Review Article - Interventional Cardiology (2014) Volume 6, Issue 1
Standardizing care in congenital heart disease: approaches in the catheterization laboratory
- Corresponding Author:
- Lisa Bergersen
Department of Cardiology,
Boston Children’s Hospital & Department of Pediatrics,
Harvard Medical School Boston, MA 02115, USA
Tel: +1 617 355 6529
Fax: +1 617 713 3808
E-mail: lisa.bergersen@cardio.chboston.org
Abstract
The recognition that practice variation can lead to higher resource utilization and negatively affect the delivery of care has led to concerted efforts to standardize care across all subspecialties in medicine. The ultimate goal of this effort is to improve patient safety and clinical outcomes. Examples of standardization tools include clinical practice guidelines, algorithms of care, templated electronic medical records, and surgical checklists. Unfortunately, for rare conditions, evidence to support ‘best clinical practice’ is often nonexistent, and clinical decision-making can become idiosyncratic.Keywords
benchmarks, catheterization, Congenital Cardiac Catheterization Project on Outcomes–C3PO, IMPACT, outcomes, SCAMP, standardization
The recognition that practice variation can lead to higher resource utilization and negatively affect the delivery of care [1–3] has led to concerted efforts to standardize care across all subspecialties in medicine. The ultimate goal of this effort is to improve patient safety and clinical outcomes. Examples of standardization tools include clinical practice guidelines, algorithms of care, templated electronic medical records, and surgical checklists [2,4–10]. Unfortunately, for rare conditions, evidence to support ‘best clinical practice’ is often nonexistent, and clinical decision-making can become idiosyncratic [11].
Efforts to standardize care in the pediatric cardiac catheterization laboratory have been limited, and are met with significant challenges inherent to any subspecialty that deals with rare diseases. However, over the past several years, important steps have been taken to start to address these challenges. In this article, we present a review of this experience and the initial efforts to standardize care in the pediatric cardiac catheterization laboratory.
Establishing benchmarks, standardizing terminology & the use of registries in interventional pediatric cardiology
One major challenge to the standardization of care in the pediatric cardiac catheterization laboratory is that the diseases treated are relatively rare and there is a wide spectrum of disease. Therefore, cardiac catheterization in pediatrics and for adults with congenital heart disease encompasses a broad range of procedures, some of which are performed infrequently. This makes it difficult for a single center to accumulate enough patients to produce data that is powerful enough to provide guidance in the standardization process.
One approach to address this issue has been the use of networks and registries that involve multiple institutions. In fact, these efforts have been very much a part of the field of pediatric cardiology from the early days. Multi-institutional studies in the 1960s and 1970s contributed enormously to improve our understanding of the natural history of congenital heart disease [12,13]. Despite these and similar efforts, most contemporary diagnostic and treatment strategies for pediatric patients with cardiovascular disease and adult patients with congenital heart disease are not supported by evidence from clinical trials, but instead are based on expert opinion, single institution observational studies, or extrapolated from adult cardiovascular medicine.
Recognizing these challenges, the National Heart, Lung and Blood Institute established the Pediatric Heart Disease Clinical Research Network (PHN) in 2001. The PHN is a cooperative network of seven clinical centers and one data coordinating center, with the mission of conducting and disseminating research leading to evidence-based treatment options for pediatric patients with congenital and acquired heart disease [14]. Significant contributions to the field of pediatric cardiology have already been made by the PHN. Although the studies directly related to transcatheter therapies from the PHN have been limited so far, the potential for important contributions from this approach are encouraging. Over the years, registries dedicated specifically to transcatheter interventions in patients with congenital heart disease have been instrumental in the development of this subspecialty and have contributed greatly to the development of transcatheter therapies for adult and pediatric patients with congenital heart disease.
▪ The Valvuloplasty & Angioplasty of Congenital Anomalies Registry
In 1983, soon after the initial reports of the use of balloon dilation to treat various congenital cardiac lesions, the Valvuloplasty and Angioplasty of Congenital Anomalies Registry was formed. The stated goal of this registry was to aid in the ‘proper, expedient and safe development’ of the different procedures [15]. The Valvuloplasty and Angioplasty of Congenital Anomalies Registry initially included seven centers and quickly expanded to include 27 centers across several countries. The goal of the registry was to collect data regarding hemodynamic, morbidity, and mortality outcomes. It did not, however, provide any guidelines concerning indications, equipment or technique for the various dilation procedures. The initial experience was published in 1990 and included common procedures such as pulmonary and aortic balloon valvuloplasties [16,17], balloon angioplasty for native and recurrent coarctation of the aorta [18,19], and branch pulmonary artery stenosis [20], as well as less common miscellaneous lesions [21]. Even though it provided important information that was used to ‘fine tune’ the technical aspects of the procedures as the initial experience developed, standardization of indications for intervention or of the technical aspects of each procedure was not part of the process and, therefore, the individual biases of each institution involved limited the widespread applicability of the data. The registry data also helped define technical success, but no widely accepted definition of procedural success has been validated prospectively with other registry data. Despite these limitations, the Valvuloplasty and Angioplasty of Congenital Anomalies Registry provided valuable data that helped shape the field of congenital cardiac interventions, including defining the safety and efficacy of each of the balloon dilation procedures performed in the 1980s. Since that time, there have been significant changes in technology and technique in the field of interventional cardiology, and the spectrum of interventions performed has expanded.
Pediatric & adult congenital cardiac catheterization registries in the current era
More recently, several multi-institutional registries have emerged with the goal of assessing outcomes after cardiac catheterization in pediatric and adult patients with congenital heart disease. The hope is to be able to use the data derived from these registries to develop benchmarks that will provide the framework for quality improvement efforts in the field of congenital interventional cardiology. These data may also provide better tools for assessing the efficacy and safety of catheterization procedures, and may provide evidence on which to base their standardization.
One major focus of pediatric cardiac catheterization registries has been procedural safety. In order to establish benchmarks or expected outcomes, and to be able to compare the safety of the procedure across different institutions and even between different operators, it is necessary to use the same methodology and terminology when reporting adverse events. It is also necessary to be able to adjust for case mix difference when performing comparisons between or within institutions. This type of risk adjustment has been used successfully for many years in the fields of adult coronary intervention [22] and pediatric cardiac surgery [23–25]. Comparatively, such efforts in pediatric interventional cardiology are in their early stages. There are currently several groups prospectively gathering the type of data necessary to allow such analysis, including the Congenital Cardiac Catheterization Project on Outcomes–C3PO [26], the Mid-Atlantic Group of Interventional Cardiology [27], and the Congenital Cardiovascular Interventional Study Consortium [28].
▪ The Congenital Cardiac Catheterization Project on Outcomes–C3PO & the assessment of risk-adjusted outcomes
The Congenital Cardiac Catheterization Project on Outcomes–C3PO is a project that sought to collect data in a uniform manner on all catheterization procedures performed at multiple institutions, including patient and procedural characteristics, as well as the occurrence of adverse events. One of the goals of this project was to provide a risk-adjusted outcome assessment that accounts for case mix differences between institutions and practitioners [26].
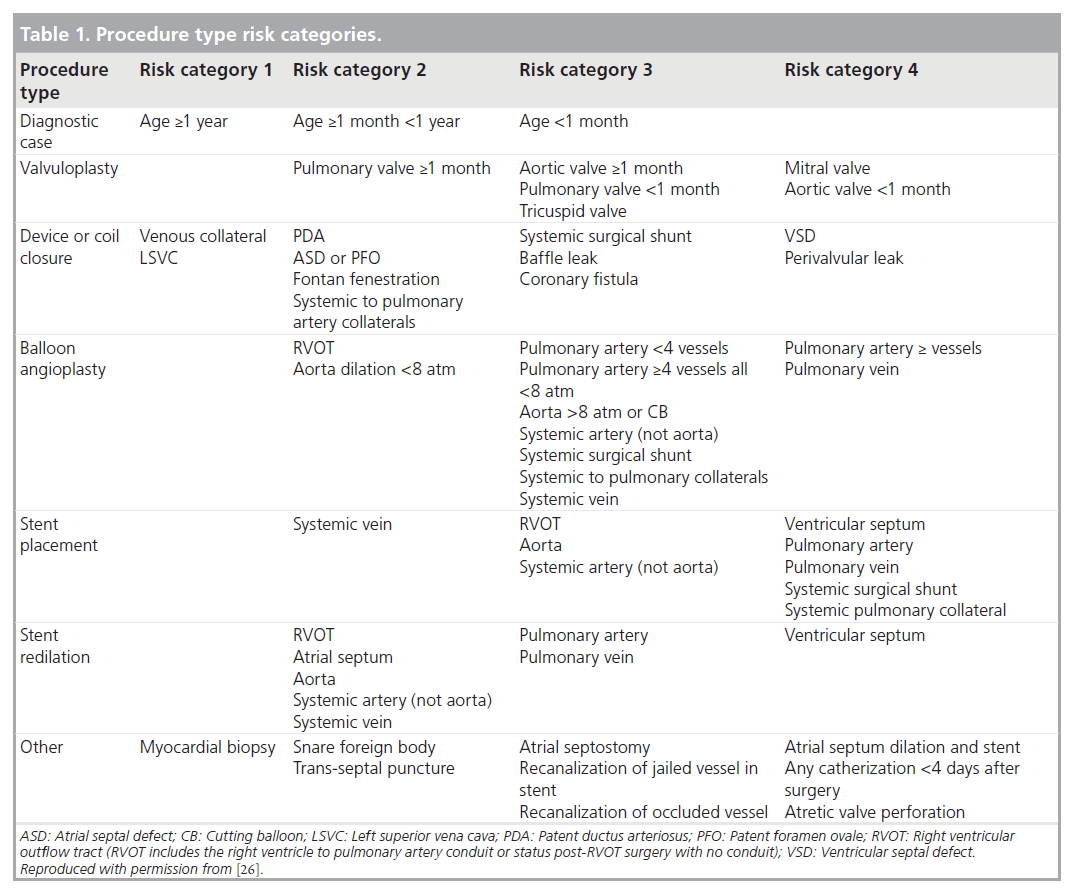
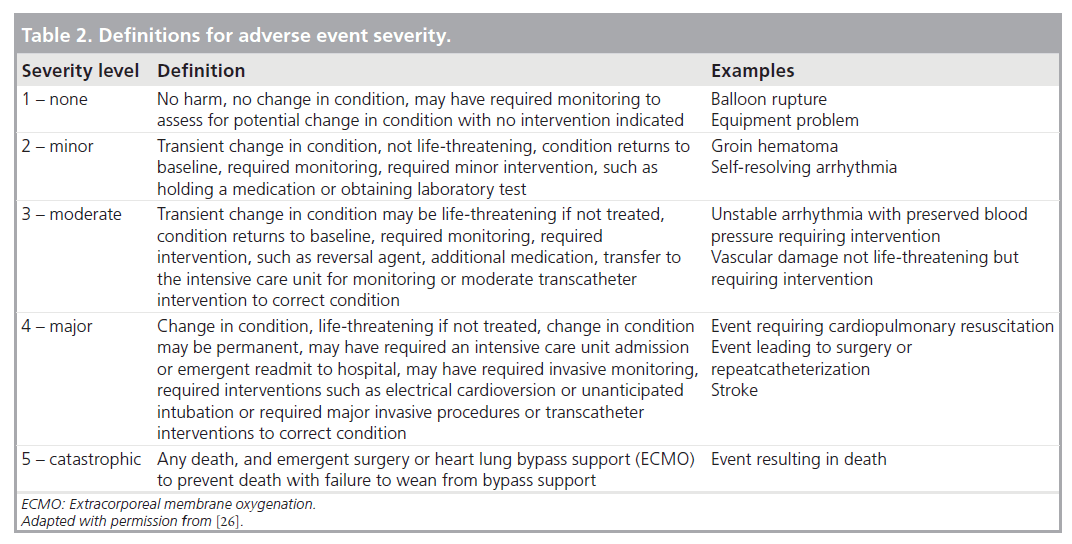
To account for procedural diversity, procedure type risk categories were developed, initially using expert consensus, then modifying the categories based on empirical methods (Table 1) [29]. Adverse events are categorized using previously established and tested definitions for adverse event severity ranging from levels 1–5 (Table 2), levels 3–5 being considered clinically important higher severity adverse events [30]. The methodology used in this registry is also unique in that it includes independent event review and validation, as well as audits to ensure event capture and completeness. Data collection started in 2007, and is recorded prospectively using a web-based data entry tool.
Using data collected in this registry, Bergersen et al. developed the catheterization for congenital heart disease adjustment for risk method, which is used to adjust adverse event rates for case mix complexity [26]. This adjustment, based on procedure type risk category, specific hemodynamic features, and the age of the patient, allows for equitable comparisons of adverse event rates among the institutions performing catheterization in pediatric and adult patients with congenital heart disease [26].
As this method is improved and undergoes further refinement based on future data, it will be an essential building block for the foundation that will be required to standardize care in the catheterization laboratory.
▪ The IMPACT Registry: improving pediatric & adult congenital treatments
Recognizing the importance of the data provided by the above mentioned registries, and recognizing their limitations in size and lack of ability to cross-communicate between the different systems, the American College of Cardiology Foundation has recently created the IMPACT Registry [31]. This national level registry created in collaboration with the Society for Cardiovascular Angiography and Interventions and under the auspices of the National Cardiovascular Data Registry, seeks to leverage expertise in the existing congenital cardiac disease registries and develop collaborations with the goal of improving the measurement of performance and outcomes. Its goal is to assess the prevalence, demographics, management, and outcomes of patients with congenital cardiac disease who are undergoing diagnostic catheterization and catheterbased intervention, and use this data to facilitate performance measurement, benchmarking, and quality improvement initiatives.
Early experience with standardization of care in a congenital cardiac catheterization laboratory
So far, formal efforts to standardize care in the pediatric cardiac catheterization laboratory have been limited. One example is the use of a Standardized Clinical Assessment and Management Plan (SCAMP). In 2008, SCAMPs were introduced into our academic center-based, pediatric cardiology practice as a quality improvement initiative. Each SCAMP targets a relatively heterogeneous patient population with a single underlying diagnosis or chief complaint, standardizing assessment and management through an iterative data collection and analysis process [32–36]. Early SCAMPs focused on outpatients [33,36], but as the tool matured and its utility became more evident, we tested the feasibility of procedural SCAMPs. So far, there have been three SCAMPs incorporated into practice that relate to the catheterization laboratory. Two of them focus on the outpatient evaluation of patients and the indications to refer the patient for catheterization, and one is a purely procedural SCAMP designed to aid in the intraprocedural decision-making process.
▪ Standardizing the indications for cardiac catheterization in patients with congenital heart disease
One major limitation to retrospective research pertaining to catheter-based interventions for congenital heart disease is that most of the interventions don’t have clear cut and widely accepted ‘thresholds’ for intervention. The indications for referring a patient to the catheterization laboratory for an intervention are highly variable from one center to the other and even within centers, from one clinician to another. Most of the existing parameters that are used to decide when to refer a patient are therefore arbitrary, and many times based on personal experience or opinion and not necessarily evidence-based. In an effort to standardize the referral process for certain congenital heart defects, we created SCAMPs for the evaluation of patients with an isolated patent ductus arteriosus (PDA) and for patients with isolated congenital aortic stenosis (AS). A brief summary of each is provided below.
Isolated PDA scamp
A PDA is estimated to account for approximately 10% of all congenital heart defects [37] and clinical consequences can vary considerably depending on the size of the PDA. The potential adverse effects of an untreated hemodynamically significant PDA are well established, and in some early series, especially those published prior to the advent of antibiotic therapy for infective endocarditis, life expectancy for the patient with a PDA was quoted as half that for an unaffected patient of the same age and cardiac anatomy [37]. Most investigators are in agreement that moderate or large PDAs, especially those with hemodynamic significance or associated infective endocarditis, should be closed. Management of the very small or clinically silent PDA is more controversial [38–43].
Based on a comprehensive review of the literature, and estimates of risk derived from those data, McElhinney et al. concluded that there is no evidence to support a superior risk:benefit balance for routine closure of the very small, hemodynamically insignif icant PDA [44]. Accordingly, they concluded that it would be difficult to justify closure of such defects simply to reduce the risk of infectious endocarditis and its complications [44]. Largely based on this analysis, a SCAMP to guide decisionmaking around indications for PDA closure in patients with an isolated PDA was created. This SCAMP is designed to include all patients over 1 year of age, with an isolated PDA on echocardiogram with no other congenital heart disease (other than bicuspid aortic valve). Patients with a history of known or suspected pulmonary arterial hypertension, history of endocarditis, documented arrhythmias, cardiomyopathy or genetic syndromes associated with development of cardiomyopathy, and patients with greater than mild left ventricular dysfunction (ejection fraction: ≤40%) are excluded. The decisionsupport diagram for this SCAMP is designed to recommend referral for closure in patients that have a hemodynamically significant PDA and patients that have an anatomically moderate or large size PDA. If there are no findings to suggest a hemodynamic load, and the PDA is small, observation only is recommended.
This SCAMP is an example of standardizing the indications for a transcatheter intervention. As with other SCAMPs, the ultimate decision is left to the clinician, but if the final decision differs from the SCAMP recommendation, this deviation and the reasons for it are documented, with the objective of learning about the decision-making process. Over time, if trends are identified or new information is revealed, the SCAMP recommendations could be changed based on these deviations. Although it is too early in the process to draw any conclusions from the available data, the hope is that this SCAMP will represent an example of how by standardizing the indications for a procedure with the goal of reducing the risk to the patient, resource utilization may be optimized.
Congential AS SCAMP
Another example of using the SCAMP methodology to standardize the indications for catheterization is the congenital AS SCAMP. The goal of this SCAMP is to guide management decisions in patients ≥6 months of age with AS (defined as echocardiographic gradient ≥15 mmHg) as the dominant hemodynamic disorder. Patients with echocardiographic gradients (defined as the maximum instantaneous gradient from the apical view or the mean gradient from the suprasternal notch view) <55 mmHg are monitored clinically at different intervals. The follow-up intervals depend on the patient’s age, left ventricular function, and degree of stenosis. Much of the focus of this SCAMP centers around identifying specific markers that can help stratify the rate of progression of AS, which is beyond the scope of this review. In patients with an echocardiographic gradient of 55 mmHg or higher, a cardiac catheterization is recommended. The goal is to reduce the number of unnecessary catheterizations, and to ensure that patients that require an intervention receive it in a timely fashion.
▪ Standardization of cardiac catheterization procedures for congenital heart disease
The balloon aortic valvuloplasty (BAV) for congenital AS SCAMP is the first purely catheterization- based procedural SCAMP. It is designed to aid in the intraprocedural decision-making process during a BAV procedure, with the goal of improving the efficacy and safety of the procedure.
The safety and efficacy of BAV for AS have been established over the last 30 years [45–60]. BAV has since become the first-line of therapy for congenital AS in neonates, children and young adults in most centers [46–50,52,54,55,57–59]. Although much has been learned about the technical aspects of BAV, and over time the procedure has become safer and more efficacious [54], little is known about how alternative intraprocedural management decisions affect long-term outcomes after the procedure.
The American Heart Association has released a scientific statement providing recommendations for the indications for BAV in congenital AS [61]. These recommendations are based on data from the Natural History studies, which showed that patients with initial catheterization-obtained gradients across the aortic valve of ≥50 mmHg were at increased risk of serious arrhythmias and possibly sudden death. Therefore, the recommendations are to perform BAV in patients with congenital AS with a catheter-obtained resting peak systolic gradient of ≥50 mmHg for asymptomatic patients, and ≥40 mmHg for patients with symptoms of angina or syncope or ischemic ST-T wave changes on electrocardiography at rest or with exercise. Consideration is also given to clinical scenarios where the patient is considering pregnancy or strenuous athletic activities. Even though intervention is not recommended for asymptomatic patients with gradients ≤40 mmHg, the Natural History studies did suggest that patients with gradients between 25 and 49 mmHg were also at increased risk of serious ventricular arrhythmia. Based on this observation, and as the procedure has become safer over the last two decades, our practice has evolved towards intervening for gradients <50 mmHg. This change in practice was incorporated into the SCAMP. The design of the SCAMP was also aimed at maximizing freedom from aortic valve replacement. Long-term follow-up after BAV reveals an ongoing, steady hazard for repeat interventions, including aortic valve replacement (AVR) [62]. It is clear that the reintervention-free survival of patients undergoing BAV depends largely on what balance of residual AS and aortic regurgitation (AR) is achieved with BAV. Patients leaving the catheterization laboratory with a lower gradient and less AR have a longer freedom from reintervention. However, there is no widely accepted definition of what constitutes acute procedural success after BAV, and different investigators have used different definitions over the years [48,52,55,58,59].
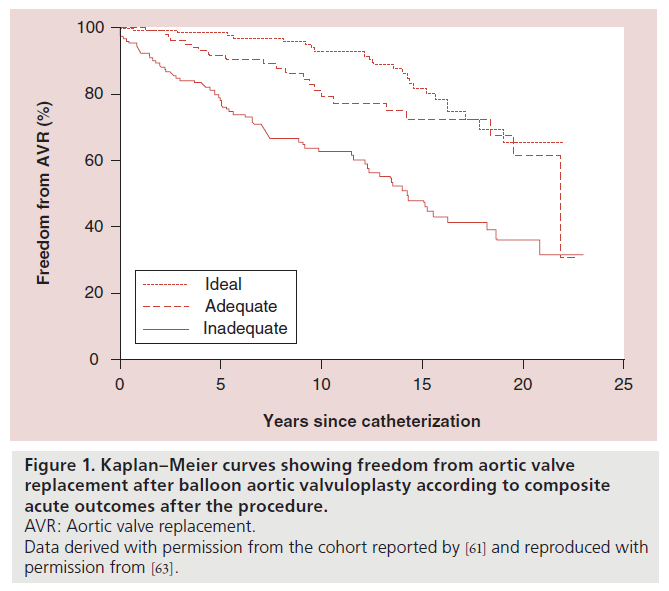
For the purposes of the SCAMP, we wanted to create a definition of acute success that would correlate with long-term outcomes after BAV, based on the available data in the literature. The largest single center experience that we could find that attempted to correlate acute outcomes with intermediate and long-term outcomes was reported by Brown et al. and included 509 patients that underwent BAV at Boston Children’s Hospital (MA, USA) between 1985 and 2008, with a median follow-up of 9.3 years [62]. In order to elucidate the interaction between the degree of residual AS and postdilation AR, as they affect referral for AVR, they modeled combinations of residual AS gradient and AR grade at the end of the catheterization procedure. Their analysis suggested that AS reduction during BAV might have greater impact than minimizing AR, with regard to delaying aortic valve surgery [62]. Based on these data, the BAV for AS SCAMP prioritized achieving a residual gradient ≤35 mmHg, while attempting to avoid moderate AR.
The acute outcomes of BAV were, therefore, classified into the following categories based on the reported post-BAV AS gradient and the angiographic assessment of post-BAV AR:
• Optimal: gradient ≤35 mmHg and trivial or no AR;
• Adequate: gradient ≤35 mmHg and mild AR;
• Inadequate: gradient >35 mmHg and/or moderate or severe AR.
This hierarchy of outcomes was based on the freedom from AVR 10 years postdilation associated with each category [62]. Freedom from AVR after BAV for the cohort reported by Brown et al. with the patients categorized into these three combined outcome categories is shown in Figure 1 [63].
Figure 1. Kaplan–Meier curves showing freedom from aortic valve
replacement after balloon aortic valvuloplasty according to composite
acute outcomes after the procedure.
AVR: Aortic valve replacement.
Data derived with permission from the cohort reported by [61] and reproduced with
permission from [63].
The SCAMP was designed specifically for patients referred for catheterization primarily for valvar AS. Therefore, several exclusion factors were identified, most of which marked patients with other left heart disease that might confound reoperation outcomes.
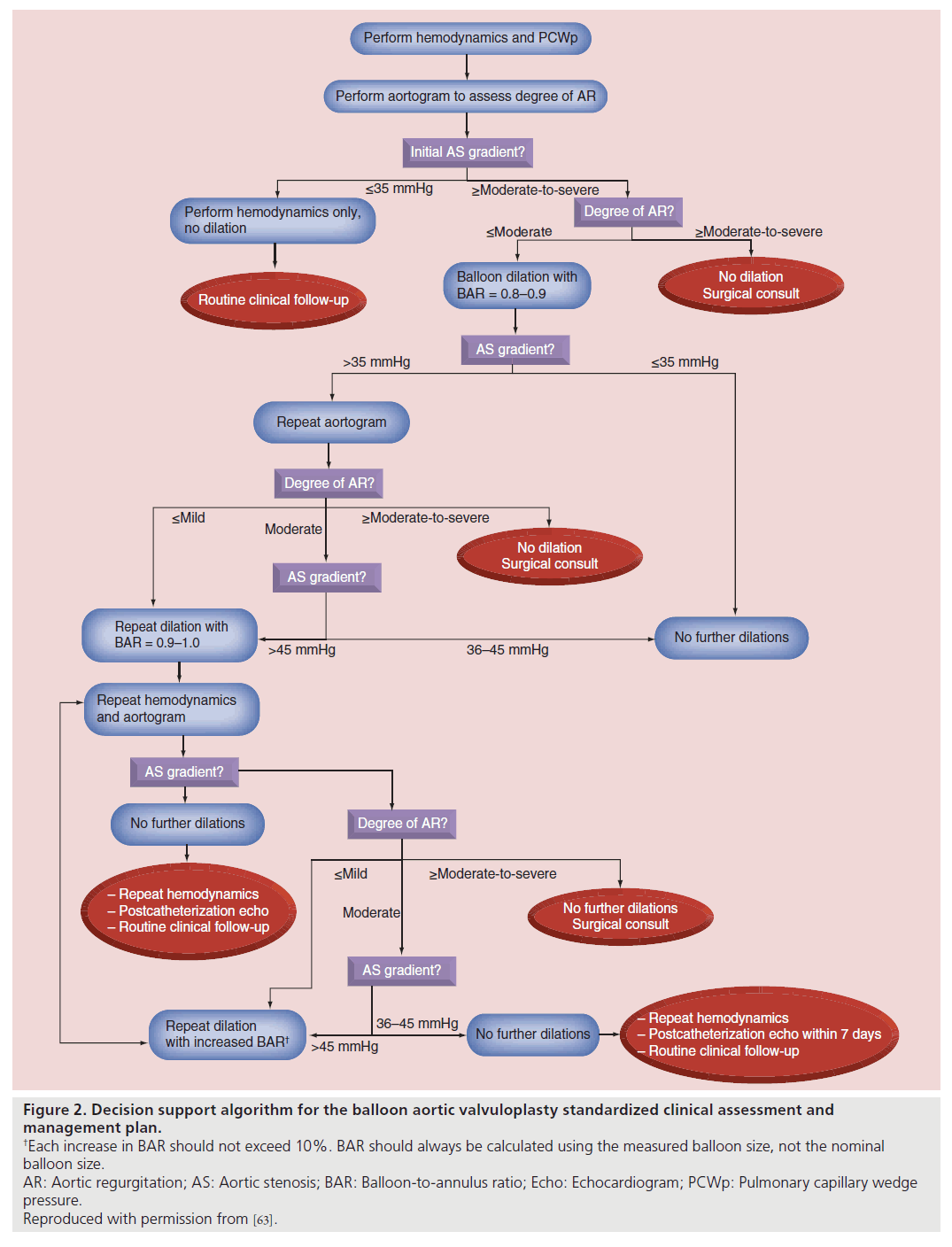
The technical details of aortic valve dilation have been described previously [48,55,59]. Our institution’s overall approach to BAV has remained largely unchanged over the last two decades. The main changes in practice incorporated into the SCAMP were: first, intervening for gradients less than 50 mmHg; second, repeated dilation to achieve residual AS gradient ≤35 mmHg, and finally, more gradual increase in the balloon to annulus ratio, increasing the measured balloon size-to-aortic valve annulus ratio (BAR) by approximately 10% with each dilation. In small children and infants, this may require taking advantage of compliance characteristics of the balloons beyond their nominal pressures to deliver specific dilating diameters.
The decision support algorithm for the SCAMP (Figure 2) provides recommendations for some of the major technical aspects of the procedure, including initial BAR, criteria for repeat dilation, incremental increase in BAR with subsequent dilations, and performance of aortic root angiograms to evaluate AR. As allowed by the design of the SCAMP, practitioners are permitted to deviate from the management recommendations, though they are asked to document the reasons for deviation to allow subsequent analysis and potential SCAMP modification.
Figure 2. Decision support algorithm for the balloon aortic valvuloplasty standardized clinical assessment and
management plan.
†Each increase in BAR should not exceed 10%. BAR should always be calculated using the measured balloon size, not the nominal
balloon size.
AR: Aortic regurgitation; AS: Aortic stenosis; BAR: Balloon-to-annulus ratio; Echo: Echocardiogram; PCWp: Pulmonary capillary wedge
pressure.
Reproduced with permission from [63].
We recently reported the results of BAV in the first 23 patients enrolled in this SCAMP, as compared with matched historical controls that underwent BAV at our institution before implementation of the SCAMP [63]. Controls were identified by a search of the Department of Cardiology database which yielded 453 patients prior to 2010 who would have met criteria for the SCAMP. From this group, and starting with the most recent patients, we performed a 4:1 match of variables that have been previously shown to affect acute procedural outcomes: age category at intervention (categories: <1 month, 1–11 months, 1–11 years and >11 years), echocardiographic AR grade before intervention (grades: none/trivial, mild, moderate or severe), AS gradient: within 15 mmHg (peak-to-peak gradient measured at catheterization), history of prior BAV, history of surgical aortic valvuloplasty, presence of left ventricular dysfunction on precatheterization echocardiogram. This design ensured that the two groups were similar in most of the categories that are known to affect acute outcomes.
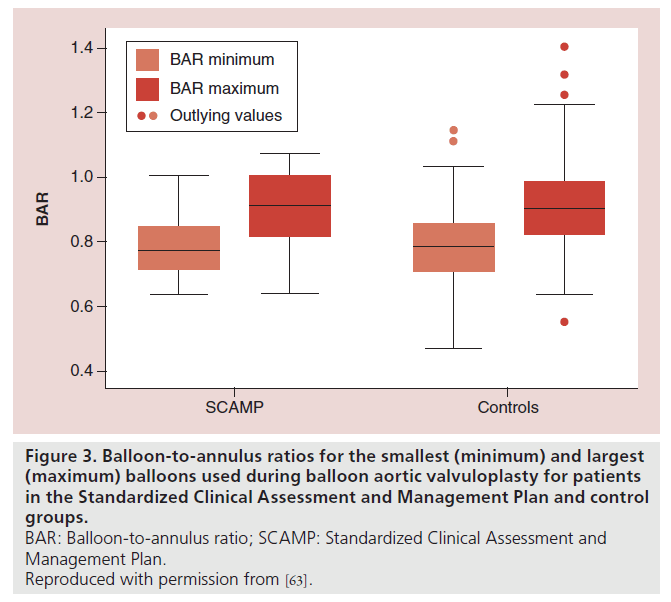
The technical aspects of the procedure did not differ significantly between the two groups. Although there was no difference in the mean of the minimum or maximum BAR in the SCAMP versus control groups, there was a trend towards less variability in the BAR in the SCAMP group (p = 0.07; Figure 3), suggesting a trend towards reduction in practice variation with use of the SCAMP.
Figure 3. Balloon-to-annulus ratios for the smallest (minimum) and largest
(maximum) balloons used during balloon aortic valvuloplasty for patients
in the Standardized Clinical Assessment and Management Plan and control
groups.
BAR: Balloon-to-annulus ratio; SCAMP: Standardized Clinical Assessment and
Management Plan.
Reproduced with permission from [63].
All 23 SCAMP patients achieved a residual AS gradient ≤35 mmHg, and the median residual AS gradient for the SCAMP group was lower (25 [10–35] mmHg) than in matched controls (30 [0–65] mmHg; p = 0.005). Importantly, the two groups did not differ with regard to degree of AR grade after BAV. This finding suggests that attempting to achieve a lower gradient using this SCAMP methodology does not necessarily result in greater AR. Compared to controls, SCAMP patients were more likely to have an optimal result, and less likely to have an inadequate result (52 vs 34% and 17 vs 45%, respectively; p = 0.02).
Deviations from the SCAMP recommendations were identified and reviewed. Overall adherence to the SCAMP decision support algorithm was 78%. This is similar to that reported for other SCAMPs, which has varied between 70 and 92% [32,34,36]. The point of deviation in all instances had to do with either balloon size or performance of aortography. The only change in the recommendations so far has been to eliminate the initial aortogram for patients that have a precatheterization echocardiogram that showed trivial or no AR. This initial angiogram was eliminated in an effort to reduce the contrast load and radiation exposure, after the observation that no patient with trivial or no AR on precatheterization echocardiogram had an angiogram that showed more significant AR before BAV.
Despite encouraging early results, these data are not conclusive and have several important limitations. Many more patients will be needed to determine conclusively whether the acute outcomes are indeed improved by using the SCAMP methodology, and whether improved acute outcomes translate into better long-term outcomes for this patient population. Until we have more conclusive data, this SCAMP should not be viewed as a widely applicable model on which to base care. In fact, as we continue to learn about BAV in patients with congenital AS and new data becomes available, we expect the SCAMP to evolve and adjust in a way that reflects the practices associated with the best outcomes. Nevertheless, the BAV for congenital AS SCAMP provides an example of how standardization of care can be incorporated into the pediatric catheterization laboratory with the goals of reducing practice variation, optimizing resource utilization and, ultimately, improving patient care.
Conclusion
Standardization of processes in medicine can lead to reductions in errors and improvement in outcomes. An excellent example of this is the implementation of surgical checklists, which has been shown to decrease morbidity and mortality in surgical patients [4]. Standardized clinical care pathways for surgical procedures providing a goal-directed approach for initial assessment, procedure selection, intraoperative management, and postoperative care have been demonstrated to reduce length of stay and costs and improve perioperative outcomes [4–8,10]. Clinical practice guidelines have also been shown to standardize care, diminish local variation of practice and improve healthcare outcomes [2]. However, there are challenges to applying these approaches in situations where the evidence in which to base ‘best clinical practices’ are scant, limited or nonexistent. The spectrum of disease treated in the pediatric catheterization laboratory is wide, and each condition is relatively rare. Despite these limitations, major leaps have been achieved in the care of patients with congenital heart disease over the last 30 years. In fact, mortality for critical congenital heart disease in the USA decreased by nearly 40% between 1979 and 1997 [64].
As summarized above, several multi-institutional registries and networks have recently emerged with the goal of assessing outcomes after cardiac catheterization in pediatric and adult patients with congenital heart disease. The data derived from these efforts will allow for the development of benchmarks that will provide the framework for quality improvement efforts in the field of congenital interventional cardiology, including more widespread standardization of care.
As more data becomes available from these registries and networks, we will need better tools for the standardization of catheterization procedures. Specifically, tools that are designed to address the challenges inherent to a field that deals with a wide spectrum of relatively uncommon conditions and procedures. Tools that have worked in other subspecialties, such as clinical practice guidelines, for example, may not be the best approach in the field of interventional pediatric cardiology. The SCAMPs developed thus far have been created and specifically designed with these challenges in mind. Importantly, the implementation of SCAMPs includes a process of iterative analysis and modification, and therefore provides a mechanism for flexibility and change based on the data obtained from the SCAMP itself, as well as data that becomes available in the literature after the creation of the management algorithms [33–36].
Another unique feature of a SCAMP is that there is active collection of data on deviations from the recommended algorithm, which takes advantage of the idiosyncratic nature of clinical practice in a heterogeneous population and provides opportunities for improving patient care [32]. This is especially important in a field like pediatric and congenital interventional cardiology, in which a creative and innovative approach is sometimes necessary to obtain the best possible outcome for a specific patient, based on the intraprocedural findings. The SCAMP methodology is not meant to replace the operator’s best judgment. It is meant to provide guidance based on available data, with the implicit acknowledgment that the data will not apply to every specific situation, and therefore adjustments will be necessary.
Although it is not likely possible or practical to develop SCAMPs for all interventional procedures, we believe that this is an important tool for the standardization of care in the catheterization laboratory. As we continue to learn about the process of standardizing care in the cardiac catheterization laboratory, we hope to improve the processes and tools used for this purpose, with the ultimate goal of improving patient care and outcomes.
Future perspective
Standardization of care in the pediatric cardiac catheterization laboratory is in its early phases, and this process is faced with significant challenges common to all subspecialties that deal with relatively uncommon diseases. There have been significant strides made in the past decade that will help us face some of these challenges, but much work remains to be done. As benchmarks are developed and evidence-based data is produced over the coming years, it will be important to continue to develop tools for standardization of care based on this data. We believe that this process will be an important component of the overall effort to improve patient safety and outcomes in the pediatric cardiac catheterization laboratory in the coming years.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Establishing benchmarks, standardizing terminology & the use of registries in interventional pediatric cardiology
• The spectrum of disease in the field of pediatric cardiology is wide, and each condition is relatively rare, making assessment of individual case outcomes challenging.
• Recently, the use of networks and registries has provided adequately powered data on which to base clinical practices. These multi-institutional endeavors will facilitate the data collection that will serve as the foundation for standardizing care in the field of pediatric and congenital cardiac catheterization.
Early experience with standardization of care in a congenital cardiac catheterization laboratory
• The Standardized Clinical Assessment and Management Plan has been developed as a quality improvement initiative, specifically designed for use in a relatively heterogeneous patient population with a single underlying diagnosis, standardizing assessment and management through an iterative data collection and analysis process.
• In the catheterization laboratory, this methodology has been used to standardize the indications for interventions in patients with isolated patent ductus arteriosus and congenital aortic stenosis, as well as to standardize intrapocedural decision-making during balloon aortic valvuloplasty procedures. Even though this experience is in its very early phases, the results to date have been encouraging.
References
Papers of special note have been highlighted as:
• of interest
- Steinberg EP. Improving the quality of care – can we practice what we preach? N. Engl. J. Med. 348(26), 2681–2683 (2003).
- Steinbrook R. Guidance for guidelines. N. Engl. J. Med. 356(4), 331–333 (2007).
- Wennberg J, Gittelsohn A. Small area variations in health care delivery. Science 182(4117), 1102–1108 (1973).
- Haynes AB, Weiser TG, Berry WR et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N. Engl. J. Med. 360(5), 491–499 (2009).
- Low DE, Kunz S, Schembre D et al. Esophagectomy – it’s not just about mortality anymore: standardized perioperative clinical pathways improve outcomes in patients with esophageal cancer. J. Gastrointest. Surg. 11(11), 1395–1402; discussion 402 (2007).
- Muehling B, Schelzig H, Steffen P, Meierhenrich R, Sunder-Plassmann L, Orend KH. A prospective randomized trial comparing traditional and fast-track patient care in elective open infrarenal aneurysm repair. World J. Surg. 33(3), 577–585 (2009).
- Munitiz V, Martinez-de-Haro LF, Ortiz A, Ruiz-de-Angulo D, Pastor P, Parrilla P. Effectiveness of a written clinical pathway for enhanced recovery after transthoracic (Ivor Lewis) oesophagectomy. Br. J. Surg. 97(5), 714–718 (2010).
- Preston SR, Markar SR, Baker CR, Soon Y, Singh S, Low DE. Impact of a multidisciplinary standardized clinical pathway on perioperative outcomes in patients with oesophageal cancer. Br. J. Surg. 100(1), 105–112 (2013).
- Shelley D, Tseng TY, Matthews AG et al. Technology-driven intervention to improve hypertension outcomes in community health centers. Am. J. Manag. Care 17(12 Spec. No.), SP103–SP110 (2011).
- Wang G, Jiang ZW, Xu J et al. Fast-track rehabilitation program vs conventional care after colorectal resection: a randomized clinical trial. World J. Gastroenterol. 17(5), 671–676 (2011).
- Darst JR, Newburger JW, Resch S, Rathod RH, Lock JE. Deciding without data. Congenit. Heart Dis. 5(4), 339–342 (2010).
- Report from the joint study on the Natural History of Congenital Heart Defects. Circulation 56(Suppl. I), I1–I187 (1977).
- DuShane JW, Weidman WH. 5 congenital cardiac defects. A study of the profile and natural history. Foreword. Circulation 32(6 Suppl.), III1–III3 (1965).
- Mahony L, Sleeper LA, Anderson PA et al. The Pediatric Heart Network: a primer for the conduct of multicenter studies in children with congenital and acquired heart disease. Pediatr. Cardiol. 27(2), 191–198 (2006).
- Allen HD, Mullins CE. Results of the Valvuloplasty and Angioplasty of Congenital Anomalies Registry. Am. J. Cardiol. 65(11), 772–774 (1990).
- Stanger P, Cassidy SC, Girod DA, Kan JS, Lababidi Z, Shapiro SR. Balloon pulmonary valvuloplasty: results of the Valvuloplasty and Angioplasty of Congenital Anomalies Registry. Am. J. Cardiol. 65(11), 775–783 (1990).
- Rocchini AP, Beekman RH, Ben Shachar G, Benson L, Schwartz D, Kan JS. Balloon aortic valvuloplasty: results of the Valvuloplasty and Angioplasty of Congenital Anomalies Registry. Am. J. Cardiol. 65(11), 784–789 (1990).
- Tynan M, Finley JP, Fontes V, Hess J, Kan J. Balloon angioplasty for the treatment of native coarctation: results of Valvuloplasty and Angioplasty of Congenital Anomalies Registry. Am. J. Cardiol. 65(11), 790–792 (1990).
- Hellenbrand WE, Allen HD, Golinko RJ, Hagler DJ, Lutin W, Kan J. Balloon angioplasty for aortic recoarctation: results of Valvuloplasty and Angioplasty of Congenital Anomalies Registry. Am. J. Cardiol. 65(11), 793–797 (1990).
- Kan JS, Marvin WJ Jr, Bass JL, Muster AJ, Murphy J. Balloon angioplasty – branch pulmonary artery stenosis: results from the Valvuloplasty and Angioplasty of Congenital Anomalies Registry. Am. J. Cardiol. 65(11), 798–801 (1990).
- Mullins CE, Latson LA, Neches WH, Colvin EV, Kan J. Balloon dilation of miscellaneous lesions: results of Valvuloplasty and Angioplasty of Congenital Anomalies Registry. Am. J. Cardiol. 65(11), 802–803 (1990).
- Peterson ED, Dai D, DeLong ER et al. Contemporary mortality risk prediction for percutaneous coronary intervention: results from 588,398 procedures in the National Cardiovascular Data Registry. J. Am. Coll. Cardiol. 55(18), 1923–1932 (2010).
- Jacobs JP, Maruszewski B. Computerized outcomes analysis for congenital heart disease. Curr. Opin. Pediatr. 17(5), 586–591 (2005).
- Mavroudis C, Gevitz M, Ring WS, McIntosh CL, Schwartz M. The Society of Thoracic Surgeons National Congenital Heart Surgery Database Report: analysis of the first harvest (1994–1997). Ann. Thorac. Surg. 68(2), 601–624 (1999).
- Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI. Consensus-based method for risk adjustment for surgery for congenital heart disease. J. Thorac. Cardiovasc. Surg. 123(1), 110–118 (2002).
- Bergersen L, Gauvreau K, Foerster SR et al. Catheterization for congenital heart disease adjustment for risk method (CHARM). JACC Cardiovasc. Interv. 4(9), 1037–1046 (2011).
- Everett AD, Ringel R, Rhodes JF et al. Development of the MAGIC congenital heart disease catheterization database for interventional outcome studies. J. Interv. Cardiol. 19(2), 173–177 (2006).
- Forbes TJ, Garekar S, Amin Z et al.; Congenital Cardiovascular Interventional Study Consortium (CCISC). Procedural results and acute complications in stenting native and recurrent coarctation of the aorta in patients over 4 years of age: a multi-institutional study. Catheter. Cardiovasc. Interv. 70(2), 276–285 (2007).
- Bergersen L, Gauvreau K, Marshall A et al. Procedure-type risk categories for pediatric and congenital cardiac catheterization. Circ. Cardiovasc. Interv. 4(2), 188–194 (2011).
- Bergersen L, Marshall A, Gauvreau K et al. Adverse event rates in congenital cardiac catheterization – a multi-center experience. Catheter. Cardiovasc. Interv. 75(3), 389–400 (2010).
- Martin GR, Beekman RH, Ing FF et al. The IMPACT Registry: improving pediatric and adult congenital treatments. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 13(1), 20–25 (2010).
- Farias M, Friedman KG, Powell AJ et al. Dynamic evolution of practice guidelines: analysis of deviations from assessment and management plans. Pediatrics 130(1), 93–98 (2012).
- Friedman KG, Kane DA, Rathod RH et al. Management of pediatric chest pain using a standardized assessment and management plan. Pediatrics 128(2), 239–245 (2011).
- Friedman KG, Rathod RH, Farias M et al. Resource utilization after introduction of a standardized clinical assessment and management plan. Congenit. Heart Dis. 5(4), 374–381 (2010).
- Rathod RH, Farias M, Friedman KG et al. A novel approach to gathering and acting on relevant clinical information: SCAMPs. Congenit. Heart Dis. 5(4), 343–353 (2010).
- Verghese GR, Friedman KG, Rathod RH et al. Resource utilization reduction for evaluation of chest pain in pediatrics using a novel standardized clinical assessment and management plan (SCAMP). J. Am. Heart Assoc. doi:10.1161/JAHA.111.000349 (2012) (Epub ahead of print).
- Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. First of two parts. N. Engl. J. Med. 342(4), 256–263 (2000).
- Balzer DT, Spray TL, McMullin D, Cottingham W, Canter CE. Endarteritis associated with a clinically silent patent ductus arteriosus. Am. Heart J. 125(4), 1192–1193 (1993).
- Cosh JA. Patent ductus arteriosus; a follow-up study of 73 cases. Br. Heart J. 19(1), 13–22 (1957).
- Marquis RM, Miller HC, McCormack RJ, Matthews MB, Kitchin AH. Persistence of ductus arteriosus with left to right shunt in the older patient. Br. Heart J. 48(5), 469–484 (1982).
- Mavroudis C, Backer CL, Gevitz M. Forty-six years of patient ductus arteriosus division at Children’s Memorial Hospital of Chicago. Standards for comparison. Ann. Surg. 220(3), 402–409; discussion 409–410 (1994).
- Lloyd TR, Beekman RH 3rd. Clinically silent patent ductus arteriosus. Am. Heart J. 127(6), 1664–1665 (1994).
- Thilen U, Astrom-Olsson K. Does the risk of infective endarteritis justify routine patent ductus arteriosus closure? Eur. Heart J. 18(3), 503–506 (1997).
- Fortescue EB, Lock JE, Galvin T, McElhinney DB. To close or not to close: the very small patent ductus arteriosus. Congenit. Heart Dis. 5(4), 354–365 (2010).
- Justo RN, McCrindle BW, Benson LN, Williams WG, Freedom RM, Smallhorn JF. Aortic valve regurgitation after surgical versus percutaneous balloon valvotomy for congenital aortic valve stenosis. Am. J. Cardiol. 77(15), 1332–1338 (1996).
- Alva C, Sanchez A, David F et al. Percutaneous aortic valvoplasty in congenital aortic valvar stenosis. Cardiol. Young 12(4), 328–332 (2002).
- Balmer C, Beghetti M, Fasnacht M, Friedli B, Arbenz U. Balloon aortic valvoplasty in paediatric patients: progressive aortic regurgitation is common. Heart 90(1), 77–81 (2004).
- Egito ES, Moore P, O’Sullivan J et al. Transvascular balloon dilation for neonatal critical aortic stenosis: early and midterm results. J. Am. Coll. Cardiol. 29(2), 442–447 (1997).
- Fratz S, Gildein HP, Balling G et al. Aortic valvuloplasty in pediatric patients substantially postpones the need for aortic valve surgery: a single-center experience of 188 patients after up to 17.5 years of follow-up. Circulation 117(9), 1201–1206 (2008).
- Hawkins JA, Minich LL, Shaddy RE et al. Aortic valve repair and replacement after balloon aortic valvuloplasty in children. Ann. Thorac. Surg. 61(5), 1355–1358 (1996).
- Keane JF, Bernhard WF, Nadas AS. Aortic stenosis surgery in infancy. Circulation 52(6), 1138–1143 (1975).
- McCrindle BW. Independent predictors of immediate results of percutaneous balloon aortic valvotomy in children. Valvuloplasty and Angioplasty of Congenital Anomalies (VACA) Registry investigators. Am. J. Cardiol. 77(4), 286–293 (1996).
- McCrindle BW, Blackstone EH, Williams WG et al. Are outcomes of surgical versus transcatheter balloon valvotomy equivalent in neonatal critical aortic stenosis? Circulation 104(12 Suppl. 1), I152–I158 (2001).
- McElhinney DB, Lock JE, Keane JF, Moran AM, Colan SD. Left heart growth, function, and reintervention after balloon aortic valvuloplasty for neonatal aortic stenosis. Circulation 111(4), 451–458 (2005).
- Moore P, Egito E, Mowrey H, Perry SB, Lock JE, Keane JF. Midterm results of balloon dilation of congenital aortic stenosis: predictors of success. J. Am. Coll. Cardiol. 27(5), 1257–1263 (1996).
- Mosca RS, Iannettoni MD, Schwartz SM et al. Critical aortic stenosis in the neonate. A comparison of balloon valvuloplasty and transventricular dilation. J. Thorac. Cardiovasc. Surg. 109(1), 147–154 (1995).
- Pedra CA, Sidhu R, McCrindle BW et al. Outcomes after balloon dilation of congenital aortic stenosis in children and adolescents. Cardiol. Young 14(3), 315–321 (2004).
- Reich O, Tax P, Marek J et al. Long term results of percutaneous balloon valvoplasty of congenital aortic stenosis: independent predictors of outcome. Heart 90(1), 70–76 (2004).
- Sholler GF, Keane JF, Perry SB, Sanders SP, Lock JE. Balloon dilation of congenital aortic valve stenosis. Results and influence of technical and morphological features on outcome. Circulation 78(2), 351–360 (1988).
- Zeevi B, Keane JF, Castaneda AR, Perry SB, Lock JE. Neonatal critical valvar aortic stenosis. A comparison of surgical and balloon dilation therapy. Circulation 80(4), 831–839 (1989).
- Feltes T, Bacha E, Beekman R et al. Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the American Heart Association. Circulation 123, 2607–2652 (2011).
- Brown DW, Dipilato AE, Chong EC, Lock JE, McElhinney DB. Aortic valve reinterventions after balloon aortic valvuloplasty for congenital aortic stenosis intermediate and late follow-up. J. Am. Coll. Cardiol. 56(21), 1740–1749 (2010).
- Porras D, Brown D, Rathod R et al. Acute outcomes after introduction of a standardized clinical assessment and management plan (SCAMP) for balloon aortic valvuloplasty in congenital aortic stenosis. Congenit. Heart Dis. doi:10.1111/chd.12142 (2013) (Epub ahead of print).
- Boneva RS, Botto LD, Moore CA et al. Mortality associated with congenital heart defects in the United States: trends and racial disparities, 1979–1997. Circulation 103(19), 2376–2381 (2001).
• Analysis of the use of surgical safety checklists to reduce morbidity and mortality shows how the development of procedural checklists can be effective for standardizing care within a field.
• The catheterization for congenital heart disease adjustment for risk method is the first risk-adjustment tool in the field and is the only quality metric for cardiac catheterization endorsed by the National Quality Forum at this time.
• First example of an inclusive national registry that will help promote the development of standardization methods within the field of congenital cardiac catheterization.
• Evidence to support the use of Standardized Clinical Assessment and Management Plans in order to improve resource utilization within the field of congenital cardiac catheterization.
• Early evidence shows the use of Standardized Clinical Assessment and Management Plans as an effective tool for standardizing care of patients with congenital heart disease who may be eligible for, or who undergo, catheterization procedures.
• A Standardized Clinical Assessment and Management Plans for balloon aortic valvuloplasty resulted in optimal acute results in half of the initial 23 patients enrolled, and outcomes in this group were better than those of matched historical controls.