.00% | 38 | 76.00% | 7.25 | 0.007 |
| LCX | 5 | 10.00% | 30 | 60.00% | 27.473 | 0.000 | |
| RCA | 23 | 46.00% | 29 | 58.00% | 1.442 | 0.229 | |
| 2nd PCI | LAD | 20 | 40.00% | - | - | - | - |
| LCX | 28 | 56.00% | - | - | - | - | |
| RCA | 14 | 28.00% | - | - | - | - | |
Note: * Chi-square test; LAD: Left Anterior Descending artery; LCX: Left Circumflex Artery; RCA: Right Coronary Artery; PCI: Percutaneous Coronary Intervention
Table 2: Comparison between group A and group B regarding coronary angiography and PCI.
The results showed no statistically significant difference between both groups regarding the pre-operative angiographic findings. Concerning the intervention vessel at index PCI, the LAD was more intervened upon in group B than group A (76% vs. 50%, respectively; p=0.007); likewise, LCX was more intervened upon in group B than A (60% vs. 10%). On the other hand, we found no statistically significant difference between both groups regarding the RCA intervention. In terms of staged PCI of non-culprit vessels, the distribution of the non-culprit vessel PCI was as follow: LCX (56%), LAD (40%), and RCA (28%), (Table 2).
The primary end point occurred in 24% of the patients in complete revascularization group and in 20% in staged revascularization group (p=0.62). The incidence of HF (14% vs. 12%; p=0.76), repeated revascularization (4% in each group), persistent angina (8% vs. 2%, p=0.16), death (2% in each group), MI (4% in each group), stent thrombosis (0% vs. 4%; p=0.15), and cerebrovascular accident (0% vs. 2%; p=0.32) was comparable between both groups (Figure 1).
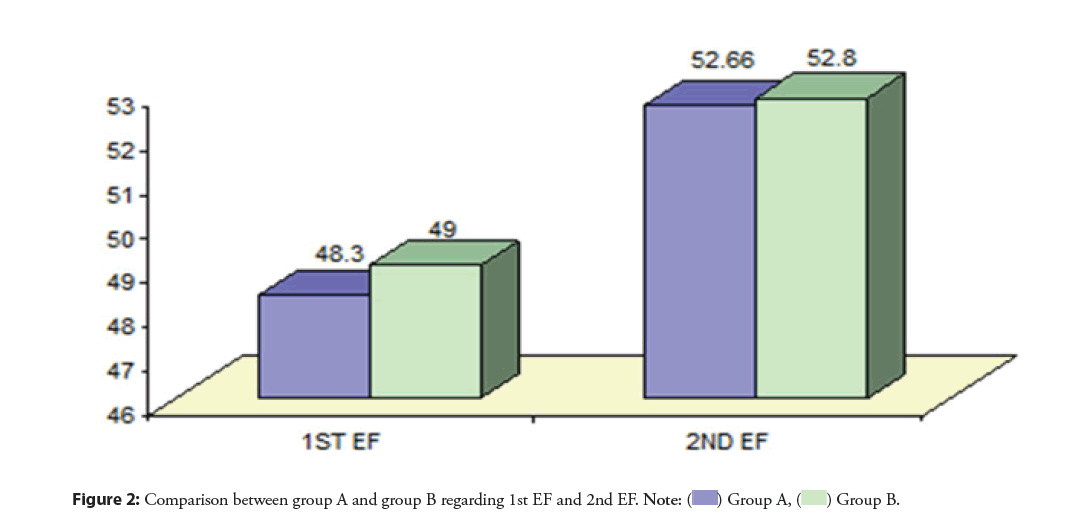
Before the PPCI, the mean Ejection Fraction (EF) was (48.30 ± 7.16 and 49 ± 7.82 for group A and B, respectively. Three months after the procedure, the mean EF increased significantly (p<0.001) in group A and B to reach 52.66 ± 5.95 and 52.8 ± 6.77, respectively. There was no statistically significant difference between group A and group B in terms of EF at the end of follow-up (p=0.92) (Figure 2).
Discussion
Clinical guidelines show controversy concerning the management approaches of the non-culprit lesions in STEMI patients with multivessel disease. The current available data demonstrates that multi-vessel PCI might be a safe alternative to culprit-only PCI and may provide comparable clinical benefits [5]. Nonetheless, only few reports directly compared the clinical outcomes of Ad-hoc complete revascularization vs. staged revascularization of STEMI patients with multivessel disease. The current clinical trial assessed the three months outcomes of complete coronary revascularization compared to staged revascularization in patients with multivessel disease undergoing PPCI.
Patients undergoing PPCI are prone to wide range of short-term complications, including mortality, re-stenosis, and need for revascularization [10]. The risk of such complications is progressively increased in patients with multivessel disease, leading to relatively worse prognosis and impaired quality of life of the affected patients [11]. Over the past few decades, a growing body of literature advocated revascularization of the non-culprit lesions, as they are biologically active, and demand approaches similar to those of unstable lesions [12]. More recently, the concept of “revascularization of the non-culprit lesions” gained momentum with the publications of major trials that demonstrated superior clinical outcomes of PCI for non-culprit lesions over PCI for culprit lesions only [13], which led the international guidelines to recommend multivessel PCI for hemodynamic stable patients [14]. However, the literature showed significant discrepancy regarding the superiority of one-time complete PCI or staged PCI in terms of short- and long-term clinical outcomes. In the present study, we found that complete Ad-hoc and staged revascularization 278had comparable short-term outcomes, with similar incidence of MACE. Such findings run in line with Saad et al., who found that staged and complete revascularization were comparable in term of 1-year MACE amongst patients with multivessel disease [15]. In another clinical trial, Politi et al., found that staged and complete revascularization had similar rates of short-term MACE [16]. These findings were consistent with the results from Tarasov, et al. [17]. However, significant controversy remains. On the contrary to our findings, a 2017 meta-analysis by Li et al., found that one-time PCI was associated with greater risk of mortality than staged revascularization [18]. This was similar to another meta-analysis by Bainey, et al. [19]. Thus,-given the current controversy in the published literature- further trials with multi-center collaboration are required.
Complete revascularization of the non-culprit lesions at the time of PPCI is advocated owing to the potential advantage of reducing the risk of early recurrence of infarction amongst patients with multivessel disease, who are highly vulnerable to recurrent ischemia in this stage. Besides, reducing the risk of vascular complications through complete revascularization at the time of PPCI carries economic benefits and minimizes healthcare expenditure [18]. However, concomitant PCI of the non-culprit lesions at the index procedure may increase the pro-thrombotic inflammatory status encountered during early STEMI and, hence, predispose to stent thrombosis and acute LV dysfunction [20]. It is also hypothesized that concomitant PCI increases the contrast dose, with subsequent higher risk of contrast-induced nephropathy [21]. On contrary, performing staged revascularization at more stable condition can lead to lower pro-thrombotic status. Besides, it was previously noted that operators usually overestimate the severity of non-culprit lesions at the time of index procedure due to spasms or endothelial dysfunction [22]. Notably, in our study, there was a numerically higher rate of persistent angina in staged revascularization group than the complete group. We hypothesized that this finding stem from the fact the nearly one-third of the patients in the staged group had three-vessel disease, with higher burden of myocardial ischemia [23]. Thus, it is advisable to select the timing of revascularization of the non-culprit lesions (Ad-hoc vs. staged) according to the readiness of the operating team, patient’s status, and lesion characteristics.
Despite that the current body of evidence largely favors revascularization of non-culprit lesions in STEMI patients over medical therapy only, there is an ongoing controversy regarding the timing of PCI of the non-culprit lesions [5]. According to a recent survey, the majority of interventional cardiologists prefer later-on PCI over concomitant procedure during revascularization of the culprit lesion. Only less than one-fourth of the responders answered that they would perform PCI during hospitalization, while the rest of responders preferred a staged procedure after at least 15 days [24]. Another survey found a similar finding [25]. In the present study, we chose to perform the staged PCI within 30 days from hospital discharge. Such decision was based on the findings of the HORIZONS-AMI trial, in which favorable 1-year outcomes was more prominent in patients underwent staged PCI with a median of 30 days after hospital discharge [26]. However, the controversy regarding the optimal time is still ongoing, with the ESC guidelines recommending revascularization before hospital discharge [27]. Thus, future studies are needed to compare the short- and long-term outcomes between staged PCI before discharge and PCI at different time points after discharge.
The current study is one of few clinical trials that directly compared the clinical outcomes of complete Ad-hoc vs. staged revascularization of STEMI patients with multivessel disease. The strengths of the present study included proper randomization and concealment allocation of the included patients, which might have reduced the selection bias. Besides, all procedures were performed in the largest two centers in Egypt with experienced interventional cardiologists to reduce the impact of personal experience on the outcomes of both studied groups.
Study limitations
However, it should be noted that the present study has some limitations. The sample size was relatively small compared to similar clinical trials. Besides, we utilized an open-label design with an inherited limitation of performance and detection bias, particularly with subjective clinical endpoints -such as the need 279for repeated revascularization. None of the included patients was hemodynamically unstable or suffered from cardiogenic shock, as this was an exclusion criterion; thus, the generalization of the study’s findings to patients with cardiogenic shock is not adequate. Another important limitation is that we did not use Fractional Flow Reserve (FFR) to evaluate the lesions severity during PCI. In The DANAMI-3–PRIMULTI trial, FFR-guided revascularization was deemed useful in lowering the incidence of short-term MACE, particularly repeated revascularization [6]. In FLOWER-MI trial, FFR-guided revascularization led to lower rate of MACE [28].
Conclusion
Staged revascularization provided comparable short-term benefits to complete revascularization in STEMI patients with multivessel disease undergoing PPCI. The present trial demonstrated that complete revascularization was associated with a trend towards higher incidence of stent thrombosis and CVA than stage revascularization. Nonetheless, both techniques had comparable short-term outcomes in terms of major adverse events. Thus, the current international guidelines should consider complete revascularization during the index procedure for STEMI patients with multivessel disease. Nonetheless, the approach is still not supported by solid evidence and -given the current controversy in the published literature- further trials with multi-center collaboration are required.
References
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 60(16): 1581-98 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Gibler WB, Cannon CP, Blomkalns AL, et al. Practical implementation of the guidelines for unstable angina/non-ST-segment elevation myocardial infarction in the emergency department: A scientific statement from the American Heart Association Council on Clinical Cardiology (Subcommittee on Acute Cardiac Care), Council on Cardiovascular Nursing, and Quality of Care and Outcomes Research Interdisciplinary Working Group, in Collaboration With the Society of Chest Pain Centers. Circulation. 111(20): 2699-710 (2005).
[CrossRef] [Google Scholar] [PubMed]
- Keeley EC, Boura JA, Grines CL. Primary angioplasty vs. intravenous thrombolytic therapy for acute myocardial infarction: A quantitative review of 23 randomised trials. Lancet. 361(9351): 13-20 (2003).
[CrossRef] [Google Scholar] [PubMed]
- Sorajja P, Gersh BJ, Cox DA, et al. Impact of multivessel disease on reperfusion success and clinical outcomes in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Eur Heart J. 28(14): 1709-16 (2007).
[CrossRef] [Google Scholar] [PubMed]
- Di Serafino L, Magliulo F, Esposito G. Functionally complete coronary revascularisation in patients presenting with ST-elevation MI and multivessel coronary artery disease. Interv Cardiol. 16: e24 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Engstrøm T, Kelbæk H, Helqvist S, et al. Complete revascularisation vs. treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3—PRIMULTI): An open-label, randomised controlled trial. Lancet. 386(9994): 665-71 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 369(12): 1115-23 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Mehta SR, Wood DA, Storey RF, et al. Complete Revascularization with multivessel PCI for myocardial infarction. N Engl J Med. 381(15): 1411-21 (2019).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Declaration of Helsinki world medical association declaration of Helsinki. Bull World Health Organ. 79(4): 373-4 (2013).
- Nicolau JC, Marin-Neto JA, Giraldez RR, et al. A comparison of percutaneous coronary intervention and surgical revascularization after fibrinolysis for acute myocardial infarction. Insights from the InTIME-2 trial. Int J Cardiol. 116(3): 383-8 (2007).
[CrossRef] [Google Scholar] [PubMed]
- Park DW, Clare RM, Schulte PJ, et al. Extent, location, and clinical significance of non-infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA. 312(19): 2019-27 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Kato K, Yonetsu T, Kim SJ, et al. Non-culprit plaques in patients with acute coronary syndromes have more vulnerable features compared with those with non-acute coronary syndromes: A 3-vessel optical coherence tomography study. Circulation Cardiovascular imaging. 5(4): 433-40 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Ahmad Y, Howard JP, Arnold A, et al. Complete revascularization by percutaneous coronary intervention for patients with st-segment–elevation myocardial infarction and multivessel coronary artery disease: An updated meta-analysis of randomized trials. J Am Heart Assoc. 9(12): 15263 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI Focused Update on Primary Percutaneous Coronary Intervention for Patients With ST-Elevation Myocardial Infarction: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 133(11): 1135-47 (2016).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Saad M, Rashed A, El-Kilany W, et al. Preliminary report on the safety and efficacy of staged vs. complete revascularization in patients with multivessel disease at the time of primary percutaneous coronary intervention. Int J Angiol. 26(3): 143-7 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Politi L, Sgura F, Rossi R, et al. A randomised trial of target-vessel vs. multi-vessel revascularisation in ST-elevation myocardial infarction: Major adverse cardiac events during long-term follow-up. Heart. 96(9): 662-7 (2010).
[CrossRef] [Google Scholar] [PubMed]
- Tarasov RS. Six month results of randomized clinical trial: Multivessel stenting in primary percutaneous coronary intervention and staged revascularization for ST-elevation myocardial infarction patients with second generation drug eluting stents. Clinical Medicine Research. 3(5): 125 (2014).
- Li Z, Zhou Y, Xu Q, et al. Staged vs. one-time complete revascularization with percutaneous coronary intervention in STEMI patients with multivessel disease: A systematic review and meta-analysis. PLoS One. 12(1): (2017).
[CrossRef] [Google Scholar] [PubMed]
- Bainey KR, Mehta SR, Lai T, et al. Complete vs. culprit-only revascularization for patients with multivessel disease undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: A systematic review and meta-analysis. Am Heart J. 167(1): 1-14.e2 (2014).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Khattab AA, Abdel-Wahab M, Röther C, et al. Multi-vessel stenting during primary percutaneous coronary intervention for acute myocardial infarction. A single-center experience. Clin Res Cardiol. 97(1): 32-8 (2008).
[CrossRef] [Google Scholar] [PubMed]
- Mager A, Assa HV, Lev EI, et al. The ratio of contrast volume to glomerular filtration rate predicts outcomes after percutaneous coronary intervention for ST-segment elevation acute myocardial infarction. Catheter Cardiovasc Interv. 78(2): 198-201 (2011).
[CrossRef] [Google Scholar] [PubMed]
- Hanratty CG, Koyama Y, Rasmussen HH, et al. Exaggeration of non-culprit stenosis severity during acute myocardial infarction: Implications for immediate multivessel revascularization. J Am Coll Cardiol. 40(5): 911-6 (2002).
[CrossRef] [Google Scholar] [PubMed]
- Mohr FW, Morice M-C, Kappetein AP, et al. Coronary artery bypass graft surgery vs. percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 381(9867): 629-38 (2013).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Dangas GD, George JC, Weintraub W, et al. Timing of staged percutaneous coronary intervention in multivessel coronary artery disease. JACC Cardiovasc Interv. 3(10): 1096-9 (2010).
[CrossRef] [Google Scholar] [PubMed]
- Hannan EL, Samadashvili Z, Walford G, et al. Culprit vessel percutaneous coronary intervention vs. multivessel and staged percutaneous coronary intervention for ST-segment elevation myocardial infarction patients with multivessel disease. JACC Cardiovasc Interv. 3(1): 22-31 (2010).
[CrossRef] [Google Scholar] [PubMed]
- Kornowski R, Mehran R, Dangas G, et al. Prognostic impact of staged vs. “one-time” multivessel percutaneous intervention in acute myocardial infarction: Analysis from the HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trial. J Am Coll Cardiol. 58(7): 704-11 (2011).
[CrossRef] [Google Scholar] [PubMed]
- Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 39(2): 119-77 (2018).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Denormandie P, Simon T, Cayla G, et al. Compared outcomes of ST-segment-elevation myocardial infarction patients with multivessel disease treated with primary percutaneous coronary intervention and preserved fractional flow reserve of non-culprit lesions treated conservatively and of those with low fractional flow reserve managed invasively: Insights From the FLOWER-MI Trial. Circulation. 14(11): e011314 (2021).
[CrossRef] [Google Scholar] [PubMed]