Short Article - Interventional Cardiology (2011) Volume 3, Issue 1
Sideguard dedicated stent system for the treatment of coronary bifurcation artery lesions
- Corresponding Author:
- Karl E Hauptmann
Division of Cardiology, Hospital of the Brothers of Mercy, Trier, Germany
Tel: +49 651 208 2784
Fax: +49 651 208 2786
E-mail: ke.hauptmann@bk-trier.de
Abstract
Keywords
coronary artery bifurcation lesions,devices,management,Sideguard® system
Introduction
Coronary artery bifurcation (CAB) disease, which is associated with the development of atherosclerotic plaques and stenosis, is diagnosed if there is a 50% or greater diameter stenosis adjacent to (<5 mm), and/or at, the ostium of both a main vessel (MV) and a side branch (SB) with a reference vessel diameter of at least 2.2 mm [1,2]. CAB disease accounts for approximately 20% of all percutaneous coronary interventions (PCIs; ~2 million PCIs/year worldwide) [3]. CAB disease is considered technically challenging since it is one of the most complex lesions (type C of the American College of Cardiology/American Heart Association class) owing to its great anatomical variability. CAB anatomy can differ in vessel size (of both the MV and SB), lesion location, eccentricity, length, morphology and plaque distribution. Moreover, most of the CAB lesions (~60%) involve the SB, making the above anatomical variation more complex due to the extent of SB disease and the angle of the origin of the SB from the MV [4]. Recently, a widely used anatomical classification of CAB lesions was suggested by Medina et al. [5], providing easily memorizable information regarding the anatomy of the CAB lesion. However, the Medina classification is incomplete owing to failure to include many important anatomical features of a given CAB lesions, such as bifurcation angle or proximal healthy segment. Furthermore, the Medina classification divides the so-called ‘true bifurcation lesions’ (meaning that both the SB and MV ostia have disease) into three subgroups (1.1.1, 1.0.1 and 0.1.1). In contrast to the Medina classification, the Movahed CAB classification includes all of the important anatomical features of a given CAB lesion, including bifurcation angle and proximal healthy segment, and it is easy to memorize using suffixes added to the letter B, which stands for bifurcation [6–8]. Moreover, the Movahed classification categorizes the so-called true bifurcation lesions into one simple category called the B2 lesions (B for bifurcation, 2 meaning that both MV and SB ostia have disease) [6].
Percutaneous coronary intervention management of CAB disease is associated with a low procedural success rate and worse clinical outcome versus non-CAB lesions. However, CAB lesions are heterogeneous groups. Many CAB lesions are low-risk lesions and are not considered, as mentioned earlier, to be true bifurcation lesions. CAB lesions with only MV disease involvement (1M lesions based on the Movahed classification: 1 for only one branch, M meaning main branch) or CAB lesions with only SB ostium disease (1S lesions based on the Movahed classification: 1 for one branch, S meaning SB) are at very low risk for complications despite being called CAB. Therefore, only B2 lesions or so-called true bifurcation lesions are considered high-risk CAB and any complex techniques or the use of dedicated bifurcation stenting should be considered only in these lesions. Furthermore, the importance of bifurcation angle and the size of the proximal segment are other important features of a given B2 lesion, which need to be considered when using different techniques or dedicated bifurcation stents.
Initial results with balloon angioplasty were poor with a high risk of acute closure of the MV or SB and a high restenosis rate [9,10]. Recently, early studies using paclitaxel-eluting balloons (PEBs) demonstrated positive results [11]. However, PEBs cannot be proposed as a stand-alone therapy for de novo coronary lesions, since paclitaxel effectively inhibits cellular proliferation but does not reduce vascular recoil, which is a major component of post-balloon-only angioplasty restenosis [12]. The introduction of bare-metal stents (BMSs) resulted in more predictable outcomes and higher success rates; however, residual stenosis at the ostium of the SB and long-term restenosis remained problems [13–15]. The use of drug-eluting stents (DESs) provided a significant reduction in the risk of repeat target lesion revascularization (TLR) by eliminating restenosis and reintervention rates in the MV [16–18]. However, despite the use of DESs, PCI outcomes for CAB disease still remained suboptimal versus those for non-CAB lesions and this has been associated with a sensibly low procedural success rate (87–100% for the MV and 76–89% for the SB), high incidence of periprocedural complications, mid-term restenosis (21–57%) and mid- and long-term major adverse cardiac events (MACEs; 2.5–12%) mainly due to uncovered SB [16,19,20]. Studies using intravascular ultrasound (IVUS) have shown that significant stenosis at the SB ostium is an important predictor of SB restenosis, occlusion and subsequent TLR after CAB PCI.
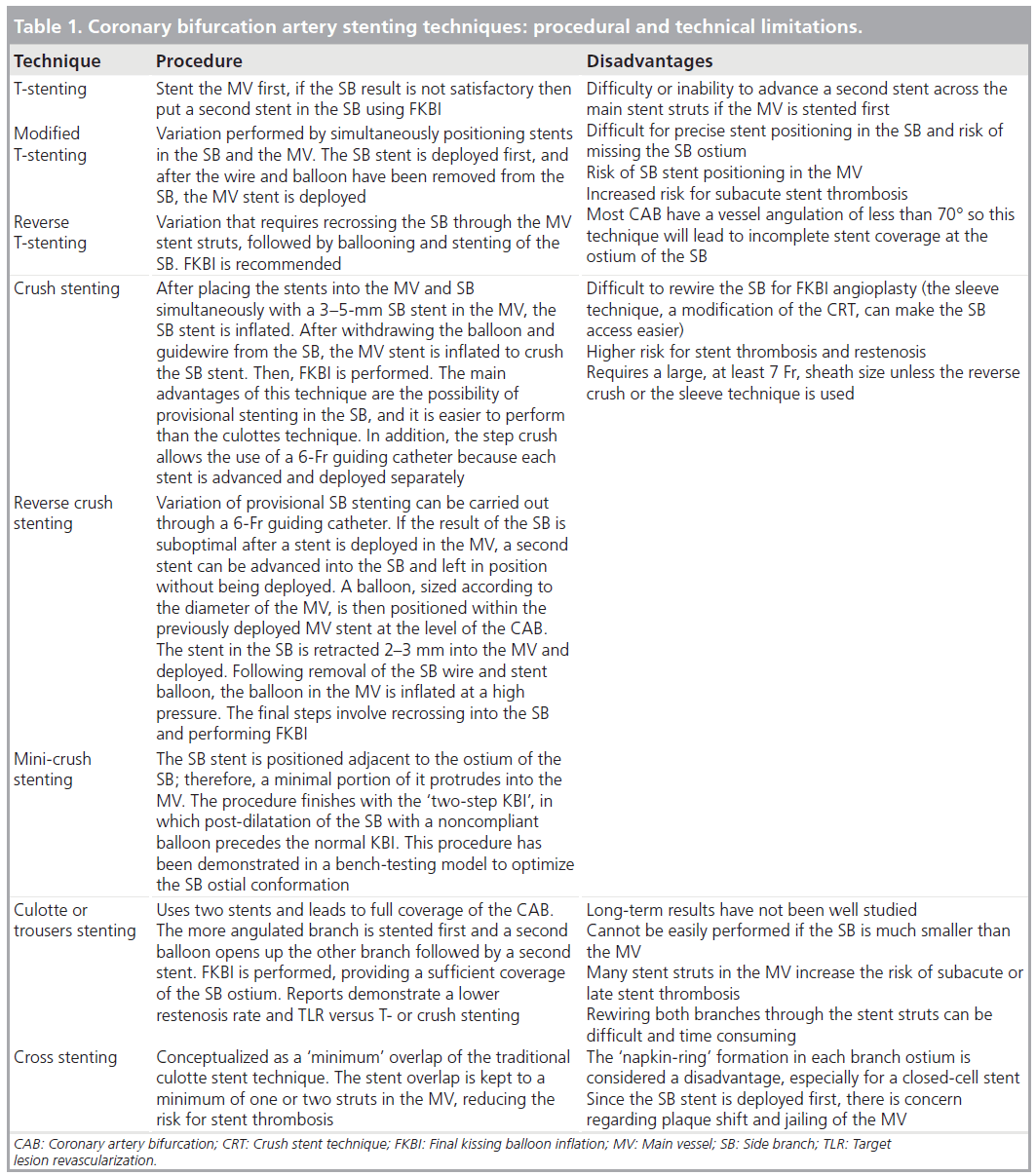
In an attempt to overcome the problem of CAB disease and to effectively cover the SB, improving angiographic and clinical outcomes has led to the introduction of several stenting techniques such as crush stenting, culotte stenting, V-stenting and T-stenting [21–23]. However, the above-mentioned complex techniques have procedural and technical limitations such as incomplete coverage of CAB, recrossing of deployed stent struts, SB jailing, failure to wire the SB through the stent struts, inability to pass the balloon through the stent struts, inability of stent structure to withstand balloon SB dilatation with restenosis at the SB ostium (one-stent approach), inability to scaffold the ostium of the SB, multiple layers of overlapping stents (two-stent approach) or incomplete stent-vessel wall apposition (Table 1) [24]. In addition, these techniques require operator skills and technical experience, and are thus technically challenging, time consuming, especially in order to achieve an optimal result, and are associated with increased x-ray and contrast-media exposure.
The above-mentioned limitations have increased the necessity to continue improving the search for effective coverage of the SB and, thus, the ideal treatment for the CAB lesions. Registries and randomized studies have shown that the MV stent-only approach (provisional stenting) is as safe and effective as the two-stent strategy, thus becoming the default treatment [3,16,25–28]. Data from the BMS era consistently demonstrated that one stent was superior to complex two-stent techniques (MACE rates: 26.7 vs 47.7%) [29], as when both the SB and MV were stented, the restenosis rate was nearly 62% [30]. Randomized studies using DESs have shown that the overall restenosis rate was higher for the two-stent versus one-stent approach (28 vs 18.7%) [16,17], irrespective of the type of DES used [31]. Recently, the Nordic collaborative group showed that there was no difference in clinical outcome between the two strategies and that the two-stent approach required significantly longer procedural and fluoroscopy times and a larger volume of contrast [3]. However, in the Nordic bifurcation trial (double stenting was achieved in 50% of cases with the crush technique, in 21% with the culotte and 29% with other techniques), SB restenosis was discovered in 11.5% of patients in the two-stent approach group and in 19.2% in the provisional stenting group (p = 0.062) [3]. MV stenting without SB protection was the most common approach utilized in the Cypher Sirolimus-Eluting Stent (STLLR) study, associated with an acceptable low incidence of adverse outcomes at 1 year [32]. In addition, the Coronary Bifurcations: Application of the Crushing Technique Using Sirolimus-Eluting Stents (CACTUS) trial failed to demonstrate the superiority of the two-stent approach. More recently, the British Bifurcation Coronary (BBC) study showed that a systematic two-stent technique resulted in higher rates of in-hospital and 9‑month MACEs, although the SB restenosis rate was similar between the two groups [33]. However, the BBC study population represented a mixed lesion subset of patients who had involvement of the small SB. Interestingly, the above-mentioned trials have major limitations, such as including all types of CAB lesions in their randomization including low-risk CAB lesions, such as 1M and 1S lesions. Randomized CAB trials involving only B2 true bifurcation lesions are needed in order to evaluate highrisk CAB intervention by using the one- or two-stent techniques.
Main vessel stenting with provisional SB stenting appears to be the first-line treatment for most CAB lesions. However, using the two-stent approach might be unavoidable depending on the individual anatomical characteristics, mainly of the SB [34]. A practical approach to CAB PCI can be summarized as follows:
▪▪Coronary artery bifurcations and guidewires: a SB guidewire could be jailed (leave the SB wire in place) depending on the operator’s preference and expertise. A jailed SB wire has the advantage of being able to be used as a marker if the SB needs to be rewired and it can change the bifurcation angle favorably [9]. On the other hand, wire detachment during removal or wire entanglement in the MV stent can lead to MACEs including the need for coronary bypass surgery. There is usually no need to remove the jailed guidewire during high-pressure stent dilatation in the MV. Instead, it has been suggested to limit the pressure to 8 atm when using a jailed wire [35]. In addition, it is preferable to avoid jailing hydrophilic guidewires as there is a risk of removing the polymer coating. Accurate handling of the guiding catheter to prevent migration into the ostium of the coronary vessel is important to allow the removal of a jailed guidewire [36].
▪▪ Main vessel provisional stenting should be the initial approach in the majority of CAB lesions. However, the strategy varies according to the size and the importance of the SB. Indeed, in cases of small and/or diffusely diseased SBs, the provisional strategy must try to keep the SB open at the end of the procedure. By contrast, in larger SBs, a strategy of recrossing the MV stent struts to perform SB balloon angioplasty and final kissing balloon inflation (FKBI) may be more appropriate, although with the risk of dissection. There is no consensus as to whether FKBI is mandatory when performing a provisional strategy. As a rule, if the SB is dilated or stented through the struts of the MV stent, a FKBI is required. Dilating through the side of the MV invariably distorts the MV’s stent architecture, dislocating the side of the stent contralateral to the SB and sacrificing stent lumen diameter [37,38]. By re-dilating the MV and SB stents simultaneously, these distortions in the MV architecture are reversed and lumen diameter is recovered. However, FKBI is difficult or impossible to perform after classical crush stenting, with a success rate of approximately 70–80% [39]. Therefore, the remaining 20–30% of patients are at a high risk of adverse outcomes after classical crush stenting.
▪▪ Treatment of a CAB lesion with two stents is performed mainly as a crossover from the provisional approach when a second stent is needed in the SB to treat a flow-limiting dissection or a suboptimal result. Using two stents as ‘intention-to-treat’ should be reserved for CAB stenosis with a SB that has a relatively large diameter (>2.5 mm) and territory of distribution and the following anatomical situations: disease in the SB that extends well beyond the ostium; if the SB has an unfavorable angle for recrossing after MB stent implantation; when the angle of the SB is such that rewiring is exceptionally difficult; and when the SB has a flow-limiting dissection after predilatation [3,40].
Overview of the market
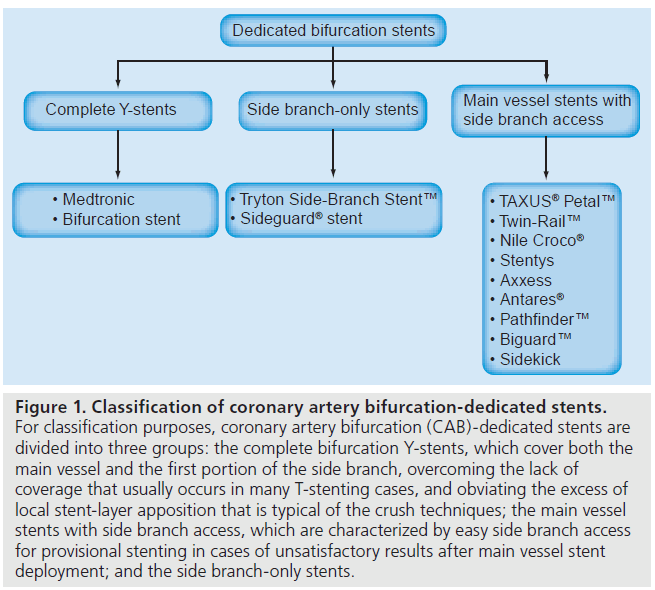
The SB ostium has become the focus of new treatment strategies because it is a common site of restenosis. As a result, novel dedicated devices have been specifically designed for CAB disease with the intention of addressing the above-mentioned shortcomings, facilitating the provisional approach and offering easy access to the SB [41]. The first generation of the dedicated CAB stents were difficult to deploy as they were stiff, thus, accurate positioning of the stent at the SB ostium was challenging. Many of these stents had larger crossing profiles and less flexibility compared with conventional stents, and were therefore difficult to deliver in tortuous or calcified arteries. Currently, most of the available CAB devices lack reliability owing to the absence of long follow-up clinical data. Other devices that were more recently marketed boast inclusion in ongoing first-in-man (FIM) registries. However, none of these stents have been approved in the USA [42]. It is hoped that the newer generation of CAB-dedicated stents will overcome these drawbacks. For classification purposes, CAB-dedicated stents can be divided into three groups (Figure 1 & Table 2) [41]:
Figure 1: Classification of coronary artery bifurcation-dedicated stents. For classification purposes, coronary artery bifurcation (CAB)-dedicated stents are divided into three groups: the complete bifurcation Y-stents, which cover both the main vessel and the first portion of the side branch, overcoming the lack of coverage that usually occurs in many T-stenting cases, and obviating the excess of local stent-layer apposition that is typical of the crush techniques; the main vessel stents with side branch access, which are characterized by easy side branch access for provisional stenting in cases of unsatisfactory results after main vessel stent deployment; and the side branch-only stents.
▪▪ Complete CAB Y-stents
▪▪ Main vessel stents with SB access
▪▪ Side branch-only stents
Introduction to the Cappella Sideguard® device
When considering the challenge of designing an easy-to-use technology for treating CAB disease, one comes to the conclusion that a one-size-fits-all approach is not reasonable. Cappella Medical Ltd. (Galway, Ireland) therefore decided to divide the treatment into two disease states: disease of the SB ostium and SB; and disease of the MV. Obviously, the SB treatment must comply and not excessively interfere with the treatment for the MV. Ostial coverage and MV interaction were considered crucial elements in the design and, for this reason, a flared self-expanding concept was adopted. The Cappella Sideguard® (SG) Coronary SB Stent and Delivery System was designed as a technology that can be used in conjunction with a MV stent in the treatment of CAB disease.
▪ Description of the stent
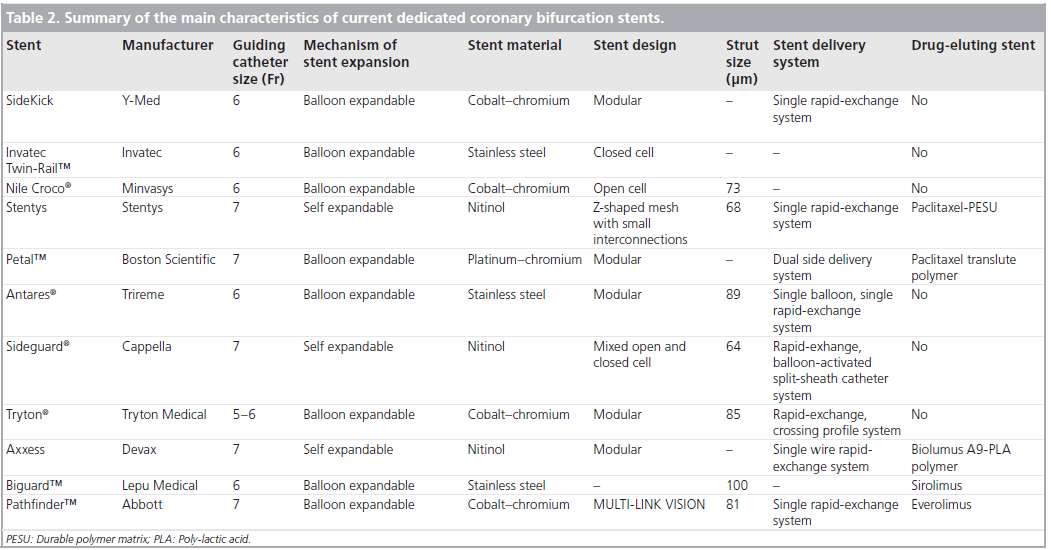
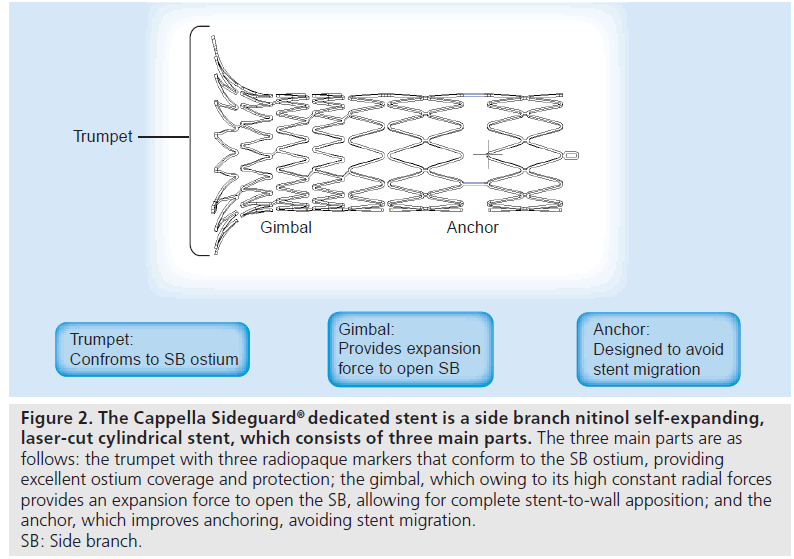
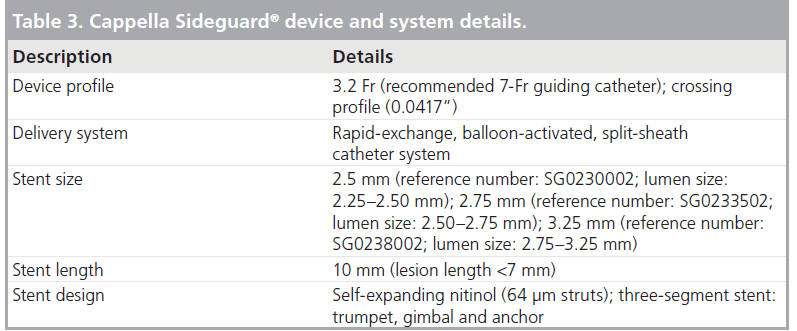
The SG stent is a SB nitinol self-expanding laser-cut cylindrical stent, which consists of three main parts: the trumpet, the gimbal and the anchor (Figure 2). The trumpet is the proximal flared end with three radiopaque markers and the trumpet-shaped design helps the stent to conform to the the 3D anatomy of the SB ostium, providing excellent ostium coverage and protection. The gimbal is the transition zone between the cup and anchor and owing to its high constant radial forces, provides expansion force to open the SB, allowing for complete stent-to-wall apposition. It also has high axial flexibility. The anchor is the main body of the stent. It is the distal ‘spacer’ region of the stent, designed to improve anchoring to avoid stent migration. A very thin strut of 64 μm is used in the SG design. The SG stent is available in 10-mm lengths, with diameters of 2.5, 2.75 and 3.25 mm (Table 3). The new SG device (SG-2) has undergone changes to the stent delivery systems and a minor change to the stent design. The SG-2 stent has a mixed open and closed cell design with a new mid-distal open cell that acts as a builtin anchoring system, preventing the SG from migrating following deployment.
Figure 2: The Cappella Sideguard® dedicated stent is a side branch nitinol self-expanding, laser-cut cylindrical stent, which consists of three main parts. The three main parts are as follows: the trumpet with three radiopaque markers that conform to the SB ostium, providing excellent ostium coverage and protection; the gimbal, which owing to its high constant radial forces provides an expansion force to open the SB, allowing for complete stent-to-wall apposition; and the anchor, which improves anchoring, avoiding stent migration. SB: Side branch.
▪ Description of the delivery system
The Cappella SG is delivered using a 7-Fr guidecompatible single-catheter delivery system that has no need for rotation. The delivery system is a rapid-exchange, balloon-delivery system composed of an interior tube that provides overall support for the device, an exterior sheath that retains the stent during positioning, minimizing device movement during deployment, and radiopaque marker bands that facilitate visibility and correct positioning of the stent at the SB ostium during stent placement and deployment. The inner lumen of the interior tube acts as the balloon inflation lumen. Deployment occurs by balloon expansion, which results in a controlled rupture of the sheath and release of the self-expanding device that utilizes a unique peel-away delivery system. The sheath remains proximally attached to the delivery system after rupture to allow safe withdrawal of the catheter delivery system. The distal end of the device has a central lumen that accommodates a 0.36-mm (0.014”) guidewire to allow rapid device exchange. The delivery system is inserted through an appropriately sized introducer sheath. Thus, the sheath prevents migration and allows accurate placement.
▪ Preparation & procedure
The procedure of positioning the Cappella SG SB stent consists of two main parts: the preparation process, and the delivering and deploying process (Figure 3A & B).
Figure 3: Preparation and procedure of positioning the Cappella Sideguard®. (A) Recommended algorithm for the use of the Cappella Sideguard (SG) dedicated stent and device. (B1) Predilated SB and MV with noncompliant balloons. (B2) Stent SB with Sideguard. The stent is deployed using a nominal pressure balloon, which helps tear a protective sheath that keeps the SG in place until deployment. Once released from the special balloon-release sheath technology, the SG self-expands into place. The delivery system and the guidewire are then removed from the SB. (B3) Stent the MV with a DES. The procedure is completed with FKBI, which is performed to move the struts of the MV stent into a more circular configuration surrounding the SB ostium. †Failure description: when inflation to 20 atm fails to open the ostium twice. DES: Drug-eluting stent; FKBI: Final kissing balloon inflation; MV: Main vessel; SB: Side branch.
Preparation process
The preparation process ensures that the superelastic properties of the nitinol SG stent will work optimally, but also alleviates the need for aggressive post-stenting dilation. Ostial SB lesions are often harder to prepare using conventional lesion preparation methods owing to the elastic nature of the ostium. More often, lesions involving the ostium of a SB will fail to yield to compliant balloon predilation, particularly when the lesions are fibrotic and/or calcified. As a result, the operator should be ready to use a more aggressive predilation strategy involving noncompliant balloons (step one), cutting balloons (step two) and, when all else fails, rotational atherectomy (step three) (Figure 3A).
Step one
Preparation involves noncompliant balloon predilation. We determined that the use of noncompliant balloons, sized 1:1 to the diameter of the SB, produce the best results. According to experts, it is important to ‘clear’ the area in and around the SB ostium in order to create a landing zone that is as free of plaque burden as possible. Cracking the lesion, and any coronary plaque located at the SB ostium, before the SG is deployed will ensure that the stent works optimally once deployed. Since it is sometimes difficult to determine coronary plaque characteristics angiographically, it is recommended to start with high-pressure dilation or, in some situations, to start with a smaller balloon (e.g., 1.5-mm diameter) to test the lesion’s compliance. The first predilation attempt should be to inflate the noncompliant balloon to greater than 20 atm for approximately 30 s [43]. Then, deflate the balloon and check with contrast to assess the lesion and vessel response. If the vessel has yielded to the predilation, it is proper to proceed to dilate the parent vessel. At this time it is also important to address any plaque that may have shifted distally into the SB before using the SG. It is recommended not to repeat the noncompliant balloon dilation a second time. Instead, move immediately to the cutting balloon. A sign that you are dealing with an unyielding fibrotic or calcified lesion is if the balloon begins to yield to the lesion instead.
Step two
In the preparation with cutting balloons, the strategy is to achieve a ‘controlled’ dissection. It is recommended to use a cutting balloon sized to the diameter of the SB (1:1) and a short balloon (6 mm). Shorter balloons are easier to work with and to use on higher-angled (>45°) CAB lesions. Another advantage of using a shorter cutting balloon is limiting the footprint of the atherotomes (cutting blades), which helps to control the amount of dissection created. It is suggested to start by positioning the cutting device so it straddles the ostium. Inflate the balloon to 8 atm and then incrementally increase the balloon pressure, as needed, up to 12 atm. Depending on the severity of the disease, it is recommended not to inflate the cutting balloon above 12 atm (the rated burst rate). Once you have managed to successfully cut/score the lesion around the ostium, relieving its hoop stress, repeat the steps proximally and distally to the ostium as required. Post-cutting balloon dilation (sequential or kissing) may be needed to address any remaining residual stenosis or dissection. If cutting balloons are not available, a scoring balloon can be used instead that can be inflated up to 14–16 atm.
Step three
Preparation with rotational atherectomy is implemented when all else fails, and rotational atherectomy can be the debulking approach of last resort. The goal is to debulk the lesion in order to increase vessel compliance. However, the downside to rotational atherectomy is that it is a time-consuming and costly process. In addition, there is an increased risk of intra-procedural events when using rotational atherectomy, including spasm, arrhythmia and slow/no reflow.
Delivering & deploying process
Radiopaque markers located at the distal and proximal ends of the SG delivery system facilitate positioning of the stent at the SB ostium. The stent is deployed using a nominal pressure balloon, which helps tear a protective sheath that keeps the SG in place until deployment. Once released from the special balloon-release sheath technology, the SG self-expands into place. The delivery system and the guidewire are then removed from the SB. Any conventional balloon-expandable MV stent is advanced over the MV guidewire, easily bypassing the SG’s trumpet-shaped tip, and deployed into place. The procedure is completed with FKBI, which is performed to move the struts of the MV stent into a more circular configuration surrounding the SB ostium.
▪ Clinical profile & postmarketing findings
The SG-I FIM study was a multicenter, prospective, nonblinded, nonrandomized trial enrolling 20 patients with de novo CAB lesions (medina CAB lesion types 1.1.1 in 56% of the patients, 1.0.1 and 0.1.1 in 32% of the patients and 1.1.0, 1.0.0 and 0.1.0 in 12%) in native coronary arteries. Additional anatomic criteria included:
▪▪ Main vessel with a reference diameter (RD) of 2.5–3.5 mm and a lesion 25 mm or less in length (visual estimate)
▪▪ Side branch RD of 2.2–2.8 mm and a lesion 7 mm or smaller in length (visual estimate)
▪▪ Coronary artery bifurcation angle of 50° or more before placement
▪▪ A lesion that could be covered with one SG stent in the SB and one sirolimus-eluting stent (SES) in the MV
Patients with recent myocardial infarction (MI; <72 h), a left main coronary artery lesion or total occlusion were excluded. All patients were clinically followed-up at 30 days and 6 months after intervention. Complete serial (after intervention and 6‑month follow-up) IVUS images were available in 11 patients. Patients were treated with predilation of the SB, SG stenting in the SB, SES stenting in the MV and FKBI. The SG stent area at the SB carina increased from 3.9 ± 1.2 mm2 to 4.6 ± 1.1 mm2 (p = 0.04), resulting in no change in lumen area (3.9 ± 1.3 mm2 vs 4.0 ± 1.3 mm2; p = 0.77) despite an intimal hyperplasia (IH) area of 0.6 ± 0.7 mm2. Six patients had minimal IH. The increase in stent area translated into an increase in lumen area of 0.4 ± 0.6 mm2. Five of the patients had relatively more IH (1.3 ± 0.4 mm2) than the other six, but the increase in stent area of 1.3 ± 0.3 mm2 compensated for the IH, resulting in no lumen decrease. By contrast, the MV stent area at the carina did not change (5.9 ± 1.1 mm2 vs 6.0 ± 1.2 mm2; p = 0.35). The serial IVUS analyses of the self-expanding nitinol SG stent indicated preserved SB ostial lumen dimensions at follow-up due to additional increases in stent area that more than compensated for IH [44]. At 6‑month follow-up technical success was achieved in 80%, which was combined with an acceptable safety profile with a MACE rate of 12.5%, a MI rate of 6.3% and a TLR rate of 12.5%, and there were no cases of stent thrombosis [45]. However, there are several limitations of this study:
▪▪ The sample was relatively small;
▪▪ The study included only patients with complete serial IVUS images (11 out of 20 or 55% of the entire SG-I FIM study), raising the possibility of selection bias;
▪▪ The study lacks preintervention IVUS analysis, hence evaluation of original lesion severities was not available;
▪▪ Only 6‑month follow-up was performed;
▪▪ There are few IVUS data on changes in this or any other self-expanding stent beyond 6 months;
▪▪ It is sometimes difficult to place an IVUS catheter perfectly coaxial to the vessel at a CAB site, thus affect serial comparisons.
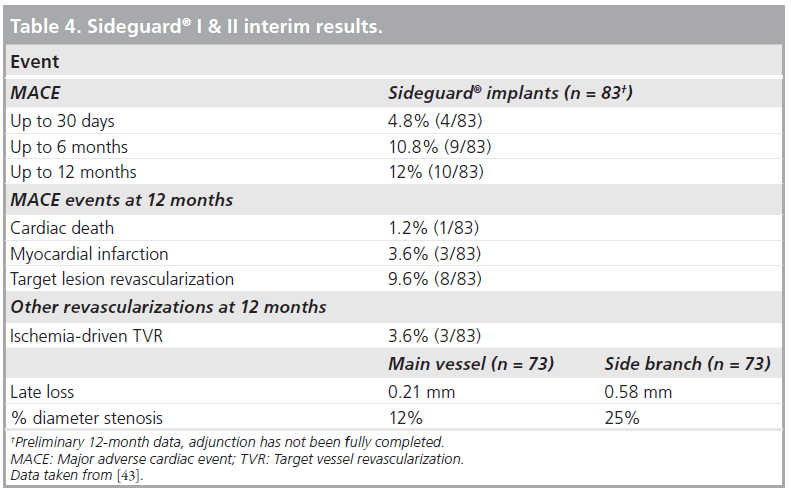
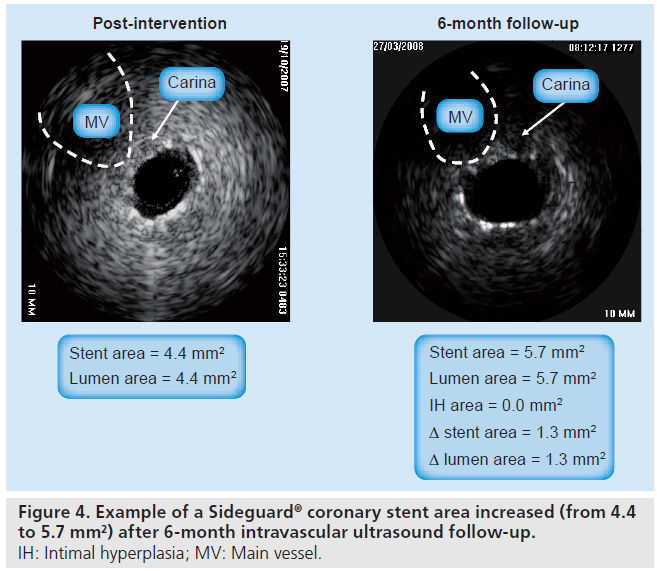
In order to overcome the above-mentioned limitations, the SG-II multicenter nonrandomized trial proceeded with the next-generation SG device. The new device has undergone changes to the stent delivery system and a minor change to the stent design. The SG-2 stent has a mixed open and closed cell design with a new middistal open cell. The SG-I and -II trial enrolled 83 patients with significantly diseased coronary arteries. Almost all patients (96%) had vascular disease involving the SB. Clinical and angiographic follow-up at 12 months was available from 83 (100%) and 73 (87.9%) patients, respectively (Table 4). The use of the SG dedicated stent resulted in a high procedure success rate (96.7%) with a low all-cause cumulative 12‑month MACE rate (12%). This was composed of a cardiac death rate of 1.2%, a MI rate of 3.6% and a TLR rate of 9.6%. The combined binary restenosis rate at 6 months was 20%, including stent thrombosis in one patient. However, this patient was in hospital for monitoring of a periprocedural dissection, which resulted in revascularization of the SB. By quantitative coronary analysis, the restenosis rate at the MV and SB ostium was found to be 12 and 25%, respectively (Table 4) [43]. The IVUS analysis showed marked suppression of neointimal hyperplasia within the stent and a significant increase in lumen volume over time within the SG stent (Figure 4). The SG investigators suggest that this is attributed to the nature of the self-expanding design of the stent, which undergoes constant expansion. In addition, there are several methodological strengths in this study that reinforce the validity of the results obtained, such as:
▪▪ Prospective enrollment
▪▪ A multicenter setting
▪▪ Total and independent monitoring of data acquisition
▪▪ Independent evaluation of the events
▪▪ Enrollment of one of the largest published cohorts of patients with CAB lesions
▪▪ The focus being on a SB stent specifically dedicated for CAB
The Cappella SG device has received CE mark approval in western Europe; however, it is not approved in the USA or Japan. Preparing to file for CE mark approval in Europe in Q4–08, the Cappella medical device company announced the start of SG-III trial. This is a prospective, multicenter single-arm trial. The primary objective of the study is to determine the change in stent area (mm2) and the corresponding vessel area change (mm2) at the carina of the SB by IVUS assessment at 6 months from the index SG stent implantation procedure. In addition, the following secondary end points will be assessed: device (delivery and deployment) success, lesion (residual diameter stenosis ≤30%) success, procedural success, angiography at 6 months, IVUS at 6 months, optical coherence tomography at 6 months for the evaluation of strut malapposition and completeness of endothelial tissue coverage of the stent struts, MACE at 6 months and stent thrombosis rate. Patients must meet all of the following (inclusion) criteria:
▪▪ Be above 18 years of age;
▪▪ Have significant ischemic heart disease;
▪▪ Undergo treatment of a single true (Movahed B2) bifurcation de novo lesion in a native vessel (Medina type 1.1.1, 1.0.1 and 0.1.1), MV lesion in a vessel with a RD of 2.8–4.0 mm and 30 mm or less in length and a SB lesion with a RD of 2.25–3.25 mm and 7 mm or less in length;
▪▪ Have a target CAB lesion that can be covered with one SG stent in the SB and one single stent in the MV;
▪▪ Have a target lesion with a CAB angle of 45o or more prior to wire placement;
▪▪The target lesion must be less than 100% occluded;
▪▪ Enrollment will occur when the Sideguard stent is introduced into the vasculature.
Enrollment will continue until there are 30 patients with good pre- and post-intervention baseline IVUS imaging of the MV and SB. A maximum of 45 patients will be enrolled to satisfy the requirement.
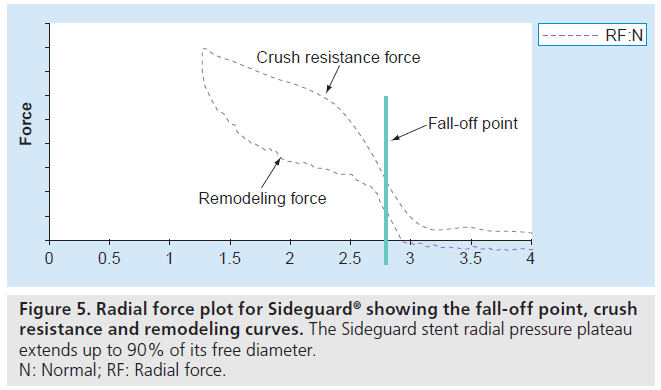
The results from the initial CE mark studies documented that the SG coronary SB Stent and Delivery System can be used in all true CAB lesions (Movahed B2 true bifurcation lesions) with CAB angles from 45 to 135° prior to wiring. The safety and efficacy data for the SG coronary SB stent and delivery system are remarkable even when compared with DES studies for treating CAB. Indeed, the self-expanding nature and the trumpet shape of the SG provide the stent with a margin of tolerance, which means that if the stent is not exactly positioned at the SB ostium, the SG will still conform to (or hug) the CAB’s anatomy. The proximal trumpet-shaped end of the stent can be flattened against the MV wall once the main stent is deployed. Also, the elastic property of nitinol enables the stent to self-expand so as to adapt to the various ostial morphologies, and the mechanical properties of nitinol sustain continuous wall apposition and scaffolding. The SG stent radial pressure plateau extends to up to 90% of its free diameter, and rapid fall off in radial pressure occurs after this point. Experimental in vivo data have demonstrated stent growing to approach this point within 30 days (Figure 5). The serial IVUS data showed that the SG stent approach reached up to 80% of its free diameter within 6 months [44]. The stent is thus able to dynamically oppose the full lumen of the SB, including the SB ostium. This dynamic apposition results in continuous contact between the stent and the vessel wall, as well as arterial expansion (positive remodeling) over time. The radiopaque markers located at the distal and proximal ends of the SG delivery system facilitate positioning of the stent at the SB ostium. The stent architecture provides a smooth transition throughout the CAB segment, most importantly, at the carinc importantly, at the carina, and there is less than a millimeter of stent strut overlap in the MV.
Another feature of the SG stent is its thin strut (64 μm) [46]. In the Intracoronary Stenting and Angiographic Results – Strut Thickness Effect on Restenosis Outcome (ISAR-STEREO) trials 1 and 2, it was reported that the use of a stent with a thinner strut is associated with a significant decrease in angiographic restenosis after coronary artery stenting [47,48]. It was demonstrated that stent strut thickness appears to be an independent predictor of restenosis after stent implantation in small coronary arteries. Indeed, strut thickness influenced restenosis in the group with a RD from 2.76 to 2.99 mm, with a restenosis rates of 23.5% in the thin-strut group versus 37% in the thick-strut group. Furthermore, the amount of IH was less with the SG stent than the radius stent (3.0 ± 1.7mm2 vs 0.8 ± 0.6 mm2), and the radius stent was 0.11 mm thicker than the SG stent [48]. Thus, we can speculate that the thin strut of the SG stent may be one of the reasons for the small amount of IH formation (0.8 ± 0.6 mm2) in this study [48]. Therefore, thin struts are characterized by limited blood f low perturbance, improved strut-to-vessel nesting, added flexibility and conformability, a lower profile, better deliverability, improved and faster endothelialization and, thus, improved clinical outcome.
Furthermore, proprietary split-sheath technology of the SG device ensures precise deployment. Placing and deploying the device is easy because SG is delivered independently from the MV stent, providing the operator the freedom to stent MVs without compromising access to the SB and without many of the problems associated with deploying multiple stents simultaneously (Figure 3B). This eliminates the risk of wire twisting (braiding) and allows the preferred stent of choice to be used for the MV. Although the deliverability of the original devices was poor, it was improved significantly in the new-generation device, as confirmed by the high level of procedural success (96.7%). Therefore, the SG can be accurately and easily positioned at the SB ostium over one wire and, once deployed, any conventional coronary stent can be positioned and deployed in the MV. Finally, the learning curve associated with SG deployment is relatively short.
Conclusion
Coronary artery bifurcation disease is considered technically challenging since it is one of the most complex lesions owing to great anatomical variability. Provisional stenting of the MV is the treatment of choice. However, despite the use of DESs and different techniques, CAB disease continues to be a challenging lesion subset. It seems that dedicated stent technology for CAB lesions in combination with site-specific drug-delivery systems offers excellent outcomes. The Cappella SG coronary system is the first self-expanding dedicated SB stent that approaches CAB disease in a compartmentalized way, allowing the independent treatment of each limb. The results from the SG-I and -II studies documented that the SG coronary SB stent and delivery system can be used in almost all true CAB lesions in a safe and technically feasible way, with very promising clinical, angiographic and IVUS short- and long-term follow-up end points.
Future perspective
Over the last 15 years, the management of CAB lesions has become a field of interest for interventional cardiologists. The management of this very challenging complex lesion subset is still far from a satisfying solution. DES employment is mandatory, and dedicated DESs, when they are available with more consistent clinical data and an easy-to-use approach that is still currently lacking, will be the solution. The Cappella SG system offers a solution that focuses on treating the SB first, rather than the MV. Thus, it is expected that in the future, the SG device may have potentially important applications in CAB lesions, mainly owing to its trumpetshaped design, its high-constant radial force, its distal anchoring design and its low profile. Furthermore, the SG-III study is intended to provide further certainty regarding those findings. Finally, it will be interesting to estimate the safety and efficacy of the SG device in distal left main disease with nonrandomized studies. Therefore, based on rapid advancement in CAB-dedicated stent technology, we will have better treatment options for CAB intervention in the future.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Coronary bifurcation lesions: the problem
▪▪ Coronary artery bifurcation (CAB) lesion management is still a controversial issue in the catheterization laboratory owing to a higher technical challenge and worse outcome when compared with standard interventions. In the last 15 years, many techniques and devices have been developed in order to accomplish comparable results with other lesion subsets; however, results seem conflicting.
CAB lesions: current treatment
▪▪ Main vessel stenting with provisional side branch stenting appears to be the first-line treatment for most CAB lesions. However, double-vessel stenting might be unavoidable depending on individual anatomical characteristics, mainly of the side branch.
Dedicated devices & related problems
▪▪ Manufacturers have recently developed a large number of dedicated devices with the aim of simplifying the procedure and ameliorating adverse outcome at medium- and long-term follow-up. However, many of these devices have been abandoned owing to failure in reaching these goals, while others still lack convincing clinical evidence.
Cappella Sideguard® dedicated device
▪▪ The Cappella Sideguard (SG) dedicated device is the first system that approaches CAB disease in a compartmentalized way, allowing the operator to treat each limb independently, offering a solution that focuses on treating the side branch first, rather than the main vessel, in a manner that is superior to provisional stenting.
Cappella Sideguard dedicated device & clinical data
▪▪ Data from the SG-I and -II trials showed that Cappella’s nitinol SG stent provides unmatched scaffolding of the entire CAB segment, excellent strut wall apposition, low injury deployment and minimum metal overlap in the main vessel. Intravascular ultrasound analysis showed marked suppression of neointimal hyperplasia within the stent and a significant increase in lumen volume over time within the SG stent.
Cappella Sideguard dedicated device & future perspective
▪▪ Preparing to file for CE mark approval in Europe in Q4 2008, the Cappella medical device company announced the start of the SG-III study to evaluate the vascular response to the SG coronary side branch stent and delivery system in de novo CAB lesions. The primary objective of the study is to determine the change in coronary stent area and corresponding change in vessel area at the carina of the side branch during the 6‑month follow-up period. Finally, it will be interesting to estimate the safety and efficacy of the SG device in distal left main disease with nonrandomized studies. Therefore, based on rapid advancement in CAB-dedicated stent technology, we will have better treatment options for CAB intervention in the future.
References
Papers of special note have been highlighted as
▪ of interest
▪▪ of considerable interest
- Lefevre T, Louvard Y, Morice MC, Loubeyre C, Piechaud JF, Dumas P: Stenting of bifurcation lesions: a rational approach. J. Interv. Cardiol. 14(6), 573–585 (2001).
- Louvard Y, Thomas M, Dzavik V et al.: Classification of coronary artery bifurcation lesions and treatments: time for a consensus! Catheter Cardiovasc. Interv. 71(2), 175–183(2008).
- Steigen TK, Maeng M, Wiseth R et al.: Randomized study on simple versus complex stenting of coronary artery bifurcation lesions: the Nordic bifurcation study. Circulation 114(18), 1955–1961 (2006).
- Leon MB: The spectrum of dedicated stents for bifurcation lesions: current status and future projections. Presented at: Angioplasty Summit TCT Asia-Pacific. Seoul, Korea,25–27 April 2007.
- Medina A, Suarez de Lezo J, Pan M: [A new classification of coronary bifurcation lesions]. Rev. Esp. Cardiol. 59(2), 183 (2006).
- Movahed MR, Stinis CT: A new proposed simplified classification of coronary artery bifurcation lesions and bifurcation interventional techniques. J. Invasive Cardiol. 18(5), 199–204 (2006).
- Movahed MR: B2 lesions are true bifurcation lesions simply categorized as one group according to the Movahed bifurcation classification. J. Invasive Cardiol. 22(5), 252 (2010).
- Movahed MR: Quantitative angiographic methods for bifurcation lesions: a consensus statement from the European Bifurcation Group. Shortcoming of the Medina classification as a preferred classification for coronary artery bifurcation lesions in comparison to the Movahed classification. Catheter Cardiovasc. Interv. 74(5), 817–818(2009).
- Weinstein JS, Baim DS, Sipperly ME, McCabe CH, Lorell BH: Salvage of branch vessels during bifurcation lesion angioplasty: acute and long-term follow-up. Cathet. Cardiovasc. Diagn. 22(1), 1–6(1991).
- Mathias DW, Mooney JF, Lange HW, Goldenberg IF, Gobel FL, Mooney MR: Frequency of success and complications of coronary angioplasty of a stenosis at the ostium of a branch vessel. Am. J. Cardiol. 67(6), 491–495 (1991).
- Fanggiday JC, Stella PR, Guyomi SH, Doevendans PA: Safety and efficacy of drug-eluting balloons in percutaneous treatment of bifurcation lesions: the DEBIUT (drug-eluting balloon in bifurcation Utrecht) registry. Catheter Cardiovasc. Interv. 71(5), 629–635 (2008).
- Zimarino M, De Caterina R: Drug-eluting balloons for percutaneous coronary interventions. Thromb. Haemost. 101(1), 9–11 (2009).
- Al Suwaidi J, Berger PB, Rihal CS et al.: Immediate and long-term outcome of intracoronary stent implantation fpor true bifurcation lesions. J. Am. Coll. Cardiol. 35(4), 929–936 (2000).
- Cervinka P, Stasek J, Pleskot M, Maly J: Treatment of coronary bifurcation lesions by stent implantation only in parent vessel and angioplasty in sidebranch: immediate and long-term outcome. J. Invasive Cardiol.14(12), 735–740 (2002).
- Assali AR, Teplitsky I, Hasdai D et al.: Coronary bifurcation lesions: to stent one branch or both? J. Invasive Cardiol. 16(9), 447–450 (2004).
- Colombo A, Moses JW, Morice MC et al.: Randomized study to evaluate sirolimus-eluting stents implanted at coronary bifurcation lesions. Circulation 109(10), 1244–1249 (2004).
- Pan M, de Lezo JS, Medina A et al.: Rapamycin-eluting stents for the treatment of bifurcated coronary lesions: a randomized comparison of a simple versus complex strategy. Am. Heart J. 148(5), 857–864 (2004).
- Sharma SK, Choudhury A, Lee J et al.: Simultaneous kissing stents (SKS) technique for treating bifurcation lesions in medium-to-large size coronary arteries. Am. J. Cardiol. 94(7), 913–917 (2004).
- Moussa I, Costa RA, Leon MB et al.: A prospective registry to evaluate sirolimus-eluting stents implanted at coronary bifurcation lesions using the ‘crush technique’. Am. J. Cardiol. 97(9), 1317–1321 (2006).
- Tanabe K, Hoye A, Lemos PA et al.: Restenosis rates following bifurcation stenting with sirolimus-eluting stents for de novo narrowings. Am. J. Cardiol. 94(1), 115–118 (2004).
- Colombo A, Stankovic G, Orlic D et al.: Modified T-stenting technique with crushing for bifurcation lesions: immediate results and 30-day outcome. Catheter Cardiovasc. Interv.60(2), 145–151 (2003).
- Ormiston JA, Webster MW, Webber B, Stewart JT, Ruygrok PN, Hatrick RI: The ‘crush’ technique for coronary artery bifurcation stenting: insights from micro-computed tomographic imaging of bench deployments. JACC Cardiovasc. Interv. 1(4), 351–357 (2008).
- Murasato Y, Hikichi Y, Horiuchi M: Examination of stent deformation and gap formation after complex stenting of left main coronary artery bifurcations using microfocus computed tomography. J. Interv. Cardiol. 22(2), 135–144 (2009).
- Ormiston JA, Webster MW, El Jack S et al.: Drug-eluting stents for coronary bifurcations: bench testing of provisional side-branch strategies. Catheter Cardiovasc. Interv. 67(1), 49–55 (2006).
- Brunel P, Lefevre T, Darremont O, Louvard Y: Provisional T-stenting and kissing balloon in the treatment of coronary bifurcation lesions: results of the French multicenter ‘TULIPE’ study. Catheter Cardiovasc. Interv. 68(1), 67–73(2006).
- Colombo A: Bifurcational lesions: searching the solution. Catheter Cardiovasc. Interv.65(1), 17–18 (2005).
- Colombo A: Contemporary treatment of coronary bifurcations with DES: part I. J. Interv. Cardiol. 18(5), 351–354 (2005).
- Ge L, Tsagalou E, Iakovou I et al.: In-hospital and nine-month outcome of treatment of coronary bifurcational lesions with sirolimus-eluting stent. Am. J. Cardiol. 95(6), 757–760 (2005).
- Al Suwaidi J, Yeh W, Cohen HA, Detre KM, Williams DO, Holmes DR Jr: Immediate and one-year outcome in patients with coronary bifurcation lesions in the modern era (NHLBI dynamic registry). Am. J. Cardiol. 87(10), 1139–1144 (2001).
- Yamashita T, Nishida T, Adamian MG et al.: Bifurcation lesions: two stents versus one stent – immediate and follow-up results. J. Am. Coll. Cardiol. 35(5), 1145–1151 (2000).
- Latib A, Cosgrave J, Godino C et al.: Sirolimus-eluting and paclitaxel-eluting stents for the treatment of coronary bifurcations. Am. Heart J. 156(4), 745–750 (2008).
- Suzuki N, Angiolillo DJ, Tannenbaum MA et al.: Strategies for drug-eluting stenttreatment of bifurcation coronary artery disease in the United States: insights from the e-Cypher S.T.L.L.R.trial. Catheter Cardiovasc. Interv. 73(7), 890–897 (2009).
- Hildick-Smith D, de Belder AJ, Cooter N et al.: Randomized trial of simple versuscomplex drug-eluting stenting for bifurcation lesions: the British Bifurcation Coronary study: old, new, and evolving strategies. Circulation 121(10), 1235–1243 (2010).
- Colombo A, Bramucci E, Sacca S et al.: Randomized study of the crush technique versus provisional side-branch stenting in true coronary bifurcations: the CACTUS (Coronary Bifurcations: Application of the Crushing Technique Using Sirolimus-Eluting Stents) study. Circulation 119(1), 71–78 (2009).
- Iakovou I, Ge L, Colombo A: Contemporary stent treatment of coronary bifurcations. J. Am. Coll. Cardiol. 46(8), 1446–1455(2005).
- Latib A, Colombo A: Bifurcation disease: what do we know, what should we do? JACC Cardiovasc. Interv. 1(3), 218–226 (2008).
- Ormiston JA, Webster MW, Ruygrok PN, Stewart JT, White HD, Scott DS: Stent deformation following simulated side-branch dilatation: a comparison of five stent designs. Catheter Cardiovasc. Interv. 47(2), 258–264(1999).
- Louvard Y, Lefevre T, Morice MC: Percutaneous coronary intervention for bifurcation coronary disease. Heart 90(6), 713–722 (2004).
- Ge L, Airoldi F, Iakovou I et al.: Clinical and angiographic outcome after implantation of drug-eluting stents in bifurcation lesions with the crush stent technique: importance of final kissing balloon post-dilation. J. Am. Coll. Cardiol. 46(4), 613–620 (2005).
- Tsuchida K, Colombo A, Lefevre T et al.: The clinical outcome of percutaneous treatment of bifurcation lesions in multivessel coronary artery disease with the sirolimus-eluting stent: insights from the Arterial Revascularization Therapies Study Part II (ARTS II). Eur. Heart J. 28(4), 433–442 (2007).
- Cortese B, Limbruno U: Coronary bifurcation lesions: innovative approaches and the future of bifurcation devices. Future Cardiol. 6(2), 221–230 (2010).
- Latib A, Colombo A, Sangiorgi GM: Bifurcation stenting: current strategies and new devices. Heart 95(6), 495–504 (2009).
- Hauptmann K: Bifurcation Management Using Cappella Technology. Euro-PCR, Paris, France(2010).
- Doi H, Maehara A, Mintz GS, Dani L, Leon MB, Grube E: Serial intravascular ultrasound analysis of bifurcation lesions treated using the novel self-expanding sideguard side branch stent. Am. J. Cardiol. 104(9), 1216–1221 (2009).
- Grube E, Wijns W, Schofer J et al.: FIM results of cappella Sideguard™ for treatment of coronary bifurcations. Presented at:Transcatheter Cardiovascular Therapeutics (TCT). Washington DC, USA, 20–25 October 2007.
- Kastrati A, Mehilli J, Dirschinger J et al.: Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation 103(23), 2816–2821 (2001).
- Pache J, Kastrati A, Mehilli J et al.: Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial. J. Am. Coll. Cardiol. 41(8), 1283–1288 (2003).
- Briguori C, Sarais C, Pagnotta P et al.: In-stent restenosis in small coronary arteries: impact of strut thickness. J. Am. Coll. Cardiol. 40(3), 403–409 (2002).
▪ Medina’s classification for the anatomy of bifurcation lesions.
▪ Provides information regarding the current management of bifurcation lesions.
▪ Interesting review of current coronary bifurcation-dedicated devices.