Special Report - Imaging in Medicine (2010) Volume 2, Issue 2
SPECT/CT in differentiated thyroid carcinoma
Torsten Kuwert† & Daniela SchmidtAuthor for correspondence: Clinic of Nuclear Medicine, Krankenhausstr. 12, 91054 Erlangen, Germany
- Corresponding Author:
- Torsten Kuwert
Clinic of Nuclear Medicine
Krankenhausstr. 12, 91054 Erlangen, Germany
Tel: +49 131 853 3411
Fax: +49 131 853 9262
E-mail: torsten.kuwert@ uk-erlangen.de
Abstract
Scintigraphic imaging of the distribution of isotopes of radioiodine is frequently performed for staging patients with differentiated thyroid carcinoma. Planar g‑camera imaging, as well as SPECT, visualize foci of radioiodine accumulation with high sensitivity. However, owing to only poor visualization of the morphology by these techniques, their diagnostic accuracy is limited. This limitation is overcome when hybrid systems integrating a SPECT camera with a x‑ray CT scanner are used. Recent evidence has demonstrated that approximately a third of patients with diagnostically unclear foci of radioiodine accumulation will benefit from the use of SPECT/CT, in terms of therapeutic management. Therefore, SPECT/CT is rapidly evolving as a new clinical tool in differentiated thyroid carcinoma.
Keywords
CT ▪ hybrid imaging ▪ radioiodine ▪ scintigraphy ▪ SPECT ▪ thyroid ▪ thyroid cancer
Neoplasms arising in the thyroid gland may be classified according to their cell of origin and differentiation (for reviews and current guidelines of management see [1–3]). Whereas medullary thyroid carcinomas (MTCs) derive from the calcitonin-producing C cells, the differentiated thyroid carcinomas (DTCs) stem from the cells synthesizing the thyroid hormones, the so-called thyrocytes. Malignant transformation of the thyrocytes is also thought to account for the anaplastic DTCs (ATCs). DTCs are tenfold more frequent than MTCs; ATCs make up only 1% of all thyroid neoplasms.
The incidence of a DTC approximates to one in 10,000 people. According to their histological appearances, DTCs can be subdivided into the papillary and follicular type, together with some rarer entities, such as the oncocytic variant. DTCs usually have retained some properties of their mother cells, such as the ability to produce the protein thyroglobulin (TG) or to accumulate iodine. Their proliferation rate is, in general, comparatively slow. Nevertheless, dedifferentiation of DTCs may occur, leading to a loss of their radioiodine-accumulating capacity and to faster growth.
New guidelines for treatment and followup of DTCs have recently been published by the American Thyroid Association [3]. Surgical removal of the primary, as well as of metastases, is the first treatment option in all DTCs. In all patients except those with low-stage papillary DTC, total thyroidectomy is mandatory. Following surgery, radioablation of thyroid remnants using the radioactive iodine isotope 131I is usually performed, at least in patients with higher tumor stages or in whom metastases are suspected. In DTCs, 131I may also be used to destroy tumor deposits later in the course of the disease. Radiation therapy and chemotherapy only have a palliative role in thyroid neoplasms. The therapeutic potential of small-molecule inhibitors of signal transduction cascades is just being explored (for a review, see [4]).
After thyroidectomy and radioablation, DTC patients are followed-up by cervical ultrasound and by measurement of the serum value of TG. TG is secreted into the bloodstream by normal and neoplastic thyroid cells and is a highly sensitive tumor marker for DTCs, in particular after removal of the thyroid gland. High serum levels of thyroid stimulating hormone (TSH) increase TG secretion, so that TG measurement is more sensitive for the detection of tumor tissue in hypothyreosis or after intramuscular injection of recombinant human TSH [5]. Furthermore, radioiodine scans using diagnostic doses of radioiodine are also part of the routine follow-up as will be described in more detail later in this article. X-ray CT as well as MRI of the neck are not usually performed in DTC patient follow-up owing to their limited accuracy in differentiating benign from malignant cervical lymph nodes and scar tissue from local recurrences. As in other tumors, these modalities are, nevertheless, indicated to diagnose distant metastases (e.g., in the lung or the skeleton).
The mean survival of DTC patients is longer than that of subjects afflicted by most other cancers [6]. As in other tumors, higher tumor stage as well as the presence of distant and/or regional metastases are associated with a worsening of prognosis [6]. In addition, the loss of differentiated features and, in particular, that of the capacity of iodine accumulation may complicate the course of disease. Accurate information on these prognostic variables early after diagnosis is essential to individually plan patient management since patients at high risk of recurrence require more intensive follow-up than those at low risk [3].
Use of radioiodine in thyroid carcinomas
Iodine is a building block of the thyroid hormones. Thyroid tissue, therefore, has the capacity to concentrate this element. The molecule mediating this accumulation is a protein cotransporting iodine and sodium, the sodium iodide symporter (NIS) [7]. NIS is also expressed by DTCs, but usually not by ATCs or MTCs. However, NIS expression in DTCs may vary as dedifferentiation of these tumors reduces its density in tumor tissue [8].
Expression of NIS is regulated by TSH via the action of the adenylate cyclase and protein kinase A [9]. Therefore, all procedures using radioactive isotopes of iodine for diagnosis and treatment of DTC are performed at high TSH levels. This may be obtained by leaving DTC patients off medication with thyroid hormone after total thyroidectomy or by intramuscular injection of recombinant human TSH prior to administration of the radiopharmaceutical.
In dedifferentiated DTCs, MTCs and most cases of ATCs, tumor deposits may fail to concentrate radioiodine, owing to a lack of NIS. This is also regularly observed in the oncocytic, so-called Hürthle’s variant of DTC. In these cases, radioiodine scintigraphy is false-negative and radiopharmaceuticals other than radioactive isotopes of radioiodine have to be used to localize metastases in patients suspected of recurrence (e.g., in the instance of elevated tumor markers). Cationic lipophilic tracers labeled by 99mTc, such as 99mTc-sestaMIBI and 99mTc-tetrofosmin, have proven valuable in this regard [10]. PET imaging of glucose metabolism or – in MTCs – of dopamine uptake may be also be tried for this purpose [11,12].
Several radioactive isotopes of iodine emit radiation suitable for medical purposes and, in particular, scintigraphy: 124I is a positron emitter and may thus be used in PET. Although promising clinical results have been obtained with this tracer [13], the comparatively high costs of PET limit its widespread use. 123I is a pure g-emitter. Its 160 keV photons are eminently suitable for conventional γ-camera imaging including SPECT [14]. Owing to the high energy of the 364 keV photons emitted by 131I, the quality of γ-camera images of 131I distribution is inferior to those depicting 123I uptake. 123I is, however, more expensive than 131I, which is the most frequently used isotope of iodine in nuclear medicine. 131I is the only isotope of iodine currently used for therapy. Its radioactive decay also produces electrons, the energy of which is deposited within 1 mm of their emergence within the tissue concentrating this radiopharmaceutical.
In DTC, there are two therapeutic indications for applying 131I [3]. First, 131I is used directly following total thyroidectomy to eliminate remaining non-neoplastic tissue left by the surgeon. The rationale for the radioablation of these benign thyroid remnants is that TG is secreted both by normal thyroid tissue and DTC recurrences. It can, therefore, only be used as tumor marker when normal thyroid tissue has been completely removed. Furthermore, radioiodine uptake in the neck can only be reliably interpreted as due to local recurrence in the absence of normal thyroid tissue. In DTC patients at high and intermediate risk of recurrence, there is no doubt regarding the necessity of radioablation. However, some controversy exists as to its performance in subjects at low risk of relapse (for a discussion see [15,16]). The second indication for the therapeutic use of 131I is the treatment of radioiodine-accumulating metastases.
After therapeutic application of 131I, its distribution in the human body is usually visualized by scintigraphy in order to gain information on the metastatic spread of the tumor. 131I may also be given at lower doses to the patient, for diagnostic purposes only (for a review see [3]). This is usually performed 6–12 months following radioablation to evaluate its success, regularly in the follow-up of patients at high risk of recurrence, and when during follow-up the suspicion of recurrence arises (e.g., at high or rising serum TG levels). In patients with low risk of recurrence and without evidence of relapse, radioiodine scintigraphy is not indicated; some controversy exists with regard to its role in the follow-up of patients with intermediate risk.
With radioiodine scintigraphy, DTC tissue is identified by its ability to accumulate radioiodine. Radioiodine is not only accumulated by tissue of thyroid origin (for a review see [17]). The salivary glands, the mucosa of the mouth, pharynx and stomach, the lactating breast as well as the hyperplastic thymus also express NIS. Furthermore, radioiodine is excreted via the kidneys and the liver. Therefore, the ureters, the bladder and the intestines may also exhibit radioiodine uptake. False-negative results of radioiodine imaging may, therefore, occur when the foci of radioiodine accumulation may be falsely attributed to uptake in one of the organs physiologically concentrating radioiodine (see also Table 1). This is the rule in scintigraphic images obtained at radioablation of thyroid remnants performed directly after thyroidectomy. On these images, radioiodine uptake in cervical lymph node metastases (LNM) usually cannot be reliably distinguished from that in benign thyroid remnants. A further cause of false-negative findings on radioiodine scans is the absence of the NIS in thyroid tumor tissue as already mentioned earlier.
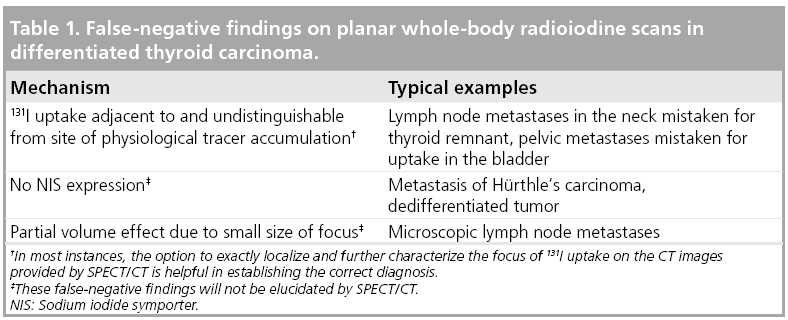
Owing to these false negatives, the sensitivity of planar radioiodine scanning for detecting DTC metastases is somewhat limited and has been reported to range between 45 and 75%, when using an elevation of the serum TG value as gold standard (for a review see [18]). However, radioiodine-positive, but TG-negative, metastases have been reported in the literature [19,20], so the validity of this gold standard could be questioned. The sensitivity of the radioiodine scan increases with the dose of radioiodine administered.
False-positive results of radioiodine imaging may occur when radioiodine uptake in the organs physiologically concentrating radioiodine is mistaken for tumor deposits (see also Table 2). This may, in particular, be the case when these exhibit anatomical variants or anomalies caused by disease. Typical examples of this mechanism are renal cysts or Meckel’s diverticula. Rarely, radioiodine uptake may also be found in tumors of nonthyroid origin or in inflammatory foci. Therefore, these may also account for false-positive findings.
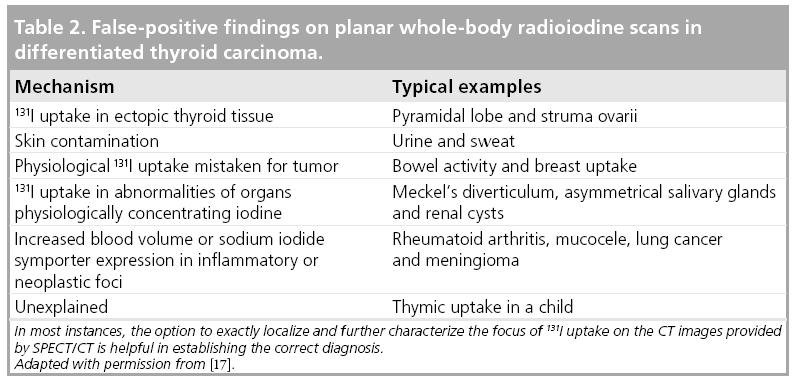
Publications reporting the specificity of planar radioiodine scanning for staging DTC are scarce since it is difficult to establish an independent gold standard in representative groups of patients. Nevertheless, specificity is believed to be higher than sensitivity [18].
SPECT/CT in DTC
Scintigraphic images of the distribution of radioiodine in the human body are poor in anatomical landmarks. This leads to problems in their interpretation. These may be overcome when registrating the molecular maps of NIS expression pixel-wise to image datasets from imaging modalities better suited to visualize morphology than radioiodine scanning, such as CT or MRI. Obviously, this is only possible when the distribution of radioiodine within the patient’s body is represented in 3D. In the case of 131I scintigraphy, this is achieved by using SPECT. Earlier attempts at image registration had to rely on software- based techniques to align independently acquired image datasets. Yamamoto et al. used this approach and reported a significant increase in diagnostic accuracy in DTC [21].
The anatomical accuracy of software-based registration of independently acquired image datasets is limited by the scarcity of common anatomical landmarks between 131I-SPECT and CT/MRI. Differences in patient positioning may also contribute to its lack in precision. A further disadvantage of software-based fusion is its high logistical demand: two examinations have to be scheduled instead of just one and the independently acquired image datasets have to be brought together in one viewing console for their joint interpretation.
These limitations can be overcome by hybrid cameras that integrate a nuclear medical detector unit with a CT or MRI scanner in one gantry. With these types of systems, the two datasets are acquired directly after each other, with the patient in the same position on the examination bed.
In 1999, the first systems combining a dual-headed SPECT camera with a low-dose nonspiral CT scanner in one gantry became commercially available. This was followed by the development of hybrid cameras equipped by multislice spiral-CT devices in 2004. Currently, an array of SPECT/CT systems incorporating CT scanners of nearly every performance level have entered the marketplace (for reviews see [22,23]). The clinical value of SPECT/CT in general has recently been reviewed [24–26]. Furthermore, a pictorial review on the value of SPECT/CT and PET/CT in thyroid carcinoma has also been published [27].
SPECT/CT allows the exact localization of foci of radioiodine uptake and thus, has the potential to differentiate reliably between uptake in metastases of thyroid cancer and that in organs physiologically accumulating this tracer.
In 2004, the first evidence on the clinical value of SPECT/CT in DTC was published: Tharp and coworkers reported a 57% increment in diagnostic accuracy of 131I-SPECT/CT compared with planar imaging in a group of 71 DTC patients from two institutions, from whom the whole, 96-image datasets had been obtained [28]. The authors were unclear on how the patients were selected. The group studied was mixed, including 17 patients examined with diagnostic doses of radioiodine and 54 subjects studied after radioiodine therapy, either given for ablation of thyroid remnants after thyroidectomy or for the treatment of metastases. The superiority of SPECT/CT over planar imaging was analyzed with regard to focus characterization and localization. The study comprised foci in the neck as well as those localized outside the neck, and reported an increment of diagnostic accuracy regardless of focus localization or treatment-related timing of the study.
Since 2004, a series of case reports have highlighted the role of 131I-SPECT/CT in a variety of clinical settings [29–36].
Currently, since 2004, nine full papers on the incremental value of 131I-SPECT/CT in DTC compared with planar imaging have appeared [37–45], besides the one mentioned previously [28]. Details of their design as well as the number of patients included are given in Table 3.
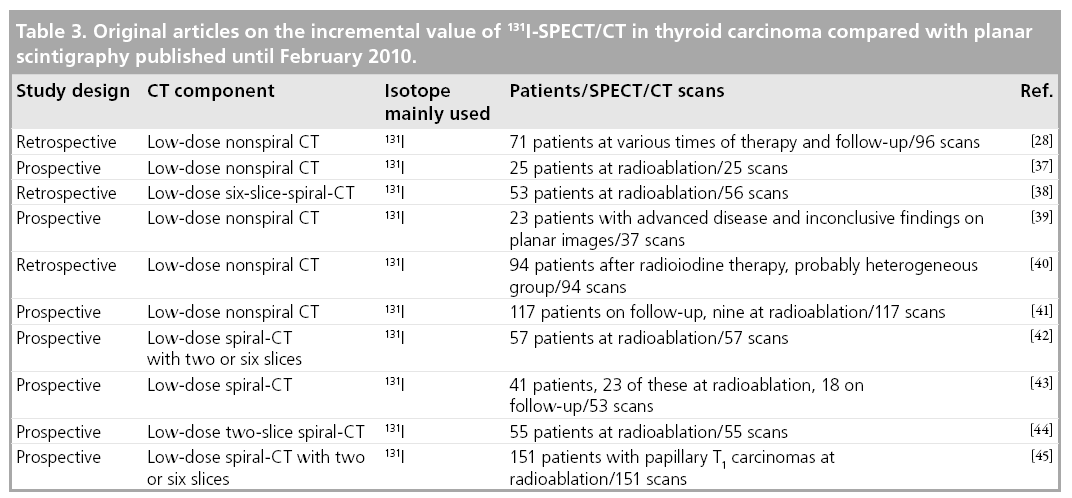
Two of these papers report heterogeneous patient groups, analogous to Tharp and coworkers. They confirm the initial results of Tharp and coworkers, with the percent increment in diagnostic accuracy ranging between 12.7 and 63.6%, depending on the site studied and the diagnostic setting [40,43]. As a result of SPECT/CT, treatment was reported to have changed in 24.4 and 23.4% of the patients studied, respectively.
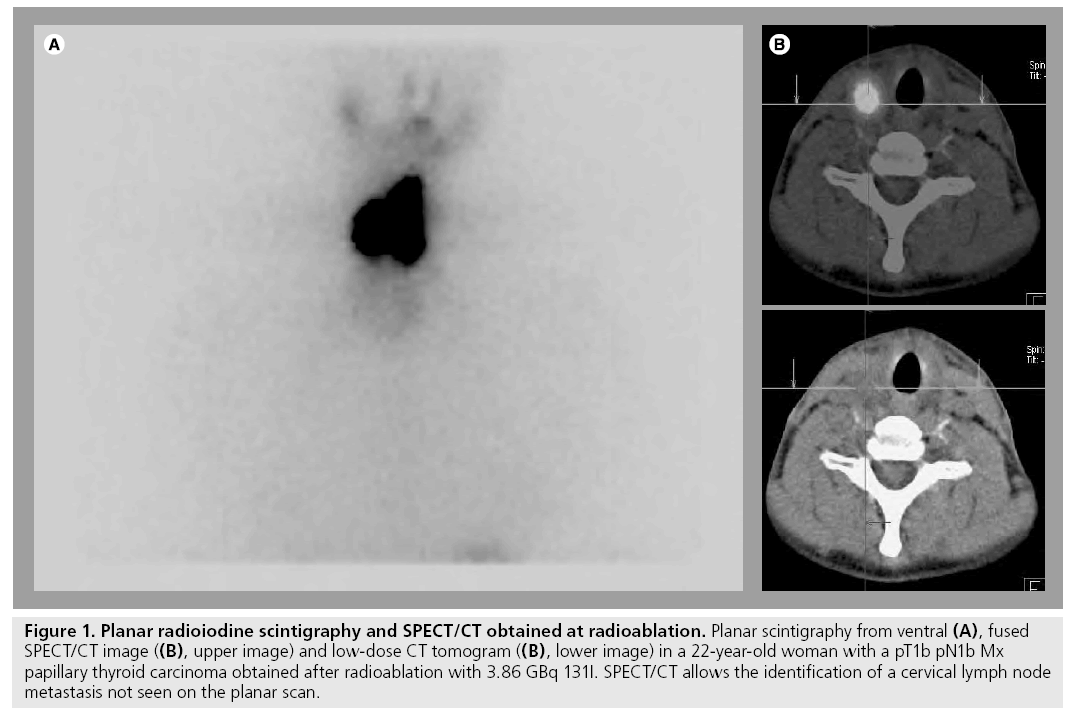
To date, the majority of the evidence available has addressed the potential role of 131I-SPECT/ CT in patients studied at radioablation [37,38,42,44,45]. With the exception of the paper by Wong et al. [38], the patients included in these studies were examined following oral application of a therapeutic dose of 131I. These publications reported that lesion-related increment in diagnostic accuracy varies between 31 and 47.6%. The benefit of SPECT/CT in this patient group is mainly due to its ability to better localize cervical foci of radioiodine uptake with regard to the thyroid bed. Thus, SPECT/CT enables a differentiation between thyroid remnants and LNM that is not possible on planar images owing to a lack of anatomical landmarks (Figure 1). However, there are two limitations of SPECT/CT in this regard: LNM in the central compartment of the neck may falsely be interpreted as thyroid remnants since the SPECT/CT criterion used in all studies for the diagnosis of lymph node metastases is their location outside this compartment, central foci of uptake are usually attributed to thyroid remnants. Furthermore, microscopic disease may also escape detection by hybrid imaging.
Figure 1: Planar radioiodine scintigraphy and SPECT/CT obtained at radioablation. Planar scintigraphy from ventral (A), fused SPECT/CT image ((B), upper image) and low-dose CT tomogram ((B), lower image) in a 22-year-old woman with a pT1b pN1b Mx papillary thyroid carcinoma obtained after radioablation with 3.86 GBq 131I. SPECT/CT allows the identification of a cervical lymph node metastasis not seen on the planar scan.
Common to all studies on the value of SPECT/ CT performed at radioablation is the lack of an independent gold standard. Techniques of structural imaging are not accurate in this clinical setting for two reasons: directly after thyroid surgery, reactively enlarged lymph nodes may be present; and LNM are frequently small and may measure well below 1 cm in diameter so that they elude detection by these techniques. Reoperation of the neck is not usually indicated directly after radioablation since the latter may eliminate the metastases in the months to come, thus making additional surgery superfluous. Clearly, the performance of neck dissections is not feasible for purely scientific purposes. To date, therefore, all papers published lack comparison to histopathology.
The impact of SPECT/CT on patient management was also analyzed in two of the previously cited papers. Schmidt et al. demonstrated that SPECT/CT leads to a revision of nodal stage in roughly 25% of all DTC patients studied [42]. In the meantime, their data were reproduced in a more homogeneous and larger group of 151 patients with a T1 papillary carcinoma pooled from two institutions [45], including 96 patients affected by microcarcinomas. Since lymph node involvement is an independent prognostic variable in papillary DTC and also in microcarcinomas, the results from SPECT/CT altered the strategy of follow-up in a quarter of DTC patients.
The considerably higher accuracy of SPECT/ CT to detect cervical LNM at radioablation opens further questions with regard to patient management. In particular, the question arises of how the LNM diagnosed by SPECT/CT should be treated. Recently, a publication reporting follow-up in patients studied by SPECT/CT at radioablation has been published. Schmidt et al. demonstrated that 18 out of 22 radioiodinepositive metastases were no longer detected 5 months following radioablation of thyroid remnants [46]. This observation argues against surgical intervention in, at least, all patients with a SPECT/CT diagnosis of LNM at radioablation. In three patients of the group studied by Schmidt et al., four radioiodine-positive LNM had persisted. The number of lesions included in this study is definitely too small to identify the variables governing the effect of radioiodine given for radioablation of thyroid remnants on LNM. Nevertheless, it should be noted that 17 out of 18 LNM eliminated by radioiodine were smaller than 0.9 ml, whereas this was the case of only one of the persisting foci.
Only one of the 61 patients staged as negative by SPECT/CT in the study published by Schmidt et al. [45] had developed a hitherto undetected radioiodine-positive cervical filia on follow-up. Wong et al. and Aide et al. have also reported a high negative-predictive value of SPECT/CT performed at radioablation with regard to tumor persistence and therapy success in their subjects [38,44]. These data suggest that SPECT/CT may also be used to stratify patients with regard to risk of recurrence or tumor persistence, thus allowing better tailoring of follow-up.
SPECT/CT was also reported to be of considerable benefit at follow-up of DTC patients (i.e., several months following radioablation): the incremental diagnostic value was 67.8 and 73.9%, respectively, in the two studies that predominantly addressed this clinical setting [39,41].
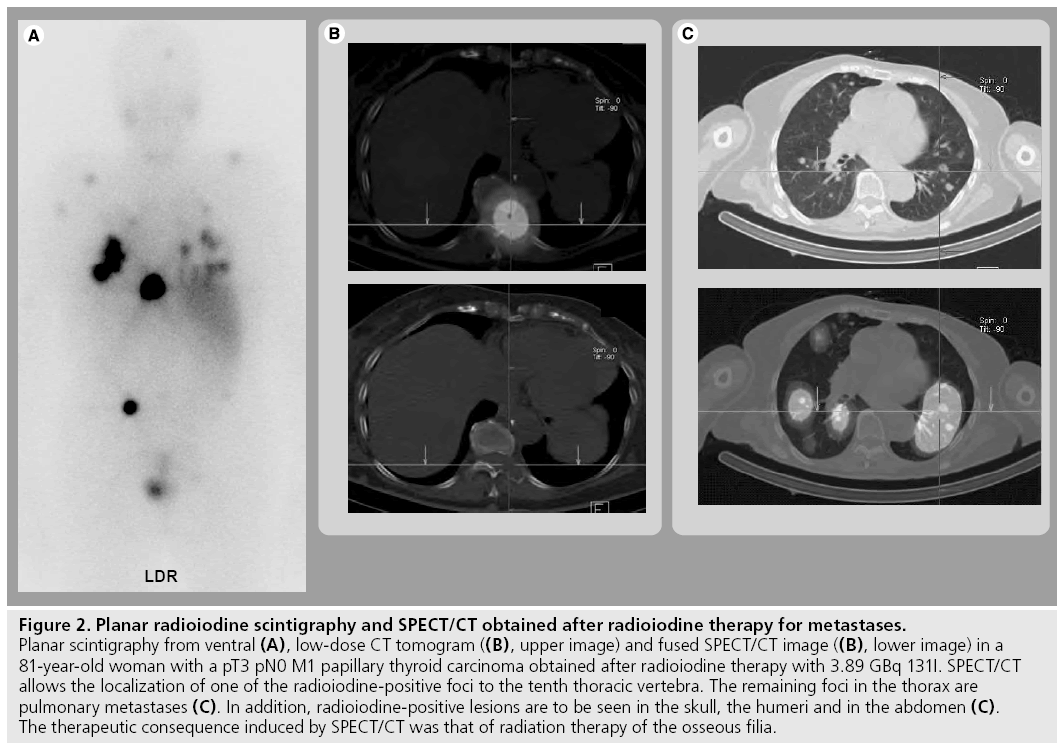
On a patient basis, this improvement in diagnostic accuracy led to a change in therapeutic strategy in 47.1% of the patients with locally advanced or metastatic disease reported by Chen et al. [39]. Spanu and coworkers reported a modification in therapeutic management caused by SPECT/CT in 35.6% of the cases with SPECT/ CT proof of malignant lesions and of 20.3% of the patients with a SPECT/CT diagnosis of benign disease [41]. Figure 2 gives an example of a patient studied by SPECT/CT to localize distant metastases of DTC and illustrates the potential of this hybrid imaging technology on follow-up.
Figure 2: Planar radioiodine scintigraphy and SPECT/CT obtained after radioiodine therapy for metastases. Planar scintigraphy from ventral (A), low-dose CT tomogram ((B), upper image) and fused SPECT/CT image ((B), lower image) in a 81-year-old woman with a pT3 pN0 M1 papillary thyroid carcinoma obtained after radioiodine therapy with 3.89 GBq 131I. SPECT/CT allows the localization of one of the radioiodine-positive foci to the tenth thoracic vertebra. The remaining foci in the thorax are pulmonary metastases (C). In addition, radioiodine-positive lesions are to be seen in the skull, the humeri and in the abdomen (C). The therapeutic consequence induced by SPECT/CT was that of radiation therapy of the osseous filia.
Potential value of SPECT/CT for dosimetry of radioiodine therapy
SPECT/CT offers the option of attenuation correction of SPECT images [47]. Furthermore, the CT information on the size of a lesion of interest could also be used to correct SPECT images for partial volume artifacts. By integrating these corrections into iterative reconstruction software, quantitation of tissue radioactivity concentration in terms of absolute values seems possible and has been demonstrated in phantom measurements [48,49] and also in patients [50]. This is true at least when tracers labeled by 99mTc are used. Papers proving this assumption for the high-energy photons of 131I are, however, still missing. Nevertheless, at least two publications have already used SPECT/CT for dosimetry of radioiodine therapy [51,52]. The accuracy of this approach and its possible impact on the dosage of 131I given for therapeutic purposes remain to be investigated, but are highly interesting issues.
Conclusion
Current evidence indicates that approximately a third of patients with diagnostically unclear foci of radioiodine accumulation benefit from the use of SPECT/CT, in terms of therapeutic management. SPECT/CT is, therefore, rapidly evolving as a new clinical tool in DTCs. Future research work will have to give more insight into its exact role in management algorithms of DTCs.
Future perspective
In DTCs, radioiodine SPECT/CT is superior to planar imaging and standalone SPECT. Therefore, this hybrid imaging technology will become indispensable for treating patients afflicted by these neoplasms. In view of the presently published evidence, the performance of SPECT/CT is recommended in all patients at radioablation and in all subjects with unclear findings in planar radioiodine scans. The potential of this technology to improve the quantitative accuracy of conventional nuclear medical imaging and also dosimetry for radioiodine therapy remains to be explored in the future.
Acknowledgements
The authors acknowledge helpful discussions with Michael Uder from the Institute of Radiolog at the University of Erlangen-Nürnberg, Germany.
Financial & competing interests disclosure
T Kuwert regularly gives lectures on behalf of Siemens Medical Solutions. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Schlumberger M, Pacini F: Thyroid Tumors. Editions Nucléon, Paris, France (2003).
- Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W: European Thyroid Cancer Task Force: European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur. J. Endocrinol. 154, 787–803 (2006).
- Cooper DS, Doherty GM, Haugen BR et al.: Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19, 1167–1214 (2009).
- Santoro M, Carlomagno F: Drug insight: small-molecule inhibitors of protein kinases in the treatment of thyroid cancer. Nat. Clin. Pract. Endocrinol. Metab. 2, 42–52 (2006).
- Weintraub BD, Szkudlinski MW: Development and in vitro characterization of human recombinant thyrotropin. Thyroid 9, 447–450, 1999.
- Greene FI, Page LI, Fleming ID et al.: AJCC Cancer Staging Handbook. Springer, NY, USA (2002).
- Dai G, Levy O, Carrasco N: Cloning and characterization of the thyroid iodide transporter. Nature 379, 458–460 (1996).
- Castro MR, Bergert ER, Goellner JR, Hay ID, Morris JC: Immunohistochemical analysis of sodium iodide symporter expression in metastatic differentiated thyroid cancer: correlation with radioiodine uptake. J. Clin. Endocrinol. Metab. 86, 5627–5632 (2001).
- Bläser D, Maschauer S, Kuwert T, Prante O: In vitro studies on the signal transduction of thyroidal uptake of 18F-FDG and 131I-iodide. J. Nucl. Med. 47, 1382–1388 (2006).
- Maxon HR: Detection of residual and recurrent thyroid cancer by radionuclide imaging. Thyroid 9, 443–446 (1999).
- Dong MJ, Liu ZF, Zhao K et al.: Value of 18F-FDG-PET/PET-CT in differentiated thyroid carcinoma with radioiodine-negative whole-body scan: a meta-analysis. Nucl. Med. Commun. 30, 639–650 (2009).
- Beheshti M, Pöcher S, Vali R et al.: The value of 18F-DOPA-PET-CT in patients with medullary thyroid carcinoma: comparison with 18FDG-PET-CT. Eur. Radiol. 19, 1425–1434 (2009).
- Freudenberg LS, Antoch G, Frilling A et al.: Combined metabolic and morphologic imaging in thyroid carcinoma patients with elevated serum thyroglobulin and negative cervical ultrasonography: role of 124I-PET/CT and FDG-PET. Eur. J. Nucl. Med. Mol. Imaging 35, 950–957 (2008).
- Ali N, Sebastian C, Foley RR et al.: The management of differentiated thyroid cancer using 123I for imaging to assess the need for 131I therapy. Nucl. Med. Commun. 27, 165–169 (2006).
- Hay ID, McDougall IR, Sisson JC: Perspective: the case against radioiodine remnant ablation in patients with welldifferentiated thyroid carcinoma. J. Nucl. Med. 49, 1395–1397 (2008).
- Verburg FA, Dietlein M, Lassmann M, Luster M, Reiners C: Why radioiodine remnant ablation is right for most patients with differentiated thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 36, 343–346 (2009).
- Shapiro B, Rufini V, Jarwan A et al.: Artifacts, anatomical and physiological variants, and unrelated diseases that might cause false-positive whole-body 131I scans in patients with thyroid cancer. Semin. Nucl. Med. 30, 115–132 (2000).
- Lind P, Kohlfürst S: Respective roles of thyroglobulin, radioiodine imaging, and positron emission tomography in the assessment of thyroid cancer. Semin. Nucl. Med. 36, 194–205, 2006.
- Ma C, Kuang A, Xie J, Ma T: Possible explanations for patients with discordant findings of serum thyroglobulin and 131I whole-body scanning. J. Nucl. Med. 46, 1473–1480 (2005).
- Caballero-Calabuig E, Cano-Terol C, Sopena-Monforte R et al.: Influence of the thyroid remnant in the elevation of the serum thyroglobulin after thyroidectomy in differentiated thyroid carcinoma. Importance of the diagnostic iodine total-body scanning. Eur. J. Nucl. Med. Mol. Imaging 35, 1449–1456 (2008).
- Yamamoto Y, Nishiyama Y, Monden T et al.: Clinical usefulness of fusion of 131I SPECT and CT images in patients with differentiated thyroid carcinoma. J. Nucl. Med. 44, 1905–1910 (2003).
- Seo Y, Mari C, Hasegawa BH: Technological development and advances in single-photon emission computed tomography/computed tomography. Semin. Nucl. Med. 38, 177–198 (2008).
- Patton JA, Townsend DW, Hutton BF: Hybrid imaging technology: from dreams and visions to clinical devices. Semin. Nucl. Med. 39, 247–263 (2009).
- Even-Sapir E, Keidar Z, Bar-Shalom R: Hybrid imaging (SPECT/CT and PET/CT) – improving the diagnostic accuracy of functional/metabolic and anatomic imaging. Semin. Nucl. Med. 39, 264–275 (2009).
- Bockisch A, Freudenberg LS, Schmidt D, Kuwert T: Hybrid imaging by SPECT/CT and PET/CT: proven outcomes in cancer imaging. Semin. Nucl. Med. 39, 276–289 (2009).
- Mariani G, Bruselli L, Kuwert T et al.: A review on the clinical uses of SPECT/CT. Eur. J. Nucl. Med. Mol. Imaging (2010) (In Press).
- Wong KK, Zarzhevsky N, Cahill JM, Frey KA, Avram AM: Hybrid SPECT-CT and PET-CT imaging of differentiated thyroid carcinoma. Br. J. Radiol. 82, 860–876 (2009).
- Tharp K, Israel O, Hausmann J et al.: Impact of 131I-SPECT/CT images obtained with an integrated system in the follow-up of patients with thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 31, 1435–1442 (2004).
- Aide N, Lehembre E, Gervais R, Bardet S: Unusual intratracheal metastasis of differentiated thyroid cancer accurately depicted by SPECT/CT acquisition after radioiodine ablation. Thyroid 17, 1305–1306 (2007).
- Macdonald W, Armstrong J: Benign struma ovarii in a patient with invasive papillary thyroid cancer: detection with I-131 SPECT-CT. Clin. Nucl. Med. 32, 380–382 (2007).
- Rachinsky I, Driedger A: Iodine-131 uptake in a menstruating uterus: value of SPECT/CT in distinguishing benign and metastatic iodine-positive lesions. Thyroid 17, 901–902 (2007).
- von Falck C, Beer G, Gratz KF, Galanski M: Renal metastases from follicular thyroid cancer on SPECT/CT. Clin. Nucl. Med. 32, 751–752 (2007).
- Wong KK, Avram AM: Posttherapy I-131 thymic uptake demonstrated with SPECT/CT in a young girl with papillary thyroid carcinoma. Thyroid 18, 919–920 (2008).
- Agriantonis DJ, Hall L, Wilson MA: Utility of SPECT/CT as an adjuct to planar whole body I-131 imaging: liver metastasis from papilary thyroid cancer. Clin. Nucl. Med. 34, 247–248 (2009).
- Qiu Z, Luo Q: Erector spinae metastases from differentiated thyroid cancer identified by I-131 SPECT/CT. Clin. Nucl. Med. 34, 137–140 (2009).
- Thust S, Fernando R, Barwick T, Mohan H, Clarke SEM: SPECT/CT identification of post-radioactive iodine treatmet false-positive uptake in a simple renal cyst. Thyroid 19, 75–76 (2009).
- Ruf J, Lehmkuhl L, Bertram H et al.: Impact of SPECT and integrated low-dose CT after radioiodine therapy on the management of patients with thyroid carcinoma. Nucl. Med. Commun. 25, 1177–1182 (2004).
- Wong KK, Zarzhevsky N, Cahill JM, Frey KA, Avram AM: Incremental value of diagnostic 131I SPECT/CT fusion imaging in the evaluation of differentiated thyroid carcinoma. AJR Am. J. Roentgenol. 191, 1785–1794 (2008).
- Chen L, Luo Q, Shen Y et al.: Incremental value of 131I SPECT/CT in the management of patients with differentiated thyroid carcinoma. J. Nucl. Med. 49, 1952–1957 (2008).
- Wang H, Fu H-L, Li J-N, Zou R-J, Gu Z-H, Wu J-C: The role of single-photon emission computed tomography/computed tomography for precise localization of metastases in patients with differentiated thyroid cancer. Clin. Imaging 33, 49–54 (2009).
- Spanu A, Solinas ME, Chessa F, Sanna D, Nuvoli S, Madeddu G: 131I SPECT/CT in the follow-up of differentiated thyroid carcinoma: incremental value versus planar imaging. J. Nucl. Med. 50, 184–190 (2009).
- Schmidt D, Szikszai A, Linke R, Bautz W, Kuwert T: Impact of 131I SPECT/spiral CT on nodal staging of differentiated thyroid carcinoma at the first radioablation. J. Nucl. Med. 50, 18–23 (2009).
- Kohlfuerst S, Igerc I, Lobnig M et al.: Post-therapeutic 131I SPECT-CT offers high diagnostic accuracy when the findings on conventional planar imaging are inconclusive and allows a tailored patient treatment regimen.Eur. J. Nucl. Med. Mol. Imaging 36, 886–893 (2009).
- Aide N, Heutte N, Rame J-P et al.: Clinical relevance of single-photon emission computed tomography/computed tomography of the neck and thorax in postablation 131I scintigraphy for thyroid cancer. J. Clin. Endocrinol. Metab. 94, 2075–2084 (2009).
- Mustafa M, Kuwert T, Weber K et al.: Regional lymph node involvement in T1 papillary thyroid carcinoma: a bicentric prospective SPECT/CT study. Eur. J. Nucl. Med. Mol. Imaging (2010) (In Press).
- Schmidt D, Linke R, Uder M, Kuwert T: Follow-up of patients with differentiated thyroid cancer examined by 131I SPECT/CT of the neck at the first radioablation. Eur. J. Nucl. Med. Mol. Imaging (2009) (Epub ahead of print).
- Schulz V, Nömayr A, Hornegger J et al.: Effect of CT-based attenuation correction on uptake ratios in skeletal SPECT. Nuklearmedizin 46, 36–42 (2007).
- Vandervoort E, Celler A, Harrop R: Implementation of an iterative scatter correction, the influence of attenuation map quality and their effect on absolute quantitation in SPECT. Phys. Med. Biol. 52, 1527–1545 (2007).
- Shcherbinin S, Celler A, Belhocine T, Vanderwerf R, Driedger A: Accuracy of quantitative reconstructions in SPECT/CT imaging. Phys. Med. Biol. 53, 4595–4604 (2008).
- Zeintl J, Vija AH, Yahil A, Hornegger J, Kuwert T: Quantitative accuracy of clinical Tc-99m SPECT/CT using OSEM with 3D resolution recovery, attenuation, and scatter correction. J. Nucl. Med. (2010) (In Press).
- Song H, He B, Prideaux A et al.: Lung dosimetry for radioiodine treatment planning in the case of diffuse lung metastases. J. Nucl. Med. 47, 1985–1994 (2006).
- Sisson JC, Dewaraja YK, Wizauer EJ, Giordano TJ, Avram AM: Thyroid carcinoma metastasis to skull with infringement of the brain: treatment with radioiodine. Thyroid 19, 297–303 (2009).
• A comprehensive textbook on all aspects of thyroid carcinomas and highly recommended as a reference text.
• Gives valuable insight into the biological background of thyroid carcinoma imaging.
• Comprehensive article on radioiodine scintigraphy in thyroid carcinoma, systematically discussing, in particular, falsepositive findings of this imaging method.
• Gives valuable insight into the technical and historical background of SPECT/CT.
• Introduces the concept of nodal staging of thyroid carcinoma by SPECT/CT performed at radioablation.
• Presents results from a well-conducted prospective study on the value of SPECT/CT performed at radioablation for thyroid carcinoma.
• Reports the largest consecutive series of differentiated thyroid carcinoma patients studied by SPECT/CT to date. It shows, in particular, that SPECT/CT is able to identify cervical lymph node metastases in a significant proportion of patients with low-stage differentiated thyroid carcinoma.
• First article to demonstrate that SPECT/CT allows the quantification of regional concentration of radioactivity in absolute terms in humans.