Research Article - Clinical Investigation (2019) Volume 9, Issue 2
Soy isoflavones (Glycine max) attenuates bilateral ovariectomy (experimental menopause) induced alteration in the hepatic and renal metabolic functions in female Wistar rats
- Corresponding Author:
- Sankar P
Department of Biochemistry
Jawaharlal Institute of Postgraduate Medical Education and Research [JIPMER]
Puducherry, India
E-mail: sankarjipmer@gmail.com
Submitted Date: 01 March 2019; Accepted Date: 19 March 2019; Published Date: 25 March 2019
Abstract
The alarming increase in the rate of premature menopause has been reported in the in India. The reason for this early menopause is still unclear, but it is a major public health problem because of its influence on the development of components of metabolic syndrome. Abnormalities in hepatic and renal metabolic functions after menopause considered as a major trigger for the postmenopausal metabolic consequences. In the present study, we investigated the effects of surgical induced menopause on hepatic and renal functions and the beneficial effects of soy isoflavones on the same. Thirty-two female Wistar rats were divided into four different groups. Bilateral ovariectomy was done to create a model of experimental menopause. Those groups of rats received soy isoflavones, the dose of 150 mg/kg BW/day was given via drinking water for the 12 weeks. Bilateral ovariectomy in rats caused impaired liver and kidney functions as indicated by increased levels of plasma functional enzymes; Aspartate Transaminase (AST), Alanine Transaminase (ALT), Alkaline Phosphatase (ALP), Lactate Dehydrogenase (LDH), AST/ALT ratio, total protein, total bilirubin, plasma urea, creatinine, uric acid, blood urea nitrogen (BUN, BUN/creatinine ratio. Treatment with soy isoflavones significantly inhibited the ovariectomy-induced changes in these liver and renal parameters, suggesting the use of this natural product as an alternative remedy in the management of liver and kidney abnormalities associated with the postmenopausal women.
Keywords
Bilateral ovariectomy, Liver function, Kidney function, Soy isoflavones
Introduction
Menopause, an age-related loss of ovarian follicular activities and estrogen production. The natural menopause occurs between 45 and 55 years of age worldwide [1]. In India, it has been estimated that the mean age of menopause between 41.9 and 49.4 [2]. The public health impacts of postmenopausal complications are substantial since women spend virtually one-third of their lives in the postmenopausal state worldwide [3]. In the direction of reports of the third consensus meeting of the Indian Menopause Society, the number of postmenopausal women in India is approximately 43 million and it may reach 103 million by 2026 [4]. These observations highlight the importance of empathizing the molecular and cellular mechanisms that underlie the pathology of metabolic complications concomitant with the postmenopausal population.
Recently, concern has been expressed that rates of premature menopause (before age 40 years) are high in India and may be increasing in certain sections of the population [4]. It has been observed that nearly four percent of women of the age group of 29-34 years attain menopause and its incidence increases to eight percent in women of age group between 35 and 39 years [4,5]. The reason for this early menopause is still unclear, but it is a major public health problem due to its influence on the development of components of metabolic syndrome.
Postmenopausal state emerged as the major risk for several metabolic diseases and is associated with a wide spectrum of liver and kidney abnormalities, known as Nonalcoholic Fatty Liver Disease (NAFLD), characterized by an excessive accumulation of fat in the hepatocytes (i.e., hepatic steatosis) and Chronic Renal Disease (CKD) [6,7]. NAFLD and CKD becoming a major public health problem due to its high prevalence and increased risk of developing metabolic syndrome and its components: Obesity, insulin resistance, type 2 diabetes mellitus, hypertension, dyslipidemia and coronary heart diseases [7-9]. Furthermore, studies have documented that the postmenopausal women three times prone to develop NAFLD and CKD is twice common in the postmenopausal state than the premenopausal state, suggesting a protective role of estrogen against NAFLD, CKD and their metabolic consequences [8-10]. Though the pathophysiology of isolated NAFLD and CKD are well established in the literature; to the best of our knowledge, there is no clearly established evidence available regarding the molecular basis of development of liver and kidney metabolic abnormalities associated with the postmenopausal state.
Hormone Replacement Therapy (HRT), a present strategy widely used in clinical practice in the management of postmenopausal related symptoms. Though HRT improves menopausal symptoms, the prolonged use of HRT is not recommended due to its adverse effects in causing a certain type of cancers such as breast cancer and ovarian cancer in addition to its negative impact on cardiovascular risks [11,12]. Hence, there is no effective pharmacological strategy is available in order to manage postmenopausal complications by causing any unwanted deleterious effects. Thus, the search for effective therapeutic strategies to tackle this problem has seen a renewed interest. Various medicinal plants, containing phytoestrogens have been used to combat this problem. One such a medicinal natural phytoestrogen is soy isoflavones from Glycine max.
Soy isoflavones, a group of naturally occurring phytoestrogens shares the structural and functional homogeneity with estrogen. It has been believed that consumption of phytoestrogens rich diet could have protective effects against postmenopausal symptoms [13]. Numerous studies including epidemiological studies to human and animal experiments have also shown anti-oxidant, anti-inflammatory, anti-diabetic and hypolipidemic effects of soy isoflavones against metabolic and cardiovascular diseases [14-18]. Consumption of SIFs also improved dyslipidemia and hepatic lipid accumulation by modulating the expression of some of the proteins involved in lipid metabolism [19-22]. In addition, recently several studies have reported the beneficial effects of SIFs on the management of fatty liver diseases, obesity and its associated metabolic complications, such as oxidative stress, insulin resistance, inflammation, and lipid abnormalities. However, the molecular mechanisms by which SIFs exhibit its beneficial effects are not clear and the molecular effect of SIFs on the pathogenesis of these metabolic complications in the model of the postmenopausal state is unknown. The beneficial effects of soy isoflavones have been studied in an animal the model; however, its efficacy in ameliorating the liver and renal functions in the postmenopausal state is largely unclear. Hence, in the present study is aimed to study the beneficial effects of soy isoflavones on liver and kidney functions in experimental menopause.
Materials and Methods
Animals and ethical approval
Thirty-two (n=32) female Wistar albino rats weighing between 100 to 120 g aged 3 months were supplied by the central animal house of Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER, Puducherry, India 605006). Rats were housed in stainless steel cages in a wellventilated room with a 12-hour light/12 hour dark cycle at ambient temperature. Experiments were carried out in accordance with the guidelines for the Care and Use of laboratory animals (National Research Council, 1985) and the study was approved by the Institutional Animal Ethical Committee with JIP/IAEC/SC/1/2010-11 reference number for notice of approval.
Experimental groups
Thirty-two female rats were divided into four groups based on their body weight. The details of treatment for the different experimental groups as follows:
• Control: Sham-operated rats fed with normal chow for 12 weeks
• Control+SIF: Ovariectomy rats fed with normal chow for 12 weeks
• Ovariectomy: Sham-operated rats fed with normal chow for 12 weeks along with soy isoflavones (150 mg/kg BW/day) via drinking water
• Ovariectomy+SIF: Ovariectomy rats fed with normal chow for 12 weeks along with soy isoflavones (150 mg/kg BW/day) via drinking water
Surgical procedure for bilateral ovariectomy
Bilateral Ovariectomy (OVX), the removal of both the ovaries have been well recognized and widely used model of human menopause to study the reproductive changes as well as the biochemical basis for the development of metabolic and endocrine complications concomitant with the postmenopausal state [23-27]. Hence, we created an experimental model of menopause by subjecting the rats to bilateral ovariectomy.
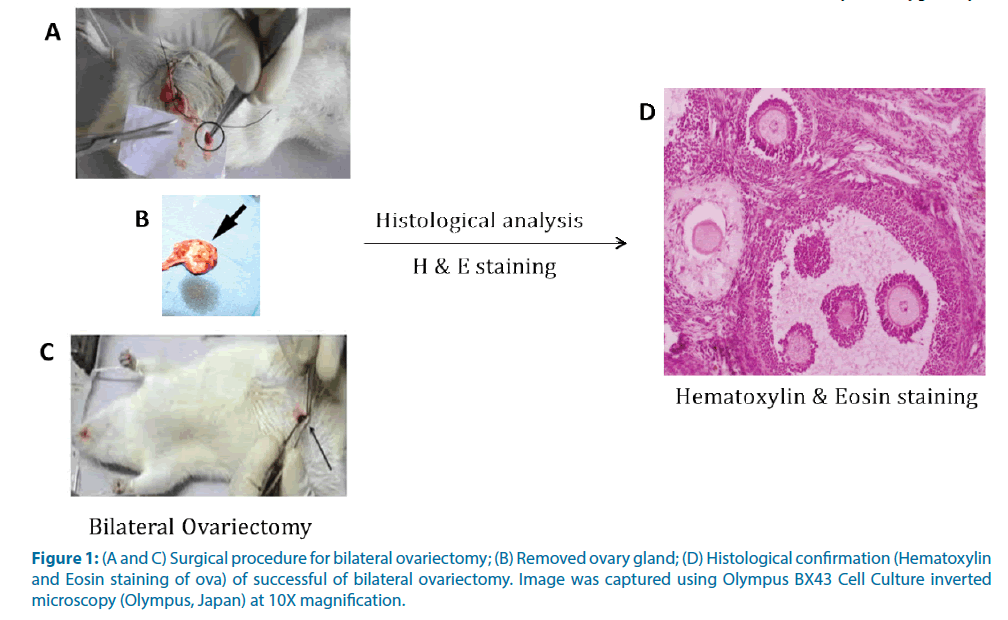
The animal was anesthetized under ketamine anesthesia (100 mg/Kg body weight). The ventral aspect of the animal was shaven and cleaned with 70% alcohol and then with sterile saline. A single midline 2-3 cm long incision was made under sterile conditions. The ovaries were located and excised. The incision was then sutured using aseptic techniques and wiped clean with sterile saline. Antibiotic cream was applied locally over the wound. The success of the bilateral ovariectomy was confirmed histologically (Figure 1). The control rats were sham operated in order to neutralize the surgical induced biochemical changes.
Figure 1: (A and C) Surgical procedure for bilateral ovariectomy; (B) Removed ovary gland; (D) Histological confirmation (Hematoxylin and Eosin staining of ova) of successful of bilateral ovariectomy. Image was captured using Olympus BX43 Cell Culture inverted microscopy (Olympus, Japan) at 10X magnification.
Methanolic extraction of soy isoflavones
Isoflavones were extracted from soybean hypocotyls according to the method described by Yoon-Bok Lee et al. [28]. The soybean hypocotyls were purchased from the local market (PAPSCO, Puducherry, India). The soybean hypocotyls were mixed with 10 volumes of 80% aqueous methanol and stirred for 4hrs at room temperature. The methanolic extract was then concentrated in a rotary evaporator at 50°C. The final step of the preparation involved freeze-drying the concentrated methanol extract.
In-vitro assessment of the anti-oxidant property of soy isoflavones extract
2,2’-dipicryl 2’-phenylhydrazine (DPPH) assay: The free radical scavenging capacity of soy isoflavones was assessed by 2,2’-dipicryl 2’-phenylhydrazine (DPPH) assay [29]. A standard graph was plotted using Gallic acid as a standard in the range of 0-100 μM/L concentration. It was found that the free radical scavenging capacity of soy isoflavones was 6.23 ± 1.04 μM/L equivalents of Gallic acid.
Route of soy isoflavones administration
In those groups of rats were isoflavones were administered, it was given mixed in drinking water at a dose of 150 mg/kg body weight/day for 12 weeks [28].
Blood sample collection and process
Fasting blood samples were collected under the deep diethyl ether anesthesia by retro-orbital vein at baseline (beginning of the study) and at the end of the study (twelve weeks). The blood samples were then centrifuged at 8,000 rpm for 10 minutes to separate the plasma. The plasma was stored at -80°C until assays were carried out. All tests were performed in accordance with the manufacturer’s instructions.
Assessment of liver functional parameters
The bio-makers of liver injury such as: Aspartate Transaminase (AST), Alanine Transaminase (ALT), Alkaline Phosphatase (ALP) and Lactate Dehydrogenase (LDH) activities in the plasma were estimated enzymatically using commercial reagent kits adapted to Olympus AU400 fully automated Clinical Chemistry Analyzer (Bayer’s Diagnostics, USA) as per the manufacturer’s instructions. The plasma total protein was estimated using commercial reagent kits adapted to Olympus AU400 fully automated Clinical Chemistry Analyzer (Bayer’s Diagnostics, USA) as per the manufacturer’s instructions.
Analysis of renal functional parameters
The biomarkers of renal injury such as: Fasting plasma urea, creatinine, uric acid were measured by Olympus AU400 fully automated Clinical Chemistry Analyzer (Bayer’s Diagnostics, USA) using commercially available kits according to the manufacturer’s instructions. Moreover, the Blood Urea Nitrogen (BUN) and BUN/creatinine ratio were calculated as follows [30]:
Blood Urea Nitrogen (BUN)=Blood urea (mg/ dL)/2.142
Statistical Analysis
Results were displayed as means ± Standard Deviation (SD). The data were analyzed statistically using Statistical Package of Social Service Windows program version 19.0 (SPSS Institute, Inc., Chicago, IL, USA) statistical packages. The One-Way Analysis of Variance (ANOVA) followed by Tukey post hoc test was used to analyze the results. A p-value of less than 0.05 (P<0.05) was considered to be significant.
Results
In-vitro anti-oxidant assay for soy isoflavones
2,2’-dipicryl 2’-phenylhydrazine (DPPH) assay: The free radical scavenging capacity of soy isoflavones was assessed by 2,2’-dipicryl 2’-phenylhydrazine (DPPH) assay. A standard graph was plotted using Gallic acid as a standard in the range of 0-100 μM/L concentration. It was found that the free radical scavenging capacity of soy isoflavones was 6.23 ± 1.04 μM/L equivalents of Gallic acid.
Effect of Soy isoflavones extract on body weight and organ weights in ovariectomized Wistar rats
In Table 1, bilateral ovariectomy in rats caused increased body weight, liver weight, kidney weight, and reduced uterus weight when compared to the sham-operated rats (control group). Treatment with soy isoflavones markedly prevented the ovariectomyinduced increase in the body weight and organ weights; liver, kidney.
| Parameters | Whole body weight (grams) |
Relative LW (g/100 g BW) | Relative KW (g/100 g BW) | Relative UW (g/100 g BW) |
|---|---|---|---|---|
| Control | 150.8 ± 7.36 | 2.24 ± 0.18 | 0.97 ± 0.08 | 0.51 ± 0.08 |
| Ovariectomy | 168.8 ± 6.13* (p 0.013) |
3.16 ± 0.22* (p 0.000) |
1.54 ± 0.10* (p 0.008) |
0.41 ± 0.04 |
| Control+SIF | 143.0 ± 9.16 | 2.17 ± 0.11 | 1.01 ± 0.21 | 0.57 ± 0.10 |
| Ovariectomy+SIF | 149.3 ± 4.37¥ (p 0.000) |
2.63 ± 0.31¥ (p 0.000) |
1.03 ± 0.08¥ (p 0.000) |
0.49 ± 0.03 |
Data were expressed as mean ± SD (n=8)
*: Comparison with control, ¥: Comparison with Ovariectomy
SIF: Soy Isoflavones; BW: Body Weight; LW: Liver Weight; KW: Kidney Weight; UW: Uterus Weight
One way analysis of variance (ANOVA) followed by Tukey post-hoc test, SPSS Version 21.0
Table 1. Soy isoflavones alleviates bilateral ovariectomy (experimental menopause) induced increment in the body weight and organ weights in female Wistar rats
Effects of soy isoflavones extract on plasma biomarkers of liver injury in ovariectomized Wistar rats
The plasma biomarkers of liver injury: Aspartate Transaminases (AST), Alanine Transaminase (ALT), Lactate Dehydrogenase (LDH), alkaline phosphatase, total bilirubin was significantly increased in response to ovariectomy. Soy isoflavones treatment markedly inhibited the increment in these plasma liver function parameters in all the experimental rats (Figures 2 and 3). In light of the above findings in the plasma, the liver tissue also displayed with the elevated levels of AST, ALT, LDH and total bilirubin in response to ovariectomy (Table 2). Soy isoflavones treatment successfully inhibited this rise (Table 2).
| Parameters | Control | Ovariectomy | Control+SIF | Ovariectomy+SIF |
|---|---|---|---|---|
| hepatic AST, IU/mg protein |
6.21 ± 0.18 | 7.89 ± 0.08* (p 0.000) |
6.14 ± 0.07 | 6.11 ± 0.24¥ (p 0.000) |
| hepatic ALT, IU/mg protein | 1.22 ± 0.03 | 2.11 ± 0.09* (p 0.031) |
1.22 ± 0.05 | 1.27 ± 0.31¥ (p 0.028) |
| hepatic LDH, IU/mg protein | 7.41 ± 1.13 | 11.14 ± 1.03* (p 0.006) |
8.01 ± 0.14 | 6.21 ± 1.07¥ (p 0.000) |
| hepatic ALP, IU/mg protein |
1.31 ± 0.02 | 2.88 ± 0.13* (p 0.000) |
1.18 ± 0.06 | 1.76 ± 0.07¥ (p 0.000) |
Data were expressed as mean ± SD (n=8)
*: Comparison with control; ¥:Comparison with Ovariectomy
SIF: Soy Isoflavones; AST: Aspartate Transaminase; ALT: Alanine Transaminase; LDH: Lactate Dehydrogenase, ALP: Alkaline Phosphatase
One way analysis of variance (ANOVA) followed by Tukey post-hoc test, SPSS Version 21.0
Table 2. Soy isoflavones ameliorates bilateral ovariectomy (experimental menopause) induced derangements in the hepatic functional parameters in the liver of female Wistar rats
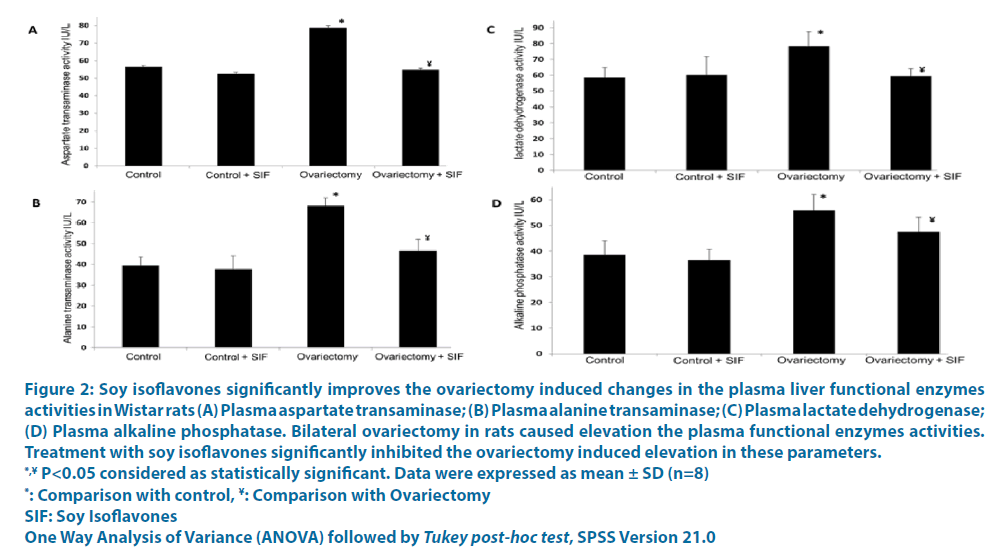
Figure 2: Soy isoflavones significantly improves the ovariectomy induced changes in the plasma liver functional enzymes
activities in Wistar rats (A) Plasma aspartate transaminase; (B) Plasma alanine transaminase; (C) Plasma lactate dehydrogenase;
(D) Plasma alkaline phosphatase. Bilateral ovariectomy in rats caused elevation the plasma functional enzymes activities.
Treatment with soy isoflavones significantly inhibited the ovariectomy induced elevation in these parameters.
*,¥ P<0.05 considered as statistically significant. Data were expressed as mean ± SD (n=8)
*: Comparison with control, ¥: Comparison with Ovariectomy
SIF: Soy Isoflavones
One Way Analysis of Variance (ANOVA) followed by Tukey post-hoc test, SPSS Version 21.0
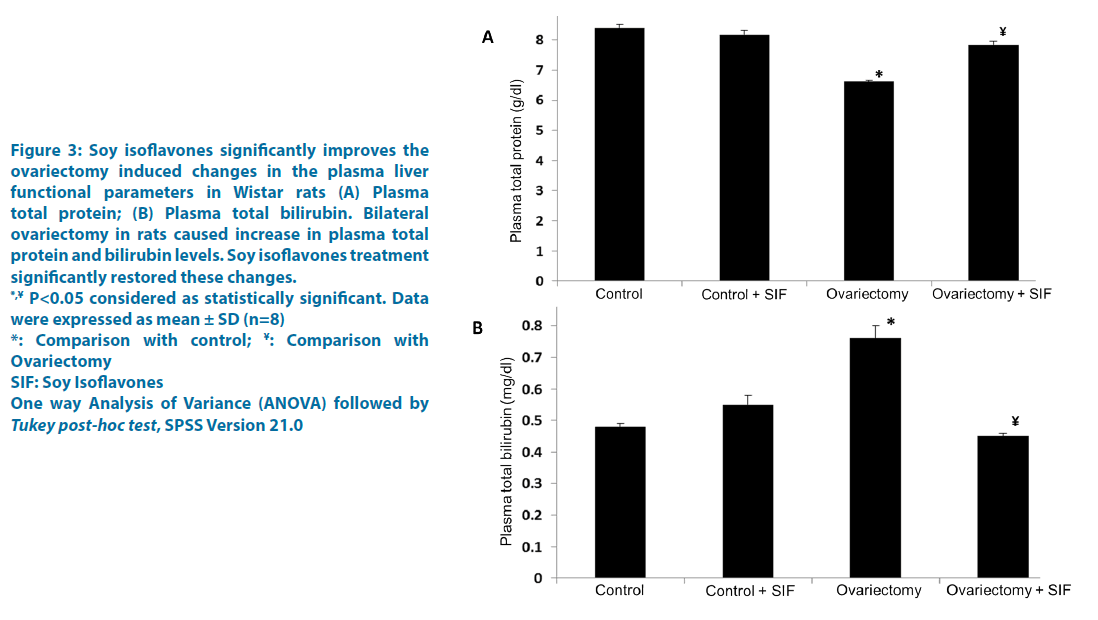
Figure 3: Soy isoflavones significantly improves the
ovariectomy induced changes in the plasma liver
functional parameters in Wistar rats (A) Plasma
total protein; (B) Plasma total bilirubin. Bilateral
ovariectomy in rats caused increase in plasma total
protein and bilirubin levels. Soy isoflavones treatment
significantly restored these changes.
*,¥ P<0.05 considered as statistically significant. Data
were expressed as mean ± SD (n=8)
*: Comparison with control; ¥: Comparison with
Ovariectomy
SIF: Soy Isoflavones
One way Analysis of Variance (ANOVA) followed by Tukey post-hoc test, SPSS Version 21.0
Effects of soy isoflavones extract on plasma biomarkers of renal injury in ovariectomized Wistar rats
The biomarkers of renal damage: Plasma urea, creatinine, and uric acid were found to be elevated in the plasma of ovariectomized rats when compared to the plasma of sham-operated control rats. Supplementation of soy isoflavones significantly inhibited the ovariectomy-induced increased in these renal parameters (Table 3). In addition, the Blood Urea Nitrogen (BUN) was found to be increased in response to ovariectomy and this increment in the calculated BUN was inhibited by soy isoflavones supplementation (Table 3).
| Parameters | Control | Ovariectomy | Control+SIF | Ovariectomy+SIF |
|---|---|---|---|---|
| Blood urea mmol/L | 5.93 ± 0.24 | 13.38 ± 0.29* (p 0.000) |
6.09 ± 0.35 | 9.53 ± 0.98¥ (p 0.000) |
| Blood Creatinine µmol/L | 26.43 ± 3.91 | 50.77 ± 4.77* (p 0.000) |
30.75 ± 1.75 | 36.50 ± 2.21¥ (p 0.002) |
| Blood Uric acid µmol/L | 185.93 ± 17.05 | 296.75 ± 23.24* (p 0.000) |
149.49 ± 58.51 | 198.58 ± 15.56¥ (p 0.000) |
| BUN mg/dL | 16.62 ± 0.67 | 37.46 ± 0.82* (p 0.000) |
17.07 ± 0.99 | 26.71 ± 2.76¥ (p 0.010) |
| BUN/Creatinine mg/dL | 56.80± 9.27 | 65.86 ± 6.91 | 49.19 ± 2.54 | 65.09 ± 9.05 |
Data were expressed as mean ± SD (n=8).
*: Comparison with control; ¥: Comparison with Ovariectomy
SIF: Soy Isoflavones; BUN: Blood Urea Nitrogen
One way analysis of variance (ANOVA) followed by Tukey post-hoc test, SPSS Version 21.0
Table 3. Soy isoflavones ameliorates bilateral ovariectomy (experimental menopause) induced derangements in the plasma renal functional parameters in female Wistar rats
Discussion
Postmenopausal women are a higher risk to develop metabolic syndrome components; oxidative stress, inflammation, insulin resistance, dyslipidemia, atherosclerosis, and cardiovascular complications when compared to the premenopausal women [31]. However, the ground for this is poorly understood. This finding forms the necessity for further research to understand the molecular basis of postmenopausal obesity complications. Through estrogen deficiency after menopause reported to accelerate metabolic syndrome complications, the derangements in the metabolic functions of liver and kidney plays a major role in the development of postmenopausal metabolic complications. Soy isoflavones are known phytoestrogens believed to possess beneficial effects against postmenopausal complications [30,31]. In the present study, we investigated the preventive effects of soy isoflavones on derangements in the metabolic activities of liver and kidney in the setting of experimental postmenopausal state.
Previous studies have reported that the postmenopausal women are more prone to liver dysfunction [32], conditions underlined by leakage of cellular enzymes: ALT, AST and LDH [33]. In the present study, the activities of these enzymes: AST, ALT, and LDH were elevated in the plasma of ovariectomized rats. This finding indicating the occurrence of liver dysfunction. This increase in the activities of these enzymes was normalized by soy isoflavones, demonstrating an attenuation effect on liver dysfunction, which is linked with the maintenance of hepatocytes membrane integrity [34]. Previously we reported the abnormal liver histology and liver inflammatory cells in response to ovariectomy and the hepatoprotective effect of soy isoflavones on the same [35-37]. From the proteomics analysis, the ovariectomy caused over-expression of pro-oxidants and pro-inflammatory proteins; NOX, MPO, HO-1, p38 MAPK, ERK 1/2, COX2, THFα, IL6 in association with suppressed expression of anti-oxidant and anti-inflammatory proteins; SOD1, Catalase, GPx, GSH in the liver [37,38]. The changes in these proteins might have caused hepatic oxidative stress, inflammatory response with the resultant liver dysfunction. Treatment with soy isoflavones significantly improves inhibited the ovariectomyinduced changes in the expression levels of these proteins and improved liver function. In the present study, we found that the soy isoflavones extract exhibited potent antioxidant property as indicated by DPPH free radical scavenging assay. The antioxidant property of soy isoflavones extract might have attributed to the hepatoprotective effects against the postmenopausal state.
Previously, we have reported that the ovariectomy in rats per se leads to the lipid metabolic derangements which ultimately leads to hypertriglyceridemia and hepatic steatosis [39]. This effect was attributed by the overexpression of lipogenic proteins, such as LXR, SREBP1, FAS, ACC, and PPARγ, in association with suppressed expression of lipolytic proteins, such as FXR, SHP, insig2 and PPARα [39]. The ovariectomy-induced hypertriglyceridemia may have caused fat accumulation in the liver leading to fatty liver. This might be reflected in the elevated plasma levels of liver function enzymes. Treatment with SIF significantly restored the lipid metabolism and improved hypertriglyceridemia and hepatic steatosis, suggesting the use of this natural phytoestrogen as an alternative strategy/remedy for relieving the lipid abnormalities concomitant with the postmenopausal population.
In addition, the surgical ovariectomy in rats caused kidney dysfunctions as evidenced by increased levels of blood urea, creatinine, uric acid, and BUN. We have previously reported the ovariectomy-induced up-regulation of renal NOX4, COX2, p38MAPD in association with down-regulation of SOD and GPx [36,38]. The overexpression of pro-oxidants proteins might have caused oxidative stress and inflammatory response leading to kidney damage. This may be reflected at plasma levels of elevated urea, creatinine, uric acid, and BUN. Treatment with soy isoflavones significantly improves these derangements and improved renal metabolic functions.
Conclusion
In the present study, we can conclude that bilateral ovariectomy (experimental menopause) in rats per se caused liver dysfunction as well as renal dysfunction. Supplementation of soy isoflavones significantly improved/reverted these dysfunctions, suggesting the use of this natural product as an alternative remedy in the management of liver and kidney metabolic dysfunctions associated with the postmenopausal state.
Conflict of Interest
We declare that we have no conflicts of interest.
Acknowledgment
The study was supported by intramural research funds sanctioned from the host institute to the corresponding author and DST INSPIRE fellowship to the first author. There was no commercial entity involved. The funding source had no role in study design, data analysis, and interpretation. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
References
- World Health Organization (WHO). Research on the menopause in the 1990s. WHO Technical Report Series, Geneva (1996).
- Kriplani A, Banerjee K. An overview of age of onset of menopause in Northern India. Maturitas 52: 199-204 (2005).
- Syamala T, Sivakami M. Menopause: An emerging issue in India. Econ Polit Wkly 4923-4930 (2005).
- Unni J. Third consensus meeting of Indian Menopause Society: A summary. J Midlife Health 1: 43-47 (2010).
- Desai S, Sinha T, Mahal A. Prevalence of hysterectomy among rural and urban women with and without insurance in Gujarat, India. Reprod Health Matters 19: 42-51 (2011).
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 346: 1221-1231 (2002).
- Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 37: 917-923 (2003).
- Gonzales CA, Bacchetti P, Khalili M. Impact of gender and menopausal status on metabolic parameters in chronic hepatitis C infection. J Viral Hepat 23: 232-239 (2016).
- Völzke H, Schwarz S, Baumeister SE, et al. Menopausal status and hepatic steatosis in a general female population. Gut 56: 594-595 (2007).
- Gutierrez-Grobe Y, Ponciano-Rodríguez G, Ramos MH, Uribe M, Méndez-Sánchez N. Prevalence of non-alcoholic fatty liver disease in premenopausal, posmenopausal and polycystic ovary syndrome women. The role of estrogens. Ann Hepatol 9: 402-409 (2010).
- McKenzie J, Fisher BM, Jaap AJ, Stanley A, Paterson K, Sattar N. Effects of HRT on liver enzyme levels in women with type 2 diabetes: A randomized placebo-controlled trial. Clin Endocrinol (Oxf) 65: 40-44 (2006).
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. J Am Med Assoc 288: 321-333 (2002).
- Lethaby A, Marjoribanks J, Kronenberg F, Roberts H, Eden J, Brown J. Phytoestrogens for menopausal vasomotor symptoms. Cochrane Database Syst Rev 12: CD001395 (2013).
- Cassidy A, Hooper L. Phytoestrogens and cardiovascular disease. J Brithish Menopause Soc 12: 49-56 (2006).
- Curran EM, Judy BM, Newton LG, et al. Dietary soy phytoestrogens and ER alpha signalling modulate interferon gamma production in response to bacterial infection. Clin Exp Immunol 135: 219-225 (2004).
- Hall WL, Vafeiadou K, Hallund J, et al. Soy-isoflavone-enriched foods and inflammatory biomarkers of cardiovascular disease risk in postmenopausal women: Interactions with genotype and equol production. Am J Clin Nutr 82: 1260-1268 (2005).
- Kagan A, Harris BR, Winkelstein W Jr, et al. Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California: Demo-graphic, physical, dietary and biochemical characteristics. J Chronic Dis 27: 345-364 (1974).
- Verdrengh M, Jonsson IM, Holmdahl R, Tarkowski. Genistein as an anti-inflammatory agent. Inflamm Res 52: 341-346 (2003).
- Badger TM, Ronis MJ, Wolff G, et al. Soy protein isolate reduces hepatosteatosis in yellow Avy/a mice without altering coat color phenotype. Exp Biol Med 233:1242-1254 (2008).
- Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem 64: 97-112 (1995).
- Kitawaki R, Nishimura Y, Takagi N, Iwasaki M, Tsuzuki K, Fukuda M. Effects of lactobacillus fermented soymilk and soy yogurt on hepatic lipid accumulation in rats fed a cholesterol-free diet. Biosci Biotechnol Biochem 73:1484-1488 (2009).
- Torre-Villalvazo I, Tovar AR, Ramos-Barragán VE, Cerbón-Cervantes MA, Torres N. Soy protein ameliorates metabolic abnormalities in liver and adipose tissue of rats fed a high fat diet. J Nutr 138: 462-468 (2008).
- Høegh-Andersen P, Tankó LB, Andersen TL, et al. Ovariectomized rats as a model of postmenopausal osteoarthritis: Validation and application. Arthritis Res Ther 6: R169 (2004).
- Hussan F, Ibraheem NG, Kamarudin TA, Shuid AN, Soelaiman IN, Othman F. Curcumin Protects against Ovariectomy-Induced Bone Changes in Rat Model. Evid-Based Complement Altern Med (2012).
- Johnston BD, Ward WE. The ovariectomised rat as a model for studying alveolar bone loss in postmenopausal women. BioMed Res Int (2015).
- Kalu DN. The ovariectomised rat model of postmenopausal bone loss. Bone Miner 15: 175-191 (1991).
- Ludgero-Correia A, Aguila MB, Mandarim-de-Lacerda CA, Faria TS. Effects of high-fat diet on plasma lipids, adiposity, and inflammatory markers in ovariectomised C57BL/6 mice. Nutrition 28: 316-323 (2012).
- Lee YB, Lee HJ, Kim KS, et al. Evaluation of the preventive effect of isoflavone extract on bone loss in ovariectomised rats. Biosci Biotechnol Biochem 68: 1040-1045 (2004).
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 28: 25-30 (1995).
- Liu Z, Zhang X, Li L, et al. Effects of lactational exposure to soy isoflavones on reproductive system in neonatal female rats. Basic Clin Pharmacol Toxicol 102: 317-324 (2008).
- Dobrek Ł, Skowron B, Baranowska A, Płoszaj K, Bądziul D, Thor P. The influence of oxazaphosphorine agents on kidney function in rats. Medicina (Kaunas) 53: 179-189 (2017).
- Eshtiaghi R, Esteghamati A, Nakhjavani M. Menopause is an independent predictor of metabolic syndrome in Iranian women. Maturitas 65: 262-266 (2010).
- Panchal SK, Poudyal H, Iyer A, et al. High-carbohydrate high-fat diet–induced metabolic syndrome and cardiovascular remodeling in rats. J Cardiovasc Pharmacol 57: 51-64 (2011).
- Poudyal H, Campbell F, Brown L. Olive leaf extract attenuates cardiac, hepatic, and metabolic changes in high carbohydrate-, high fat-fed rats. J Nutr 140: 946-953 (2010).
- Abdelrazek H, Mahmoud M, Tag HM, Greish SM, Eltamany DA, Soliman MT. Soy isoflavones ameliorate metabolic and immunological alterations of ovariectomy in female Wistar rats: Antioxidant and Estrogen Sparing Potential. Oxid Med Cell Longev (2019).
- Srinivasan M, Sudheer AR, Menon VP. Ferulic Acid: Therapeutic potential through its antioxidant property. J Clin Biochem Nutr 40: 92-100 (2007).
- Sankar P, Zachariah B, Vickneshwaran V, Jacob SE, Sridhar MG. Amelioration of oxidative stress and insulin resistance by soy isoflavones (from Glycine max) in ovariectomized Wistar rats fed with high fat diet: The molecular mechanisms. Exp Gerontol 63: 67-75 (2015).
- Panneerselvam S, Packirisamy RM, Bobby Z, Sridhar MG (2016) Protective effect of soy isoflavones (from Glycine max) on adipose tissue oxidative stress and inflammatory response in an experimental model of post-menopausal obesity: The molecular mechanisms. Biochem Anal Biochem 5: 2161-1009 (2016).
- Panneerselvam S, Packirisamy RM, Bobby Z, Jacob SE, Sridhar MG. Soy isoflavones (Glycine max) ameliorate hypertriglyceridemia and hepatic steatosis in high fat-fed ovariectomized Wistar rats (an experimental model of postmenopausal obesity). J Nutr Biochem 38: 57-69 (2016).