Research Article - Neuropsychiatry (2017) Volume 7, Issue 6
Social Network Cultivation and Diurnal Cortisol Profiles in Healthy Chinese Elders
- Corresponding Author:
- Dr. Julian Lai
Department of Applied Social Sciences
City University of Hong Kong
Tel: +852 34424306
Fax: +852 34420283
Abstract
Abstract
Objective: Psychobiological research on aging in humans is confounded by individual differences that remain to be characterized more precisely. The present study was designed to address this issue by examining the impact of Network Cultivation, a behavioral tendency to strengthen one’s social ties on diurnal cortisol profiles in healthy Chinese elders in Hong Kong.
Methods:Authors performed secondary analysis of data reported by Lai [1] using multilevel modelling. Seventy-eight healthy seniors provided saliva samples over two consecutive days at immediately, 3, 6, 9, and 12 hours after waking. Cortisol levels were assayed by using an enzyme-linked immunosorbent assay kit (ELISA) developed for use in saliva. Social network cultivation was measured with the Cultivation subscale of the Support Network Scale (SNS) that had been validated in prior studies with Chinese participants. The relationship between network cultivation and diurnal cortisol rhythms was examined using a mixed effect model.
Results:Cortisol levels declined significantly from waking to 12 hours thereafter and the rate of decline decreased significantly over time. In comparison to socially proactive participants, those having lower scores in Network Cultivation exhibited a higher diurnal cortisol level and a less efficient or flatter decline. The effect of Network Cultivation remained significant after controlling the influences of gender, age, waking time, and socioeconomic status.
Conclusions: An attenuated behavioural tendency in cultivating social ties in the elders is associated with a higher cortisol level and a flatter diurnal decline, which may increase susceptibility to agerelated diseases. Further research is warranted to uncover the psychophysiological mechanisms translating social network cultivation into altered functioning of the hypothalamic-pituitaryadrenocortical axis.
Keywords
Salivary cortisol, Diurnal decline, Network cultivation, Chinese elders
Introduction
The hypothalamic-pituitary-adrenocortical (HPA) axis is crucial for homeostatic and allostatic adjustments to internal and external challenges. However, how aging affects this neuroendocrine axis in humans is not completely understood [1,2]. The end-product of the HPA axis, cortisol has been studied extensively in relation to aging in last two decades. There is now evidence showing that aging is associated with a higher level of diurnal cortisol [3,4] whereas a lower diurnal output of cortisol is correlated with longevity. However, an accurate characterization of the diurnal cortisol profiles associated with aging or maladjustment in the elderly is still elusive. This could be attributed to weak correlation between age and cortisol levels and the moderation of the age-cortisol relationship by a number of psychological and behavioral factors, For instance, the positive correlation between age and 24-hour plasma free cortisol has been found to be moderate (r=0.37) [5]. Moreover, the relationship between aging and cortisol has been shown to be modulated by psychosocial (e.g., anxiety levels, [6]) and behavioral (e.g., sleep duration, [7]) factors in that elders with lower anxiety levels or sleeping longer exhibit attenuation in their cortisol outputs. As pointed out in prior studies conducted by the authors [8], some people may age more successfully than others such that they are able to remain biologically ‘younger’ than their peers.
In an original attempt to examine the associations of factors integral to the construct of successful aging with cortisol, the authors [1] found that Chinese elders who spend more time and effort cultivating their social relationships with family, relatives, friends, and neighbours (Network Cultivation) exhibit a more salient cortisol awakening response (CAR) and a steeper diurnal decline of salivary cortisol from 30 minutes to 12 hours after waking. The significance of this finding is twofold. First, this specific diurnal rhythm of cortisol, characterized by a more salient CAR and a steeper decline, has been observed in younger age groups [9] and in elders who are better adjusted [9] or cognitively more competent [11]. As it has been shown that cultivation of social ties predicts successful aging in a longitudinal study with 2,120 Chinese elders in Hong Kong [12], the aforementioned findings can be taken to imply that those who grow old more successfully may be biologically younger than their peers. Second, the measure of Network Cultivation (NC) was developed in an original attempt to address the limitations associated with the use of conventional measures of network size and support seeking to assess social support in the elderly [12]. The construct of Network Cultivation distinguishes itself from the concepts of network size and support by its proactive nature. It denotes a “proactive” behavioral tendency to maintain or strengthen existing networks so as to enhance resilience in old age. In this regard, the aged individuals are construed as agents proactively cultivating their social ties rather than passive receivers of support from available networks. This proactive feature may explain the prior finding that only Network Cultivation, but not network size and emotional support emerged as the significant predictor of diurnal cortisol rhythms in Chinese elders [1].
Despite the significance of findings reported by Lai [1], their impact is curtailed by a number of limitations which are worthwhile to further address given the novelty of the construct of Network Cultivation and its potentially beneficial effect on diurnal cortisol profiles in elders. The first issue is related to the method used by Lai [1], to analyze the association between Network Cultivation and diurnal decline. The effect of Network Cultivation on diurnal decline was revealed by dichotomizing the participants into two groups with high versus low in Network Cultivation at the median and comparing the diurnal slopes of cortisol between these two groups. Although this method has been used commonly in geriatric research [13], it precludes the analysis of nonlinear trends in diurnal decline, which have been shown to be a central feature of the diurnal cortisol rhythm in Chinese [14] and Western [15] populations. In addition, dichotomizing participants into two groups at the median may also obscure important differences between participants with average scores and those with either high or low scores on Network Cultivation.
The second issue concerns the use of conventional linear models such as RM-ANOVA in the analyses. As pointed out in a number of recent studies, RM-ANOVA is restricted to single-level analyses, and the assumptions of independence of observations and compound symmetry inherent to GLM rarely hold in reality [16,17]. It has been demonstrated that multilevel modelling of data (e.g., growth curve model) is a better alternative to RM-ANOVA, especially for repeated measures designs because it allows researchers to examine simultaneously within- and between-individual phenomena that contribute to change over time [18]. Moreover, multilevel modelling provides much more flexibility in examining situations characterized by missing data and more complex error structures [19], and is able to delineate quadratic changes in the diurnal cortisol cycle more accurately than RM-ANOVA (e.g.,[20]).
In response to the aforementioned issues and considerations, the association between Network Cultivation and diurnal cortisol profiles was reexamined by applying multilevel modelling to data collected from 78 community-dwelling elders from Hong Kong by Lai [1]. This secondary analysis is justified in view of a recent review that provides useful guidelines for saliva sampling in research on cortisol [21]. Moreover, secondary analysis in psychiatric research has become increasingly common [22-24] and has made substantial contributions to mental health research (e.g., [25]). We focused specifically on diurnal decline of cortisol to minimize the impact of non-compliance which is most relevant to the assessment of cortisol concentrations in the first hour post-awakening due to the volatile nature of this hormone during this period [21]. Moreover, compared to the cortisol awakening response, later circadian components of cortisol secretion are associated more with stable than transient factors, as suggested by Chellew [26]. Because Network Cultivation has been conceived as a relatively stable behavioral tendency in Chinese elders by Chong [12], focusing on the diurnal concentration of cortisol aligns with the objective of the present study and serves to improve the interpretability of findings.
Method
▪ Participants
The sample consisted of 78 Chinese elders recruited from community elderly centers in Hong Kong, with an equal number of men and women (mean age=73.09 yrs.). The ages of participants ranged from 62 yrs. to 86 yrs., with 91% older than 65 yrs. Participants were screened to ensure that they were not habitual smokers, had no prior or current diagnoses of psychiatric disorders, cancers, cardiovascular disorders, and were not currently on medications such as estrogen, synthetic glucocorticoids, antisteroid drugs, and antiseizure drugs that would potentially affect cortisol concentrations. The procedure was identical to that adopted in prior studies with Hong Kong Chinese [8,27] and was approved by the relevant approving body of the City University of Hong Kong. In particular, the researcher briefed participants in the first home visit, and provided them with a study pack containing instructions for saliva collection using saliva sampling tubes (Salivettes Sarstedt AG & Co., Nümbrecht, Germany), questionnaires, and a comprehensive description of the procedure of the study. Participants were instructed to collect one saliva sample using the Salivettes on each day for two consecutive days for 5 times at immediately, 3, 6, 9 and 12 hours after waking. They were also asked to refrain from eating, drinking anything other than water, smoking and brushing of teeth for at least one hour prior to saliva sampling. Saliva samples were stored in participants’ home freezers until the researcher came to collect them during the second home visit. Participants were asked to complete a scale assessing network cultivation and provide demographic information. The saliva samples were stored at −20°C in the laboratory until they were thawed for cortisol assays. To minimize seasonal effect on cortisol concentrations, data collection was completed within 3 months.
▪ Instruments
Network cultivation was measured by the Cultivation subscale of the Support Network Scale (SN-Cultivation Subscale, Chong [12]). This measure consisted of 4 items asking respondents how much time and efforts they invested in cultivating their relationships with (1) family members, (3) relatives, (3) friends, and (4) neighbours, respectively, using a 5-point scale: 1=not at all; 5=very much. A Network Cultivation score was computed by adding ratings of the 4 items. Scores on this scale thus range from the lowest of 4 to the highest of 20. The Cultivation scores of the present sample ranged from 4 to 18 with a mean of 10.49 (SD=2.94), which did not deviate substantially from that of a population-based Hong Kong Chinese sample with ages ranging from 40 yrs. to 74 yrs. (N=2970, mean=11.72, SD=2.76, Chong [12]). The mean scores of the tertiles from the lowest to the highest are 7.27, 10.46, and 13.73, respectively. This measure has been shown to have acceptable internal consistency in prior studies (e.g., Cronbach alpha=0.64, Chong [12]) and in the present sample (Cronbach alpha=0.70).
We assessed Socioeconomic status (SES) with a subjective measure used previously by Wright & Steptoe [28]. Participants were asked to indicate where they stood in society on a drawing of a ladder of ten rungs, with the top rung (10) representing people who are the best off and the bottom one (1) those who are the worst off. The mean of this measure was 5.68 (SD=2.32) for the present sample.
▪ Cortisol assays
Details of biochemical assays of cortisol have been reported by the authors in Lai [1]. Specifically, saliva samples were thawed and centrifuged at 3000 rpm for 15 min at room temperature. Clear supernatant was used for analysis. An enzymelinked immunosorbent assay kit (ELISA) (Salimetrics, LLC, State College, PA, USA) was used to determine cortisol concentrations. The assay sensitivity for the kit was 0.2 nmol/l. Intraassay and inter-assay coefficients of variation were 3% and 10%, respectively.
Statistical Analysis
Among the 84 participants who consented to take part in this study, 75 provided complete data on both Network Cultivation and cortisol at 0, 3, 6, 9, and 12 hours post-awakening. For the remaining 9 participants, those who had one or more missing cortisol values on both days were excluded. Those who had complete cortisol data on one day but with two or more missing values on the other day were also excluded. Participants who had missing value at the immediately postawakening sampling time on one of the two days were also excluded. As a result, 3 more participants were included, making the final sample 78. Application of the aforementioned inclusion criteria resulted in 6 missing cortisol values which were imputed by mean values.
Cortisol data from the 78 participants were transformed to reduce skewness. Extreme values were winsorized at two standard deviations to reduce the impact of outliers. At the low end, 0.2 nmol/l replaced values smaller than 0.2 nmol/l. A 4th root transformation was then applied to the final set of data before it was subject to further analyses using a mixed effect model with ML estimation (IBM SPSS 24). Distribution of residuals (skewness=-.11, SE=.09) was normalized by the 4th root transformation. Day and saliva sampling time were treated as repeated measures, with gender, age, waking time, and socioeconomic status as covariates. This method models decline of cortisol levels over the course of a day, determined the shape of change, and examined systematic differences in changes in terms of differences in Network Cultivation between participants. Unlike conventional analysis using RM-ANOVA, this method allows researchers to determine and adopt the most appropriate covariance structures.
Results
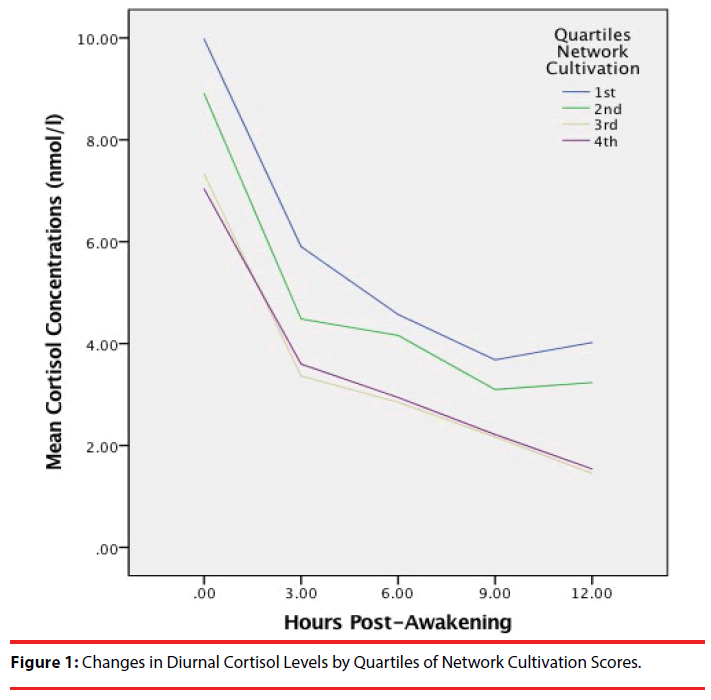
Table 1 summarizes cortisol concentrations over the course of a day in participants in each quartile defined by their Network Cultivation scores. Diurnal decline in cortisol concentrations was defined by cortisol levels at 0, 3, 6, 9, 12 hours post-awakening. Results summarized in Table 2 show that there was a significant linear decline of cortisol, but this was modulated by a significant quadratic growth such that the rate of decline decreased gradually over time. Network Cultivation was negatively associated with diurnal cortisol levels in that participants with lower scores in Network Cultivation exhibited a higher level in comparison to their peers with higher scores on the same measure. The crosslevel interaction between Network Cultivation and quadratic change in cortisol was also significant. As illustrated in Figure 1, the decline in cortisol level from immediately to 3 hours post-awakening among participants with lower scores in Network Cultivation (non-Cultivators) was similar to that in their peers scoring higher on Network Cultivation (Cultivators). However, the decline of cortisol from 3 to 12 hours post-awakening in non-Cultivators was flatter in comparison to that in the Cultivators. This pattern of findings suggests that a lower behavioral tendency in cultivating one’s social ties is associated with a higher diurnal cortisol level and a less efficient decline in cortisol, which may have important health implications. The difference in diurnal cortisol profile between non-Cultivators and Cultivators is similar to that between older people (mean age=73.2 yrs.) and that of a younger age group (mean age=26.7 yrs.) observed in a prior study using an intensive protocol of 24-hour blood sampling [29]. This can be taken to imply that a lower behavioral tendency in cultivating one’s social ties may accentuate the increase in basal activity and flattening of diurnal amplitude of cortisol that characterize an aging HPA axis.
| Saliva Sampling Times: Hours Post-Awakening | |||||
|---|---|---|---|---|---|
| Quartiles in Network Cultivation | 0 | 3 | 6 | 9 | 12 |
| First | 9.98 (.87) | 5.90 (.41) | 4.57 (.42) | 3.68 (.33) | 4.02 (.29) |
| Second | 8.91 (.67) | 4.48 (.53) | 4.16 (.38) | 3.10 (.32) | 3.23 (.32) |
| Third | 7.33 (.65) | 3.36 (.24) | 2.85 (.31) | 2.16 (.29) | 1.45 (.14) |
| Fourth | 7.04 (.65) | 3.60 (.27) | 2.94 (.24) | 2.21 (.27) | 1.54 (.10) |
Note: Means of Network Cultivation scores of the quartiles are 6.69, 9.46, 11.56, and 14.23, respectively.
Table 1: Means (SEMs) of Diurnal Cortisol Levels (nmol/l) Aggregated across Two Days in 78 Healthy Chinese Seniors.
| Network Cultivation and Diurnal Decline (+0, +3, +6, +9 & +12 hours post-awakening, N = 78) | ||||
|---|---|---|---|---|
| Fixed Effects | Coefficient | SE | t (df) | p |
| Average wakeup cortisol level: Intercept |
1.65 | .07 | 25.23 (241.63) | .000 |
| Day | -.02 | .03 | -.70 (458.90) | .482 |
| Linear decline of cortisol | -.08 | .02 | -4.99 (648.38) | .000 |
| Quadratic growth rate of cortisol | .004 | .0013 | 2.93 (648.20) | .003 |
| Day* linear decline | .006 | .011 | .55 (643.26) | .586 |
| Day* quadratic growth | -.0004 | .0009 | -.41 (647.39) | .679 |
| Network Cultivation | -.024 | .007 | -3.57 (171.35) | .000 |
| NC*linear growth rate of cortisol | .003 | .002 | 1.67 (660.18) | .095 |
| NC*quadratic growth rate of cortisol | -.0004 | .0002 | -2.39 (614.40) | .017 |
| Gendera | .01 | .031 | .31 (77.66) | .756 |
| Age | .0022 | .0025 | .88 (77.66) | .383 |
| Socioeconomic Status (SES) | .002 | .007 | .33 (77.66) | .739 |
| Waking Timeb | .02 | .01 | 1.79 (77.66) | .077 |
a, male = 1, female = 2. b, average waking times across the 2 days were used (mean = 05:32). Age, Network Cultivation, SES, and Waking Time were centered before the analysis.
Table 2: Multilevel Models of Cortisol Parameters associated with Diurnal Decline and Network Cultivation.
Discussion
Using multilevel modelling and a more appropriate operationalization of diurnal decline of cortisol, the present study has generated new information that supplements prior findings. Present findings not only demonstrate a significant linear diurnal decline in cortisol, but also reveal that this decline becomes flatter over time. This specific pattern of diurnal rhythm is consistent with findings reported in recent studies with samples from different cultures [10,14]. Present findings provide additional support to the advantages of using multilevel modelling in designs where repeated measurement of cortisol concentration over time is a central feature.
With respect to the impact of the cultivation of social ties, present findings also extend prior data [1] by demonstrating a significant effect of Network Cultivation on both the diurnal level of cortisol and the efficiency of decline. Using RMANOVA, Lai [1] found that the diurnal decline from 30 minutes to 12 hours post-awakening in the Cultivators was steeper than that in the non-Cultivators, but this difference was not observed when diurnal decline was defined more appropriately by change in cortisol levels from immediately to 12 hours post-awakening. The pattern of diurnal decline of cortisol in elders who are socially less proactive approximates that observed typically in maladjusted populations. These points to the possibility that a lower behavioral tendency in cultivating social ties may accentuate cortisol secretion and decelerate diurnal decline, which in turn promotes ill health and maladjustment in the elderly. Given that aging is associated with an elevated cortisol secretion (e.g., [30]) and the linkage between overproduction of this hormone and depression in the elderly (e.g., [31]), a lower motivation in network cultivation may exacerbate hypercortisolism in old age and leads to increased morbidity. Further research is warranted to illuminate the mechanisms whereby this specific psychosocial variable is translated into health outcomes in the aging populations.
In addition to the aforementioned implications, our findings also confirm the sensitivity of salivary cortisol to variation in positive psychosocial attributes such as cultivation of social ties and point to the utility of cortisol as a biomarker of adjustment in the relatively healthy. This assertion is supported by a growing body of evidence demonstrating the attenuating effects of positive psychosocial attributes on various components of the diurnal cortisol cycles (e.g., optimism, [27]; positive well-being, [32]; positive affect [33]). The significant effect of Network Cultivation on diurnal cortisol profiles reiterates the importance of proactivity in the social lives of older people, which has been demonstrated in Japanese seniors [34]. These prior data and present findings converge to provide support to the important health implications of proactive social motivations in old age.
Despite the significant implications of present findings, their impact is moderated by a number of limitations. The first issue is that adherence in participants has not been monitored by using proper electronic devices in the present study. This issue has been addressed recently in a number of studies using different methods to monitor waking and saliva sampling times [18,35]. As summarized in a recent review [21], the issue of compliance is more relevant to cortisol concentrations in the first hour post-awakening because of the volatile nature of cortisol levels during this period of time. With respect to the use of proper electronic devices to monitor saliva sampling, it has been pointed out that the use of this kind of devices may complicate the protocol for elderly participants and does not necessarily result in enhanced reliability [13]. Although we were not able to completely eliminate the influence of nonadherence, its effect has been minimized by focusing primarily on diurnal cortisol levels 3 hours after waking.
In addition to the aforementioned issue, the psychological mechanisms whereby Network Cultivation is translated into the observed neuroendocrine effect remain to be uncovered. The construct of Network Cultivation has been validated in relationship to variables integral to the concept of successful aging in a panel study with Chinese elders by Chong [12], but psychological or behavioral mechanisms have not been examined. A low tendency in network cultivation may limit social contacts and as a result, undermines the feeling of social embeddedness. In a similar vein, a lower tendency in cultivating social ties could accentuate loneliness, which has been shown to be an important factor exacerbating the agerelated decline in physiological resilience [36]. This has important policy implications as a better understanding of mediating mechanisms will inform the formulation and implementation of effective interventions to reduce ill health and/or promote proactive aging in the elderly. Increased attention to this issue in future geriatric research is warranted. Although our findings only point to an association between Network Cultivation and diurnal cortisol without any implications for causal connection, these data provide an empirical basis for further research to address the issue of causal connection using more vigorous designs.
Acknowledgement
Support of this study was provided by City University Research Grants (project numbers. 7002199 & 7004519). Parts of the data reported in this paper have been presented at the Annual Conference of the International Society of Psych neuroendocrinology, 8 - 11 September 2015, Edinburgh, Scotland.
References
- Lai JC, Chong AM, Siu OT, et al. Social network characteristics and salivary cortisol in health older people. Scientific. World. J (2012).
- Chahal HS, Drake WM. The endocrine system and aging. J. Pathol 211(2), 173-180 (2007).
- Ice GH. Factors influencing cortisol level and slope among community dwelling older adults in Minnesota. J. Cross. Cult. Gerontol 20(2), 91-108 (2005).
- Kumari M, Badrick E, Sacker A, et al. Identifying patterns in cortisol secretion in an older population: Findings from the Whitehall II study. Psychoneuroendocrinology 35(7), 1091-1099 (2010).
- Hartaigh B, Loerbroks A, Thomas GN, et al. Age-dependent and –independent associations between depression, anxiety, DHEAS, and cortisol: From the MIPH Industrial Cohort Studies (MICS). Psychoneuroendocrinology 37(7), 929-936 (2012).
- Rueggeberg R, Wrosch C, Miller GE. Sleep duration buffers cortisol increases in older adulthood. Psychoneuroendocrinology 37(7), 1029-1038 (2012).
- Lai JC, Chong AM, Siu OT, et al. Humor attenuates the cortisol awakening response in healthy older men. Biol. Psychol 84(2), 375-380 (2010).
- Heaney JL, Phillips AC, Carroll D. Ageing, depression, anxiety, social support and the diurnal rhythm and awakening response of salivary cortisol. Int. J. Psychophysiol 78(3), 201-208 (2010).
- Zilioli S, Imami L, Slatcher RB. Life satisfaction moderates the impact of socioeconomic status on diurnal cortisol slope. Psychoneuroendocrinology 60(1), 91-95 (2015).
- Evans P, Hucklebridge F, Loveday C, et al. The cortisol awakening response is related to executive function in older age. Int. J. Psychophysiol 84(2), 201-204 (2012).
- Chong AML, Cheung CK, Woo J, et al. Availability, use, and cultivation of support networks as predictors of the well-being of middle-aged and older Chinese: A panel study. Scientific. World. J (2012).
- Kraemer HC, Giese-Davis J, Yutis M, et al. Design decisions to optimize reliability of daytime cortisol slopes in an older population. Am. J. Geriatr. Psychiatry 14(4), 325-333 (2006).
- Hsiao FH, Yang TT, Ho RT, et al. The self-perceived symptom distress and health-related conditions associated with morning to evening diurnal cortisol patterns in outpatients with major depressive disorder. Psychoneuroendocrinology 35(4), 503-515 (2010).
- Vedhara K, Miles J, Bennett P, et al. An investigation into the relationship between salivary cortisol, stress, anxiety and depression. Biol. Psychol 62(2), 89–96 (2003).
- Blackwell E, de Leon CF, Miller GE. Applying mixed regression models to the analysis of repeated-measures data in psychosomatic medicine. Psychosom. Med 68(6), 870-878 (2006).
- Quené H, van den Bergh H. On multi-level modeling of data from repeated measures designs: a tutorial. Speech. Commun 43(1), 103-121 (2004).
- Smyth N, Thorn L, Hucklebridge F, et al. Detailed time course of the cortisol awakening response in healthy participants. Psychoneuroendocrinology 62(1), 200-203 (2015).
- Hayes AF. A primer on multilevel modeling. Human. Commun. Res 32(4), 385-410 (2006).
- Heck RH, Thomas SL, Tabata LN. Multilevel and longitudinal modelling with IBM SPSS. New York: Routledge, USA (2010).
- Stalder T, Kirschbaum C, Kudielka BM, et al. Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology 63(1), 414-432 (2016).
- Addolorato G, Leggio L, Ferrulli A, et al. Dose-response effect of Bacllfen in reducing daily alcohol intake in alcohol dependence: Secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol. Alcohol 46(3), 312-317 (2011).
- Hien DA, Kiang H, Campbell ANC, et al. Do treatment improvements in PTSD severity affect substance use outcomes? A secondary analysis from a randomized clinical trial in NIDA’s Clinical Trials Network. Am. J. Psychiatry 167(1), 95-101 (2010).
- Katz IR, Rupnow M, Kozma C, et al. Risperidone and falls in ambulatory nursing home residents with dementia and psychosis or agitation. Am. J. Geriatr. Psychiatry 12(5), 499-508 (2004).
- Cheng HG, Phillips MR. Secondary analysis of existing data: opportunities and implementation. Shanghai. Arch. Psychiatry 26(6), 371-375 (2014).
- Lai JC, Evans PD, Ng SH, et al. Optimism, positive affectivity, and salivary cortisol. Br. J. Health. Psychol 10(4), 467-484 (2005).
- Epel ES. Psychological and metabolic stress: A recipe for accelerated cellular aging? Hormones 8(1), 7-22 (2009).
- Wolkowitz OM, Epel ES, Reus VI, et al. Depression gets old fast: Do stress and depression accelerate cell aging? Depress. Anxiety 27(4), 327-338 (2010).
- Evans P, Forte D, Jacobs C, et al. Cortisol secretory activity in older people in relation to positive and negative well-being. Psychoneuroendocrinology 32(8-10), 922-930 (2007).
- Steptoe A, Gibson EL, Hamer M, et al. Neuroendocrine and cardiovascular correlates of positive affect measured by ecological momentary assessment and by questionnaire. Psychoneuroendocrinology 32(1), 56-64 (2007).
- Sugisawa H, Liang J, Liu X. Social networks, social support, and mortality among older people in Japan. J. Gerontol 49(1), S3-S13 (1994).
- Smyth N, Clow A, Thorn L, et al. Delays of 5 – 15 min between awakening and the start of saliva sampling matter in assessment of the cortisol awakening response. Psychoneuroendocrinology 38(9), 1476-1483 (2013).
- Hawkley LC, Cacioppo JT. Aging and loneliness: Downhill quickly? Curr. Direct. Psychol. Sci 16(4), 187-191 (2007).